Abstract
The ovarian steroid hormones, estradiol and progesterone, and their nuclear receptors (estrogen receptor [ER] and progesterone receptor [PR]), are involved in breast cancer development. As ER-positive/PR-positive tumors progress, they are likely to become steroid hormone-resistant/independent, yet often retain expression of their steroid receptors. Notably, up to 40% of women with steroid receptor-positive tumors exhibit de novo resistance or eventually fail on estrogen- or ERα-blocking therapies (acquired resistance). Indeed, most of the research on this topic has centered on mechanisms of ER ‘escape’ from endocrine therapy and the design of better ER-blocking strategies; signaling pathways that mediate endocrine (i.e., anti-estrogen) resistance are also excellent therapeutic targets. However, serious consideration of PR isoforms as important drivers of early breast cancer progression and ER modulators is timely and significant. Indeed, progress has been hindered by ER-centric experimental approaches. This article will focus on defining a role for PR in breast cancer with hopes of providing a refreshing PR-focused perspective.
Keywords: breast cancer, estrogen receptor, hormone replacement therapy, mammary gland biology, progesterone receptor, protein kinases, stem cells
Progesterone receptor isoforms are multifunctional transcription factors
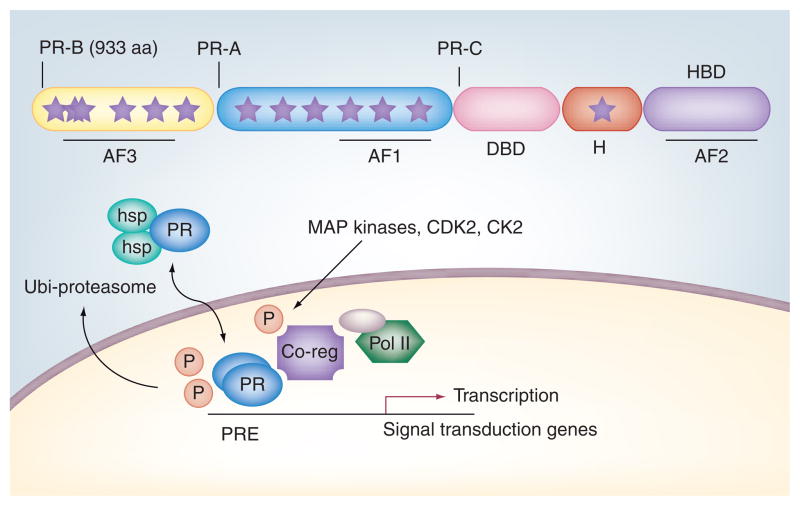
Progesterone receptors (PRs) are ligand-activated transcription factor members of the steroid hormone receptor (SR) subfamily of nuclear receptors (Figure 1). Two common isoforms (A and B) are created from the same gene via alternate translational start sites; PR-B refers to the full-length receptor, while PR-A is an N-terminally truncated version (missing the first 164 amino acids found in PR-B). The PR gene is differentially regulated by two independent (isoform-specific) promoters. A and B isoforms can act as homo- (A:A or B:B) or heterodimers (A:B) and are capable of binding DNA at progesterone response elements [1] and/or via tethering to other transcription factors (signal transducers and activators of transciption [STATs], specificity protein 1 [SP1] and activator protein 1) [2–5]. PR-A and -B can regulate the same or different (isoform-specific) sets of target genes and exhibit both ligand-dependent and -independent activities [6,7]; these PR functions are heavily influenced by cross-talk/input from peptide growth factor-initiated signal transduction pathways [8]. A third PR isoform termed PR-C is truncated still further downstream by use of an additional AUG codon within the DNA-binding domain; this highly tissue-specific receptor inhibits the actions of PR-B in the uterus and is important for the induction of labor [9].
Figure 1. Progesterone receptor isoforms are sensors for growth factor-induced signaling.
PR-B and truncated PR-A are substrates for mitogenic protein kinases, including CDK2 (up to eight sites, including Ser400), MAPKs (Ser294 and Ser345) and CK2 (Ser81). Phosphorylated receptors and/or coregulators of transcription (such as steroid receptor coactivators) mediate promoter selection and sensitivity of PR target genes to progesterone and other hormones, including peptide growth factors (EGF, FGF receptor or IGF). Up to 14 sites (stars) in PR-B are phosphorylated either basally and/or in response to hormone action; MAPK- or CDK2-dependent phosphorylation of PR Ser294 facilitates ligand-dependent nuclear export and receptor downregulation via targeting to the ubiquitin–proteasome pathway.
AF: Activation function; DBD: DNA-binding domain; H: Hinge; HBD: Hormone-binding domain; hsp: Heat-shock protein; P: Phosphorylation; Pol II: RNA polymerase II; PR: Progesterone receptor; PRE: Progesterone response element.
Steroid hormone receptors function as signal transduction molecules. PRs function not only as critical regulators of transcription but also to activate signal transduction pathways, many of which are involved in pro-proliferative signaling in the breast. Because normal (cycling) mammary epithelial cells are devoid of estrogen receptor (ER) and PR, studies on the biochemistry of PR action have largely employed ER-positive (ER+)/PR-positive (PR+) human breast cancer cell lines (MCF-7, T47D and ZR-75). Emerging in vitro data suggest that PR extranuclear (nongenomic) actions lead to rapid activation of protein kinases (MAPK, PI3K/Akt and c-Src) in part by a ligand-induced interaction between PR and c-Src kinase [10–12]. Seminal work from Migliaccio et al. demonstrated that synthetic progesterone treatment rapidly activated c-Src and ERK2 (MAPK) in breast cancer cells (T47D), and this MAPK activation translated into an increase in T47D cell growth (Figure 2) [10]. These data showed that c-Src activation was dependent upon an interaction between PR, c-Src and, surprisingly, ERα; treatment with anti-estrogens blocked progesterone-induced MAPK activation. Interestingly, in these studies, no direct interaction between PR and c-Src was observed, implicating ER as a linker molecule in heterotrimeric signaling complexes. Subsequent work from this group identified two ER-interacting domains within PR that are responsible for mediating PR/ER/c-Src interactions [13]. Complementary work from Boonyaratanakornkit et al. reached a similar conclusion; rapid activation of c-Src/MAPK was observed following treatment with progestins [11]. However, in vitro, signaling occurred independently of PR interaction with ER. These researchers iden-tified a direct interaction between an N-terminal proline-rich region of PR and the SH3-domain of c-Src. In contrast to what was previously observed (described earlier), progestin-induced MAPK levels were low (25% of EGF-treated positive control), and did not translate to increased cell growth; this group observed a drop in progesterone-induced cell growth inhibition in PR-null normal mammary epithelial cells (MCF10A) stably expressing mutant PR-B incapable of interacting with c-Src (relative to cells expressing wild-type PR-B) [11].
Figure 2. Progesterone receptor-B, but not progesterone receptor-A, and estrogen receptor-α participate in membrane-tethered protein complexes capable of rapidly activating c-Src and MAPKs.
Progesterone/PR and estrogen/ER transactivate EGFR and/or ErbB2; phosphorylated steroid hormone receptors and signaling molecules, including protein kinases and surface receptors, enter the nucleus and participate in transcription complexes at selected gene promoters.
E2: Estradiol; EGFR: EGF receptor; ER: Estrogen receptor; P: Phosphorylation; P4: Progesterone; PR: Progesterone receptor; Shc: Src homology domain II containing.
The rapid signaling and transcriptional activities of PR are integrated events. Although the rapid signaling actions of SRs take place independently of transcription (i.e., in seconds to minutes), it is becoming increasingly clear that membrane-initiated and nuclear functions of SRs are fully integrated events (Figure 2). For example, Faivre et al. first demonstrated a mechanism of progestin/PR-induced autocrine signaling in which rapid signaling complexes (containing PR and c-Src) are required for subsequent expression of PR-target genes (including EGF receptor [EGFR] and WNT1) [14]. In response to progestins, secreted WNT1 activates frizzled receptors on the cell surface, leading to matrix metalloproteinase production and cleavage of heparin-binding EGF molecules (i.e., to produce free EGF). Progestin-dependent transactivation of EGFR ultimately induces sustained MAPK activation, cyclin D1 expression, and increased cell proliferation and survival [14]. In this model, rapid or membrane-associated PR signaling induces c-Src- and MAPK-dependent phosphorylation of PR Ser345 [15]. Phosphorylation of PR Ser345 is required for PR tethering to SP1, a transcription factor mediator of progestin-responsive genes, such as p21 and EGFR. These data demonstrated that PR-containing rapid signaling complexes function to transmit specific information (i.e., in the form of phosphorylation events) to genomic transcriptional complexes. Related to this concept, intriguing new data from Béguelin et al. defined a novel model for PR cross-talk with signaling complexes that involved progestin-induced activation and nuclear translocation of ErbB2, a membrane-associated receptor tyrosine kinase [16]. Once localized in the nucleus, ErbB2 formed a transcriptional complex with PR and STAT3, serving as a transcriptional coactivator for STAT3 and controlling genes such as cyclin D1. Inhibiting formation of this transcriptional complex prevented progestin-driven PR/ErbB2-positive tumor growth in mouse models. Taken together, these data support a novel role for PR involving a hybrid of extranuclear and genomic actions: ligand-activated PR induces EGFR [14] or ErbB2 [16] transactivation and subsequent transcriptional complex formation, with nuclear PR being a critical component of this protein complex at selected gene promoters.
Whereas the protein complex components that are critical to support progestin-induced MAPK activation remain somewhat controversial (discussed earlier), all models tend to agree that rapid activation of MAPKs by progestins is mediated by membrane-associated PR, either directly or indirectly. Notably, SRs (ER, PR and androgen receptor) traffic to the plasma membrane, in part via heat-shock protein 27-dependent tethering, where they are reversibly palmitoylated in order to facilitate and prolong membrane location and function [17]. Work from these groups and others [12] underscored the important extra-nuclear role that SRs play in the rapid activation of cytoplasmic or membrane-associated protein kinases (c-Src and PI3K/Akt), and downstream signaling cascades (MAPKs). Importantly, these kinases modify regulatory sites on SRs, including ER and PR [15], and their coregulators [18], thereby integrating both rapid signaling and genomic actions.
Like other SRs, PRs are significantly post-translationally modified by phosphorylation, acetylation, sumoylation and ubiquitination [19–23]. These modifications are often ligand dependent, but can also occur independently of ligand binding (primarily in response to protein kinase activation), and significantly alter receptor stability, localization, tethering interactions, transcriptional activity and promoter selectivity [24]. For example, MAPK and cdk2 have previously been demonstrated to phosphorylate and modulate the activity of both liganded and unliganded PR [21,25–27]. Phospho-PRs are targeted to specific gene subsets, and subsequent specific transcriptional profiles depend on the phosphorylation status of PR [15,19,28]. Thus, a feed-forward loop between progestin-activated protein kinases and subsequent phosphorylation of PRs (by those same kinases) underlies the profound effects that activated kinases have on the nuclear functions of PR, particularly with regard to promoter selectivity [14,15,28]. With the exception of K303A ERα, a hyperactive mutant ER found in a subset of human breast cancers [29], one reason that ER and PR are seldom mutated is because these receptors are subject to intense epigenetic regulation (i.e., phosphorylation most often translates to gain of function) by the same protein kinases that are most often upregulated or constitutively activated in breast cancer. Because a myriad of post-translational inputs are capable of driving receptor and/or coregulator behaviors, there may be little pressure for adaptive mutations that accomplish that same task (however, the receptors are frequently overexpressed).
Growth factor- or SR-induced rapid signaling provides a mechanism for PR promoter selection. These types of data underscore the concept that so-called rapid signaling actions of SRs simply constitute a required step in the pathway to gene regulation and, specifically, promoter selection (i.e., by the very same receptors). That is, rapid and dynamic shuttling of SRs between the cytoplasmic and nuclear compartments allows for constant interaction with protein kinases; SRs are in fact sensors for the actions of growth factors and signaling molecules stationed within and at the plasma membrane. Thus, although extranuclear PR actions are often considered to be functionally distinct from downstream genomic PR events (they are most often studied separately), cytoplasmic and nuclear receptors are probably part of the same dynamic or ‘fluxing’ population (Figure 2). In response to hormonal cues, cycling populations of transiently membrane-localized PRs rapidly activate appropriate protein kinase cascades. These kinases phosphorylate nearby substrates (i.e., membrane-tethered PRs and cytoplasmic coregulators). Entire complexes containing steroid receptor phosphospecies, coregulators and signaling molecules (including kinases) then associate dynamically with regulatory regions/enhancers in DNA to activate or inhibit gene expression. This scheme explains why some SR-dependent promoters are exquisitely sensitive to alterations in protein kinase activities (a minority of receptors are membrane associated at any given time), while others are much more tightly regulated by steroid hormone alone [15]. Overall, kinase signaling (including SR-dependent rapid signaling) is a mechanism for promoter selection; it provides a means of quickly altering hormone responsiveness at some, but not all, promoters. This is an important facet of PR action and explains why PR gene signatures differ in normal versus neoplastic mammary epithelial cells [30]; under the influence of signal transduction pathways commonly activated in breast cancer cells, PR signaling and thus promoter selection, differs dramatically, resulting in altered cell/tumor biology.
Progesterone is a potent breast mitogen. Once a controversial notion, it is now well accepted that progesterone acts as a proliferative hormone in the breast, although it is paradoxically inhibitory in the reproductive tract and ovaries. A primary function of progesterone/PR is to mediate the massive expansion of epithelial-derived mammary alveoli (alveologenesis and organization of alveoli into lobules) during puberty and pregnancy in preparation for lactation. Increased serum levels of progesterone during the luteal phase of the menstrual cycle are coincident with a high proliferative index of epithelial cells in the milk duct system [31]. Likewise, during diestrus in mice, when progesterone levels rise by approximately fourfold, an increase in ductal structures is visible in mammary gland whole mounts [32]. Mouse knockout studies demonstrated that PR-B, rather than PR-A, is specifically required for the epithelial cell proliferation that is the basis of extensive mammary gland ductal side branching and alveologenesis [33]. Studies in receptor activator of NF-κB ligand (RANKL) and cyclin D1 (i.e., both major downstream effectors of PR)-deficient mice show similar blocks in alveologenesis [34,35], while receptor of activator of NF-κB (RANK)-transgenic mice express increased cyclin D1 and undergo increased hormone-driven proliferation and mammary tumor formation [36]. In contrast to PR-B, ERα is required for mammary ductal elongation prior to pregnancy when the gland is highly responsive to estrogen, but relatively unresponsive to progesterone [37]. Estrogen/ER also contributes to alveolar development, in part via induction of PR expression [38].
Steroid receptor action is required for normal mammary gland development. Like ERα, PR isoforms are found in a minority of mammary epithelial cells (MECs). These receptors are most often coexpressed, occurring in only approximately 10–20% of luminal epithelial cells in the normal mammary gland [37]. Multiple studies have concluded that SR-negative (SR-) cells comprise the majority of the proliferating (nonpregnant) normal MEC cohort [39–41]. Thus, in response to progesterone, it has been proposed that PR+ cells provide mitogenic paracrine signals that direct neighboring SR- cells to divide (Figure 3) [42]. Recently, Beleut et al. described two distinct mechanisms of progesterone-induced MEC proliferation that occurred in waves following progesterone administration to adult ovariectomized mice [43]. Initially peaking approximately 24 h post-treatment, a subset of PR+ cells (5% of MECs) in the luminal compartment were stimulated to divide. Cyclin D1, a PR target gene, was required for this cell-autonomous proliferative response. After approximately 3 days of progesterone treatment, there was a second wave of proliferation peaks (27% of MECs); this fraction of cells is PR null but dependent upon the PR-induced paracrine factor, RANKL, for mitogenic stimulation (Figure 3). Similarly, WNT4, another paracrine mitogen induced by PR, is required for progesterone-induced side-branching during the development of mammary ducts [44]. Other studies performed in mice and rats also illustrate that a small percentage of PR-B, but not PR-A, expressing MECs actively undergo cell division, as measured by bromodeoxyuridine incorporation and PR co-staining; proliferation of PR-B containing cells becomes extensive during pregnancy [45]. Regulation of PR isoform expression is poorly understood in humans. However, in rodent models, estrogen induced PR-A expression, while progesterone alone or estrogen plus progesterone were required for significant PR-B expression [37]. In summary, in the normal breast, estrogen/ER may primarily act to increase PR-A expression [37], while progesterone/PR-B initiates a series of potent proliferative factors (WNT4, cyclin D1 and RANKL) for exquisitely timed expansion of the mammary gland.
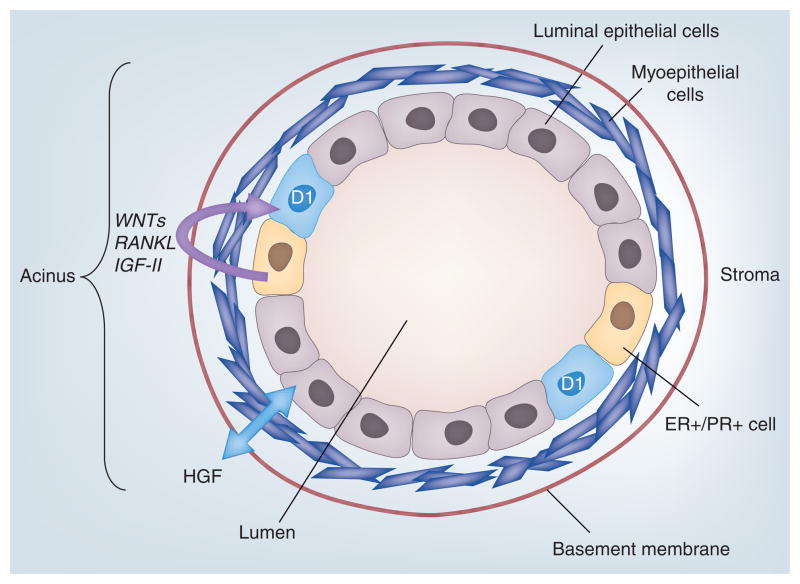
Figure 3. Proliferating cells in the normal (non-pregnant) mammary gland are typically steroid hormone receptor null.
ER and PR isoforms are coexpressed in a minority population of mammary epithelial cells that lie adjacent to proliferating (cyclin D1-positive) steroid receptor-negative cells. Progesterone/PR-dependent paracrine factors (WNTs, RANKL and IGF-II) induce neighboring (PR-null) cells to undergo proliferation. An early switch to autocrine signaling mechanisms occurs in the development of ER-positive/PR-positive breast cancers.
ER: Estrogen receptor; PR: Progesterone receptor.
Hormone-dependent breast cancers undergo an early switch to autocrine growth signaling. Despite the relatively low abundance of MECs in the normal (i.e., nonpregnant) breast that express SRs, the majority of breast cancers are ER+/PR+ upon initial diagnosis [46]. Numerous models, both in vitro and in vivo, demonstrate that progesterone/PR remains a strong mitogenic and prosurvival stimulus within the context of breast cancer [8]. PR, in the presence and absence of ligand, induced anchorage-independent growth and increased survival in breast cancer cell lines [14,28,47]. In mouse models, mammary tumors induced by chemical carcinogens and genetic disruption of the tumor suppressor, BRCA1, were dependent on PR action [48,49]. In addition, administration of medroxyprogesterone acetate induced mammary carcinogenesis in multiple species, including mice [50]. Furthermore, in rats, CDB-4124, a clinically used (for uterine fibroids and endometriosis) antiprogestin/PR modulator (PRM), inhibited the appearance of spontaneous preneoplastic mammary lesions and N-methyl-N-nitrosourea-induced (ER+) mammary tumors, primarily via suppression of proliferation and induction of apoptosis [51]. A few small clinical trials have used additional PRMs to target PR in breast cancer with good success, despite cross-reactivity with glucocorticoid receptors [52,53]. Finally, large clinical trials have demonstrated that progestin added to hormone replacement therapy significantly increased the incidence and grade of breast tumors in post-menopausal women [54]. No increased risk was associated with estrogen alone [54,55], and estrogen-only hormone replacement therapy may be protective in some women. Synthetic progestins used in hormone replacement therapy clinical trials and progesterone have overlapping effects on PR [50]; therefore, progesterone is not considered an entirely safe alternative.
Interestingly, gene-expression analysis of normal human MECs cultured in 3D relative to similarly cultured T47D human breast cancer cells showed distinct genetic profiles upon progestin treatment, indicating that progesterone-induced proliferative programs differ between normal and cancer cells [30]. This is not entirely surprising, considering that in the normal (non-pregnant) breast, the majority of proliferating cells are devoid of SRs and instead primarily divide in response to paracrine signals; in SR-positive breast tumors, PR-containing cells proliferate, presumably via autocrine mechanisms that may be WNT1-, EGFR- and cyclin D1-dependent [14]. In addition, mitogenic protein kinases (CDK2, c-Src, CK2 and MAPK), often upregulated in breast cancer, drive PR hypersensitivity to ligand and ligand-independent activity, and can also redirect phospho-PR to alternate promoters ([10,15,19,28,56]; discussed further later).
Progesterone mediates mammary gland stem cell self-renewal. Lifetime exposure to steroid hormones (either exogenous or endogenous) is a critical risk factor for the development of breast cancer. For example, a greater number of menstrual cycles (experienced over an individual’s lifetime) is correlated with increased breast cancer incidence [57]. Accumulating evidence implicates progesterone/PR in the maintenance and expansion of breast stem and progenitor cells. It has been proposed that mammary stem cells (MaSCs) comprise a population of putative primary targets for transformation to breast malignancies [58,59]. Quiescent MaSCs are thought to be activated during periods of glandular expansion, such as puberty and pregnancy [59–61], when progesterone levels are high. Early reports described hormone receptor-positive (30–40%) and -negative cells that divide asymmetrically (as measured by DNA labeling) in mice undergoing puberty, and proliferate again in adulthood upon hormone administration [61–63]. Others reported mouse MaSCs to be ER-/PR- cells surrounded by myoepithelial and luminal cells, some of which express both ER and PR [64]. Similarly, in humans, the cell populations enriched for MaSCs have been reported to be both SR+ [65] and SR- [66]. It is likely that MaSCs are SR-, yet require local SR+ cells to provide paracrine signals [58]. Shackelton et al. were able to generate functional mammary glands from MaSCs isolated from a niche in the basal epithelial layer [60].
Recently, progesterone was shown to induce basal MaSC (CD49fhi) expansion in the diestrus phase of cycling female mice [32]. The authors suggest that PR induction of WNT4 and RANKL in the luminal compartment act in a paracrine manner to enrich the basal MaSC population. Genetically engineered mice with RANK deleted from mammary epithelial cells were resistant to progestin-induced epithelial proliferation and expansion of CD49hi stem cells; these mice also exhibited sensitization to DNA-damaging agents [67]. While these are intriguing results, the contribution of RANK to human breast development and cancer awaits confirmation [68]. In primary human breast cultures, Graham et al. described an increase in progenitor cell populations in response to progesterone treatment [30]. Recent work in human MECs showed that WNT1, a progesterone-regulated gene [14], is located upstream of Notch signaling [69], which is implicated in affecting stem cell self-renewal and lineage-specific differentiation in the mammary gland [70]. It is thus reasonable to predict that progesterone may also drive the expansion of breast cancer progenitor cells, a hypothesis examined by Horwitz et al. [71,72]. In these studies, T47D human breast cancer cell xenografts were reported to contain a rare population of basal-like CD44+ tumor-initiating cells (ER−PR−CK5+), an intermediate cell population (ER−PR−CK5−) and an expanding population of luminal-like cells (ER+PR+CK5−). Upon treatment with progestin, ER+PR+CK5+ cells were observed and ER−PR−CK5+ cells were enriched. The authors propose that the ER+PR+CK5+ cells comprise a transitional cell population present in tumors that may retrogress to ER−PR−CK5+ cells in response to progestins [71,72]. As a result, progesterone maintenance and expansion of MaSCs may have implications for breast tumor stem cell populations; these cells are likely to be more resistant to traditional cancer therapies due to their ability to undergo quiescence, a state characterized by a high degree of resistance to apoptosis and agents that primarily target properties of rapidly dividing cells (i.e., classical chemotherapies). Going forward, it will be critical to delineate important similarities and differences between the various models used to study these hormone-dependent aspects of mammary gland biology; significant differences exist between mice (the primary genetic model employed in breast cancer research), humans and rats. The inclusion of more rat models may provide further insight into steroid receptor biology in mammary gland development and tumor progression (reviewed in [37]).
Expert commentary
Is the action of PRs in breast cancer a missed opportunity? Owing to a convergence of factors, PR action in breast cancer has been almost entirely overlooked. First, the topic is complex. The natural hormone, progesterone, has opposing effects according to target tissue and cell context. Progestins are mitogenic in the breast, but inhibitory in the uterus and ovaries; the basis for this divergence is still unknown. Human breast cancer cells cultured in 2D (plastic dishes) exhibit a biphasic pattern of growth in response to progesterone (when subjected to continuous progesterone treatment, they undergo one or more rounds of cell division and are then growth inhibited [73]). In addition, genetically engineered mice are the primary animal models used in breast cancer research. As both ER and PR are required for mammary gland development, interpretation of studies using ER- or PR-knockout mice are limited in that these animals lack the structures/cells that give rise to breast cancer (i.e., mammary gland development is severely impaired). Unlike the human breast, the mouse mammary gland does not fully develop until pregnancy; virgin glands are relatively unresponsive to progesterone and primarily express PR-A, but contain very little PR-B. Indeed, few genetic mouse models develop ER+/PR+ mammary tumors [74]. Furthermore, studies of human breast oncogenes (i.e., transgenic mouse models) frequently evaluate virgin animals, making it impossible to implicate PR-B (i.e., the proliferative receptor) in tumor biology. This may partly explain why treatment of well-established animal tumor models with progestin (agonists) rarely augments tumor biology (although the use of antiprogestin [antagonists] is often inhibitory; discussed later). Antiprogestins were rejected in early human clinical trials not because they were not highly effective [53], but because they had significant cross-reactivity with their glucocorticoid receptor close cousins, resulting in intolerable side effects (reviewed in [75]). Finally, considerable political resistance has discouraged mainstream use of antiprogestins within the USA for any indication (i.e., the antiprogestin, RU486, is clinically known as ‘the abortion pill’); drug companies avoid the development of agents perceived to be unpopular or not sufficiently lucrative/patentable. For these unfortunate reasons (few of which are relevant to peer-reviewed science on this topic), PR isoforms are grossly understudied relative to ERα in the breast and breast cancer. In fact, experts suggest that PR is a highly relevant SR with respect to both normal and neoplastic breast epithelial cell proliferation [30], early breast cancer progression [51,76,77] and, more recently, mammary gland stem-cell biology [32]. Like ER, PR mutations are not commonly seen in the majority of breast cancers, although the normal 1:1 ratio of PR-A to PR-B is frequently altered [78]; the significance of this finding is unknown but probably relates to altered homeostasis and rapidly changing patterns of gene expression during early tumor development [30].
Why study progesterone/PR in the breast? ER is the first example and the primary focus of very successful ‘targeted’ breast cancer therapies. However, the actions of ER and PR are intimately linked in biology. PR is an important ER target gene and thus acts as a major downstream effector of estrogen action. As mentioned previously, historically, progesterone was assumed to have little to no effect on breast tumorigenesis, partly owing to its well-established inhibitory and differentiative role in the uterus and reproductive organs. However, more recently, progesterone has been implicated as a proliferative hormone in the normal breast [30] and a lifelong risk factor for breast cancer [55,79–84]. Notably, as with ER, there is extensive cross-talk between PR and the same signal transduction pathways that are required for mammary gland development and are most often elevated in breast cancer. For example, the proliferative effects of progesterone are highly dependent upon tyrosine kinase growth factor receptors (EGFR family members) and their downstream protein kinase effectors (c-Src and MAPKs); these effects (i.e., cell proliferation) map to direct phosphorylation of PR-B, but not PR-A [14]. Cross-talk between PR-B and the EGFR pathway provides a basis for understanding mechanisms of transcriptional synergy between progestins and EGF on numerous endogenous genes that are highly relevant to breast cancer biology [85]. PR target genes, such as WNTs [14,44], are secreted factors that may contribute to paracrine and autocrine proliferation signals during progression to malignant transformation [69]. The physiological significance of EGF-induced PR-B hyperactivation relates to the key role of both molecules, along with ERα, as mediators of massive alveolar proliferation during mammary gland development/early pregnancy [86]. This interplay between growth factors and both SR (ER/PR) functions (inappropriately) during breast cancer progression, when tyrosine kinase activities are elevated and hyperactive SRs are still present and functional (although frequently at low abundance; discussed further later). For this reason, targeted therapies against ER and ErbB (EGFR/ErbB2) family members are now a clinical mainstay, but their success can be limited by mechanisms of tumor progression. The addition of PR-blocking therapies to this list could be life saving; anti-progestins are predicted to severely impair the process of tumor progression (i.e., by blocking PR-induced upregulation of signaling pathway intermediates that include known mediators of endocrine resistance), which invariably occurs upon exposure to anti-estrogens or estrogen blockers [87–89]. Indeed, this is a missed opportunity for women facing fewer and fewer treatment options as they fail classical endocrine therapies.
More abundant PR may not translate to increased transcriptional activity. An early event in tumor development includes an altered ratio of coexpressed PR-A to PR-B (normally observed to be 1:1), with loss of PR-B (i.e., apparent predominance of PR-A) occurring most often [78,90]. The natural assumption is that PR-A is thus the dominant isoform, perhaps even driving tumor phenotype. However, it is also well appreciated that liganded SRs are rapidly downregulated relative to their inactive forms. Thus, the expression of phosphorylated receptors (namely PR-B) may appear to be low in PR-driven tumors due to increased phospho-PR ubiquitinylation and rapid protein ‘loss’ by proteasome-mediated turnover of activated receptors [21]; growth factors also ultimately lower PR mRNA expression via reversible mechanisms [21,91,92]. SR proteins and their coregulators are direct targets of growth factor-activated cytoplasmic protein kinases. Thus, a ‘vicious cycle’ is created, wherein growth factors induce phosphorylated and transcriptionally hyperactive PRs that turn over even more rapidly, making low-abundance receptors nearly ‘invisible’ at the protein level. However, their robust nuclear activity is clearly detected in reporter gene assays and at endogenous genes using subphysiologic hormone concentrations [19,21,85]. In fact, apparent ‘loss’ of PR is an excellent clinical marker of high growth factor receptor expression and activity [92]. This high-kinase condition is responsible for phosphorylating PR and increasing both its transcriptional activity and rate of turnover. Hyperactive PR protein may be relatively undetectable by clinically employed antibody-binding assays; when protein levels are measured, clinicians may mistakenly conclude that apparently ‘PR-null’ tumors have escaped hormonal regulation. Instead, ‘loss’ of PR-B may in fact be an excellent early marker of PR-B-driven biology; similar mechanisms have been reported for ER in breast cancer cells containing activated c-Src kinase [93]. Importantly, we reported that hyperactive (deSUMOylated) phospho-PR-B is capable of driving breast cancer cell proliferation and survival via the transcriptional regulation of novel PR target genes that are not known to be particularly responsive to progestin alone, but are very responsive to high kinase activities [94]. Surprisingly, these genes include novel phospho-PR-regulated genes and ER-regulated genes. Because hyperactive phospho-PR-B is largely deSUMOylated [19], it also fails to transrepress ER [23]; we suspect that the two receptors (PR and ER) cooperate at many of the same genes.
The development of the ER+/PR-null tumor phenotype may be PR driven. There is considerable functional overlap between ER and PR. Notably, many ER-regulated genes are also PR regulated (including c-myc, cyclin D1, c-fos, STATs and IGF pathway components), and these receptors even tether to the same transcription factors (activator protein 1 and SP1) to regulate nonclassical target genes (which contain no hormone-responsive element). ERα and PR-B also participate in similar membrane-associated, cytoplasmic (or ‘rapid’) signaling complexes (discussed previously) in association with EGFR and c-Src kinase upstream of the ERK1/2 MAPK module [10]. ERα or PR-B localized near the cell membrane are both capable of transactivating EGFR [14,95]. In fact, steroid hormone-induced rapid activation of MAPK appears to be most robust when both ERα and PR-B are coexpressed in model cell lines [10,13]. The end point of MAPK signaling is most often the regulation of nuclear transcription factors. Indeed, ER and PR are direct targets (substrates) of mitogenic protein kinases, including MAPK. This cross-talk even extends to the regulation of ER/PR interactions (Figure 4). In response to progesterone binding, SUMOylated PR isoforms (both A and B) transrepress ER [23]; MAPK-dependent phosphorylation events (namely PR Ser294) lift this repression by blocking PR SUMOylation [19]. ER and PR are most often coexpressed in early-stage breast cancer. Loss of PR mRNA and protein can indicate a functional loss of ER (ER+/PR-low or -null); this is a common assumption. However, an alternative pathway exists in which phospho-PR is under-SUMOylated and thus no longer able to transrepress ER (Figure 4). Phospho-PR instead behaves as a hyperactive or constitutive (i.e., ligand-independent) transcription factor at selected gene promoters, including those classically regulated by ER [28].
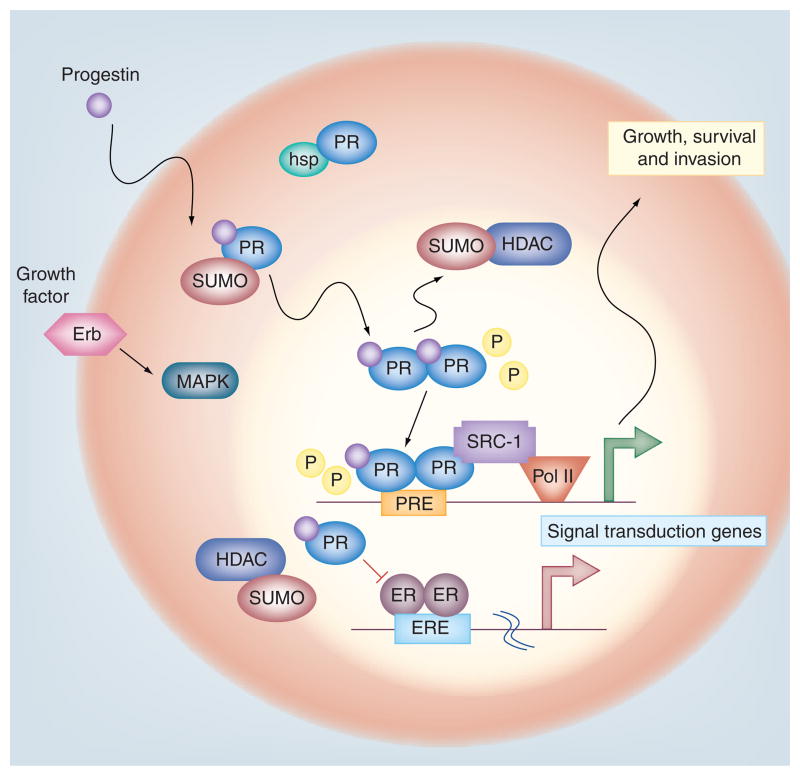
Figure 4. Reversible progesterone receptor Ser388 SUMOylation provides a mechanism for rapid changes in hormone responsiveness according to extracellular cues.
PRs are rapidly SUMOylated in response to progesterone binding. SUMOylated PR species are tenfold less active on selected gene promoters and capable of ER transrepression (by unknown mechanisms). Growth factor-induced MAPK activation leading to phosphorylation of PR Ser294 prevents PR Ser388 SUMOylation, thereby lifting SUMO-dependent repression of both PR and ER transcriptional activities. Phosphorylated and deSUMOylated PR-B drives breast cancer cell proliferation and survival.
ER: Estrogen receptor; Erb: Erythroblastic leukemia viral oncogene homolog; ERE: Estrogen response element; HDAC: Histone deacetylase; hsp: Heat-shock protein; P: Phosphorylation; Pol II: RNA polymerase II; PR: Progesterone receptor; PRE: Progesterone response element; SRC: Steroid receptor coactivator; SUMO: Small ubiquitin-related modifier.
Five-year view
Future studies should focus on the goal of defining the contribution of protein kinase inputs to PR-dependent signaling and PR/ER cross-talk in breast cancers that are classically believed to be ‘ER driven’ but are resistant to anti-estrogen therapy (and may in fact be PR driven). SR-specific gene signatures, rather than protein levels (often limited to a small sampling of the tumor), should be used clinically to assess hormone responsiveness. With the development of more selective antiprogestins [51], the opportunity to understand and target the ER+/PR ‘loss’ phenotype as a means of combating early progression to hormone-refractory breast cancer is within reach; this phenotype can be clearly defined by the presence of a phospho-PR-B gene signature, predicted to be a sensitive and reliable readout of PR activity when PR protein levels appear to be reduced. Related to this idea, we recently defined a phospho-PR gene signature that includes both ligand-dependent and -independent PR-regulated genes; our signature predicts a high likelihood of rapid progression to breast cancer metastasis [Knutson T, Lange C, Unpublished Data]. It will now be important to validate this exciting finding in preclinical models of human breast cancer.
A wealth of basic and clinical studies have implicated PR action in breast cancer. However, only a fraction of information is known compared with what is known about ER, which was the first example of highly successful targeted therapy. A few tenants of PR action have emerged: PRs behave quite differently with regard to isoform specificity and cellular context (i.e., breast vs uterus or normal vs neoplastic cells); altered PR behavior is in large part conferred by the actions of activated protein kinases; PR hypersensitivity that approaches ligand independence is driven by phosphorylation events and may be significant in certain contexts; and phospho-PR may precede/mark the near complete loss of PR protein and later growth factor-driven suppression of PR mRNA that occurs during the development and progression of endocrine-resistant luminal B-type (ER+/PR−) breast cancers. Indeed, the most appropriate use for PRMs may be during early breast cancer development or very early tumor progression (i.e., before PR levels drop precipitously). There is an increasingly recognized need to prevent or reverse the development of early lesions (i.e., that may or may not ever progress); this is a largely untapped area that warrants intense scrutiny of PRs as potentially important drivers of an early switch from SR-dependent paracrine to autocrine signaling mechanisms. The ultimate degree of aggressiveness of progressing tumors may be determined early on, partly dictated by the influence of progesterone/PR on the stem-cell compartment. An increased understanding of PR function and cross-talk with ER in normal, pre-neoplastic and neoplastic settings, as well as stronger advocacy from scientist-, clinician- and patient/survivor-led groups are needed to overcome remaining resistance to the goal of including PR-targeted strategies as part of the repertory of mainstream endocrine/ER-based therapies.
Key issues.
While mouse models have significantly expanded our knowledge of breast disease, through the development and utilization of rat models we may achieve a more balanced understanding of steroid receptor regulation in breast cancer. Such models provide insight into the complex hormone-driven mechanisms of human breast cancer development and early progression, which represents a significant gap in our knowledge.
Clinicians need to consider progesterone receptor (PR)-A and -B isoform-specific expression and action in human tumors (rather than total PR levels). Assay of well-characterized phosphorylated residues on both estrogen receptor (ER) and PR may predict clinical outcome more accurately; incorporation of steroid receptor-specific gene signatures as indicators of transcriptional activity and thus steroid receptor-driven biology is timely and feasible, and may provide the ultimate readout of endocrine status.
Important cross-talk between growth factors and PR and between both PR isoforms and ER exists (and is the subject of highly valuable targeted therapies); PR action has been widely overlooked in this scheme. Scientists and clinicians need to work together on the development of preclinical models that clearly evaluate PR action and PR cross-talk with ER, with the goal of advancing towards routine use of PR-targeted therapies as a significant and life-saving improvement to classical endocrine therapy.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
This work was supported by a grant from the National Institutes of Health (NIH/National Cancer Institute): R01 CA123763 (formerly R01 DK53825; NIH/National Institute of Diabetes and Digestive and Kidney Diseases). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
of interest
of considerable interest
- 1.Tsai SY, Carlstedt-Duke J, Weigel NL, et al. Molecular interactions of steroid hormone receptor with its enhancer element: evidence for receptor dimer formation. Cell. 1988;55(2):361–369. doi: 10.1016/0092-8674(88)90059-1. [DOI] [PubMed] [Google Scholar]
- 2.Richer JK, Lange CA, Manning NG, et al. Convergence of progesterone with growth factor and cytokine signaling in breast cancer. Progesterone receptors regulate signal transducers and activators of transcription expression and activity. J Biol Chem. 1998;273(47):31317–31326. doi: 10.1074/jbc.273.47.31317. [DOI] [PubMed] [Google Scholar]
- 3.Owen GI, Richer JK, Tung L, Takimoto G, Horwitz KB. Progesterone regulates transcription of the p21(WAF1) cyclin-dependent kinase inhibitor gene through Sp1 and CBP/p300. J Biol Chem. 1998;273(17):10696–10701. doi: 10.1074/jbc.273.17.10696. [DOI] [PubMed] [Google Scholar]
- 4.Proietti C, Salatino M, Rosemblit C, et al. Progestins induce transcriptional activation of signal transducer and activator of transcription 3 (Stat3) via a Jak- and Src-dependent mechanism in breast cancer cells. Mol Cell Biol. 2005;25(12):4826–4840. doi: 10.1128/MCB.25.12.4826-4840.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tseng L, Tang M, Wang Z, Mazella J. Progesterone receptor (hPR) upregulates the fibronectin promoter activity in human decidual fibroblasts. DNA Cell Biol. 2003;22(10):633–640. doi: 10.1089/104454903770238102. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsen BM, Richer JK, Sartorius CA, Horwitz KB. Expression profiling of human breast cancers and gene regulation by progesterone receptors. J Mammary Gland Biol Neoplasia. 2003;8(3):257–268. doi: 10.1023/b:jomg.0000010028.48159.84. [DOI] [PubMed] [Google Scholar]
- 7.Richer JK, Jacobsen BM, Manning NG, et al. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277(7):5209–5218. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- 8.Lange CA, Sartorius CA, Abdel-Hafiz H, et al. Progesterone receptor action: translating studies in breast cancer models to clinical insights. Adv Exp Med Biol. 2008;630:94–111. [PubMed] [Google Scholar]
- 9.Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-κB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20(4):764–775. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- 10.Migliaccio A, Piccolo D, Castoria G, et al. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 1998;17(7):2008–2018. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boonyaratanakornkit V, Scott MP, Ribon V, et al. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8(2):269–280. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- 12.Saitoh M, Ohmichi M, Takahashi K, et al. Medroxyprogesterone acetate induces cell proliferation through up-regulation of cyclin D1 expression via phosphatidylinositol 3-kinase/Akt/nuclear factor-κB cascade in human breast cancer cells. Endocrinology. 2005;146(11):4917–4925. doi: 10.1210/en.2004-1535. [DOI] [PubMed] [Google Scholar]
- 13.Ballare C, Uhrig M, Bechtold T, et al. Two domains of the progesterone receptor interact with the estrogen receptor and are required for progesterone activation of the c-Src/Erk pathway in mammalian cells. Mol Cell Biol. 2003;23(6):1994–2008. doi: 10.1128/MCB.23.6.1994-2008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;27(2):466–480. doi: 10.1128/MCB.01539-06. Biochemical characterization of progesterone receptor (PR)-dependent autocrine proliferative signaling. Provides novel evidence of the integration of rapid and genomic PR signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faivre EJ, Daniel AR, Hillard CJ, Lange CA. Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol Endocrinol. 2008;22(4):823–837. doi: 10.1210/me.2007-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Béguelin W, Diaz Flaque MC, Proietti CJ, et al. Progesterone receptor induces ErbB-2 nuclear translocation to promote breast cancer growth via a novel transcriptional effect: ErbB-2 function as a coactivator of Stat3. Mol Cell Biol. 2010;30(23):5456–5472. doi: 10.1128/MCB.00012-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razandi M, Pedram A, Levin ER. Heat shock protein 27 is required for sex steroid receptor trafficking to and functioning at the plasma membrane. Mol Cell Biol. 2010;30(13):3249–3261. doi: 10.1128/MCB.01354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narayanan R, Adigun AA, Edwards DP, Weigel NL. Cyclin-dependent kinase activity is required for progesterone receptor function: novel role for cyclin A/Cdk2 as a progesterone receptor coactivator. Mol Cell Biol. 2005;25(1):264–277. doi: 10.1128/MCB.25.1.264-277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daniel AR, Faivre EJ, Lange CA. Phosphorylation-dependent antagonism of sumoylation derepresses progesterone receptor action in breast cancer cells. Mol Endocrinol. 2007;21(12):2890–2906. doi: 10.1210/me.2007-0248. [DOI] [PubMed] [Google Scholar]
- 20.Daniel AR, Gaviglio AL, Czaplicki LM, et al. The progesterone receptor hinge region regulates the kinetics of transcriptional responses through acetylation, phosphorylation, and nuclear retention. Mol Endocrinol. 2010;24(11):2126–2138. doi: 10.1210/me.2010-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci USA. 2000;97(3):1032–1037. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weigel NL, Bai W, Zhang Y, et al. Phosphorylation and progesterone receptor function. J Steroid Biochem Mol Biol. 1995;53(1–6):509–514. doi: 10.1016/0960-0760(95)00098-k. [DOI] [PubMed] [Google Scholar]
- 23.Abdel-Hafiz H, Takimoto GS, Tung L, Horwitz KB. The inhibitory function in human progesterone receptor N termini binds SUMO-1 protein to regulate autoinhibition and transrepression. J Biol Chem. 2002;277(37):33950–33956. doi: 10.1074/jbc.M204573200. [DOI] [PubMed] [Google Scholar]
- 24.Ward RD, Weigel NL. Steroid receptor phosphorylation: assigning function to site-specific phosphorylation. Biofactors. 2009;35(6):528–536. doi: 10.1002/biof.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Beck CA, Poletti A, et al. Phosphorylation of human progesterone receptor by cyclin-dependent kinase 2 on three sites that are authentic basal phosphorylation sites in vivo. Mol Endocrinol. 1997;11(6):823–832. doi: 10.1210/mend.11.6.0006. [DOI] [PubMed] [Google Scholar]
- 26.Pierson-Mullany LK, Lange CA. Phosphorylation of progesterone receptor serine 400 mediates ligand-independent transcriptional activity in response to activation of cyclin-dependent protein kinase 2. Mol Cell Biol. 2004;24(24):10542–10557. doi: 10.1128/MCB.24.24.10542-10557.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knotts TA, Orkiszewski RS, Cook RG, Edwards DP, Weigel NL. Identification of a phosphorylation site in the hinge region of the human progesterone receptor and additional amino-terminal phosphorylation sites. J Biol Chem. 2001;276(11):8475–8483. doi: 10.1074/jbc.M009805200. [DOI] [PubMed] [Google Scholar]
- 28•.Daniel AR, Lange CA. Protein kinases mediate ligand-independent derepression of sumoylated progesterone receptors in breast cancer cells. Proc Natl Acad Sci USA. 2009;106(34):14287–14292. doi: 10.1073/pnas.0905118106. Describes a mechanism whereby kinase signals activate phospho-PR transcriptional activity in the absence of ligand to upregulate growth-promoting genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui Y, Zhang M, Pestell R, et al. Phosphorylation of estrogen receptor α blocks its acetylation and regulates estrogen sensitivity. Cancer Res. 2004;64(24):9199–9208. doi: 10.1158/0008-5472.CAN-04-2126. [DOI] [PubMed] [Google Scholar]
- 30•.Graham JD, Mote PA, Salagame U, et al. DNA replication licensing and progenitor numbers are increased by progesterone in normal human breast. Endocrinology. 2009;150(7):3318–3326. doi: 10.1210/en.2008-1630. Demonstrates that progesterone increases mammary epithelial cell proliferation and increases progenitor cell populations using a normal human breast primary cell culture model. In addition, PR gene signatures differed between similarly cultured normal and cancer cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18(4):502–519. doi: 10.1210/edrv.18.4.0308. [DOI] [PubMed] [Google Scholar]
- 32••.Joshi PA, Jackson HW, Beristain AG, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465(7299):803–807. doi: 10.1038/nature09091. Demonstrates that progesterone/PR is required for mammary stem cell proliferation. [DOI] [PubMed] [Google Scholar]
- 33.Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ. Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol Cell Endocrinol. 2001;1(1–2):97–103. doi: 10.1016/s0303-7207(01)00465-8. [DOI] [PubMed] [Google Scholar]
- 34.Fata JE, Kong YY, Li J, et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 2000;103(1):41–50. doi: 10.1016/s0092-8674(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 35.Sicinski P, Donaher JL, Parker SB, et al. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82(4):621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Suarez E, Jacob AP, Jones J, et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468(7320):103–107. doi: 10.1038/nature09495. [DOI] [PubMed] [Google Scholar]
- 37.Kariagina A, Aupperlee MD, Haslam SZ. Progesterone receptor isoform functions in normal breast development and breast cancer. Crit Rev Eukaryot Gene Expr. 2008;18(1):11–33. doi: 10.1615/critreveukargeneexpr.v18.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brisken C, O’Malley B. Hormone action in the mammary gland. Cold Spring Harb Perspect Biol. 2010;2(12):a003178. doi: 10.1101/cshperspect.a003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57(22):4987–4991. [PubMed] [Google Scholar]
- 40.Russo J, Ao X, Grill C, Russo IH. Pattern of distribution of cells positive for estrogen receptor α and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res Treat. 1999;53(3):217–227. doi: 10.1023/a:1006186719322. [DOI] [PubMed] [Google Scholar]
- 41.Seagroves TN, Lydon JP, Hovey RC, Vonderhaar BK, Rosen JM. C/EBPβ (CCAAT/enhancer binding protein) controls cell fate determination during mammary gland development. Mol Endocrinol. 2000;14(3):359–368. doi: 10.1210/mend.14.3.0434. [DOI] [PubMed] [Google Scholar]
- 42.Brisken C, Park S, Vass T, et al. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci USA. 1998;95(9):5076–5081. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Beleut M, Rajaram RD, Caikovski M, et al. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci USA. 2010;107(7):2989–2994. doi: 10.1073/pnas.0915148107. Delineates separate mechanisms for progestin-induced proliferation of PR-positive (cell-autonomous) and PR-negative (paracrine-stimulated) mammary epithelial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brisken C, Heineman A, Chavarria T, et al. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000;14(6):650–654. [PMC free article] [PubMed] [Google Scholar]
- 45•.Kariagina A, Aupperlee MD, Haslam SZ. Progesterone receptor isoforms and proliferation in the rat mammary gland during development. Endocrinology. 2007;148(6):2723–2736. doi: 10.1210/en.2006-1493. Characterizes PR-A and -B in rat mammary glands and demonstrates that PR-B mediates proliferation in this model. [DOI] [PubMed] [Google Scholar]
- 46.Arpino G, Weiss H, Lee AV, et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005;97(17):1254–1261. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 47.Moore MR, Conover JL, Franks KM. Progestin effects on long-term growth, death, and Bcl-xL in breast cancer cells. Biochem Biophys Res Commun. 2000;277(3):650–654. doi: 10.1006/bbrc.2000.3728. [DOI] [PubMed] [Google Scholar]
- 48.Lydon JP, Ge G, Kittrell FS, Medina D, O’Malley BW. Murine mammary gland carcinogenesis is critically dependent on progesterone receptor function. Cancer Res. 1999;59(17):4276–4284. [PubMed] [Google Scholar]
- 49.Poole AJ, Li Y, Kim Y, et al. Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science. 2006;314(5804):1467–1470. doi: 10.1126/science.1130471. [DOI] [PubMed] [Google Scholar]
- 50.Lanari C, Lamb CA, Fabris VT, et al. The MPA mouse breast cancer model: evidence for a role of progesterone receptors in breast cancer. Endocr Relat Cancer. 2009;16(2):333–350. doi: 10.1677/ERC-08-0244. [DOI] [PubMed] [Google Scholar]
- 51.Wiehle RD, Lantvit DD, Yamada T, Christov K. CDB-4124, a progesterone receptor modulator, inhibits mammary carcinogenesis by supressing cell proliferation and inducing apoptosis. Cancer Prev Res (Phila) 2011;4(3):414–424. doi: 10.1158/1940-6207.CAPR-10-0244. [DOI] [PubMed] [Google Scholar]
- 52.Perrault D, Eisenhauer EA, Pritchard KI, et al. Phase II study of the progesterone antagonist mifepristone in patients with untreated metastatic breast carcinoma: a National Cancer Institute of Canada Clinical Trials Group study. J Clin Oncol. 1996;14(10):2709–2712. doi: 10.1200/JCO.1996.14.10.2709. [DOI] [PubMed] [Google Scholar]
- 53.Robertson JF, Willsher PC, Winterbottom L, Blamey RW, Thorpe S. Onapristone, a progesterone receptor antagonist, as first-line therapy in primary breast cancer. Eur J Cancer. 1999;35(2):214–218. doi: 10.1016/s0959-8049(98)00388-8. [DOI] [PubMed] [Google Scholar]
- 54••.Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684–1692. doi: 10.1001/jama.2010.1500. Large-scale clinical trial linking the progesterone component of hormone replacement therapy to increased risk of invasive breast cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 56.Hagan CR, Regan TM, Dressing GE, Lange CA. Ck2-dependent phosphorylation of progesterone receptors (PR) on Ser81 regulates PR-B-isoform-specific target gene regulation in breast cancer cells. Mol Cell Biol. 2011 doi: 10.1128/MCB.01246-10. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernstein L. Epidemiology of endocrine-related risk factors for breast cancer. J Mammary Gland Biol Neoplasia. 2002;7(1):3–15. doi: 10.1023/a:1015714305420. [DOI] [PubMed] [Google Scholar]
- 58.Brisken C, Duss S. Stem cells and the stem cell niche in the breast: an integrated hormonal and developmental perspective. Stem Cell Rev. 2007;3(2):147–156. doi: 10.1007/s12015-007-0019-1. [DOI] [PubMed] [Google Scholar]
- 59.Harmes DC, DiRenzo J. Cellular quiescence in mammary stem cells and breast tumor stem cells: got testable hypotheses? J Mammary Gland Biol Neoplasia. 2009;14(1):19–27. doi: 10.1007/s10911-009-9111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439(7072):84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 61.Smith GH. Label-retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development. 2005;132(4):681–687. doi: 10.1242/dev.01609. [DOI] [PubMed] [Google Scholar]
- 62.Booth BW, Boulanger CA, Smith GH. Selective segregation of DNA strands persists in long-label-retaining mammary cells during pregnancy. Breast Cancer Res. 2008;10(5):R90. doi: 10.1186/bcr2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Booth BW, Smith GH. Estrogen receptor-α and progesterone receptor are expressed in label-retaining mammary epithelial cells that divide asymmetrically and retain their template DNA strands. Breast Cancer Res. 2006;8(4):R49. doi: 10.1186/bcr1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Asselin-Labat ML, Shackleton M. Stingl J mouse mammary stem cells. J Natl Cancer Inst. 2006;98(14):1011–1014. doi: 10.1093/jnci/djj267. [DOI] [PubMed] [Google Scholar]
- 65.Clarke RB. Isolation and characterization of human mammary stem cells. Cell Prolif. 2005;38(6):375–386. doi: 10.1111/j.1365-2184.2005.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36(Suppl 1):59–72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schramek D, Leibbrandt A, Sigl V, et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 2010;468(7320):98–102. doi: 10.1038/nature09387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanos T, Brisken C. High hopes for RANKL: will the mouse model live up to its promise? Breast Cancer Res. 2011;13(1):302. doi: 10.1186/bcr2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ayyanan A, Civenni G, Ciarloni L, et al. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc Natl Acad Sci USA. 2006;103(10):3799–3804. doi: 10.1073/pnas.0600065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dontu G, Jackson KW, McNicholas E, et al. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6(6):R605–R615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71•.Horwitz KB, Dye WW, Harrell JC, Kabos P, Sartorius CA. Rare steroid receptor-negative basal-like tumorigenic cells in luminal subtype human breast cancer xenografts. Proc Natl Acad Sci USA. 2008;105(15):5774–5779. doi: 10.1073/pnas.0706216105. Describes a rare population of tumor-initiating cells in T47D breast cancer cell xenografts that are enriched upon progestin administration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Horwitz KB, Sartorius CA. Progestins in hormone replacement therapies reactivate cancer stem cells in women with preexisting breast cancers: a hypothesis. J Clin Endocrinol Metab. 2008;93(9):3295–3298. doi: 10.1210/jc.2008-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Groshong SD, Owen GI, Grimison B, et al. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1) Mol Endocrinol. 1997;11(11):1593–1607. doi: 10.1210/mend.11.11.0006. [DOI] [PubMed] [Google Scholar]
- 74.Allred DC, Medina D. The relevance of mouse models to understanding the development and progression of human breast cancer. J Mammary Gland Biol Neoplasia. 2008;13(3):279–288. doi: 10.1007/s10911-008-9093-5. [DOI] [PubMed] [Google Scholar]
- 75.Lange CA, Yee D. Progesterone and breast cancer. Womens Health. 2008;4(2):151–162. doi: 10.2217/17455057.4.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benakanakere I, Besch-Williford C, Ellersieck MR, Hyder SM. Regression of progestin-accelerated 7, 12-dimethylbenz[a]anthracene-induced mammary tumors in Sprague–Dawley rats by p53 reactivation and induction of massive apoptosis: a pilot study. Endocr Relat Cancer. 2009;16(1):85–98. doi: 10.1677/ERC-08-0069. [DOI] [PubMed] [Google Scholar]
- 77.Cerliani JP, Giulianelli S, Sahores A, et al. Mifepristone inhibits MPA-and FGF2-induced mammary tumor growth but not FGF2-induced mammary hyperplasia. Medicina (B Aires) 2010;70(6):529–532. [PubMed] [Google Scholar]
- 78.Graham JD, Yeates C, Balleine RL, et al. Characterization of progesterone receptor A and B expression in human breast cancer. Cancer Res. 1995;55(21):5063–5068. [PubMed] [Google Scholar]
- 79.Anderson E. The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res. 2002;4(5):197–201. doi: 10.1186/bcr452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clarke CA, Glaser SL, Uratsu CS, et al. Recent declines in hormone therapy utilization and breast cancer incidence: clinical and population-based evidence. J Clin Oncol. 2006;24(33):e49–e50. doi: 10.1200/JCO.2006.08.6504. [DOI] [PubMed] [Google Scholar]
- 81.Hofseth LJ, Raafat AM, Osuch JR, et al. Hormone replacement therapy with estrogen or estrogen plus medroxyprogesterone acetate is associated with increased epithelial proliferation in the normal postmenopausal breast. J Clin Endocrinol Metab. 1999;84(12):4559–4565. doi: 10.1210/jcem.84.12.6194. [DOI] [PubMed] [Google Scholar]
- 82.Lee S, Kolonel L, Wilkens L, et al. Postmenopausal hormone therapy and breast cancer risk: the Multiethnic Cohort. Int J Cancer. 2006;118(5):1285–1291. doi: 10.1002/ijc.21481. [DOI] [PubMed] [Google Scholar]
- 83.Ross RK, Paganini-Hill A, Wan PC, Pike MC. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst. 2000;92(4):328–332. doi: 10.1093/jnci/92.4.328. [DOI] [PubMed] [Google Scholar]
- 84.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 85.Shen T, Horwitz KB, Lange CA. Transcriptional hyperactivity of human progesterone receptors is coupled to their ligand-dependent down-regulation by mitogen-activated protein kinase-dependent phosphorylation of serine 294. Mol Cell Biol. 2001;21(18):6122–6131. doi: 10.1128/MCB.21.18.6122-6131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. Dev Cell. 2001;1(4):467–475. doi: 10.1016/s1534-5807(01)00064-8. [DOI] [PubMed] [Google Scholar]
- 87.Fan P, Yue W, Wang JP, et al. Mechanisms of resistance to structurally diverse antiestrogens differ under premenopausal and postmenopausal conditions: evidence from in vitro breast cancer cell models. Endocrinology. 2009;150(5):2036–2045. doi: 10.1210/en.2008-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Massarweh S, Osborne CK, Creighton CJ, et al. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008;68(3):826–833. doi: 10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- 89.Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr Relat Cancer. 2004;11(4):643–658. doi: 10.1677/erc.1.00776. [DOI] [PubMed] [Google Scholar]
- 90.Mote PA, Bartow S, Tran N, Clarke CL. Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat. 2002;72(2):163–172. doi: 10.1023/a:1014820500738. [DOI] [PubMed] [Google Scholar]
- 91.Cui X, Schiff R, Arpino G, Osborne CK, Lee AV. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol. 2005;23(30):7721–7735. doi: 10.1200/JCO.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 92.Cui X, Zhang P, Deng W, et al. Insulin-like growth factor-I inhibits progesterone receptor expression in breast cancer cells via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway: progesterone receptor as a potential indicator of growth factor activity in breast cancer. Mol Endocrinol. 2003;17(4):575–588. doi: 10.1210/me.2002-0318. [DOI] [PubMed] [Google Scholar]
- 93.Chu I, Arnaout A, Loiseau S, et al. Src promotes estrogen-dependent estrogen receptor α proteolysis in human breast cancer. J Clin Invest. 2007;117(8):2205–2215. doi: 10.1172/JCI21739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Daniel AR, Knutson TP, Lange CA. Signaling inputs to progesterone receptor gene regulation and promoter selectivity. Mol Cell Endocrinol. 2009;308(1–2):47–52. doi: 10.1016/j.mce.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Levin ER. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol. 2003;17(3):309–317. doi: 10.1210/me.2002-0368. [DOI] [PubMed] [Google Scholar]