Abstract
Laboratory training and testing of auditory recognition skills in animals is important for understanding animal communication systems that depend on auditory cues. Songbirds are commonly studied because of their exceptional ability to learn complex vocalizations. In recent years, mounting interest in the perceptual abilities of songbirds has increased the demand for laboratory behavioural training and testing paradigms. Here, we describe and demonstrate the success of a method for auditory discrimination experiments, including all the necessary hardware, training procedures and freely-available, versatile software. The system can run several behavioural training and testing paradigms, including operant (go-nogo, stimulus preference, and two-alternative forced choice) and classical conditioning tasks. The software and some hardware components can be used with any laboratory animal that learns and responds to sensory cues. The peripheral hardware and training procedures are designed for use with songbirds and auditory stimuli. Using the go-nogo paradigm of the training system, we show that adult zebra finches learn to recognize and correctly classify individual female calls and male songs. We also show that learning the task generalizes to new stimulus classes; birds that learned the task with calls subsequently learned to recognize songs faster than did birds that learned the task and songs at the same time.
Keywords: Auditory perception, Operant discrimination, Songbirds, Zebra Finches, Go/nogo, Automated training paradigms, Neuroethology
The sensory and perceptual functions of the auditory system can be investigated by measuring behavioural responses to sounds. Among non-human animals, sound processing and perception are of particular interest in birds because many species communicate using complex vocalizations and exhibit the rare capability of vocal learning (Doupe & Kuhl 1999; Dooling et al. 2000; Brenowitz & Woolley 2004; Dooling & Lohr 2006). Songbirds that learn to produce and recognize complex vocalizations are commonly used in laboratory studies of natural sound processing, vocal learning and vocal motor production (Williams 2008). Laboratory studies examining the role of vocalizations in reproductive behaviour also commonly use songbirds because of the importance of song in sexual competition and mate choice (Hauber et al. 2010). A history of auditory training and testing techniques using small birds exists (Hulse 1995; Dooling et al. 2000), but the increasing popularity of the songbird for studying the perception of learned vocalizations suggests that an automated, well described and fully tested method for training and testing small birds on auditory recognition tasks will facilitate studies investigating vocal perception.
Songbirds offer several practical advantages to the study of vocal communication. Many species can be bred and raised in captivity, allowing for highly-controlled developmental manipulations, detailed analyses of behavioural development and comparisons across species. Some species develop rapidly (<100 days), allowing laboratory experiments to be conducted throughout the life span of an individual and across generations (Campbell and Hauber 2009; Feher et al. 2009). Furthermore, behavioural and neurophysiological experiments can be combined; the neural circuits that control song processing, learning and production are well described (Nordeen & Nordeen 2008).
The zebra finch (Taeniopygia guttata), an Australian estrildid finch, is the most commonly studied songbird in the laboratory (Williams 2008; Warren et al. 2010). Only males sing and use their songs to court females (Zann 1996). Females use the acoustic properties of male songs for mate selection (Forstmeier & Birkhead 2004; Spencer et al. 2005; Boogert et al. 2008; Holveck & Riebel 2007; 2010). Males and females also produce calls, which are learned in males but not in females (Vicario 2004; Vignal et al. 2004; 2008). Both males and females can distinguish between the songs and calls of different individuals, as demonstrated by operant conditioning experiments (Okanoya & Dooling 1991; Cynx & Nottebohm 1992; Riebel 2009). In zebra finches, operant conditioning experiments have examined the temporal and spectral characteristics of calls and songs that are necessary for discrimination (Cynx et al. 1990; Lohr & Dooling 1998; Dooling & Lohr 2006; Riebel 2009; Nagel et al. 2010), developmental (Braaten et al. 2006) and experiential (Benny & Braaten 2000) changes in song discrimination, and female preferences for male songs as they relate to mate choice (Riebel 2009). Several studies have shown that female song preferences measured using operant testing paradigms predict which songs evoke female sexual responses such as copulation solicitation displays and mating (Riebel and Slater 1998; Holveck and Riebel 2007, 2010; Anderson 2009).
Behavioural auditory training and testing paradigms that are manually controlled are impractical and vulnerable to data collection and analysis errors. Commercially available training and testing systems that are automated are expensive and use hardware and software that may not have the flexibility to accommodate a wide range of experimental designs. For example, depending on the number of input and output lines necessary for a given paradigm, a single interface module may only control one or two training chambers, requiring the user to purchase multiple modules. Additionally, commercially available hardware components that are necessary for successful discrimination training, such as feeders, are often unsuitable for small birds (Njegovan et al. 1994). To overcome these issues, laboratories implement custom-designed software and/or hardware systems (Okanoya & Dooling 1988; Schraff et al. 1998; Houx & ten Cate 1999; Gentner et al. 2000; Sturdy & Weissman 2006; van Heijningen et al. 2009; Nagel et al. 2010). These custom-designed solutions are not available for all scientists and are challenging to develop and employ, especially for small birds.
We have developed an auditory recognition training system (ARTSy) that uses inexpensive hardware and custom software that is flexible, high-throughput and freely available to scientists. ARTSy can be used to train and test any animal species that learns to recognize and respond to sensory cues. For example, the hardware can be modified to address questions regarding the discrimination of visual or olfactory cues. Here, we describe the system with hardware designed for training small birds to recognize and respond to individual sounds or sound sequences, how to assemble the hardware, and use the software. We demonstrate the success of the system by describing the recognition training and testing of male and female zebra finches using female calls and male songs. Results also show that task learning in adult zebra finches generalizes to other sound classes.
METHODS
Overview
The training system can be used for a variety of behavioural paradigms, including operant (go-nogo, two-alternative forced choice, preference) and classical conditioning tasks. Here, we tested the go-nogo paradigm with male and female zebra finches. The basic parameters that we define and the procedures we describe for go-nogo are similar for the other training paradigms. In the go-nogo paradigm, the subject is rewarded for responding to the correct (go) sound and punished for responding to the incorrect (nogo) sound. Subjects are rewarded with food and punished with a period of complete darkness. Zebra finches typically learn the go-nogo task after 600 to 3,000 trials, which is consistent with the results from studies using systems designed for use in individual laboratories (Cynx & Nottebohm 1992; Cynx 1995; Braaten et al. 2006). First, we trained subjects on the go-nogo task with female long calls. We reasoned that training birds with one class of sounds that is separate from the class of sounds that are of experimental interest is advantageous because learning the go-nogo task and learning to recognize and discriminate between specific sounds are two separate problems that the subjects must solve. Dissociating the learning of the task and the sound recognition learning that is required for discrimination allows the experimenter to directly compare discrimination performance on different types of experimental stimuli. After subjects reached a criterion of 80% or better correct responses to long calls (Fig. 1A), we then tested their ability to generalise the go-nogo task to zebra finch songs (Fig. 1B). We also trained a separate group of birds using songs and compared task learning using long calls as stimuli to task learning using songs as stimuli because these stimuli differ in duration, acoustic complexity and salience. We predicted that the automated system would successfully train birds to discriminate between different sounds in a comparable number of trials as previously described in the literature and that task learning would generalise to another sound class such that birds would learn to recognize songs faster if they learned them after learning the task on calls than if they learned the songs and the task simultaneously.
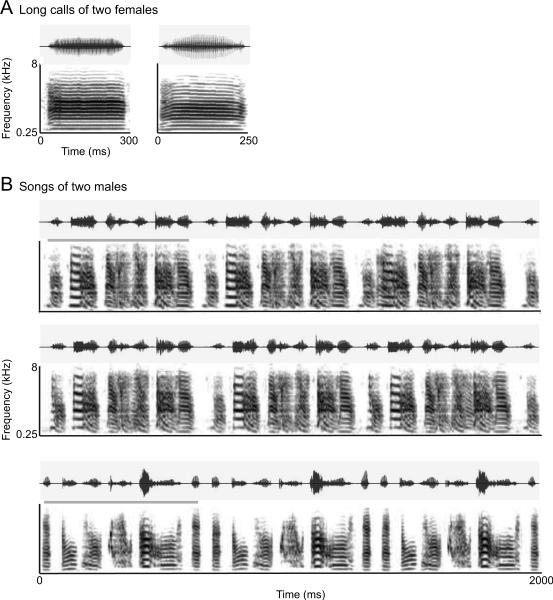
Figure 1.
Oscillogram (top) and spectrogram (bottom) of example training sounds. A) Two example long calls, each recorded from a different female. B) Three example songs. The top two are exemplars of the same bird's song and the bottom is a song from a different bird. The gray lines above the top and bottom song spectrograms highlight motifs which are repeated within the 2 s stimulus.
Subjects
We trained 9 (2 male, 7 female) adult zebra finches (Taeniopygia guttata) that were hatched and reared in the breeding colony in the Department of Psychology at Columbia University. All birds were naive to discrimination training. Birds were raised in 6 different family cages (some subjects were from the same lineage) and housed as adults in same-sex aviaries so that they could see and hear other zebra finches of both sexes in the room. The colony photoperiod was maintained on a 14 hr light/10 hr dark cycle. Colony room temperature was maintained between 23 and 29 degrees Celsius. Mixed seed, water, cuttlebone and grit were freely available; and the birds were supplemented three times a week with lettuce and twice a week with eggs and shells. We trained two separate cohorts of adult zebra finches with different sounds on the initial go-nogo task: The first cohort (N = 4, 1 male, 3 female) was trained with songs, and the second cohort (N = 5, 1 male, 4 female) was trained with long calls. Subjects in cohort 2 were tested for their ability to generalise the task to songs after they were trained to recognize long calls. The ages of subjects in cohort 1 (420 ± 8.49 days, N = 4) and 2 (227.20 ± 101.92 days, N = 5) were recorded at the start of the experiment. The mean weight of the females in cohort 2 recorded at the start of the experiment was 14.18 ± 0.80 g, N = 4; the weight of the male was 16.64 g. All animal procedures were approved by the Columbia University Institutional Animal Care and Use Committee (IACUC) and met all applicable state and federal guidelines.
Apparatus
Training events were conducted in a custom-built wire-cage (43.18 cm × 30.48 cm × 30.48 cm) housed in a sound isolation cubicle (78.74 cm × 53.34 cm × 53.34 cm outside and 67.31 cm × 41.91 cm × 44.45 cm inside) lined with acoustic foam (Fig. A4; H10-24A, Coulbourn Instruments, Allentown, PA, USA). A custom-built, acrylic response panel (30.48 cm × 30.48 cm) replaced one wall of the cage. An infrared slot sensor was mounted on the left side of the response panel (SLO30VB6Y, Banner Photoelectric Sensors, Prime Inc., Oradell, NJ, USA). A custom-built solenoid-driven feeder delivered food reinforcement during training (Fig. A5). A fluorescent lamp (137076, www.jcwhitney.com) mounted on the back of the isolation chamber was used for light reinforcement during training. An overhead fluorescent lamp (13707G, www.jcwhitney.com) was used to illuminate the chamber when the subjects were not training. A speaker (KFC-1377, Kenwood USA Corporation, Kenwood, CA) was placed on the top of the wire cage, centred over the response panel. During training sessions, a camera with microphone (KPC600, Bollide, B & H, www.bhphotovideo.com) placed on the top of the cage recorded training events. Two perches and a bamboo nest cup were mounted inside the cages.
Sound Stimuli
Songs and long calls were recorded while birds were housed in custom-built sound isolation booths (Fig. 1). Songs were recorded by placing a male and a female in the same cage inside the booth. Female long calls were recorded by placing a single female inside the booth, immediately following exposure to another female. A microphone (SRO, Earthworks) was placed in the top, centre of the cage. Sounds were digitally recorded at a sampling rate of 44.1 kHz (EDIROL Audio Capture FA-66, www.roland.com; Sound Analysis Pro Recorder software, Ofer Tchernichovski, City College, CUNY). Background noise was reduced by bandpass filtering raw sound files to include frequencies that ranged from 0.25 to 8 kHz (Goldwave, Goldwave Inc.). Power in all sound files was rebalanced in RMS intensity, and a 5 ms amplitude ramp was applied to the onset and offset of each file to remove potential acoustic artifacts that resulted from the editing process (Matlab, www.mathworks.com).
Long calls
Zebra finches emit long (or distance) calls when they are isolated or immediately separated from other birds (Zann 1996). Female long calls have a characteristic frequency- and amplitude-modulated harmonic structure with a fundamental frequency of 0.4 to 0.5 kHz (Vignal et al. 2008). They typically range from 150 to 250 ms in duration (Okanoya & Dooling 1991). Long calls recorded from 4 adult females housed in our colony were used to create 3 exemplar sound files of each female's long call. Long calls were a mean duration of 193.75 ± 51.11 ms, N = 4. Examples of long call stimuli are shown is Figure 1A.
Songs
Zebra finch song is composed of individually identifiable syllables that are produced in stereotyped sequences called motifs (Zann 1996). Syllables are acoustically distinct units of continuous sound, separated by silence. Sequences of acoustically simple introductory notes often precede the production of multiple, repeating motifs. For cohort 1, we used database recordings of songs from birds housed at the Department of Psychology, Hunter College (Vyas et al. 2009) to generate stimuli that were unfamiliar to subjects before training began (Cynx & Nottebohm 1992). Three exemplars of each male's song were made from a library of recorded songs. Similarly, for cohort 2, we created unfamiliar song stimuli from database recordings of 15 adult males that we no longer housed in our colony. Six song exemplars were created from unique song recordings of each male. All song exemplars included approximately 3 motifs, omitted introductory notes, and were 2 seconds in duration. Example song stimuli are shown in Figure 1B.
Procedures
Software
All experimental events were controlled and recorded by a custom-written Matlab program (David Schneider, Columbia University) that we have made available (www.commneuro.psych.columbia.edu). The core of our software was designed to present sounds and/or deliver reinforcement for multiple behavioural training paradigms (Appendix, Supplementary Material). Here, we describe how to use the program to successfully acclimate birds to the feeder, shape birds to peck the sensor for food, and to train birds in a typical go-nogo task.
Behavioural training
1. Acclimation
One week prior to the start of training, subjects were moved from the colony room to isolation booths so they could acclimate to the training chamber. Subjects were housed individually, and remained in the isolation booths for the duration of the experiments. Booth photoperiod, temperature and food and water availability were the same as the colony room conditions. We found that subjects performed better when they were housed continuously in training chambers, and were not repeatedly handled and transported between the colony room and testing chambers.
2. Training to peck the sensor for food
Training the birds to peck the sensor occurred in 3 stages. During these stages the birds were food restricted. We removed seed cups before the start of the dark cycle (21:00), and began training sessions at the start of the light cycle (07:00). Sessions lasted 4 to 6 hours depending on subjects' performance level (subjects were carefully monitored during these sessions because the amount of food they received depended on how well they performed). Subjects had access to cuttlebone, grit and water at all times. In the first stage, subjects were introduced to the feeder by eating from it when it was stationary. The lip of the feeder was positioned to protrude under the response panel so that seed was visible and accessible near the mesh flooring. Subjects mastered this task in 1 session. In the second stage, birds were familiarised with the sound and motion of the feeder with the acclimation program, which was designed to present the feeder at regular intervals. Two parameters defined the timing of an acclimation trial in the program: reward duration and inter-trial-interval (ITI). Reward duration defined how long the feeder was available to subjects. The ITI defined the duration between trials or feeder activations. ITIs are bound by minimum and maximum values. This allows the user to choose an inconsistent ITI to prevent temporal pattern learning. During feeder acclimation, the parameters were set as follows: reward duration = 10 s; ITI minimum = 30 s; and ITI maximum = 60 s. Therefore, the feeder was activated every 30 to 60 s, presenting seed to subjects for 10 s intervals. Subjects typically mastered this stage in 1–2 training sessions. Some subjects startled when the feeder moved, preventing them from approaching it. In these cases, we paused the program to let them eat briefly from the stationary feeder before resuming training. When we observed consistent startling, we repeated training, regardless of whether subjects regularly ate from the feeder, until the startle behaviour subsided. In the third and final stage, subjects were shaped (Appendix, 3.1) to peck the portion of the response panel outlined by the sensor (Fig. A8). When subjects pecked the response panel or moved their heads inside the sensor, its infrared beam was broken causing feeder activation. At this point, subjects initiated all trials. We used the shaping program to monitor the sensor and drive the feeder. Reward duration was the only defined parameter (10 s). On the first shaping day, we placed a piece of tape encrusted with seeds on the panel inside the sensor. Subjects first triggered the feeder when eating seed from the tape. When the ration of seed on the tape decreased, birds investigated and ate from the feeder. Subjects eventually ate from the feeder after pecking the sensor. Subjects stayed at this training stage until they consistently pecked the sensor and ate from the feeder. Some subjects stopped pecking the sensor once the seed on the tape was gone. In these cases, we intervened by re-seeding the tape and/or temporarily increasing the reward duration by 5–10 s. Stage 3 learning required 1–4 sessions, depending on the subject. Many birds learned the task in 1 session, but we trained them for an additional session, starting with tape only, to ensure that they learned the task. All stages progressed sequentially on independent days and usually totalled 4–6 training days overall.
3. Training to go-nogo
We first trained birds on the go-nogo task with either female long calls (cohort 1) or songs (cohort 2) (Fig. A9). Two go sounds and 2 nogo sounds, 3 exemplars each, were assigned to each bird. Sound presentation was counterbalanced so that go and nogo sounds were not the same for all the subjects. Food cups were removed the night before training sessions, and birds started training immediately following lights-on in the morning. The subjects initiated all trials. Seven parameters defined the timing of a go-nogo trial in the program: 1) start delay; 2) pre-response duration; 3) response duration; 4) reward duration; 5) punishment duration; 6) null duration; and 7) ITI. The start delay determined the time between trial initiation and stimulus playback. The pre-response duration defined the time after stimulus playback before subjects could respond and successfully activate the feeder. The response duration is the time subjects had to respond to a stimulus before the response is registered as “null” and a null period ensues. Punishment duration defines how long the light goes out and subjects were in darkness. Null outcome duration defines a set period of no outcome after a stimulus.
To initiate a trial, the birds had to first peck in the sensor. Tripping the sensor resulted in sound playback, and then the birds had 2 s to respond by pecking in the sensor again. For cohort 2, there was a 0.5 s delay (pre-response duration) after the call stopped playing before subjects could respond (Appendix, 3.1). If the call that played was a go call, then the birds received a 6 s seed reward (hit). If the call that played was a nogo call and the birds pecked the sensor, then the light turned off for 16 s (false alarm). If the birds did not respond to a go call (miss), a 6 s null period elapsed before the birds could initiate a new trial. Similarly, no response to a nogo long call (correct rejection) was followed by a 6 s null period. Birds learned to respond by pecking the sensor after hearing go long calls and to withhold a response after hearing nogo long calls. All long calls were delivered in repeating sets of 12 (4 long calls, 3 exemplars of each), but the order of long call presentation in each set was random. The behavioural results from each day were concatenated into a single data file, and percent correct scores were calculated for individuals every 100 trials, even when the 100 trials spanned two days. Consistent with similar behavioural studies, we stopped training when birds performed at 80% (or better) for two consecutive blocks of 100 trials (Dent et al. 2008). During go-nogo training, we rarely intervened. Individual birds varied in the number of trials they performed per day, but if an individual's characteristic performance markedly declined (i.e. they were much less active than on previous days or inactive during sessions), we suspended training for a day. Subjects increased their activity after a one day break from training.
Task generalization (transfer)
In cohort 2, after training using long calls, the go-nogo stimuli were switched to zebra finch songs. Trial timing parameters and performance criterion were identical to the baseline training that used calls as stimuli. Birds were randomly assigned 2 go and 2 nogo songs.
Data analysis
Data were automatically recorded by the program in a format that is easily accessible in Excel spreadsheets. Data sheets include the type of response (response = 1, or no response = 0), the stimulus, the time the stimulus began, the time the stimulus stopped, and duration from the offset of the stimulus to when the bird responded (if there was no response, then the duration is equal to the defined response duration). For all subjects (cohorts 1 and 2), we calculated the mean number of trial blocks to reach criterion on baseline (long calls or songs) and transfer (songs) training phases, the mean number of days (or sessions) to criterion, and the mean number of trials per day. Paired t tests were used to evaluate if there were differences in cohort 2 between these measures on baseline (calls) and transfer (songs) training phases. Independent t tests were used to determine if there were differences between cohorts 1 and 2 on these measures. Data were analysed using Matlab (Mathworks Inc.) and GraphPad (Prism V.5, GraphPad Software Inc.).
RESULTS
Baseline training
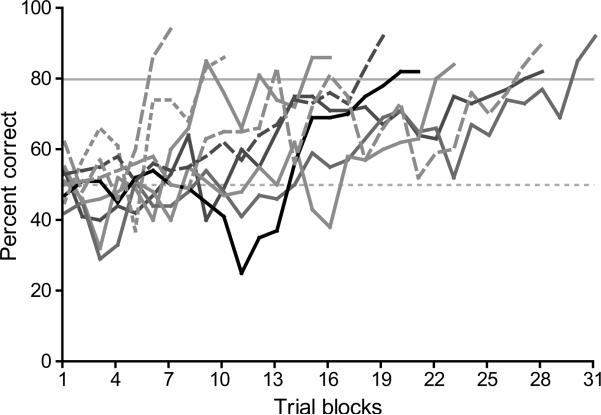
Both cohorts learned the baseline task. Birds trained on the baseline task with long calls reached criterion in a mean of 23.8 ± 5.89 (N = 5) trial blocks (each block was 100 trials). Birds trained on the baseline task with songs reached criterion in a mean of 16 ± 9.49 (N = 4) blocks. A comparison between the two groups on the mean number trial blocks to criterion was not significant (two-tailed independent samples t test: t7 = 1.522, P = 0.1719). The cumulative baseline performance of both training groups over trials to criterion is shown in Figure 2. The mean number of days for birds to reach criterion was 6.75 ± 4.57 for birds trained on songs and 12.60 ± 5.59 for birds trained on long calls. Correspondingly, birds trained on songs performed 315.4 ± 152 trials per day on average while birds trained on long calls performed 213.8 ± 64.8 trials per day. Differences between training groups on the mean number of days to criterion were not significant (two-tailed independent t test: t7 = 1.683, P = 0.1363), nor were differences in the mean number of trials per day (two-tailed independent t test: t7 = 1.369, P = 0.2132). The number of days to criterion and the mean number of trials per day on the baseline task are shown for individuals in Table 1.
Figure 2.
Performance of all birds on baseline training. Cumulative performance of birds trained on long calls (solid lines) and songs (dashed lines) over 100-trial blocks to criterion. Each line shows the performance for an individual bird. All birds performed similarly during baseline training, regardless of the stimulus type (N = 9). The black curve is the performance of an individual bird representative of the median (Mdn ± IQR = 21 ± 15, N = 9) number of trial blocks to criterion for cohorts 1 and 2 combined. The dashed gray line represents 50% correct responses or chance performance; the solid gray line represents criterion (80%) correct performance.
Table 1.
Training performance per day
| Bird | Sex | Group | Days to criterion | Mean trials per day (X ± SD) | ||
|---|---|---|---|---|---|---|
| Baseline |
Transfer |
Baseline |
Transfer |
|||
| 1 | F | 1 | 6 | - | 182 ± 146 | - |
| 2 | F | 1 | 6 | - | 342 ± 192 | - |
| 3 | F | 1 | 13 | - | 219 ± 86 | - |
| 4 | M | 1 | 2 | - | 519 ± 284 | - |
| 5 | F | 2 | 11 | 3 | 257 ± 94 | 459 ± 78 |
| 6 | F | 2 | 7 | 3 | 232 ± 76 | 422 ± 55 |
| 7 | F | 2 | 18 | 6 | 176 ± 95 | 227 ± 146 |
| 8 | F | 2 | 19 | 8 | 123 ± 143 | 188 ± 79 |
| 9 | M | 2 | 8 | 4 | 281 ± 78 | 305 ± 127 |
Transfer
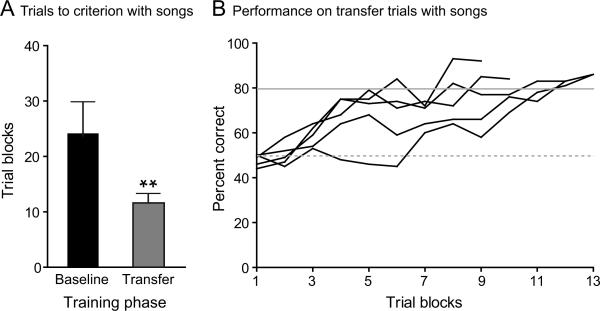
Birds transferred to songs reached criterion in significantly fewer trial blocks (11.40 ± 1.82, N = 5) than during baseline training with long calls (two-tailed paired t test: t4 = 4.7, P = 0.0093) (Fig. 3A). The cumulative performance of birds to criterion on the transfer task is shown in Figure 3B. Accordingly, all birds reached criterion in significantly fewer days (4.8 ± 2.17, N = 5) during transfer training than during baseline training (two-tailed paired t-test: t4 = 4.628, P = 0.0098). We compared the mean number of trials per day on baseline and transfer training phases to assess whether the decrease in latency to criterion was accompanied by an increase in the mean number of trials performed in a day. Birds performed significantly more trials per day during transfer to songs (320.2 ± 118.4, N = 5) than during baseline training with calls (two-tailed paired t test: t5 = 2.866, P = 0.0457). The number of days to criterion and the mean number of trials per day on the transfer task are shown for individuals in Table 1.
Figure 3.
Performance of cohort 2 on baseline training with calls and transfer training with songs. A) Mean number of trial blocks to criterion on baseline (calls) and transfer (songs). Birds reached criterion in fewer trial blocks during the transfer training compared to the baseline training, P = 0.0093, N = 5. Error bars represent one SD. B) Cumulative performance of birds on transfer training over trials to criterion, N = 5.
Response bias
During behavioural training, many animals exhibit response biases that affect the rate at which they learn to perform a given behaviour. The analysis of response biases can be methodologically useful for adjusting rewards and punishments to maximize learning. For the go-nogo training described here, birds chose to either activate a sensor (go) or to refrain from activating a sensor (nogo) on each trial. To determine if birds showed response biases to activate the sensor or to refrain from activating the sensor regardless of the stimulus presented, we measured the degree to which birds showed a bias toward either the go or nogo behaviour, and how their responses changed over the course of learning.
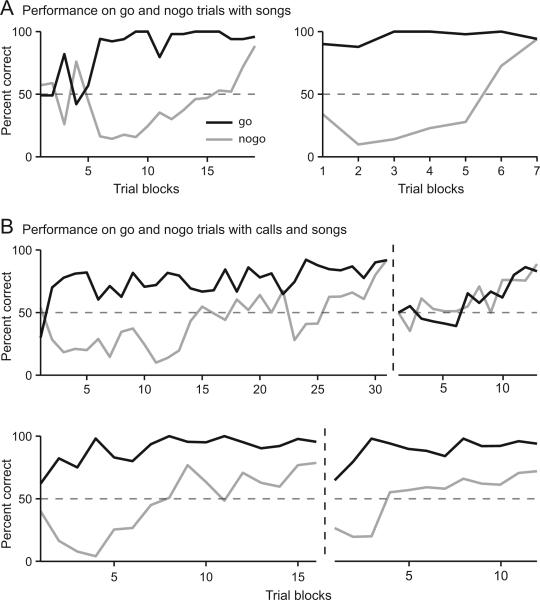
Across both cohorts, all but one bird had a bias to activate the sensor during both go and nogo trials, resulting in a greater percent correct performance for go stimuli than for nogo stimuli. Throughout baseline learning for both go and nogo stimuli, birds activated the sensor (go) on 70 % of trials (N = 8), which is substantially greater than would be predicted with no response bias (50 %). The overall percent correct increased over the course of training because the response bias attenuated. For example, go responses in 8 out of 9 birds decreased from 74 % during trial blocks 2 through 4 to 59 % during the last 2 blocks before reaching criterion performance. Figure 4A shows the learning curves for two birds that were baseline trained on songs. The bird in the left panel showed a tendency for activating the sensor which emerged after approximately 500 trials and maintained, but decreased this tendency until percent correct performance reached nearly 100 percent. The bird in the right panel began training with a strong response bias to activate the sensor and maintained the strength of this bias until the last trial block.
Figure 4.
Performance on go and nogo trials. A) Mean performance on go (black) and nogo (gray) 100-trial blocks for birds that were baseline trained with songs. The left and right panels show data from two different birds. Most birds showed a consistent response bias to activate the sensor, resulting in a larger percent correct for go than for nogo trials. B) Mean performance on go and nogo trials for birds that were baseline trained on calls and transferred to songs. Data to the left of the vertical dashed line show discrimination performance for calls. Data to the right of the vertical dashed line show discrimination performance after transfer to songs.
Of the birds that were baseline trained on calls, some maintained the strength of their response biases after transfer to songs whereas in others their response biases attenuated. The top of Figure 4B shows the learning curve for a bird that had a response bias to activate the sensor during baseline training on calls, but displaced the bias and learned the go and nogo stimuli simultaneously after being transferred to training with songs. The bottom panel of Figure 4B shows the learning curve for a bird that maintained its response bias after being transferred to training with songs.
DISCUSSION
Auditory recognition training and testing using both calls and songs show that all birds successfully learned the go-nogo operant task using the behavioural training system and procedures described here. Learning occurred in a number of trials that was comparable to the number of trials required to learn auditory recognition tasks in other studies (Cynx & Nottebohm 1992; Cynx 1993; Cynx 1995; Benney & Braaten 2000; Braaten et al. 2006). The mean number of trials required for birds to learn the go-nogo task was not statistically different between cohorts, regardless of stimulus type. Therefore, learning the task appears to be consistent for stimuli that differ in length, complexity and salience. Additionally, birds were able to generalise the task to new stimuli in fewer trials than on baseline training, and their cumulative performance (percent correct) was less variable across trial blocks during transfer training compared to baseline training (Figs. 2 & 3). This indicates that, once the go-nogo task was learned using one class of sounds, the skill can be applied to discriminating among sounds of different classes. These findings demonstrate that, although it may not be possible to completely separate task learning from recognition learning, training birds to recognize and correctly classify sounds that differ in acoustics and salience from the sounds that are of primary interest aids in the dissociation of the two types of learning
Our training results are consistent with studies that trained zebra finches to discriminate among natural songs using a go-nogo task (Cynx & Nottebohm 1992; Cynx 1993; Cynx 1995; Benney & Braaten 2000; Braaten et al. 2006), but training with synthetic vocalizations may take up to 2,000 more trials (Sturdy & Weismann 2006; Verzijden et al. 2007). This is not surprising considering that recognizing natural vocalizations is behaviourally and ecologically relevant for songbirds. However, we also observed differences between the behaviour of subjects trained with long calls and songs; birds trained on both calls and songs performed significantly more trials per day during song learning than during call learning and we observed a trend for birds to perform more trials per day during baseline song training than during baseline call training (Table 1). Correspondingly, learning curves showed a trend for birds trained on songs to reach criterion in fewer days and in fewer trials than birds trained on calls (Table 1). These results suggest that songs are more rewarding to hear and/or easier to discriminate among and recognize than are calls, potentially because they are longer in duration, more acoustically distinct and/or more socially relevant than calls. To the best of our knowledge, this is the first demonstration that zebra finches recognize and correctly classify natural female long calls in a learning paradigm. This may be relevant for future studies when choosing stimuli in order to reduce the overall time that is required to train and test birds on stimulus sets containing multiple go and nogo stimuli.
We found that nearly all birds (8 of 9) showed a strong response bias to activate the sensor (go) during both go and nogo trials. This suggests consistent differences in the effectiveness of the reward and punishment, residual effects from shaping, and/or motivational influences (e.g. hunger). For example, preceding go-nogo training, birds were trained to “go” for food, possibly creating or increasing a go response bias by rewarding the pecking behaviour. A simple way to counteract response biases is to alter the reward or punishment duration. For example, increasing the duration of the lights-out after a false alarm response could attenuate the bias for birds to “go” by making the punishment more effective. The software allows the user to change the duration of the reward and punishment to optimize learning as training progresses.
In both cohorts, we observed individual differences in learning acquisition rates, response profiles (Figs. 2; 3B; 4) and activity levels (trials/day) (Table 1). With the exception of response biases to peck the sensor, we did not find systematic trends in response behaviour during training that accounted for performance changes. Some birds showed abrupt transitions in performance by dramatically decreasing misses and/or false alarms over few training trials (Fig. 4A), while other birds showed gradual increases in performance for both go and nogo stimuli (Fig. 4B). The variability in the learning curves was likely due to individual differences in learning strategies, decision thresholds and/or the number of trials completed per unit time (e.g. how many trials a bird initiated each day), all of which can affect the individual rate of performance improvement (Gallistel et al. 2004).
The go-nogo method described here is designed to test hypotheses regarding perceptual invariance and signal detection in songbirds. For example, what spectrotemporal features of vocalizations serve as acoustic hallmarks for individual recognition? How good are songbirds at detecting target signals in background scenes? Once subjects learn training stimuli, probe stimuli that differ from training stimuli in the acoustic characteristics of interest can be introduced. Hypotheses can be tested with this system by altering aspects of a stimulus that are predicted to be essential for its recognition, with the expectation that the manipulation of behaviourally-salient features will result in performance differences in learned discrimination tasks. If a stimulus is modified beyond recognition or discriminability, criterion performance should not recover on the probe discrimination task. We include two training phases (baseline and transfer) in our go-nogo method so that we can compare the latency to criterion between the probe and training phases because, after baseline training, subjects are similarly proficient on the go-nogo task. A finer measure of task difficulty that is currently absent in our system is reaction time. Although we did not measure reaction time here, it can be measured using ARTSy (see Appendix).
Although we specifically demonstrated the success of ARTSy for training zebra finches to classify vocalizations, ARTSy is designed for use with any amenable species and stimuli that investigate sensory perception in any modality. The detectors, actuators, stimulus presentation, reward and punishment can be modified independently to optimize the system for a wide range of behavioural experiments. For example, the infrared detectors can be replaced with physical detectors such as switches, buttons, levers, or lickometers. The digital control of the feeder and booth light can be used to control other rewards and punishments such as liquid reward or air puff punishment. The stimulus control and presentation can be modified for use in olfactory or visual experiments, and the sound card used here (see Appendix) could be substituted with a video card to display complex visual stimuli.
ARTSy is designed to conduct multiple, simultaneous behavioural experiments and/or simultaneous behaviour and electrophysiology experiments. The open-source code is written in Matlab, which is broadly used in animal behaviour and neuroscience research. Currently, four training and testing booths can be independently controlled using one computer, but this number could be increased with straightforward modifications to the system. Minimizing the number of computers needed to control multiple training booths allows for high-throughput training and testing, and minimizes the physical space needed to operate a training and testing system.
All of the features in our programs (e.g. timing parameters, training protocols) are standardized for ease of use. The Appendix provides comprehensive descriptions of the other automated training protocols and programs including classical conditioning, two-alternative forced choice training and auditory preference tests.
Conclusions
A central goal of neuroethology is to elucidate the neural and behavioural mechanisms that enable organisms to recognize and discriminate among communication signals that are essential to survival and reproduction. Our auditory recognition training and testing system produces high-throughput, dependable data and provides reliable results among multiple subjects trained on different stimulus types that are consistent with the results of studies using similar methods (Cynx & Nottebohm 1992; Cynx 1993; Cynx 1995; Benney & Braaten 2000; Braaten et al. 2006). The software and hardware components can be easily modified by the user to meet the demands of different behavioural paradigms and analyses. General support for these modifications and instructions for building and running our system are provided in the Appendix. Use of this system will facilitate laboratory studies that contribute to understanding sensory perception in animals.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Woolley lab for feedback on the ARTSy design, Rebekka Dohme for expert technical assistance, Ofer Tchernickovski for help trouble-shooting the software, Virginia Wohl and and Paul Curtin for comments on the manuscript. This work was supported by the National Institute on Deafness and Other Communication Disorders (F31-DC010301, DMS; R01-DC009810, SMNW), the National Science Foundation (IOS-0920081, SMNW) and the National Organization for Hearing Research (SMNW and AV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson R. Operant conditioning and copulation solicitation display assays reveal a stable preference for local song by female swamp sparrows Melospiza georgiana. Behavioral Ecology and Sociobiology. 2009;64:215–223. [Google Scholar]
- Benney KS, Braaten RF. Auditory scene analysis in estrildid finches (Taeniopygia guttata and Lonchura striata domestica): a species advantage for detection of conspecific song. Journal of Comparative Psychology. 2000;114:174–182. doi: 10.1037/0735-7036.114.2.174. [DOI] [PubMed] [Google Scholar]
- Boogert N, Giraldeau L, Lefebvre L. Song complexity correlates with learning ability in zebra finch males. Animal Behaviour. 2008;76:1735–1741. [Google Scholar]
- Braaten RF, Petzoldt M, Colbath A. Song perception during the sensitive period of song learning in zebra finches (Taeniopygia guttata) Journal of Comparative Psychology. 2006;120:79–88. doi: 10.1037/0735-7036.120.2.79. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Woolley SMN. The avian song control system: a model for understanding changes in neural structure and function. In: Parks TN, Rubel EW, Fay RR, Popper AN, editors. Plasticity of the Auditory System. Springer-Verlag; New York: 2004. pp. 228–284. [Google Scholar]
- Campbell DL, Hauber ME. Conspecific-only experience during development reduces the strength of heterospecific song discrimination in zebra finches (Taeniopygia guttata): a test of the optimal acceptance threshold hypothesis. Journal of Ornithology. 2009;151:379–389. [Google Scholar]
- Cynx J. Conspecific song perception in zebra finches (Taeniopygia guttata) Journal of Comparative Psychology. 1993;107:395–402. doi: 10.1037/0735-7036.107.4.395. [DOI] [PubMed] [Google Scholar]
- Cynx J. Similarities in absolute and relative pitch perception in songbirds (starling and zebra finch) and a nonsongbird (pigeon) Journal of Comparative Psychology. 1995;109:261–267. [Google Scholar]
- Cynx J, Nottebohm F. Role of gender, season, and familiarity in discrimination of conspecific song by zebra finches (Taeniopygia guttata) Proceedings of the National Academy of Sciences. 1992;89:1368–1371. doi: 10.1073/pnas.89.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynx J, Williams H, Nottebohm F. Timbre discrimination in zebra finch song syllables. Journal of Comparative Psychology. 1990;104:303–308. doi: 10.1037/0735-7036.104.4.303. [DOI] [PubMed] [Google Scholar]
- Dent ML, Welch TE, Mcclaine EM, Shinn-Cunningham BG. Species differences in the identification of acoustic stimuli by birds. Behavioural Processes. 2008;77:184–190. doi: 10.1016/j.beproc.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Lohr B. Auditory temporal resolution in the Zebra Finch (Taeniopygia guttata): a model of enhanced temporal acuity. Ornithological Science. 2006;5:15–22. [Google Scholar]
- Dooling RJ, Lohr B, Dent ML. Hearing in birds and reptiles. In: Dooling RJ, Popper AN, Fay RR, editors. Comparative Hearing: Birds and Reptiles. Springer-Verlag; New York: 2000. pp. 308–359. [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annual Review of Neuroscience. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Feher O, Wang H, Saar S, Mitra PP, Tchernichovski O. De novo establishment of wild-type song culture in the zebra finch. Nature. 2009;459:564–568. doi: 10.1038/nature07994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmeier W, Birkhead TR. Repeatability of mate choice in the zebra finch: consistency within and between females. Animal Behaviour. 2004;68:1017–1028. [Google Scholar]
- Gallistel CR, Fairhurst S, Balsam P. The learning curve: implications of a quantitative analysis. Proceedings of the National Academy of Sciences. 2004;101:13124–13131. doi: 10.1073/pnas.0404965101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner T, Hulse SH, Bentley GE, Ball GF. Individual vocal recognition and the effect of partial lesions to HVc on discrimination, learning, and categorization of conspecific song in adult songbirds. Journal of Neurobiology. 2000;42:117–33. doi: 10.1002/(sici)1097-4695(200001)42:1<117::aid-neu11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Hauber ME, Campbell DLM, Woolley SMN. The functional role and female perception of male song in zebra finches. EMU. 2010;110:209–218. [Google Scholar]
- Holveck M, Riebel K. Preferred songs predict preferred males: consistency and repeatability of zebra finch females across three test contexts. Animal Behaviour. 2007;74:297–309. [Google Scholar]
- Holveck M, Riebel K. Low-quality females prefer low-quality males when choosing a mate. Proceedings of The Royal Society B: Biological Sciences. 2010;277:153–160. doi: 10.1098/rspb.2009.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houx A, ten Cate C. Song learning from playback in zebra finches: is there an effect of operant contingency? Animal Behaviour. 1999;57:837–845. doi: 10.1006/anbe.1998.1046. [DOI] [PubMed] [Google Scholar]
- Hulse SH. The discrimination-transfer procedure for studying auditory perception and perceptual invariance in animals. In: Klump GM, Dooling RJ, Fay RR, Stebbins WC, editors. Methods in Comparative Psychoacoustics. Birkhäuser Verlag; Basel: 1995. pp. 319–330. [Google Scholar]
- Lohr B, Dooling RJ. Detection of changes in timbre and harmonicity in complex sounds by zebra finches (Taeniopygia guttata) and budgerigars. Journal of Comparative Psychology. 1998;112:36–47. doi: 10.1037/0735-7036.112.1.36. [DOI] [PubMed] [Google Scholar]
- Lohr B, Dooling RJ. Auditory temporal resolution in the Zebra Finch (Taeniopygia guttata): A model of enhanced temporal acuity. Ornithological Science. 2006;5:15–22. [Google Scholar]
- Nagel KI, McLendon HM, Doupe AJ. Differential influence of frequency, timing and intensity cues in a complex acoustic categorization task. Journal of Neurophysiology. 2010;104:1426–1437. doi: 10.1152/jn.00028.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njegovan M, Hilhorst B, Ferguson S, Weisman R. A motor-driven feeder for operant training in song birds. Behavior Research Methods, Instruments & Computers. 1994;26:26–27. [Google Scholar]
- Nordeen EJ, Nordeen KW. Circuits and cellular mechanisms of sensory acquisition. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Cambridge University Press; Cambridge: 2008. pp. 256–270. [Google Scholar]
- Okanoya K, Dooling RJ. Obtaining acoustic similarity measures from animals: a method for species comparisons. Journal of the Acoustical Society of America. 1988;83:1690–1693. doi: 10.1121/1.395927. [DOI] [PubMed] [Google Scholar]
- Okanoya K, Dooling RJ. Perception of distance calls by budgerigars (Melopsittacus undulatus) and zebra finches (Poephila guttata): assessing species-specific advantages. Journal of Comparative Psychology. 1991;105:60–72. doi: 10.1037/0735-7036.105.1.60. [DOI] [PubMed] [Google Scholar]
- Riebel K. Song and female mate choices in zebra finches: a review. In: Naguib M, Janik V, Clayton N, Zuberbuhler K, editors. Advances in the Study of Behavior. Academic Press; London: 2009. pp. 197–238. [Google Scholar]
- Riebel K, Slater PJB. Testing female chaffinch preferences by operant conditioning. Animal Behaviour. 1998;56:1443–1453. doi: 10.1006/anbe.1998.0933. [DOI] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F, Cynx J. Conspecific and heterospecific song discrimination in male zebra finches with lesions in the anterior forebrain pathway. Journal of Neurobiology. 1998;36:81–90. [PubMed] [Google Scholar]
- Spencer KA, Wimpenny JH, Buchanan KL, Lovell PG, Goldsmith AR, Catchpole CK. Developmental stress affects the attractiveness of male song and female choice in the zebra finch (Taeniopygia guttata) Behavioral Ecology and Sociobiology. 2005;58:423–428. [Google Scholar]
- Sturdy CB, Weisman RG. Rationale and methodology for testing auditory cognition in songbirds. Behavioural Processes. 2006;72:265–272. doi: 10.1016/j.beproc.2006.03.007. [DOI] [PubMed] [Google Scholar]
- van Heijningen CA, de Visser J, Zuidema W, ten Cate C. Simple rules can explain discrimination of putative recursive syntactic structures by a songbird species. Proceedings of the National Academy of Sciences. 2009;106:20538–20543. doi: 10.1073/pnas.0908113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzijden MN, Etman E, van Heijningen C, van der Linden M, ten Cate C. Song discrimination learning in zebra finches induces highly divergent responses to novel songs. Proceedings of The Royal Society B: Biological Sciences. 2007;274:295–301. doi: 10.1098/rspb.2006.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario D. Using learned calls to study sensory-motor integration in songbirds. Annals of the New York Academy of Sciences. 2004;1016:246–262. doi: 10.1196/annals.1298.040. [DOI] [PubMed] [Google Scholar]
- Vignal C, Mathevon N, Mottin S. Audience drives male songbird response to partner's voice. Nature. 2004;430:448–451. doi: 10.1038/nature02645. [DOI] [PubMed] [Google Scholar]
- Vignal C, Mathevon N, Mottin S. Mate recognition by female zebra finch: analysis of individuality in male call and first investigations on female decoding process. Behavioural Processes. 2008;77:191–198. doi: 10.1016/j.beproc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Vyas A, Harding C, Borg L, Bogdan D. Acoustic characteristics, early experience, and endocrine status interact to modulate female zebra finches' behavioral responses to songs. Hormones and Behavior. 2009;55:50–50. doi: 10.1016/j.yhbeh.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Warren WC, Clayton DF, Ellegren H, Arnold AP, Hillier LW, Künstner A, Searle S, White S, Vilella AJ, Fairley S, Heger A, Kong L, Ponting CP, Jarvis ED, Mello CV, Minx P, Lovell P, Velho TA, Ferris M, Balakrishnan CN, Sinha S, Blatti C, London SE, Li Y, Lin YC, George J, Sweedler J, Southey B, Gunaratne P, Watson M, Nam K, Backström N, Smeds L, Nabholz B, Itoh Y, Whitney O, Pfenning AR, Howard J, Völker M, Skinner BM, Griffin DK, Ye L, McLaren WM, Flicek P, Quesada V, Velasco G, Lopez-Otin C, Puente XS, Olender T, Lancet D, Smit AF, Hubley R, Konkel MK, Walker JA, Batzer MA, Gu W, Pollock DD, Chen L, Cheng Z, Eichler EE, Stapley J, Slate J, Ekblom R, Birkhead T, Burke T, Burt D, Scharff C, Adam I, Richard H, Sultan M, Soldatov A, Lehrach H, Edwards SV, Yang SP, Li X, Graves T, Fulton L, Nelson J, Chinwalla A, Hou S, Mardis ER, Wilson RK. The genome of a songbird. Nature. 2010;464:757–762. doi: 10.1038/nature08819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H. Birdsong and singing behaviour. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Cambridge University Press; Cambridge: 2008. pp. 32–49. [Google Scholar]
- Zann RA. The zebra finch: a synthesis of field and laboratory studies. Oxford University Press; Oxford: 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.