Abstract
Glycogenic hepatopathy (GH ) is a rare cause of serum transaminase elevations in type 1 diabetes mellitus. We describe a 13-year-old male with a history of poorly controlled type 1 diabetes mellitus who presented with hepatomegaly and severe transaminase flares. Liver histology confirmed GH. Treatment consists of improving glycemic control. Hepatomegaly due to excess glycogen storage in poorly controlled type 1 diabetics has been associated with younger patients with poor glycemic control, occurring about 2-4 weeks after starting insulin treatment, and resolving upon glucose stabilization. We conclude that glycogenic hepatopathy can cause hepatomegaly and significant transaminase elevations in individuals with type I diabetes mellitus, The recovery of severe transaminase elevations in this patient illustrates the more benign course of GH, which is a condition with a far better prognosis. Clinician awareness of GH should prevent diagnostic delay and will provide better insight into the prevalence of GH.
Glycogen hepatopathy (GH) can present with different clinical symptoms and signs, the most dramatic being a syndrome first described by Mauriac in 1930 of growth retardation, hepatomegaly, cushingoid features, and delayed puberty.1 Whereas the Mauriac syndrome was first described, the histologic findings of GH remain underrecognized. We present a case of GH in a patient with poorly controlled type 1 diabetes mellitus. The recovery of severe transaminase elevations in this patient illustrates the more benign course of GH. The aim of this study is to describe the clinical characteristics and pathologic features of GH to improve wider recognition of this disease.
CASE
A 13-year-old male with a 3-year history of type 1 diabetes mellitus. He was treated with glargine and lispro insulin, with an average requirement was 1.2 units/kg/day. His hemoglobin A1c ranged from 7.0% to 13.0% of total hemoglobin. He developed severe increases in transaminase levels that were followed by recovery to normal levels during periods of better metabolic control (Figure 1). Physical examination revealed hepatomegaly. Laboratory analysis was compatible with major aminotransferase disturbances (Figure 1), with concurrent increases in gamma-glutamyl transferase 187 U/L (normal <35 U/L) and alkaline phosphatase 179 U/L (normal <120 U/L). Liver synthetic capacity as measured by serum albumin 33 g/L (normal 34 to 52 g/L), total bilirubin 9.7 umol/L and coagulation tests (activated partial prothrombin time 28.8 seconds (normal <29 seconds) and international normalization ratio 0.9 ( normal <0.9). Total cholesterol 5.74 mmol/L, triglyceride 1.55 mmol/L, low density lipoprotein 2.7mmol/L, Thyroglobulin antibodies 1550 IU/mL (normal<115 IU/mL), microsomal antibodies 56.7 IU/mL (normal < 34 IU/mL). Immunoglobulins A, G, M and E were normal. Serology tests for HIV, hepatitis C and B, cytomegalovirus and infectious mononucleosis were negative. Serum alpha-1 antitrypsin, ceruloplasmin, copper, iron and ferritin levels were in normal ranges. Liver kidney microsomal antibodies, smooth muscle antibodies (ASMA) and mitochondrial antibodies (AMA) were negative. Ultrasound and CT scans of the abdomen showed that the liver measured 19.4 cm in diameter with increased echogenicity and an inhomogenous texture. Liver biopsy showed that there was glycogen accumulation, characterized by hepatocyte swelling, accentuation of cell membranes due to cytoplasmic rarefaction and a strongly positive periodic acid Schiff (PAS, stains polysaccharides) staining (Figure 2). After diastase digestion, which selectively degrades glycogen, PAS staining was no longer positive, confirming that glycogen accumulation was responsible for the findings (Figure 2).
Figure 1.
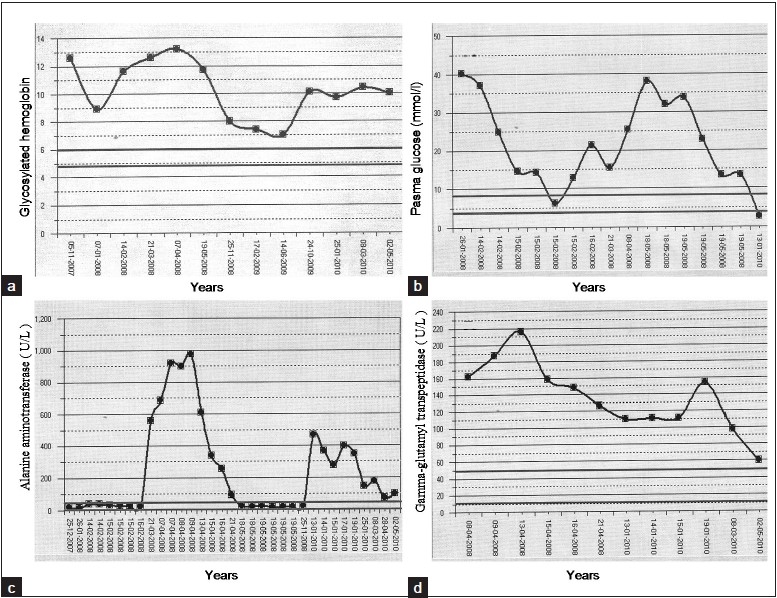
Intermittent rises in glycosylated hemoglobin (a), plasma glucose (mmol/L ) (b), alanine aminotransferase (U/L) (c) and gamma-glutamyl transpeptidase (U/L) (d)
Figure 2.
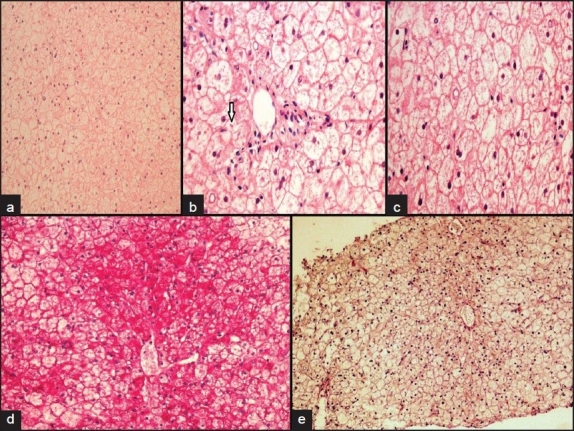
Liver histopathology showed diffuse hepatocyte swelling with rarefaction of cytoplasm and compressed sinusoids (a), intracytoplasmic giant mitochondria seen as round, red to pink globules (arrow) (hematoxylin and eosin stain, ×10) (b), prominent hepatocellular membranes (hematoxylin and eosin stain ×40) (c), abundant cytoplasmic glycogen deposits are demonstrated by a PAS stain ×40 (d), glycogen removed by diastase digestion (10×) (e)
DISCUSSION
The most common liver function tests include the serum aminotransferases such as alanine aminotransferase (ALT) which measures the concentration of intracellular hepatic enzymes that have leaked into the circulation and serve as a marker of hepatocyte injury and gamma-glutamyl transpeptidase, which acts as a marker of biliary function and cholestasis. Children with type 1 diabetes are frequently investigated for hepatic abnormalities. The prevalences of elevated ALT in type 1 (9.5%) and type 2 (12.1%) diabetes patients were both considerably higher than the 2.7% expected in the general population and higher than 5.6% reported at baseline in clinical trials.2–4 This suggests that about 10% of diabetes patients under regular review in secondary care may need further investigation for the causes of elevated ALT. In Egypt, 692 patients with type 1 diabetes showed elevated ALT in 3.9% and abnormal hepatic ultrasound in 4.5%.5 Elevated ALT in type 1 diabetes was slightly more common in males, and appeared to show some association with microalbuminuria and dyslipidemia.2 Diabetes mellitus is associated with non-alcoholic fatty liver disease including its severe form, non-alcoholic steatohepatitis.6,7 Elevated serum transaminases in type 1 as well as type 2 diabetes are most frequently caused by non-alcoholic fatty liver disease.8 In a small case series where patients with liver test abnormalities were investigated in detail, marked accumulation of glycogen and steatohepatitis were demonstrated on liver biopsy.9,10 Glycogen loading of the liver was first documented as a component of Mauriac syndrome in 1930.1 The liver defects observed in Mauriac syndrome can occur without the syndromal features in adults with type 1 diabetes.11–12
The key finding in GH is glycogen accumulation in the liver causing hepatomegaly and elevated liver enzymes, especially transaminases. Hepatomegaly and elevated transaminases are very frequent findings in GH.5,12–19 All patients with GH are on insulin therapy and virtually all patients have type 1 diabetes. The etiology for hepatomegaly is less clear. Hepatomegaly can be a complication of diabetes. Frequent episodes of hyperglycemia and subsequent treatment with insulin cause hepatomegaly due to hepatic glycogen and lipid accumulation in type 1 diabetics.18 Inactivation of glycogen phosphorylase due to hyperglycemia causes inhibition of glycogenolysis and activation of glycogen synthase. This results in glycogen synthesis. Insulin activates glycogen synthase and results in further glycogen accumulation.19 Glycogen production persists for some time after insulin levels have declined. Hepatomegaly due to excess glycogen storage in poorly controlled type 1 diabetics has been associated with younger patients with poor glycemic control, occurring about 2 to 4 weeks after starting insulin treatment, and resolving upon glucose stabilization. Our case show similar clinical and histological features to those described by others.5,12–19
In conclusion, intermittent elevated liver transaminases in patients with type 1 diabetes can be due to GH, a condition with a far better prognosis. Clinician awareness of GH should prevent diagnostic delay and will provide better insight into the prevalence of GH.
REFERENCES
- 1.Mauriac P. Gros ventre, hepatomegalie, troubles de las croissance chez les enfants diabetiques traits depuis plusieurs annes par l’insuline. Gax Hebd Med Bordeaux. 1930;26:402–410. [Google Scholar]
- 2.West J, Brousibl J, Gazis A, Jackson L, Mansell P, Bennett A, et al. Elevated serum alanine transaminase in patients with type 1 or type 2 diabetes mellitus. Q J Med. 2006;99:871–876. doi: 10.1093/qjmed/hcl116. [DOI] [PubMed] [Google Scholar]
- 3.Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh II. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. Br Med J. 2004;328:983–7. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebovitz HE, Kreider M, Freed MI. Evaluation of liver function in type 2 diabetic patients during clinical trials. Diabetes Care. 2002;25:815–21. doi: 10.2337/diacare.25.5.815. [DOI] [PubMed] [Google Scholar]
- 5.El-Karaksy HM, Anwar G, Esmat G, Mansour S, Sabry M, Helmy H, et al. Fouad H. Prevalence of hepatic abnormalities in a cohort of Egyptian children with type 1 diabetes mellitus. Pediatr Diabetes. 2009 Dec 23; doi: 10.1111/j.1399-5448.2009.00627.x. [DOI] [PubMed] [Google Scholar]
- 6.Marchesini G, Brizi M, Morselli-Labate A, Blanchi G, Bugianesi E, McCullogh A, et al. Association of non-alcoholic fatty liver disease and insulin resistance. Am J Med. 1999;107:450–5. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 7.Matteoni C, Younossi Z, Gramlich T, Bopari N, Liu Y, McCullough N. Nonalcoholic fatty liver disease: a spectrumof clinical and pathological severity. Gastroenterology. 1999;116:1413–19. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 8.Angulo P. Nonalcoholic fatty liver disease. N Engl J M. (1) 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 9.Olsson R, Wesslau C, William-Olsson T, Zettergren L. Elevated aminotransferases and alkaline phosphatases in unstable diabetes mellitus without ketoacidosis or hypoglycemia. J Clin Gastroenterol. 1989;11:541–545. doi: 10.1097/00004836-198910000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Munns CF, McCrossin RB, Thomsett MJ, Batch J. Hepatic glycogenosis: reversible hepatomegaly in type 1 diabetes. J Paediatr Child Health. 2000;36:449–452. doi: 10.1046/j.1440-1754.2000.00547.x. [DOI] [PubMed] [Google Scholar]
- 11.Torbenson M, Chen YY, Brunt E, Cummings OW, Gottfried M, Jakate S, et al. Glycogenic hepatopathy: an underrecognized hepatic complication of diabetes mellitus. Am J Surg Pathol. 2006;30:508–13. doi: 10.1097/00000478-200604000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Chatila R, West AB. Hepatomegaly and abnormal liver tests due to glycogenosis in adults with diabetes. Medicine (Baltimore) 1996;75:327–33. doi: 10.1097/00005792-199611000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Carcione L, Lombardo F, Messina MF, Rosano M, De Luca F. Liver glycogenosis as early manifestation in type 1 diabetes mellitus. Diabetes Nutr Metab. 2003;16:182–184. [PubMed] [Google Scholar]
- 14.Evans RW, Littler TR, Pemberton HS. Glycogen storage in the liver in diabetes mellitus. J Clin Pathol. 1955;8:110–113. doi: 10.1136/jcp.8.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamuta M, Ohashi M, Goto K, Tanabe Y, Hiroshige K, Nawata H. Diabetes mellitusassociated glycogen storage hepatomegaly: report of a case and review of the Japanese literature. Fukuoka Igaku Zasshi. 1993;84:354–358. [PubMed] [Google Scholar]
- 16.Torres M, Lopez D. Liver glycogen storage associated with uncontrolled type 1 diabetes mellitus. J Hepatol. 2001;35:538. doi: 10.1016/s0168-8278(01)00132-5. [DOI] [PubMed] [Google Scholar]
- 17.Van den Brand M, Elving LD, Drenth JP, van Krieken JH. Glycogenic hepatopathy: a rare cause of elevated serum transaminases in diabetes mellitus. Neth J Med. 2009;67:394–6. [PubMed] [Google Scholar]
- 18.Abaci A, Bekem O, Unuvar T, Ozer E, Bober E, Arslan N, et al. Hepatic glycogenosis: A rare cause of hepatomegaly in type 1 diabetes mellitus. Journal of Diabetes and Its Complications. 2008;22:325–8. doi: 10.1016/j.jdiacomp.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Van Steenbergen W, Lanckmans S. Liver disturbances in obesity and diabetes mellitus. International Journal of Obesity. 1995;S3:S27–S36. [PubMed] [Google Scholar]


