Abstract
In 2009, consensus guidelines were published on intensification of insulin therapy using the premix analog biphasic insulin aspart (BIAsp) 30 in the treatment of type 2 diabetes, based on the recommendations of an international, independent expert panel. The guidelines included recommendations and titration algorithms for intensification from basal insulin once (OD) or twice daily (BID) to BIAsp 30 BID, from OD BIAsp 30 to BID, and from BID BIAsp 30 to three times daily (TID). Building on these recommendations, the objective was to develop similar, simple and effective guidelines for intensification switch from a BIAsp 30 to a mid-/high-ratio premix regimen for the vast majority of patients with type 2 diabetes. A panel of independent experts with extensive clinical experience in premix analog therapy met in October 2009 to review the therapeutic role of mid- and high-ratio premixes (BIAsp 50 and 70, respectively). The panel outlined a series of algorithms for intensifying BIAsp 30 BID and TID with mid-/high-ratio premixes, along with practical suggestions relating to intensification for individual patients. A simple tool to aid dose adjustment was also developed. The guidelines suggested here should assist physicians in introducing mid-/high-ratio premixes to optimize the insulin therapy of patients with type 2 diabetes who are failing to achieve glycemic targets on a BIAsp 30 BID or TID regimen.
Keywords: BIAsp 30, 50 and 70; high-ratio premix; insulin analog; insulin intensification; mid-ratio premix; premix insulin; type 2 diabetes
INTRODUCTION
The progressive nature of type 2 diabetes makes achieving and maintaining glycemic control a challenge for both patients and physicians. The gradual loss of endogenous insulin secretion due to deterioration of beta-cell function and the associated insulin resistance results in the majority of patients being unable to maintain A1C targets on a treatment regimen of lifestyle changes or oral antidiabetic drugs (OADs). The addition of insulin therapy is an important step towards achieving long-term glycemic control and minimizing the risk of micro- and macrovascular complications and all-cause mortality.
However, even with insulin therapy, suboptimal glycemic control persists, with many patients remaining on unmodified treatment regimens for too long. Guidelines emphasize the importance of modifying treatment regimens when A1C goals have not been attained; and that modifications should be implemented before loss of glycemic control occurs and should be individually tailored to patient needs. When treatment is actively titrated, assessed and intensified, patients are more likely to achieve glycemic targets.[1]
Insulin premixes are commonly prescribed in the treatment of diabetes, addressing both prandial and basal insulin needs with a single product; however, international recommendations for intensification of insulin therapy using premix analogs are limited. In 2009, consensus guidelines were published on intensification of insulin therapy using the premix analog biphasic insulin aspart (BIAsp) 30 in the treatment of type 2 diabetes, based on the recommendations from an international, independent expert panel.[2] Building on these recommendations, a review of the therapeutic role of mid- and high-ratio premixes (BIAsp 50 and 70, respectively) followed, with the objective of developing similar simple and effective guidelines for intensification switch from a BIAsp 30 to a mid-/high-ratio premix regimen for the vast majority of patients with type 2 diabetes.
INSULIN THERAPY AND THE IMPORTANCE OF POSTPRANDIAL PLASMA GLUCOSE REGULATION
A1C levels continue to be the most widely used predictor of future risk of diabetes-related complications, but highly variable postprandial surges in plasma glucose levels may contribute to worse clinical outcomes than relatively stable plasma glucose levels with the same A1C value.[3] Monitoring of plasma glucose levels is therefore essential to detect glycemic excursions leading to hyper- or hypoglycemia. The importance of controlling fasting plasma glucose (FPG) to maintain A1C at recommended levels is well established,[4] but there is accumulating evidence for the role of elevated postprandial plasma glucose (PPG) as an independent risk factor for cardiovascular events.[5,6] There is also evidence for an association between post-meal hyperglycemia and oxidative stress, inflammation, carotid intima-media thickness and endothelial dysfunction, all of which are known markers of cardiovascular disease,[7] retinopathy[8] and cognitive dysfunction.
An analysis of the diurnal glycemic profiles of patients with type 2 diabetes with poor glycemic control (A1C>10%) showed that PPG accounted for 30% of hyperglycemia, with this proportion increasing to 50–70% in patients with A1C values of <7.3%, highlighting the need for targeted control of postprandial peaks of hyperglycemia if overall tight glycemic control is to be achieved.[5] Reaching target A1C with type 2 diabetes is frequently impossible without addressing PPG. As A1C approaches normal values and the relative contribution of PPG to A1C increases, treatment of both prandial and fasting glucose is required at all times for optimal glycemic control.[9]
WHY ARE MID-/HIGH-RATIO PREMIXES NEEDED?
Modern premix insulin formulations, with a fast-acting insulin component in addition to a basal component, are designed to address postprandial excursions and overall glycemic control, and thereby enhance achievement of A1C targets.[1] While a basal–bolus injection regimen, comprising a rapid-acting insulin analog administered with meals together with a long-acting insulin taken once or twice daily, is a popular strategy for intensifying insulin therapy in the treatment of type 2 diabetes, the number of injections (≥4 daily) required can be considered too intrusive or too complex by some patients, particularly the elderly and those with busy lifestyles. Premix insulin analogs such as biphasic insulin aspart can offer effective and simpler treatment options and are becoming widely used in many countries.
BIAsp combines rapid-acting soluble insulin aspart (IAsp), which addresses prandial insulin needs, with protamine crystallized insulin aspart, which has a delayed action of intermediate duration, for both mealtime and basal insulin requirements. For many patients with type 2 diabetes, good glycemic control can be attained on a regimen of twice- or three-times-daily BIAsp 30.[1] This low-ratio formulation of premix insulin analog comprises 30% IAsp and 70% protaminated aspart. For some patients taking low-ratio premixes, however, glycemic targets are still not achieved, which may reflect the increasing need to address the contribution of PPG as A1C improves.[5] Uptitration of the dose of BIAsp 30 in these patients would increase the amount of protaminated insulin with each additional injection and may induce hypoglycemia. There is therefore a requirement for premix analogs that can be injected three times daily and can effectively address poorly controlled PPG levels.
Two BIAsp preparations have been formulated with increased proportions of soluble, rapid-acting IAsp: BIAsp 50 and BIAsp 70, comprising 50% and 70% soluble IAsp respectively (the remainder of each being protaminated aspart). This range of formulations allows scope for individualizing insulin therapy, and for targeting poor postprandial glycemic control in patients who require more prandial insulin in order to reach glycemic targets.
TARGETS FOR GLYCEMIC CONTROL
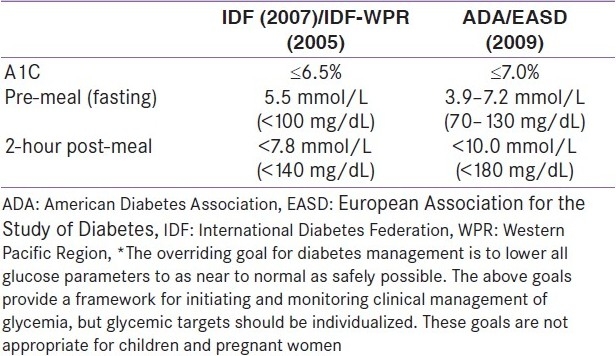
Current glycemic targets for type 2 diabetes for Europe (set by the International Diabetes Federation and the EASD) and the USA (set by the ADA) are challenging [Table 1]. These targets reflect the proven association between reductions in A1C and reduced risk of diabetic complications.
Table 1.
Glycemic goals for clinical management of diabetes*
CLINICAL EVIDENCE FOR MID-/HIGH-RATIO PREMIX ANALOGS FOR INTENSIFICATION OF INSULIN THERAPY
A detailed review of the pharmacology and clinical performance of mid- and high-ratio premix formulations and how they differ from the low-ratio premix insulins has been discussed in a recent publication.[10] In order to establish the boundaries of the guidelines to be developed, the panel of experts considered the following evidence.
Pharmacology of mid-/high-ratio premix analogs
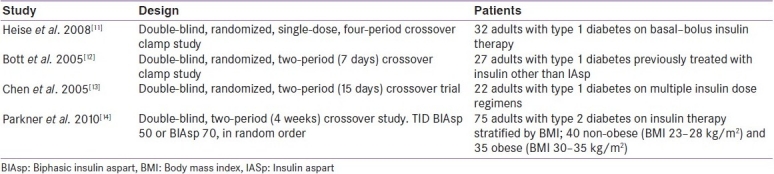
Table 2 outlines the studies comparing the pharmacokinetic and pharmacodynamic properties of BIAsp 30, 50 and 70 reviewed and discussed prior to the development of the guidelines.
Table 2.
Studies comparing the pharmacokinetic and pharmacodynamic properties of BIAsp 30, 50 and 70 reviewed for guideline development
The pharmacodynamics and pharmacokinetics of BIAsp 30, 50 and 70 and IAsp were compared in a glucose clamp trial on 32 patients with type 1 diabetes on basal–bolus therapy.[11] Each patient underwent four glucose clamps and received a single dose of 0.4 U/kg BIAsp 30, 50 or 70 and IAsp. As the percentage of rapid-acting, soluble IAsp increased in the BIAsp formulations, ‘early-phase’ (area under the curve glucose infusion rate [AUCGIR] 0–6 h: post-injection) metabolic activity increased (ratio BIAsp 50/BIAsp 30 = 1.28 [P<0.001]; BIAsp 70/BIAsp 50 = 1.18 [P<0.001]; IAsp/BIAsp 70 = 1.15 [P<0.01]). Conversely, ‘late-phase’ metabolic activity decreased, shown by significantly lower ratios of AUCGIR 12–28 h (BIAsp 50/BIAsp 30 = 0.17 [P<0.01]; BIAsp 70/BIAsp 50 = 0.21 [P<0.05]). This was a result of the smaller percentages of intermediate-acting protaminated insulin aspart in these preparations [Figure 1].[11]
Figure 1.
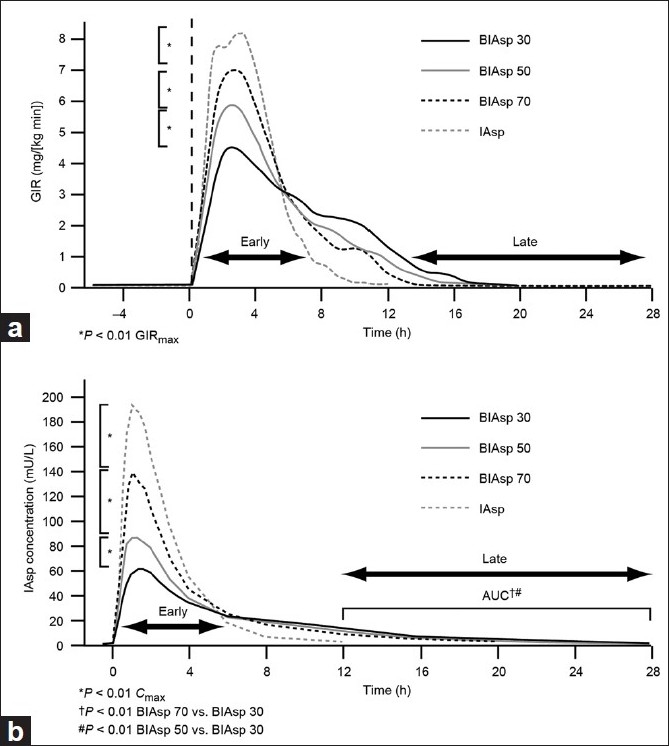
Pharmacodynamic and pharmacokinetic profiles for biphasic insulin aspart (BIAsp) 30, 50 and 70 and insulin aspart (IAsp). (a) Glucose infusion rate (GIR) profiles reflect the ratio of IAsp/protaminated IAsp in the formulations. (b) The early IAsp level increases and the late IAsp level decreases with a higher percentage of soluble aspart in the formulation. AUC, area under the curve; Cmax, maximum concentration Reprinted with permission from Heise T, Eckers U, Kanc K, Nielsen JN, Nosek L. The pharmacokinetic and pharmacodynamic properties of different formulations of biphasic insulin aspart: a randomized, glucose clamp, crossover study. Diabetes Technol Ther 2008;10:479-485. The publisher for this copyrighted material is Mary Ann Liebert, Inc. publishers
Similar results were reported when comparing three-times-daily (TID) BIAsp 30 with BIAsp 70 during a 7-day, two-period crossover clamp study, also in patients with type 1 diabetes.[12] The larger fraction of soluble IAsp in BIAsp 70 compared with BIAsp 30 resulted in greater metabolic effect during the initial phase (AUCGIR0–6 h: ratio BIAsp 30/BIAsp 70 = 0.86; 95% CI [0.76, 0.97]; P 0.016) and decreased activity during the late phase (AUCGIR6–12 h: 1.90; 95% CI [1.37, 2.64]; P 0.001). AUCGIR 0–12 h did not differ between formulations over 8 days but slightly higher IAsp levels over the days also resulted in higher GIR peaks, indicating the accumulation of metabolic effect.[12]
A comparison of the pharmacokinetic characteristics of BIAsp 30 and BIAsp 70 during 15 days of multiple dosing (TID) reported early postprandial phase insulin AUC0–6 h to be significantly higher for BIAsp 70 than for BIAsp 30 (ratio BIAsp 30/BIAsp 70 = 0.72; 95% CI [0.63, 0.83]; P<0.001). AUC6–14 h was markedly lower for BIAsp 70 than for BIAsp 30 (BIAsp 30/BIAsp 70 = 1.9; 95% CI [1.42, 2.55]; P<0.002).[13] The time to maximum insulin concentration (Tmax) was significantly shorter after all three meals with BIAsp 70 (P<0.05). Peak levels for both BIAsp 30 and BIAsp 70 were seen to increase with each daily injection over this 15-day period, suggesting a degree of accumulation that needs to be taken into consideration if a TID dose is to be prescribed.[13]
A further comparison in patients with type 2 diabetes also reported the profiles of BIAsp 50 and BIAsp 70 to be as expected based on their respective content of protamine-bound IAsp (50% and 30%).[14] The study groups were stratified by body mass index (BMI) [Table 2] and AUCGlu0–24 h: BIAsp 50/BIAsp 70 were 0.97; 95% CI [0.90, 1.05]; P 0.49 for non-obese and 0.98; 95% CI [0.92, 1.05]; P 0.55 for obese patients. In both BMI groups FPG was high, significantly more so with BIAsp 70 than with BIAsp 50 (P<0.01), suggesting that BIAsp 30 should be considered as the evening dose for a TID regimen.[14]
In conclusion, the pharmacological profiles of BIAsp 30, 50 and 70 differ significantly, reflecting the different proportions of free and protaminated IAsp in the formulations, with a greater proportion of free IAsp resulting in higher early-phase metabolic effect and lower late-phase metabolic effect. These pharmacokinetic properties of BIAsp 30 and 70 h ave been shown to remain constant during multiple dosing. These differences in metabolic activity may offer the potential to address individual patient requirements, but how does this translate into clinical practice?
CLINICAL EVIDENCE FOR MID-/HIGH-RATIO PREMIX ANALOGS: ADDRESSING THE NEED FOR MORE PRANDIAL INSULIN
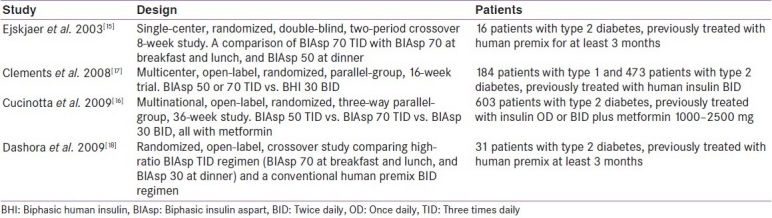
Twice-daily (BID) biphasic human insulin (BHI) 30 therapy or once-daily (OD) or BID basal insulin therapy are widely used in the treatment of type 2 diabetes, but a number of studies have reported that lower mean daily blood glucose levels can be achieved with mid-/high-ratio premix analog regimens. The clinical studies reviewed prior to guideline development discussion are shown in Table 3.
Table 3.
Studies comparing the clinical properties of BIAsp 30, 50 and 70, reviewed for guideline development
In a proof-of-concept study, 16 patients with type 2 diabetes maintaining stable glucose control on prestudy treatment of BID BHI 30 therapy were randomized to treatment of BIAsp 70 TID or BIAsp 70–70–50, for 26–30 days, dividing their prestudy dose into three equal doses. There were no significant differences between treatments in mean serum glucose level; however, AUCGlu 0–24 h was lower with the TID BIAsp 70 regimen compared with the BIAsp 50 evening dose regimen (not significant). The effect of changing the dinner insulin dose to BIAsp 50 did not alter overall glucose control significantly from that provided with an evening dose of BIAsp 70. Exploratory analysis comparing pre- and post-study serum insulin levels showed that the high-ratio premix treatments provided lower daytime glucose excursions and improved daytime control compared with BID BHI, but morning fasting glucose levels indicated that an evening dose of low-ratio premix may optimize overnight glycemic control.[15]
In a treat-to-target trial, adults with type 2 diabetes previously treated with OD or BID insulin (human or analog) for ≥3 months, plus metformin, were randomized to treatment with BIAsp 70 TID, BIAsp 50 TID or BIAsp 30 BID for 36 weeks (all in combination with metformin).[16] Patients switched dose 1:1 from previous total insulin dose, split 25%–25%–50% (BIAsp 30: 50%–50%). Insulin in all groups was titrated based on pre-meal plasma glucose measurements. Patients being treated with BIAsp 50 or 70 switched to BIAsp 30 at dinnertime after 12 weeks if pre-breakfast plasma glucose was >7.0 mmol/L (43% in the BIAsp 50 and 54% BIAsp 70 group). All three BIAsp preparations effectively lowered A1C from baseline and after 36 weeks of treatment A1C was significantly lower in the BIAsp 50:50:50(30) arm than in the BIAsp 30:30 arm: –0.3; 95% CI [–0.47, –0.14]; P 0.0004. No significant differences were found between BIAsp 70:70:70(30) and BIAsp 30:30. Reductions in plasma glucose were demonstrated with all three treatments, but greater reductions were seen with the high-ratio BIAsp preparations together with lower prandial increments than with BIAsp 30.[16]
In another trial, adults with type 1 diabetes or type 2 diabetes previously treated with human premix insulin BID for ≥3 months (±OADs) were randomized to receive BIAsp 50 or BIAsp 70 TID (BIAsp 50 if BMI >30 kg/m2; BIAsp 70 if BMI ≤30 kg/m2) or BHI 30 BID for 16 weeks.[17] A1C, the primary efficacy endpoint, was shown to be significantly lower for the BIAsp treatment group (pooled BIAsp 50 and BIAsp 70 data) compared with the BHI 30 treatment group (–0.32; 95% CI [–0.48, –0.16]; P 0.0001). Average blood glucose level was significantly lower in the BIAsp group compared with the BHI 30 group (mean –0.79; 95% CI [–1.17, –0.40]; P 0.0001).[17]
During a 60-day crossover study, patients with type 2 diabetes were randomized to BIAsp TID before meals: BIAsp 70 before breakfast and lunch, and BIAsp 30 before dinner (BIAsp 70, 70, 30) or to BHI 30 BID before meals.[18] A 24-hour in-patient plasma glucose profile was performed at the end of each 30-day treatment period. Compared with the human premix BID treatment, the BIAsp TID regimen showed lower plasma glucose concentration, reduced post-lunch glucose excursions and a better pharmacokinetic profile.[18]
Overall, these study results demonstrate that daily blood glucose profile and A1C in subjects with type 2 diabetes can be significantly improved with BIAsp 50 or 70 compared with human premix; switching from BHI 30 to a mid- or high-ratio premix analog may therefore be an attractive option for patients with type 2 diabetes in need of treatment optimization. BIAsp 30 was generally found to be more suitable for evening administration.
A TID BIAsp regimen has also been compared with basal-bolus therapy using TID IAsp plus OD NPH insulin.[19] Following 4 months of treatment, glycemic control was found to be comparable between regimens. Treatment intensification can be associated with an increased risk of hypoglycemia; however, the incidence of hypoglycemia and adverse events was similar for both treatment groups in this study, as was end-of-study weight gain (~2 kg for both treatment groups).
As the numbers of studies reporting the use of mid-/high-ratio premix formulations are few, data on infrequent events such as hypoglycemic and adverse events are far from being conclusive. However, the available data do indicate that tighter postprandial glycemic control can be achieved with these formulations when compared with premix human insulin, without compromising safety.
CONSIDERATIONS FOR GUIDELINE DEVELOPMENT
While there is a need for further clinical data, there is sufficient evidence for the development of treatment guidelines that are currently lacking for this class of insulin.
The pharmacological profiles of BIAsp 50 and BIAsp 70 differ significantly, reflecting the different proportions of free and protaminated IAsp in the formulations; a greater proportion of free IAsp results in higher early-phase metabolic effect and lower late-phase metabolic effect.
Evidence from clinical studies on mid- and high-ratio premix formulations suggests that the effects of these two treatments cannot be considered as interchangeable due to their different pharmacokinetic/pharmacodynamic profiles, although neither showed superiority.
For the purposes of the guidelines, the assumption was made that treatment would have the same effect on individuals with type 1 or type 2 diabetes, although primary users are anticipated to be patients with type 2 diabetes.
Differences with respect to the risk of hypoglycemia have been reported for the mid-/high-ratio premix formulations compared with those experienced with low-ratio premix. Hypoglycemic risk could be addressed by the inclusion of BIAsp 30 as the evening dose.
-
Patients with poorly controlled glucose levels on a regimen of BID or TID premix insulin were considered likely to benefit from switching to mid-/high-ratio premix formulations.
-
–Patients with normal FPG but elevated PPG may derive the most benefit from BIAsp 70 therapy, as this has the greatest proportion of prandial insulin.
-
–Patients with elevated FPG and PPG levels may benefit most from BIAsp 50 therapy, as it has equal proportions of prandial and basal insulin analog.
-
–Allocation to treatment on the basis of BMI appears arbitrary; thus there are no clear benefits to including consideration of obesity in development of the guidelines. Physicians should therefore continue to use their judgement and treat each case on an individual basis.
-
–Patients are often switched from other premix insulins on a 1:1 basis, but new strategies may be required depending on the needs of the individual patient and their prior regimen.
-
–What is the best practice for dividing the daily dose between meals?
-
–
PRACTICAL GUIDELINES FOR INSULIN INTENSIFICATION WITH MID-/HIGH-RATIO PREMIX THERAPY
Box 1 outlines points to consider before intensification with mid-/high-ratio premix therapy.
Box 1.
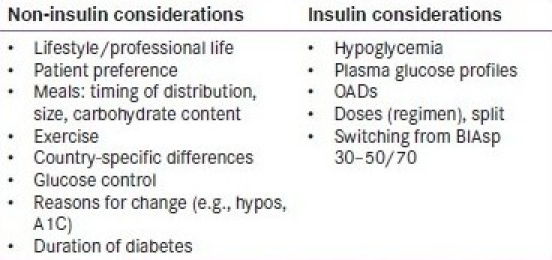
Checklist of useful points to consider prior to intensifying a patient's therapy from BIAsp 30 BID or three-times-daily with mid-/high-ratio premix.
Box 2 provides some practical guidance for intensifying a patient's therapy from BIAsp 30 BID or TID with high-ratio premix.
Box 2.

Practical guidance for intensifying from BIAsp 30 BID or three-times-daily to high-ratio premixes.
Intensifying BIAsp 30 BID with mid-/high-ratio premix insulin analogs
Figure 2 shows a simple algorithm for intensification of BIAsp 30 BID with mid- or high-ratio premix. In this algorithm, a starting A1C of 10% is taken as the intensification trigger value, as recommended in the ADA guidelines. A two-step intensification protocol can be used where PPG >10 mmol/L, switching to a daily regimen of OD BIAsp 50 + OD BIAsp 30. If this option is taken, it is logical to change the breakfast dose rather than the evening dose to BIAsp 50, since dosing with the mid-ratio premix in the evening has the potential to increase FPG as per the clinical data.[15]
Figure 2.
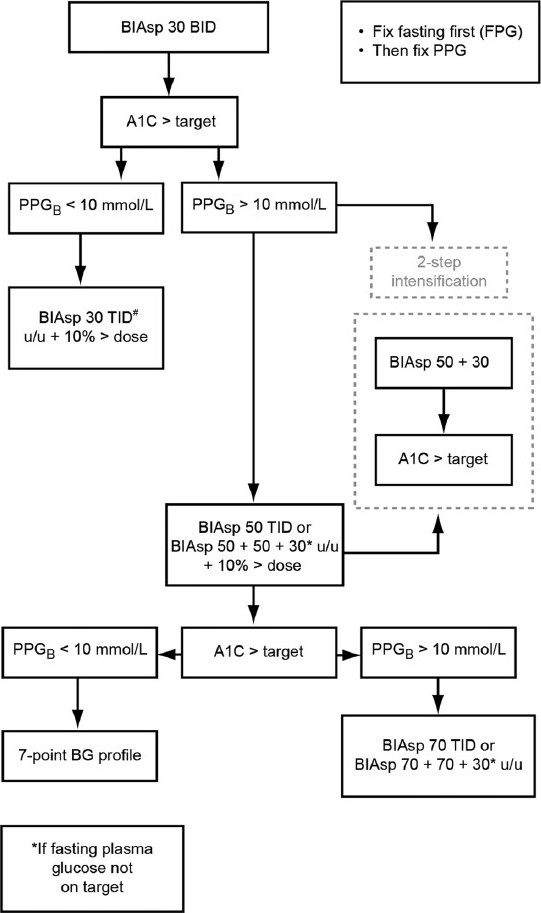
A simple algorithm for the intensification of biphasic insulin aspart (BIAsp) 30 twice daily (BID) with high-ratio premix. BG, blood glucose; FPG, fasting plasma glucose; PPG, postprandial blood glucose; PPGB, post-breakfast value; PPGL, post-lunch value; TID, three times daily; u/u, unit/ unit; u/u + 10% > dose, unit/unit plus 10% of total dose (added at lunch). #Most probable final dose
Intensifying BIAsp 30 TID with mid-/high-ratio premix insulin analogs
Figure 3 shows an algorithm for the recommendations for intensification of BIAsp 30 TID with mid-/high-ratio premix. Options are to switch all the patient's daily injections at once, or to concentrate on one mealtime injection switch at a time and then optimize this particular dose. In subjects on the high-mix regimens of BIAsp 50–50–50 with insufficient nightly control, as reflected in an FPG level >7.0 mmol/L (126 mg/dL), the dinner injection can be substituted with BIAsp 30. This decision is to be made at the physician's discretion; seven-point blood glucose profiles, if available, are a useful tool to help determine mealtime dosing.
Figure 3.
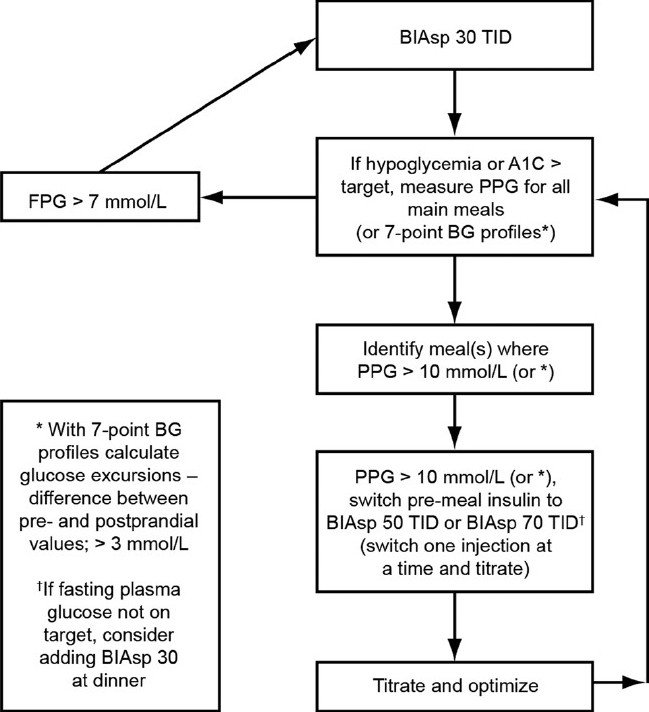
Recommendations for the intensification of biphasic insulin aspart (BIAsp) 30 three times daily (TID) with high-ratio premix. BG, blood glucose; FPG, fasting plasma glucose; PPG, postprandial blood glucose. *Excursions: difference between pre- and postprandial values
Titration of mid-/high-ratio premix insulin analogs
The algorithm illustrated in Table 4a is adapted from the NovoMix® 30 expert panel meeting, October 2009.[2,20] When using this algorithm to intensify from BIAsp 30 BID or TID, the following guidelines are recommended. Notable distinctions from the 2009 guidelines for intensification with BIAsp 30 are that no further dose increments are recommended over a pre-meal plasma glucose of >6.2 mmol/L (>111 mg/dL), and that a PPG algorithm has been included [Table 4b].
Table 4.
Algorithms for titration of mid-/high-ratio biphasic insulin aspart premixes (a) Titration based on pre-meal plasma glucose of mid-/high-ratio premixes. Adapted with permission from the low-ratio premix (NovoMix® 30) international expert panel meeting and consensus statement[2] and from the NovoMix® 30 Summary of Product Characteristics[20]
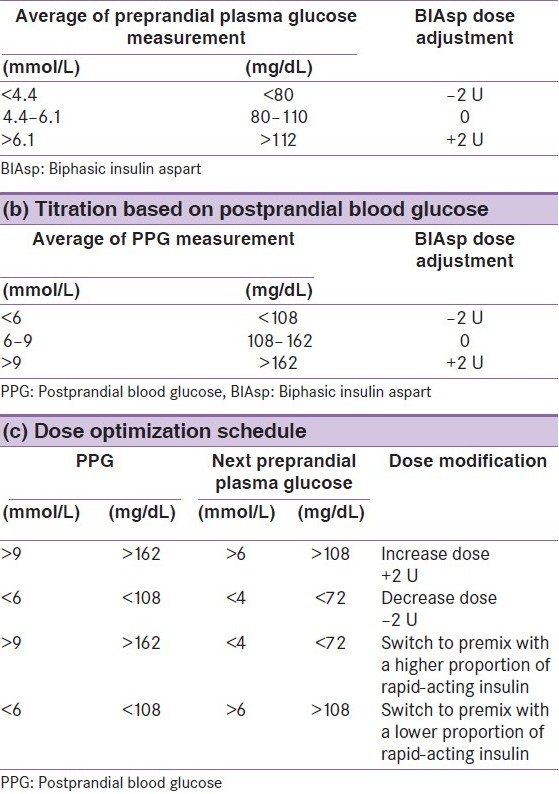
Titrate all three doses according to the algorithm in Table 4, changing only one dose at a time. Weekly titration is recommended (Box 2).
For pre-meal measurement, change the insulin dose prior to the preceding meal.
For post-meal measurement (PPG), change insulin prior to that particular meal.
Table 4c shows the dose optimization schedule.
In patients whose PPG is above target but whose FPG is acceptable, physicians may consider switching to a premix insulin with a higher proportion of rapid-acting insulin. Conversely, when PPG is falling below target measurements and FPG is above them, switching patients to a premix with a lower proportion of rapid-acting insulin is recommended.
RECOMMENDATIONS FOR POSTPRANDIAL PLASMA GLUCOSE MANAGEMENT
Incremental glucose peaks are frequent in type 2 diabetes and occur for most patients (95%) within 1 hour post-meal; the timing of these peaks is therefore not influenced by treatment intervention, be it lifestyle or pharmaceutical. The timing of PPG measurement is recommended to be not more than 120 minutes from the start of the meal and within 90 minutes of its end.
Post-meal plasma glucose should not exceed 9 mmol/L (162 mg/dL) as long as hypoglycemia is avoided.
Self-monitoring of blood glucose should be encouraged because it is currently the most practical method for monitoring post-meal glycemia.
Efficacy of treatment regimens should be monitored as frequently as required to guide therapy towards achieving post-meal plasma glucose target.
CONSIDERATIONS FOR DOSING AND TITRATION BIASP 50 AND BIASP 70
Down-titration is recommended if major or recurrent minor hypoglycemia occurs. Table 4 illustrates dose adjustments based on pre-meal FPG values.
Guidance is aimed at the typical patient with type 2 diabetes and assumes no metabolic decompensation (diabetic ketoacidosis, extreme hyperglycemia, fluctuating glucose levels).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Garber AJ, Wahlen J, Wahl T, Bressler P, Braceras R, Allen E, et al. Attainment of glycaemic goals in type 2 diabetes with once-, twice-, or thrice-daily dosing with biphasic insulin aspart 70/30 (the 1-2-3 study) Diabetes Obes Metab. 2006;8:58–66. doi: 10.1111/j.1463-1326.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- 2.Unnikrishnan AG, Tibaldi J, Hadley-Brown M, Krentz AJ, Ligthelm R, Damci T, et al. Practical guidance on intensification of insulin therapy with BIAsp 30: A consensus statement. Int J Clin Pract. 2009;63:1571–7. doi: 10.1111/j.1742-1241.2009.02192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceriello A. Postprandial hyperglycemia and diabetes complications: Is it time to treat? Diabetes. 2005;54:1–7. doi: 10.2337/diabetes.54.1.1. [DOI] [PubMed] [Google Scholar]
- 4.International Diabetes Federation. 2007 Guideline for Management of Postmeal Glucose. [Last accessed on 2010 Dec 9]. Available from: http://www.idf.org/webdata/docs/Guideline_PMG_final.pdf .
- 5.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: Variations with increasing levels of HbA(1c) Diabetes Care. 2003;26:881–5. doi: 10.2337/diacare.26.3.881. [DOI] [PubMed] [Google Scholar]
- 6.Cavalot F, Petrelli A. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: Lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab. 2006;91:813–9. doi: 10.1210/jc.2005-1005. [DOI] [PubMed] [Google Scholar]
- 7.Hanefeld M, Koehler C, Schaper F, Fuecker K, Henkel E, Temelkova-Kurktschiev T. Postprandial plasma glucose is an independent risk factor for increased carotid intima-media thickness in non-diabetic individuals. Atherosclerosis. 1999;144:229–35. doi: 10.1016/s0021-9150(99)00059-3. [DOI] [PubMed] [Google Scholar]
- 8.Shiraiwa T, Kaneto H, Miyatsuka T, Kato K, Yamamoto K, Kawashima A, et al. Post-prandial hyperglycemia is an important predictor of the incidence of diabetic microangiopathy in Japanese type 2 diabetic patients. Biochem Biophys Res Commun. 2005;336:339–45. doi: 10.1016/j.bbrc.2005.08.158. [DOI] [PubMed] [Google Scholar]
- 9.Monnier L, Colette C, Dunseath GJ, Owens DR. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care. 2007;30:263–9. doi: 10.2337/dc06-1612. [DOI] [PubMed] [Google Scholar]
- 10.Christiansen JS, Liebl A, Davidson JA, Ligthelm RJ, Halimi S. Mid- and high-ratio premix insulin analogues: Potential treatment options for patients with type 2 diabetes in need of greater postprandial blood glucose control. Diabetes Obes Metab. 2010;12:105–14. doi: 10.1111/j.1463-1326.2009.01144.x. [DOI] [PubMed] [Google Scholar]
- 11.Heise T, Eckers U, Kanc K, Nielsen JN, Nosek L. The pharmacokinetic and pharmacodynamic properties of different formulations of biphasic insulin aspart: A randomized, glucose clamp, crossover study. Diabetes Technol Ther. 2008;10:479–85. doi: 10.1089/dia.2008.0019. [DOI] [PubMed] [Google Scholar]
- 12.Bott S, Tusek C, Heinemann L, Friberg HH, Heise T. The pharmacokinetic and pharmacodynamic properties of biphasic insulin aspart 70 (BIAsp 70) are significantly different from those of biphasic insulin aspart 30 (BIAsp 30) Exp Clin Endocrinol Diabetes. 2005;113:545–50. doi: 10.1055/s-2005-872852. [DOI] [PubMed] [Google Scholar]
- 13.Chen JW, Lauritzen T, Christiansen JJ, Jensen LH, Clausen WH, Christiansen JS. Pharmacokinetic profiles of biphasic insulin aspart 30/70 and 70/30 in patients with type 1 diabetes: A randomized double-blinded crossover study. Diabet Med. 2005;22:273–7. doi: 10.1111/j.1464-5491.2004.01404.x. [DOI] [PubMed] [Google Scholar]
- 14.Parkner T, Dyrskog SE, Laursen T, Chen JW, Mouritzen U, Brondsted L, et al. Obesity does not influence the unique pharmacological properties of different biphasic insulin aspart preparations in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:414–20. doi: 10.1111/j.1463-1326.2009.01178.x. [DOI] [PubMed] [Google Scholar]
- 15.Ejskjaer N, Rasmussen M, Kamp N, Lindholm A, Christiansen JS. Comparison of thrice daily ‘high’ vs. ‘medium’ premixed insulin aspart with respect to evening and overnight glycaemic control in patients with type 2 diabetes. Diabetes Obes Metab. 2003;5:438–45. doi: 10.1046/j.1463-1326.2003.00299.x. [DOI] [PubMed] [Google Scholar]
- 16.Cucinotta D, Smirnova O, Christiansen JS, Kanc K, Le Devehat C, Wojciechowska M, et al. Three different premixed combinations of biphasic insulin aspart: Comparison of the efficacy and safety in a randomized controlled clinical trial in subjects with type 2 diabetes. Diabetes Obes Metab. 2009;11:700–8. doi: 10.1111/j.1463-1326.2009.01035.x. [DOI] [PubMed] [Google Scholar]
- 17.Clements MR, Tits J, Kinsley BT, Rastam J, Friberg HH, Ligthelm RJ. Improved glycaemic control of thrice-daily biphasic insulin aspart compared with twice-daily biphasic human insulin: A randomized, open-label trial in patients with type 1 or type 2 diabetes. Diabetes Obes Metab. 2008;10:229–37. doi: 10.1111/j.1463-1326.2006.00687.x. [DOI] [PubMed] [Google Scholar]
- 18.Dashora U, Ashwell S, Home P. An exploratory study of the effect of using high-mix biphasic insulin aspart in people with type 2 diabetes. Diabetes Obes Metab. 2009;11:680–7. doi: 10.1111/j.1463-1326.2008.01024.x. [DOI] [PubMed] [Google Scholar]
- 19.Ligthelm RJ, Mouritzen U, Lynggaard H, Landin-Olsson M, Fox C, le Devehat C, et al. Biphasic insulin aspart given thrice daily is as efficacious as a conventional basal-bolus insulin regimen with four daily injections: A randomised open-label parallel group four months comparison in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2006;114:511–9. doi: 10.1055/s-2006-924424. [DOI] [PubMed] [Google Scholar]
- 20.NovoMix® 30 Summary of Product Characteristics. [Last accessed on 2010 Sep 23]. Available from: www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/000308/WC500029441.pdf .


