Abstract
Objective:
To assess the validity of Diabetes in Pregnancy Study Group India (DIPSI) guidelines, a modified version of the WHO criterion to diagnose gestational diabetes mellitus (GDM).
Materials and Methods:
A total of 1 463 consecutive pregnant women in the second and third trimester of pregnancy underwent 75 g oral glucose tolerance test (OGTT) and 2-h plasma glucose (PG) was measured by the glucose oxidase-peroxidase (GOD-POD) method. GDM was diagnosed with 2-h PG ≥ 7.8 mmol/L (WHO criteria) and the rest were classified as normal glucose tolerant (NGT) women. GDM women were advised medical nutrition therapy (MNT) for two weeks. Those who failed to reach the target glycemic level of FPG < 5.0 mmol/L and 2-h PG < 6.67 mmol/L with MNT were advised insulin. All of them were followed throughout pregnancy until delivery. Birth weight of 90th percentile (> 3.45 kg) in the neonates was considered as macrosomia (primary outcome).
Results:
The mean maternal age and body mass index were 23.60±3.32 years and 21.5±4.06 kg/m2 respectively. The mean gestational age was 27.9±5.56 weeks. DIPSI criterion identified 196 women (13.4%) as GDM and the rest as NGT. Insulin was required in 19 (9.7%) women with GDM. Macrosomia was observed in 9.9% GDM women with intervention and 9.8% in NGT (P = 1.000).
Conclusion:
DIPSI criterion is a one step-cost effective and evidence-based procedure to diagnose GDM in any socio-economic setting.
Keywords: Diabetes in pregnancy study group India, gestational diabetes mellitus, World Health Organization
INTRODUCTION
Gestational diabetes mellitus (GDM) is characterized by carbohydrate intolerance of varying severity with onset or first recognition during pregnancy.[1] Women with a history of GDM are at increased risk of future diabetes, predominantly type-2 diabetes, as are their children.[2] The extent of this risk depends on diagnostic criteria used to identify GDM.[2] Studies conducted in different populations and with different methodologies, consistently reported an increase in GDM in all race/ethnicity groups, suggesting that there is an increase in GDM prevalence.[3] A true increase in the prevalence of GDM aside from its adverse consequences for the infant in the newborn period might reflect or contribute to the ongoing pattern of increasing diabetes and obesity.[3] This implies that universal screening and care of GDM is of paramount public health priority,[4] rather than risk factor screening.[5] To standardize the diagnosis of GDM, the World Health Organization (WHO) has proposed using a 2-h 75 g OGTT, with a threshold plasma glucose concentration of greater than 7.8 mmol/L at 120 min, similar to that for impaired glucose tolerance (IGT) outside pregnancy.[6] A number of studies have documented that the treatment of gestational diabetes as defined by WHO criterion reduced serious perinatal morbidity and also improved the woman's health-related quality of life.[7–9] Diabetes in Pregnancy Study Group India (DIPSI) diagnostic criterion of 2-h PG ≥ 7.8mmol/L with 75 g oral glucose load is a modified version of WHO, in that the WHO procedure requires women to be in the fasting state, whereas DIPSI procedure is performed in the fasting/nonfasting state irrespective of the last meal timing.[10] Hence, this prospective study was undertaken to ascertain the validity of DIPSI criterion to diagnose GDM based on pregnancy outcome in Indian population.
MATERIALS AND METHODS
The study was initiated with the approval of the Institutional Ethics Committee. The sample size was determined based on the overall prevalence of GDM in Indian population (13.9%)[11] and with 90% statistical power, a total of 1,463 consecutive pregnant women were recruited into the study between April 2009 and February 2010. A standardized questionnaire was used and details pertaining to their anthropometrics, family history, medical, and obstetric history, and other relevant information were collected. Their body mass index (BMI) and blood pressure were recorded. After obtaining the informed consent, pregnant women were given 75 g oral glucose load irrespective of their last meal timing[10,12–14] and venous plasma was drawn at 2 h. The plasma glucose was estimated in the central laboratory by the glucose oxidase peroxidase (GOD-POD) method. Pregnant women with 2-h PG ≥ 7.8 mmol/L (DIPSI criterion)[6,10] were diagnosed as GDM and rest were classified as normal glucose tolerant (NGT) women. GDM women were advised medical nutrition therapy (MNT) for two weeks and those who did not respond to MNT by maintaining FPG ~ < 5.0 mmol/L and peak post meal ~ < 6.7 mmol/L were advised insulin.[15] All of them were followed until delivery. The end point of the primary outcome was the birth weight of the neonates, since the most common and significant neonatal complication clearly associated with gestational diabetes is macrosomia.[16] Birth weight of 90th percentile (>3.45 kg) was considered as macrosomia in Indian population.
Statistical analysis
To compare the mean values between the groups independent t-test was used and for proportions, Chi-square test was employed. Logistic regression was used to examine the level of association of macrosomia with maternal age, gestational age, family history of diabetes, BMI, and GDM status. Analysis was two tailed and P-value < 0.05 was considered statistically significant. Using SPSS package Version 16.0 we performed the statistical analysis.
RESULTS
The mean maternal age of the 1 463 pregnant women was 23.60 ± 3.32 years and BMI was 21.5 ± 4.06 kg/m2. The mean gestational age was 27.9±5.56 weeks. Using the DIPSI criterion of 2-h PG ≥ 7.8 mmol/L, 196 women (13.4%) were diagnosed as GDM. Pregnant women who had family history of diabetes were 18.3%. The percentage of pregnant women who came to the prenatal clinic in the second trimester was 52% and in the third trimester was 48%. Insulin was given in 19 (9.7%) women with GDM, who failed to respond to MNT.
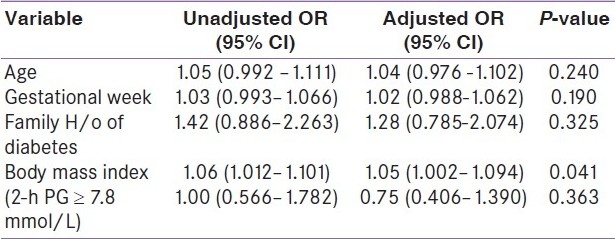
Out of 1 463 women enrolled in the study, the birth weight of the neonates was available for 1108 women [(956/1267) 76% for NGT women and (152/196) 78% for GDM women]. The mean birth weight of neonates born to GDM and NGT women was 2.86±0.46 and 2.84±0.43 kg, respectively. There was no statistically significant difference in the mean birth weight of neonates born to women in the two groups (P=0.705). Macrosomia defined as birth weight greater than 3.45 kg (90th percentile) was observed in 9.9% of GDM women with intervention and 9.8% of the NGT women, respectively (P = 1.000). The birth weight distribution was also similar (P = 0.942), in both the groups [Figure 1]. We examined the level of association between macrosomia and GDM status after controlling the factors: maternal age, gestational age, family history of diabetes, and BMI. It was found that, the GDM status (2-h PG ≥ 7.8 mmol/L) of the pregnant women after intervention was not associated with macrosomia [adjusted Odds Ratio (OR) = 0.752; 95% Confidence Interval (CI) (0.406-1.390); P=0.363] [Table 1]. No neonatal morbidity was observed.
Figure 1.
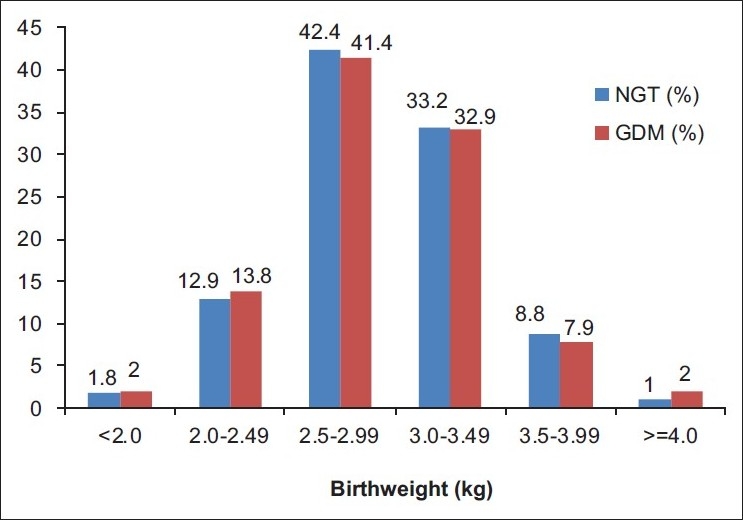
Neonate birth weight distribution of women with normal glucose tolerant and gestational diabetes mellitus
Table 1.
Unadjusted and adjusted OR With 95% CI for macrosomia according to risk factors
DISCUSSION
In this study, women were given 75 g oral glucose load irrespective of their last meal timing and 2-h PG ≥ 7.8 mmol/L were diagnosed as GDM. The rationale is that, after a meal, a normal glucose tolerant woman would be able to maintain euglycemia despite glucose challenge due to brisk and adequate insulin response. Whereas, a woman with GDM who has impaired insulin secretion, her glycemic level increases with a meal and with glucose challenge, the glycemic excursion exaggerates further.[10,17,18] This cascading effect is advantageous as this would not result in false positive diagnosis of GDM.[10]
On follow-up, the birth weight greater than 3.45 kg (90th percentile) was observed in 9.9% of GDM women and 9.8% of the NGT women, respectively. There was no significant difference in the two groups in terms of pregnancy outcome (P = 1.000). This was due to the intervention with MNT and/or insulin in maintaining FPG ~ 5.0 mmol/L and 2-h post meal ~ 6.7 mmol/L in GDM women. Intervention helped in maintaining the pregnancy outcome in GDM women equivalent to that of NGT women. Gayle C et al. also observed that diagnosis of GDM with OGTT 2-h PG ≥ 7.8 mmol/L and treatment in a combined diabetes antenatal clinic is worthwhile with a decreased macrosomia rate and fewer emergency cesarean section.[8] In our study, the distribution of birth weight of neonates born to GDM and NGT women were similar [Figure 1], indicating that the intervention given to pregnant women with 2-h PG ≥ 7.8 mmol/L had a significant effect in obtaining neonatal birth weight appropriate for gestational age. We also found that there was no association between macrosomia and the GDM status (P=0.363) with intervention after controlling factors such as maternal age, gestational week, family history of diabetes, and BMI. The results of our study are unlikely to be vitiated as we had a follow up data of 76% NGT and 78% GDM women.
In pregnancy, the decision to perform a placebo controlled trial requires clinical equipoise.[19] Hence, in this study, we did not have a control group of untreated pregnant women with 2-h PG ≥ 7.8 mmol/L, as there are publications confirming that treatment of GDM women as defined by WHO criterion (2-h PG ≥ 7.8 mmol/L) was associated with reduced risk of pregnancy outcome.[7,16] Recently a study performed by Wahi et al. also documented the advantages of adhering to DIPSI guidelines in the diagnosis (2-h PG ≥ 7.8 mmol/L) and management of GDM for a significantly positive effect on pregnancy outcome.[9] Hence, the policy of not treating women with 2-h PG ≥ 7.8 mmol/L amounts to deliberately exposing the pregnant mothers to unphysiological glycemic level despite our extensive knowledge of the benefits of treatment of mild hyperglycemia during pregnancy.[11,20–22]
In India more than 70% of population live in rural settings and facilities for diagnosing diabetes itself is limited. In this scenario, performing OGTT recommended by other associations [e.g., American Diabetes Association, National Diabetes Data Group, International Association of Diabetes and Pregnancy Study Groups] to diagnose GDM is not possible as the cost involved is prohibitive to perform three blood tests and thus not favored by both health care providers and seekers. This may be one of the reasons why the program for universal screening for all pregnant women is not implemented. Most importantly detection and care of GDM has become a public health priority as the still birth rate is high in India and one of the causes is gestational diabetes mellitus.[23] Hence, the need is for a simple and economical test to diagnose GDM. In this context, DIPSI procedure of estimating plasma glucose from one blood sample is cost effective and evidence based as revealed by the pregnancy outcome in this study and as well as by Wahi et al.[9] Even if the test is to be repeated in each trimester, the cost of performing DIPSI procedure will be 66% less than the cost of performing any other diagnostic procedures. “Clinical wisdom dictates that type of screening, universal or selective, and threshold selection should be performed in conjunction with the population-specific profile. This practical, cost-effective approach will address patient needs and remove from the stage an artificial controversy that leads to sophistry and pontification at public expense.”[24]
CONCLUSION
DIPSI criterion requires estimation of plasma glucose in one blood sample to diagnose GDM. This cost-effective and evidence-based procedure meets our responsibility of offering “a single-step definitive glucose test” to every pregnant woman belonging to any socio-economic status. This study has validated the credibility of DIPSI criterion. Further studies are warranted to substantiate this suggestion.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop Conference on Gestational Diabetes Mellitus: The Organizing Committee. Diabetes Care. 1998;21(Suppl 2):B161–7. [PubMed] [Google Scholar]
- 2.Dornhost A, Rossi M. Risk and prevention of Type 2 Diabetes in women with gestational diabetes. Diabetes Care. 1998;21(Suppl 2):B43–9. [PubMed] [Google Scholar]
- 3.Ferrara A. Increasing prevalence of Gestational Diabetes Mellitus - A Public Health Perspective. Diabetes Care. 2007;30(Suppl 2):S141–6. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- 4.Shamsuddin K, Mahdy ZA, Siti Rafiaah I, Jamil MA, Rahimah MD. Risk factor screening for abnormal glucose tolerance in pregnancy. Int J Gynaecol Obstet. 2001;75:27–32. doi: 10.1016/s0020-7292(01)00468-4. [DOI] [PubMed] [Google Scholar]
- 5.Simmons D, Devers MC, Wolmarans L, Johnson E. Difficulties in the use of risk factors to screen for GDM. Diabetes Care. 2009;32:e8. doi: 10.2337/dc08-1313. [DOI] [PubMed] [Google Scholar]
- 6.Alberti KG, Zimmett PZ. Definition, diagnosis and classification of diabetes mellitus and its complications, Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 7.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, et al. Effect of treatment of gestational diabetes mellitus. N Engl J Med. 2005;352:2477–86. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 8.Gayle C, Germain S, Marsh MS, Rajasingham D, Brackenridge A, Carroll P, et al. Comparing pregnancy outcomes for intensive versus routine antenatal treatment of GDM based on a 75 gm OGTT 2- h blood glucose (>140 mg/dl) Diabetologia. 2010;53:S435. [Google Scholar]
- 9.Wahi P, Dogra V, Jandial K, Bhagat R, Gupta R, Gupta S, et al. Prevalence of Gestational Diabetes Mellitus (GDM) and its Outcomes in Jammu Region. J Assoc Physicians India. 2011;59:227–30. [PubMed] [Google Scholar]
- 10.Anjalakshi C, Balaji V, Balaji MS, Ashalata S, Suganthi S, Arthi T, et al. A Single test procedure to diagnose gestational diabetes mellitus. Acta Diabetol. 2009;46:51–4. doi: 10.1007/s00592-008-0060-9. [DOI] [PubMed] [Google Scholar]
- 11.Seshiah V, Balaji V, Balaji MS, Paneerselvam A, Arthi T, Thamizharasi M, et al. Prevalence of gestational diabetes mellitus in South India (Tamil Nadu) - a community based study. J Assoc Physicians India. 2008;56:329–33. [PubMed] [Google Scholar]
- 12.Seshiah V, Sahay BK, Das AK, Shah S, Banerjee S, Rao PV, et al. Gestational Diabetes Mellitus - Indian Guidelines. (804-6).J Indian Med Assoc. 2009;107:799–802. [PubMed] [Google Scholar]
- 13.Pettitt DJ, Bennett PH, Hanson RL, Narayan KM, Knowler WC. Comparison of World Health Organization and National Diabetes Data Group procedures to detect abnormalities of glucose tolerance during pregnancy. Diabetes Care. 1994;17:1264–8. doi: 10.2337/diacare.17.11.1264. [DOI] [PubMed] [Google Scholar]
- 14.Pettitt DJ, Bennett PH, Saad MF, Charles MA, Nelson RG, Knowler WC. Abnormal glucose tolerance during pregnancy in Pima Indian women: Long term effects on the offspring. Diabetes. 1991;40(Suppl 2):126–30. doi: 10.2337/diab.40.2.s126. [DOI] [PubMed] [Google Scholar]
- 15.Jovanovic-Peterson L, Bevier W, Peterson CM. The Santa Barbara County Health Care Services Program: birth weight change concomitant with screening for and treatment of glucose intolerance of pregnancy: A potential cost-effective intervention. Am J Perinatal. 1997;14:221–8. doi: 10.1055/s-2007-994131. [DOI] [PubMed] [Google Scholar]
- 16.Jovanovic L. What is so bad about a big baby? Diabetes Care. 2001;24:1317–8. doi: 10.2337/diacare.24.8.1317. [DOI] [PubMed] [Google Scholar]
- 17.Kuhl C. Insulin Secretion and insulin resistance in pregnancy and GDM.Implications for diagnosis and management. Diabetes. 1991;40(Suppl 2):18–24. doi: 10.2337/diab.40.2.s18. [DOI] [PubMed] [Google Scholar]
- 18.Catalano PM, Tyzbir ED, Wolfe RR, Calles J, Roman NM, Amini SB, et al. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol. 1993;264:E60–67. doi: 10.1152/ajpendo.1993.264.1.E60. [DOI] [PubMed] [Google Scholar]
- 19.Gifford F. Uncertainty about clinical equipoise.Clinical equipoise and the uncertainty principles both require further scrutiny. BMJ. 2001;322:795. [PMC free article] [PubMed] [Google Scholar]
- 20.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339–48. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bevier WC, Fischer R, Jovanovic L. Treatment of women with an abnormal glucose challenge test (but a normal oral glucose tolerance test) decreases the prevalence of macrosomia. Am J Perinatol. 1999;16:269–75. doi: 10.1055/s-2007-993871. [DOI] [PubMed] [Google Scholar]
- 22.Negrato CA, Jovanovic L, Tambascia MA, Calderon Ide M, Geloneze B, Dias A, et al. Mild gestational hyperglycemia as a risk factor for metabolic syndrome in pregnancy and adverse perinatal outcomes. Diabetes Metab Res Rev. 2008;24:324–30. doi: 10.1002/dmrr.815. [DOI] [PubMed] [Google Scholar]
- 23.Pattinson R, Kerber K, Buchmann E, Friberg IK, Belizan M, Lansky S, et al. Stillbirths: how can health systems deliver for mothers and babies? Lancet. 2011;377:1610–23. doi: 10.1016/S0140-6736(10)62306-9. [DOI] [PubMed] [Google Scholar]
- 24.Yogev Y, Metzger BE, Hod M. Establishing diagnosis of gestational diabetes mellitus: Impact of the hyperglycemia and adverse pregnancy outcome study. Semin Fetal Neonatal Med. 2009;14:94–100. doi: 10.1016/j.siny.2009.01.001. [DOI] [PubMed] [Google Scholar]


