Abstract
We present here a rare case of synchronous adrenocortical carcinoma (ACC) and renal cell carcinoma (RCC). A 27-year-old woman presented with gradual abdominal distension, hematuria, and loss of weight of 3-months duration. She gave a history of treatment for hypertension. The computed axial tomography (CT) scan revealed a large retroperitoneal mass. Her urinary VMA was slightly elevated. Her 24-h urinary metanephrine level was normal. The patient underwent left adrenalectomy, left nephrectomy, spleenectomy, and distal pancreactomy with segmental colonic resection. Postoperative pathology revealed ACC of left suprarenal measuring 22 × 19 × 18 cm3 and RCC involving the left upper pole of kidney measuring 3 × 2 × 1 cm3.
Keywords: Adrenalectomy, adrenocortical carcinoma, renal cell carcinoma
INTRODUCTION
Adrenocortical carcinomas (ACCs) are rare malignant tumors with an estimated incidence of 0.05–0.2% of all malignancies. ACCs often have a poor prognosis.[1] Because of the juxtaposition of the adrenal gland to the kidney, it is not uncommon for ACC to involve the renal parenchyma.[2] Occasionally renal cell carcinoma (RCC) may metastasize to the contralateral adrenal gland.[3,4] Synchronous ACC and RCC are very rare and only one such case has been reported in the English medical literature.[5] We present here another case of synchronous ACC and RCC.
CASE REPORT
A 27-year-old woman presented with a history of gradual abdominal distension, hematuria, and loss of weight of 3-months duration. She gave a history of hypertension on treatment and cold abscess in childhood. On examination, her pulse rate was 84/min and blood pressure was 140/100 mmHg. She had a large mass palpable in left hypochondrium, left lumber region, and epigastrium crossing the midline. Her central nervous, cardio-vascular and respiratory systems were clinically normal. She had no supraclavicular lymphadenopathy. Both breasts and axillae were normal. Multiple healed scars were found in the neck. Her complete blood count was within normal limits. Blood biochemistry was normal except for elevated serum LDH (1693 International Units per Litre). She had normal GFR on the right side and decreased GFR on the left side (13.7 ml/min). Urinary VMA for 24 h was 16.9 mg (normal 8 mg/day) and 24 h urinary metanephrine was 5.7 mg/day (normal 5.5 mg/day). The computed tomography (CT) scan revealed a large retroperitoneal mass measuring 22 × 19 × 18 cm3. Left upper pole appeared indented. CT chest was normal. A clinical diagnosis of pheochromocytoma was made. The patient underwent left adrenalectomy along with left nephrectomy, splenectomy, and distal pancreactomy with a portion of colon (splenic flexure). On examination, the tumor measured 22 19 × 18 cm3 and was well capsulated. The left kidney was surrounded by the tumor, but not infiltrated by it. It showed large areas of degeneration, necrosis, and hemorrhage. The left kidney also showed a well-defined tumor near the upper pole measuring 3 × 2 × 1 cm3 with whitish cut surface. The spleen, pancreas, and colon were free from tumor infiltration.
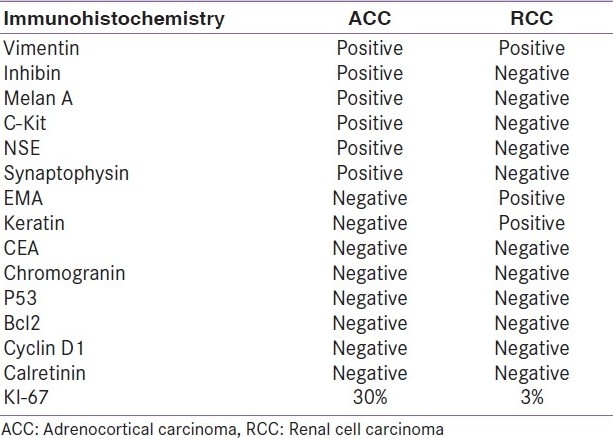
Microscopic examination of the adrenal tumor showed a cellular tumor [Figure 1a–d] composed of cells arranged diffusely, in sheets, trabeculae and peritheliomatous arrangement supported by delicate fibrovascular stroma. Large areas of degeneration, necrosis [Figure 1b], and hemorrhage were seen. Most of the cells were large, round, oval to polygonal with eosinophilic cytoplasm. Some of them showed clear cytoplasm. The nuclei were hyperchromatic and pleomorphic. Few large multinucleate tumor giant cells were seen [Figure 1a]. Mitotic figures varied from 15 to 20/50 high power field (HPF) [Figure 1c]. A focus of vascular invasion was seen [Figure 1d]. No capsular infiltration was seen. Immunohistochemistry (IHC) revealed positivity for Vimentin, Inhibin [Figure 2a], Melan-A [Figure 2d], C-Kit [Figure 2c], NSE, Synaptophysin and negative reaction for EMA, Keratin, CEA, Chromogranin, P-53, Bcl-2, Cyclin-D1, and Calretinin.[6–9] Ki-67 index was 30% [Figure 2b]. Ki-67 labelling index more than 2.5 is considered as malignant.[6] The adrenal tumor displayed 6 of 9 Weiss criteria[8] for malignancy: abundant necrosis, high mitotic count, high nuclear grade, a diffuse architecture, eosinophilic tumor cell cytoplasm, and vascular invasion. The tumor did not exhibit the remaining criteria: sinusoidal invasion, atypical mitotic figure, and capsular invasion. Examination of the kidney showed a tumor in the cortex. The cells were arranged in sheets separated by delicate vascular stroma. They were polygonal and most of them showed clear cytoplasm. Some of them showed eosinophilic granular cytoplasm. Moderate cellular and nuclear pleomorphism were seen [Figures 3a and b]. Mitotic figures were rare. The renal capsule was intact. IHC revealed positivity for EMA and Keratin [Figures 3c and d]. Ki-67 index was 3% [Table 1]. A diagnosis of synchronous ACC and RCC was made.
Figure 1.
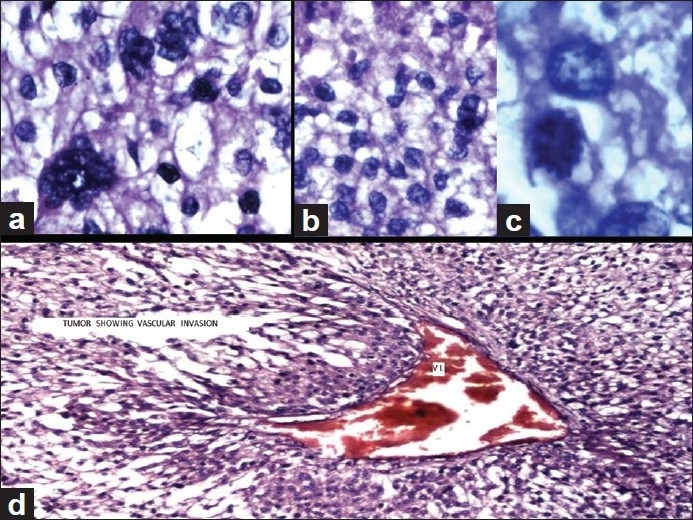
Adrenocortical carcinoma (H and E): (a) Pleomorphic tumor cells showing bizarre hyperchromatic nuclei (×40). (b) Sheet of pleomorphic tumor cells showing necrosis at the top (×40), (c) Mitotic activity by tumor cells (×100), and (d) tumor shows vascular invasion, marked as VI (×20)
Figure 2.
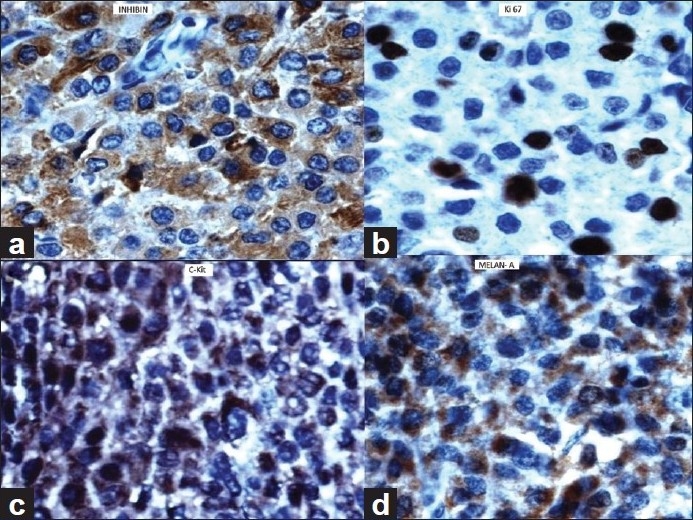
Adrenocortical carcinoma (immunehistochemistry). Tumor cells showing positive reaction for (a) Inhibin (×40), (b) Ki 67 (×40), (c) C-Kit (×40), (d) Melan A (×40)
Figure 3.
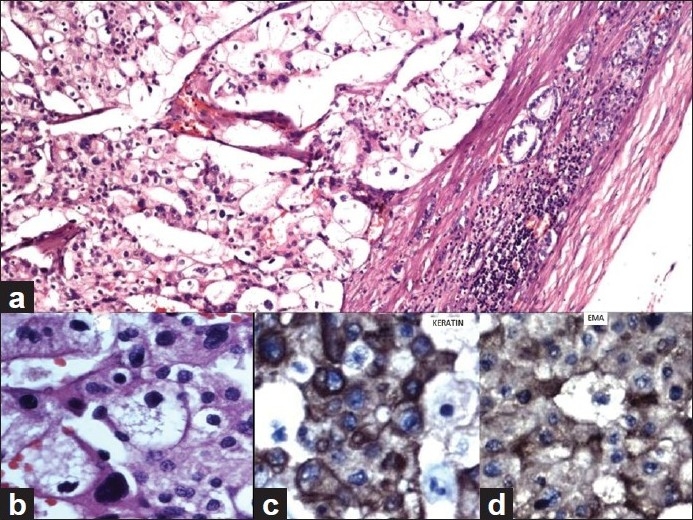
Renal cell carcinoma (H and E). (a) Large tumor cells with mostly clear cytoplasm are seen. Renal capsule and few renal tubules are seen at the periphery (H and E, ×10). (b) Large pleomorphic tumor cells both with clear cytoplasm and eosinophilic granular cytoplasm (H and E, ×40). (c) Pleomorphic tumor cells showing positivity for Keratin (IHC, ×40) and (d) Tumor cells showing positivity for EMA (IHC, ×40).
Table 1.
Immunohistochemical findings of adrenocortical carcinoma and renal cell carcinoma
DISCUSSION
Because of anatomical proximity of adrenal glands and kidneys, it is possible that the ACC may involve the renal parenchyma. Similarly, RCC may involve adrenals or metastasise to adrenal glands. RCC are especially notorious for metastasizing to unusual sites, and the primary tumor is often clinically silent. As a result, these metastases tend to be confused with primary tumors of the organs in which they lodge. This is often the case when the RCC metastasize to the contralateral adrenal gland. It is therefore necessary to carefully evaluate the simultaneous detection of these two tumors.
Discerning malignancy in resected adrenocortical neoplasms can pose diagnostic difficulty. Macroscopic examination is the first important step toward diagnosis and should include accurate measurement of the specimens and description of the cut surface of the tumors. It is also important to sample the specimens for histological diagnosis near foci of hemorrhage and/or necrosis. Histological scoring systems evaluating multiple parameters, especially the criteria of Weiss, have been shown to be reliable in differential diagnosis.[6] There are nine histological criteria according to Weiss system of grading.[10,11] They are: (i) high nuclear grade, (ii) mitotic rate greater than five per 50 HPF, (iii) atypical mitotic figures, (iv) eosinophilic tumor cell cytoplasm (greater than 75% tumor cells), (v) diffuse architecture (greater than 33% of tumor), (vi) necrosis, (vii) venous invasion, (viii) sinusoidal invasion, and (ix) capsular invasion. A tumor is labelled malignant when it meets three or more of these histological criteria.[10,11]
ACC and RCC can be differentiated on IHC. ACC are often positive for Vimetin, Calretinin, Inhibin, Melan-A, C-kit, and negative to CEA and keratin.[2,6–9] RCC on the other hand are positive for EMA and keratin. Recently, kit kinase inhibitors have been shown to have promising therapeutic effect on c-kit-expressing tumors.[9] With this development, testing ACC for C-kit is important from the point of management of the patient. Besides these molecular findings, adrenocortical malignancy is consistently associated with the increased cell proliferation. An assessment of neoplastic cell proliferation using immunostains of cell cycle-associated nuclear antigen such as Ki-67 is a very useful auxiliary method of evaluating malignancy in resected adrenocortical neoplasms. Ki-67 index of more than 2.5% is considered malignant.
The ACC in the present case had six histological criteria according to Weiss system of grading and hence was considered malignant. The Ki-67 index was also high (30%). We differentiated the ACC and RCC based on IHC. The patient was advised chemotherapy for ACC. No further treatment was required for RCC as the tumor was small and confined to the kidney.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Peppa M, Pikounis V, Papaxoinis G, Macheras A, Economopoulos T, Raptis SA, et al. Adrenocortical carcinoma secreting cortisol, androgens and aldosterone: A case report. Cases J. 2009;2:8951. doi: 10.4076/1757-1626-2-8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wick MR, Cherwitz DL, McGlennen RC. Adrenocortical carcinoma.An immunohistochemical comparison with renal cell carcinoma. Am J Pathol. 1986;122:343–52. [PMC free article] [PubMed] [Google Scholar]
- 3.Foucar E, Dehner LP. Renal cell carcinoma occurring with contralateral adrenal metastasis: A clinical and pathologic trap. Arch Surg. 1979;114:955–63. doi: 10.1001/archsurg.1979.01370320091019. [DOI] [PubMed] [Google Scholar]
- 4.Ishida M, Kojima K, Ohtomo K. Renal cell carcinoma with double synchronous contralateral adrenal metastases. Korean J Urol. 2010;51:879–81. doi: 10.4111/kju.2010.51.12.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jani P, Nasr AL, Demellawy DE. Synchronous renal cell carcinoma and adrenocortical carcinoma: A rare case report and clinicopathologic approach. Can J Urol. 2008;15:4016–9. [PubMed] [Google Scholar]
- 6.Sasano H, Suzuki T, Moriya T. Recent advances in histopathology and immunohistochemistry of adrenocortical carcinoma. Endocr Pathol. 2006;17:345–54. doi: 10.1007/s12022-006-0006-0. [DOI] [PubMed] [Google Scholar]
- 7.Zhang PJ, Genega EM, Tomaszewski JE, Pasha TL, LiVolsi VA. The role of calretinin, inhibin, melan-A, BCL-2, and C-kit in differentiating adrenal cortical and medullary tumors: An immunohistochemical study. Mod Pathol. 2003;16:591–7. doi: 10.1097/01.MP.0000073134.60541.E8. [DOI] [PubMed] [Google Scholar]
- 8.Stojadinovic A, Brennan MF, Hoos A, Omeroglu A, Leung DH, Dudas ME, et al. Adrenocortical Adenoma and Carcinoma: Histopathological and molecular comparative analysis. Mod Pathol. 2003;16:742–51. doi: 10.1097/01.MP.0000081730.72305.81. [DOI] [PubMed] [Google Scholar]
- 9.Gross DJ, Munter G, Bitan M, Siegal T, Gabizon A, Weitzen R, et al. Israel Glivec in Solid Tumors Study Group.The role of imatinib mesylate (Glivec) for treatment of patients with malignant endocrine tumors positive for c-kit or PDGF-R. Endocr Relat Cancer. 2006;13:535–40. doi: 10.1677/erc.1.01124. [DOI] [PubMed] [Google Scholar]
- 10.Jain M, Kapoor S, Mishra A, Gupta S, Agarwal A. Weiss criteria in large adrenocortical tumors: A validation study. Indian J Pathol Microbiol. 2010;53:222–6. doi: 10.4103/0377-4929.64325. [DOI] [PubMed] [Google Scholar]
- 11.Aubert S, Wacrenier A, Leroy X, Devos P, Carnasille B, Proye C, et al. Weiss system revisited: A clinicopathologic and immunohistochemical study of 49 adrenocortical tumors. Am J Surg Pathol. 2002;26:1612–9. doi: 10.1097/00000478-200212000-00009. [DOI] [PubMed] [Google Scholar]


