Abstract
Objective
To define and characterize the subpopulation of platelets capable of regulating the functional interactions of factors Va (FVa) and Xa (FXa) on the thrombin-activated platelet surface.
Methods and Results
Flow cytometric analyses were used to define and characterize platelet subpopulations. At a concentration of thrombin known to elicit maximal platelet activation, platelet-derived FVa release, and prothrombinase assembly/function, only a subpopulation of platelets were positive for FVa and FXa binding. An additional subpopulation bound lower levels of FVa, but little, if any, FXa. Fluorescence microscopy analyses confirmed these data. Phenotypically, platelets capable of binding FXa were more highly reticulated and demonstrated significantly increased expression of several key adhesion molecules, including P-selectin, GPIbα, and integrins αIIb and β3. This platelet subpopulation was also defined by the expression of a non-dissociable, membrane-bound pool of functional platelet-derived FVa which comprised ~35–50% of the total membrane-bound cofactor.
Conclusions
The ability of activated platelets to support thrombin generation is defined by a subpopulation of platelets expressing a non-dissociable pool of platelet-derived FVa and increased adhesive receptor density. This subpopulation is hypothesized to play a significant role in regulating both normal hemostasis and pathological thrombus formation since the adherent properties of platelets and their ability to mount and sustain a procoagulant response are crucial steps in both of these processes.
Keywords: platelets, thrombin, factor V, adhesion molecules, coagulation
Thrombin generation is pivotal to many physiologic and pathophysiologic processes, including, but not limited to, hemostasis,1 thrombosis,1 wound healing,2 and atherosclerosis.3 Thrombin is formed via prothrombinase, a Ca2+-dependent, stoichiometric complex of the cofactor factor Va (FVa) and the serine protease factor Xa (FXa), assembled on an appropriate membrane surface.1 Even though FXa effects the cleavage of prothrombin to thrombin, its ability to do so in a physiologically-relevant time frame is absolutely dependent upon the presence of both FVa and the membrane surface. Activated platelets not only provide this membrane surface in vivo, but also express and release FVa.4 The platelet-derived pool of FVa originates from megakaryocyte endocytosis of the plasma-derived procofactor.5, 6 Subsequent to its endocytosis, factor V is phenotypically modified7 to produce a cofactor with increased and sustained procoagulant activity, distinguishing it from its plasma-derived counterpart.8, 9
The amount of thrombin produced via prothrombinase assembled on the surface of activated platelets is dependent upon both the level of platelet activation,10 which is defined by the agonist used, and the quantity and quality of bound FVa. While it has long been known that platelets are heterogeneous with respect to volume, density, lipid peroxidation, and metabolism,11, 12 heterogeneity in the ability of activated platelets to bind coagulation factors has only recently been observed.13–19 Alberio and colleagues were the first to demonstrate that only a fraction of platelets activated simultaneously with low concentrations of thrombin (<5nM) and convulxin, an agonist of the collagen receptor GPVI, retain high levels of platelet-derived factor V/Va at their membrane surface13 in a transglutaminase-dependent manner.15 Studies done by Kempton and colleagues16 demonstrate the formation of two subpopulations of activated platelets differentiated by their ability to bind the constituents of both intrinsic tenase and prothrombinase. Subsequent studies by Panteleev, et al. demonstrated that a unique platelet subpopulation, formed subsequent to thrombin-induced activation, binds high levels of factors IXa, VIIIa, and X.17
Even though all of these studies have described heterogeneity in the ability of activated platelet subpopulations to bind coagulation factors, these subpopulations have not been characterized extensively, regarding their ability to generate thrombin via assembly of a functional prothrombinase, nor their expression of other membrane properties important for platelet function. In contrast to previous studies using low dose thrombin and convulxin/collagen as platelet agonists, thrombin was used as the sole agonist in this study as it has been shown to effect maximal prothrombinase assembly and function at the activated platelet surface. Our data indicate that subsequent to thrombin-catalyzed activation, not all platelet-bound FVa molecules are capable of supporting FXa binding. Rather, the ability of activated platelets to generate thrombin via prothrombinase is defined by a subpopulation of platelets expressing both non-dissociable and dissociable pools of platelet-derived FVa, each capable of binding FXa, and expressing an increased density of adhesive receptors at their activated membrane surface. Since the adherent properties of platelets and their ability to mount and sustain a procoagulant response are crucial steps in effecting normal hemostasis as well as pathological thrombus formation, the identified platelet subpopulation should be significant in regulating and contributing to these processes.
METHODS
Materials purchased, antibody descriptions, preparation of coagulation proteins, and methods with which we have substantial experience are detailed in the supplemental materials (available online at http://atvb.ahajournals.org). Techniques and protocols new to our laboratory and essential to this work are detailed here.
Generation and characterization of procoagulant platelet subpopulations
Platelets were isolated from human venous blood collected from healthy non-medicated individuals as detailed previously.20 Washed platelets (1×108/mL) were activated with thrombin (50nM) in the presence of RGDS (1mM) to prevent platelet aggregation. Alternatively, platelets were activated with a combination of low dose thrombin (5nM) and convulxin (0.5µg/mL). In some experiments, platelets were activated in the presence of dansylcadaverine (≤200µM). Following the addition of hirudin (75nM), activated platelets were incubated with FXa (5nM). In some experiments, plasma-derived FVa (5nM) was also added to ensure saturation of all available prothrombinase sites. Following fixation to cross-link platelet-bound FVa and FXa, platelets expressing FVa, FXa, P-selectin, integrin β3, GPIbα, and/or integrin αIIb, were visualized by flow cytometry using specific or control fluorophore-conjugated monoclonal antibodies. In some experiments, the platelets were incubated with Retic-COUNT™ thiazole orange (TO) reagent prior to flow cytometric analyses. Platelet-bound proteins were also visualized by fluorescence microscopy. Flow cytometry and fluorescence microscopy protocols are detailed in supplemental materials.
Determination of a functional, non-dissociable, platelet-derived factor Va pool
Platelet suspensions (1×109/mL) containing RGDS (5mM) and GPRP (2mM) were activated with thrombin (50nM). CaCl2 was omitted from the buffer in order to disrupt Ca2+-dependent interactions between the FVa heavy and light chains. The platelets were subsequently washed by centrifugation 1–5 times in buffer containing RGDS (5mM) and lacking Ca2+. At each wash, platelet-bound FVa activity was determined using a one-stage factor V clotting assay, and retention of the FVa light and heavy chains was assessed by western blotting. In other experiments, retention of the FVa heavy chain was assessed by flow cytometry. Details are provided in supplemental materials.
Statistical analyses
For Tables 1 and 2, unpaired t-tests were used to calculate two-tail P values. All calculations were performed using GraphPad Prism software. Data are expressed as mean±SEM.
Table 1.
Procoagulant platelets express increased adhesive receptor density*
| MFI × 10−3 |
||||
|---|---|---|---|---|
| Receptor | CD# | FXa Negative | FXa Positive | Fold Increase |
| Integrin β3 | 61 | 1.6 ± 1.9 | 9.0 ± 3.0† | 5.8 ± 1.6 |
| P-selectin | 62P | 5.1 ± 2.9 | 22.7 ± 4.2‡ | 4.5 ± 0.8 |
| GPIbα | 42b | 14.3 ± 2.6 | 87.5 ± 18.9‡ | 6.1 ± 2.4 |
| Integrin αIIb | 41 | 35.8 ± 1.4 | 150 ± 5.4§ | 4.2 ± 0.3 |
FXa binding and platelet adhesion receptor expression were detected using appropriate fluorophore-conjugated monoclonal antibodies as described in Methods and shown in Figure 2. Fold increases in receptor density were determined as the ratio of mean fluorescence intensity (MFI) in the FXa positive platelets to MFI in the FXa negative platelets. Values are expressed as mean±SEM, n=2–109;
P<0.01;
P<0.001;
P<0.02 compared with FXa negative platelets.
Table 2.
Age-related expression of adhesive platelet membrane receptors*
| MFI × 10−3 |
||||
|---|---|---|---|---|
| Receptor | CD# | Mature (TO−) | Young (TO+) | Fold Increase |
| Integrin β3 | 61 | 2.5 ± 1.1 | 14.7 ± 1.9† | 6.0 ± 1.0 |
| P-selectin | 62P | 4.2 ± 1.5 | 24.8 ± 2.1† | 6.0 ± 0.7 |
| GPIbα | 42b | 7.7 ± 3.3 | 52.7 ± 4.8† | 6.8 ± 0.9 |
Platelet adhesion receptor density was determined as described. Platelet age was assessed in all samples by analysis of thiazole orange (TO) staining as detailed in Methods. Fold increases in receptor density were determined as the ratio of MFI in the young (TO positive) platelets to MFI in the mature (TO negative) platelets. Values are expressed as mean±SEM, n=26–52;
P<0.001 compared with mature platelets.
RESULTS
FXa binding defines a unique, procoagulant platelet subpopulation dependent upon, but not described by, FVa binding
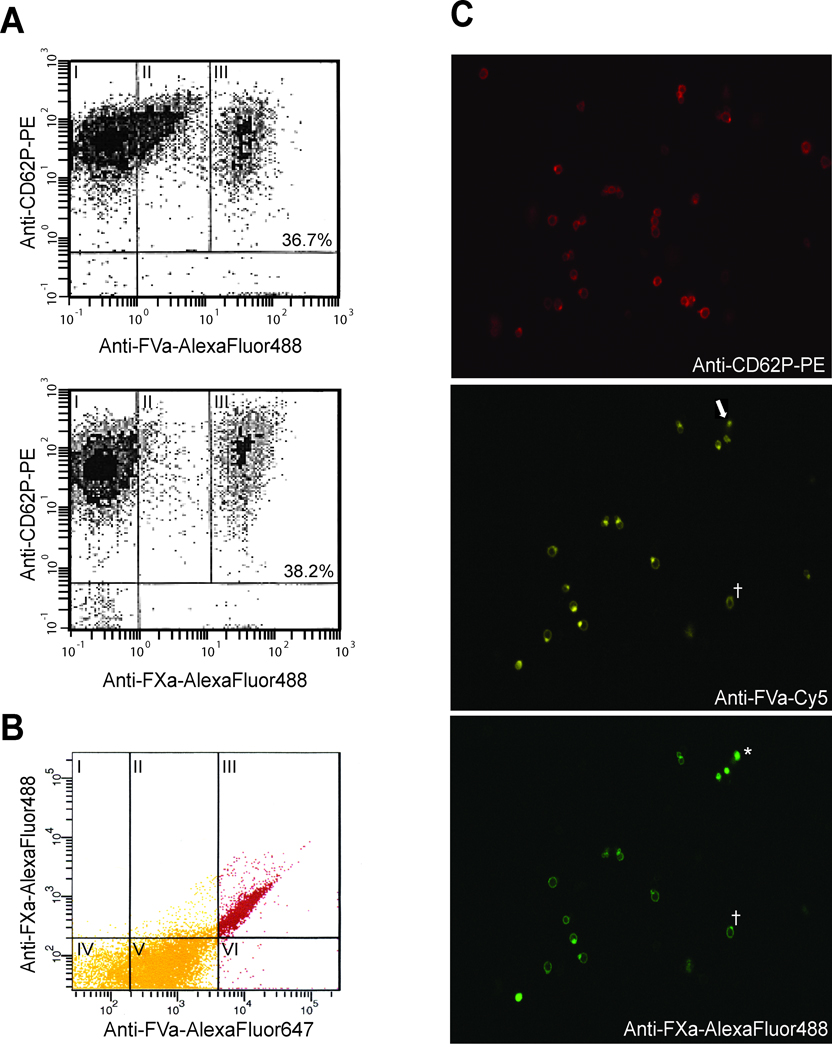
In the current study, thrombin was used at a concentration (50nM) previously shown to elicit a number of activation-dependent events regulating prothrombinase assembly and function.21 Under these conditions, the maximal release, activation, and binding of platelet-derived FVa is effected.10 However, as the concentration of the platelet-derived cofactor is not sufficient to saturate all prothrombinase binding sites, the addition of purified plasma-derived FVa is required. When bound to the activated platelet membrane, both cofactor pools support the subsequent binding of FXa. Previously reported kinetic analyses of thrombin generation at the activated platelet membrane confirmed that human platelets, in the presence of exogenous factors Va and Xa, become functionally saturated with factors Va and Xa when activated with approximately 50nM thrombin.10 In this study, even though the kinetics of prothrombin activation indicated that saturation of the platelet prothrombinase binding sites had been achieved, flow cytometric analyses indicated that only a subset of the activated platelets were able to bind factors Va and Xa (Figure 1A). Representative analyses demonstrated that FVa binding to maximally activated platelets (upper panel) segregates into three distinct subpopulations – those demonstrating no binding (I), an intermediate level of binding (II), or substantial (i.e. “high”) FVa binding (III). The mean fluorescence intensity of the platelets binding “intermediate” levels of FVa was consistently 5–8% of that expressed by those platelets binding “high” levels of FVa (5.8 ± 1.5%, n=28). In duplicate reaction mixtures, assessment of FXa binding characteristics (lower panel), indicated that platelets segregate into only two populations – those demonstrating substantial FXa binding (III) and those demonstrating no binding whatsoever (I). Similar analyses, with 28 different platelet donors (age 33–91 years old; 54% female), indicated that the percentage of platelets capable of binding FXa varied from 8–65% (mean = 31.1 ± 14.7%) (Supplemental Figure I), and was comparable to the subset defined by “high” FVa binding (mean = 33.5 ± 13.6%) for each individual. Neither donor gender nor age showed a correlation with the percentage of platelets comprising the procoagulant subpopulation. As the monoclonal antibodies against both FVa and FXa had the same fluorescence to protein ratio and yielded similarly sized subpopulations, it appears that those platelets defined as “high” binders may bind factors Va and Xa equivalently.
Figure 1. FXa binding defines a unique procoagulant platelet subpopulation dependent upon, but not described by, FVa binding.
Washed platelets were activated with thrombin (50 nM) and subsequently incubated with saturating concentrations of plasma-derived FVa and FXa (5 nM) as detailed in Methods. (A) Following fixation, platelets were stained with PE-conjugated anti-CD62P to identify activated platelets (y-axes) and specific AlexaFluor488-labeled antibodies to detect FVa (upper panel) and FXa (lower panel) binding (x-axes). The subpopulations of activated platelets that I) do not bind FVa or FXa, II) bind intermediate levels of FVa, or III) bind substantial amounts of FVa or FXa (“high binders”), are indicated by their relative levels of immunostaining. The percent of platelets binding “high” levels of FVa or FXa is indicated in the corresponding panel. (B) Similarly prepared platelets were simultaneously labeled with anti-CD62-PE, anti-FVa-AlexaFluor647, and anti-FXa-AlexaFluor488 to quantify FVa and FXa binding to the CD62+ platelet population. Panels I-III = FXa binding; panels II and V = “intermediate” FVa binding; panels III and VI = “high” FVa binding. For confocal microscopic analyses (C), platelets were activated as above, and cytocentrifuge specimens were prepared and visualized as described in Methods. These images represent a qualitative assessment of the ability of activated platelets to bind FVa and FXa. The laser intensity of the confocal microscope was set to a low level such that only those platelets capable of binding “high” levels of FVa were visualized. Shown here is a single field simultaneously immunostained with three different antibodies to define platelet activation (top panel), “high” FVa binding (middle panel), and FXa binding (bottom panel). The asterisk (*) indicates a platelet which stains for FVa and FXa, without evidence of P-selectin staining, reflecting the variance in P-selectin expression in the procoagulant population subpopulation (Supplemental Figure V). Also indicated are a platelet which expressed “high” levels of FVa but did not bind FXa (arrow), and an apparent anti-FXa-AlexaFluor488 antibody aggregate (†).
To determine whether the platelets capable of “high” FVa binding comprised the same platelet subpopulation as those capable of binding FXa, two approaches were taken. First, flow cytometric analyses of activated platelets, dual-labeled to assess both FVa (anti-FVa-AlexaFluor647) and FXa (anti-FXa-AlexaFluor488) binding, yielded similar platelet subpopulations (Supplemental Figure II) as those shown in Figure 1A in that separate subpopulations bound “intermediate” and “high” levels of FVa while a single subpopulation bound “high” levels of FXa. Additional quantification of factors Va and Xa binding to the CD62+ platelet population (Figure 1B) indicated that 88.0 ± 1.1% (n=6) of platelets capable of binding FXa also showed “high” levels of FVa binding (Panel III), and represented the majority of the procoagulant platelet population. Likewise, the vast majority of activated platelets binding “high” levels of FVa (93 ± 0.5 %; n=6) were defined by FXa binding. A small percentage of those platelets originally defined as binding intermediate levels of FVa, were able to bind FXa (mean = 11.8 ± 1.1%; n=6) (panel II), but these platelets represent those binding the greatest amount of FVa in that population. Likewise, a small percentage of those platelets defined as “high” factor Va binders do not appear to bind FXa (mean = 7.0 ± 0.5%; n=6) (panel VI). Similar data were obtained in three additional experiments each using platelets from different donors (data not shown).
Confocal microscopic analyses of activated platelets immunostained with anti-FV#2-Cy5, anti-FXa-AlexaFluor488, and anti-CD62-PE confirmed these data (Figure 1C). A cell by cell comparison of these three images indicated that, although not all activated platelets bound FVa and FXa, significant levels of FVa and FXa binding colocalized to the same subset of activated platelets, in support of the concept that, with few exceptions (Figure 1C arrow), the majority of platelets expressing “high” levels of FVa are those that bind FXa. These combined data indicate that the assembly of a functional prothrombinase is primarily defined by a discrete subpopulation of activated platelets, and, equally as important, that all platelet-bound FVa molecules are not equal in their ability to support FXa binding.
Additional experiments were done to determine whether this subset of procoagulant platelets mimicked that previously reported by Alberio et al.13 Indeed, a similar subset could be generated with the simultaneous addition of low dose thrombin (5nM) and the GPVI agonist convulxin (data not shown); however, the addition of the transglutaminase inhibitor dansylcadaverine (≤ 200µM) was without effect on FVa binding to the activated platelet (Supplemental Figure III), suggesting a different mechanism for procoagulant subpopulation formation than that proposed by Dale et al.15
Characterization of the adhesive properties of the procoagulant platelet subpopulation
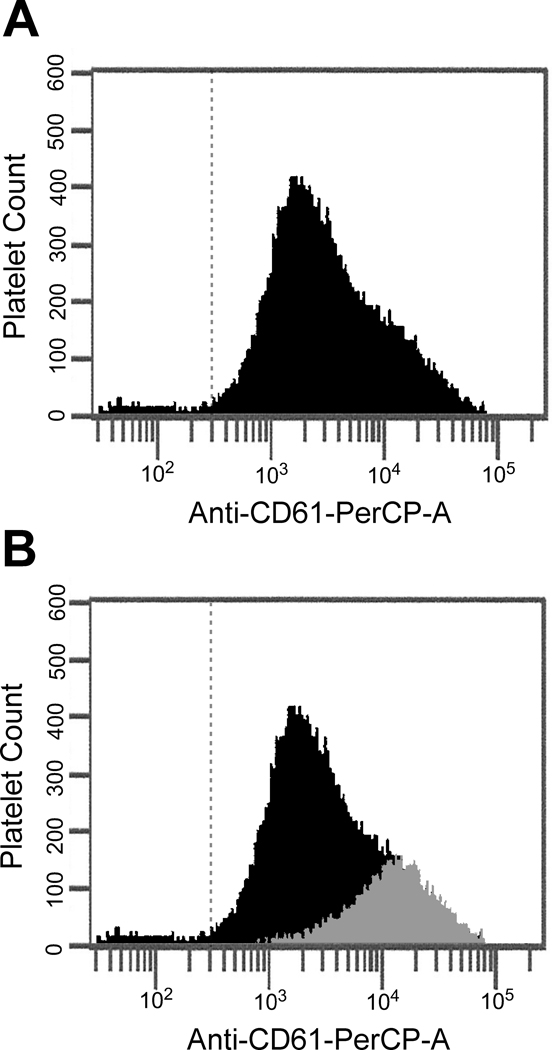
Experiments were done to characterize the adhesive properties of the procoagulant platelet subpopulation capable of binding a functional prothrombinase by quantifying the membrane densities of P-selectin (CD62P) and GPIbα(CD42b) as well as integrins αIIb(CD41) and β3(CD61) using specific fluorophore-conjugated monoclonal antibodies (Supplemental Figure IV). These experiments were performed in the presence of added plasma-derived FVa to define the entire population of platelets capable of binding FXa. In a representative experiment (Figure 2), procoagulant platelets demonstrated a 5.8 ± 1.6-fold (n=93) higher level of integrin β3 expression as compared to those platelets unable to bind FXa. Likewise, higher levels of expression were observed for P-selectin (4.5 ± 0.8-fold, n=109), integrin αIIb (4.2 ± 0.3-fold, n=2), and GP1bα (6.1 ± 2.4-fold, n=18) (Table 1). In additional flow cytometric analyses, which simultaneously compared both FVa and FXa binding characteristics, the increased expression of P-selectin previously seen in the FXa positive platelets was also seen in those platelets capable of binding “high” levels of FVa (Supplemental Figure V), consistent with the existence of a single procoagulant platelet subpopulation expressing increased adhesive receptor density.
Figure 2. Integrin β3 expression is increased on the surface of procoagulant platelets.
(A) Activated platelets capable of binding FXa were identified as described in Figure 1. Adhesive receptor density in the entire activated platelet population was defined using anti-CD61-PerCP. (B) To determine the portion of total integrin β3 expression contributed by the subpopulation of platelets capable of binding FXa, FXa-positive platelets were defined using anti-FXa-AlexaFluor488 and analyzed using anti-CD61-PerCP. The integrin β3 expression of the FXa-positive platelets (grey histogram) is shown as an overlay over the integrin β3 expression of the entire activated platelet population.
Thiazole orange (TO), a dye frequently used to stain nucleic acid and previously shown to identify the youngest platelets in circulation,22 was used to evaluate the effect of platelet age on FVa and FXa binding to, as well as adhesive receptor density expressed by, activated platelets. Dual-labeling studies demonstrated that 56.3% ± 5.4% (n=16) of TO-positive platelets were able to bind high levels of FVa, while 63.2% ± 2.8% (n=20) were able to bind FXa. Conversely, 75.1% ± 5.4% (n=16) of platelets able to bind high levels of FVa, and 68.7% ± 3.8% (n=20) of platelets able to bind FXa, were TO-positive. Evaluation of adhesive receptor expression as a function of platelet age demonstrated a 5.9 ± 0.7-fold (n=52) higher level of P-selectin expression in the youngest (TO-positive) platelets as compared to their more mature (TO-negative) counterparts (Table 2). In addition, expression of integrin β3 and GPIbα were increased 6.0 ± 1.0-fold (n=26) and 6.8 ± 0.9-fold (n=26) in TO-positive platelets, respectively (Table 2).
The procoagulant platelet subpopulation is characterized by a non-dissociable pool of platelet-derived FVa
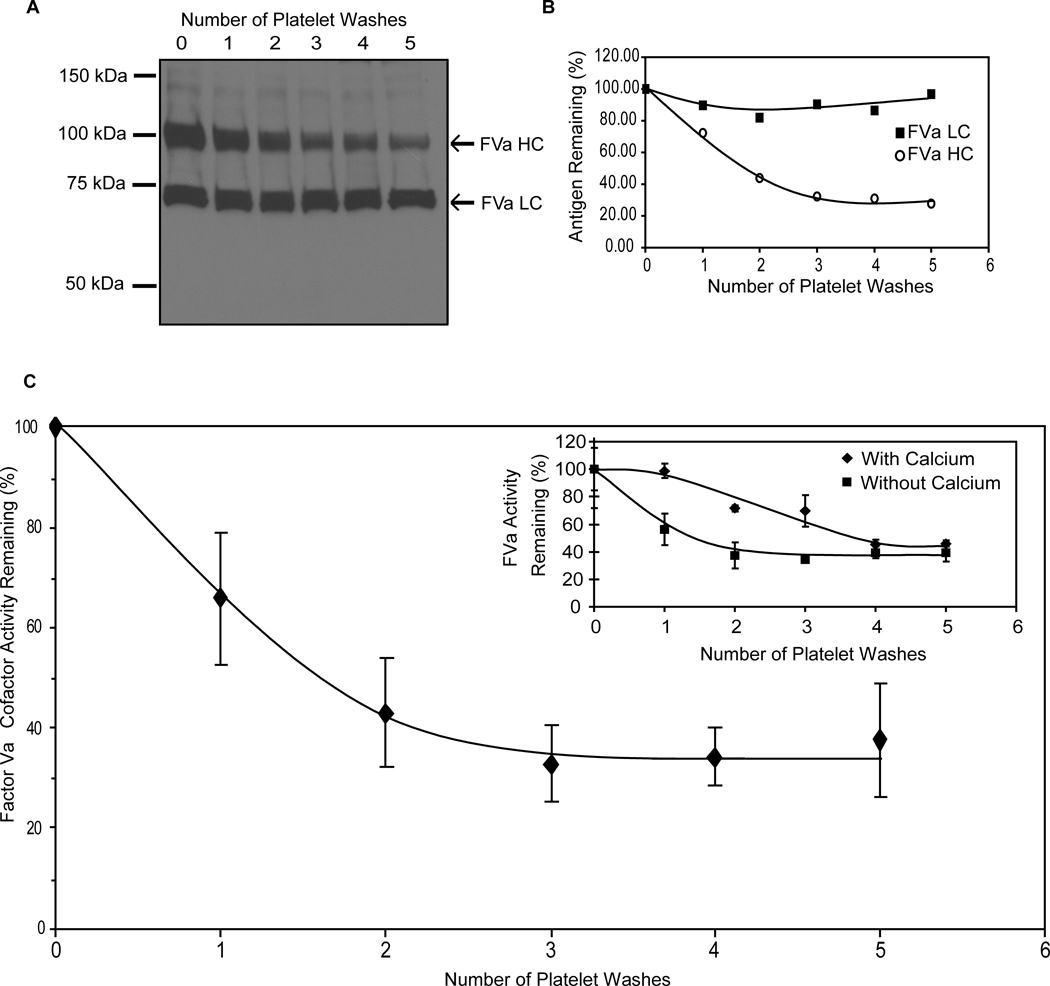
During the course of these studies, observations were made indicating that a significant fraction of the membrane-bound, platelet-derived FVa pool expressed subsequent to platelet activation could not be removed from the platelet membrane despite repeated washing. Thrombin-catalyzed activation of washed human platelets in the presence of RGDS and GPRP, to prevent platelet aggregation and polymerization of platelet-derived fibrin, allowed repeated washing of the activated platelets, providing RGDS was included in the wash buffers. When washing was done in the presence of EDTA to disrupt the Ca2+-dependent interaction between the FVa heavy and light chains, flow cytometric analyses using a monoclonal antibody specific for the FVa heavy chain indicated that approximately 35% of the heavy chain remained bound to the activated platelet membrane even though its interaction with the light chain had been abolished (Supplemental Figure VI). The amount of bound light chain remained unchanged, consistent with its Ca2+-independent membrane binding characteristics (data not shown).1 Near identical results were obtained when retention of the platelet-derived FVa heavy and light chains was assessed by Western blotting (Figure 3A&B). Densitometric analyses indicated that the majority of the light chain remained associated with the platelet surface, as did ~30% of the heavy chain. Thus, platelet activation resulted in the formation of a substantial, non-dissociably bound pool of FVa. This non-dissociably bound cofactor pool was capable of binding FXa since clotting assays indicated that 37.6 ± 11.2% (range = 14.6 – 62.0%, n=7) of the FVa cofactor activity remained associated with the activated platelet membrane following five washes (Figure 3C), either in the presence or absence of Ca2+ (Figure 3C inset).
Figure 3. Identification of a functional, non-dissociable, platelet-derived FVa pool.
Activated platelets were washed repeatedly (0 – 5 times) in the absence of Ca2+ as described in Methods. The remaining platelet-bound FVa heavy chain (HC) and light chain (LC) were evaluated by immunoblotting under reducing conditions (A) and quantified by densitometric analyses (B). Subsequent to washing, the remaining platelet-bound FVa activity was determined using a prothrombin time-based clotting assay specific for factor V (C). Data are expressed as the mean ± SEM (n =7) of the % FVa activity remaining after each wash as compared to the membrane-bound activity assessed immediately following platelet activation. Inset: Activated platelets were washed in the presence (♦) or absence (■) of Ca2+, and FVa activity was determined as above (n=1).
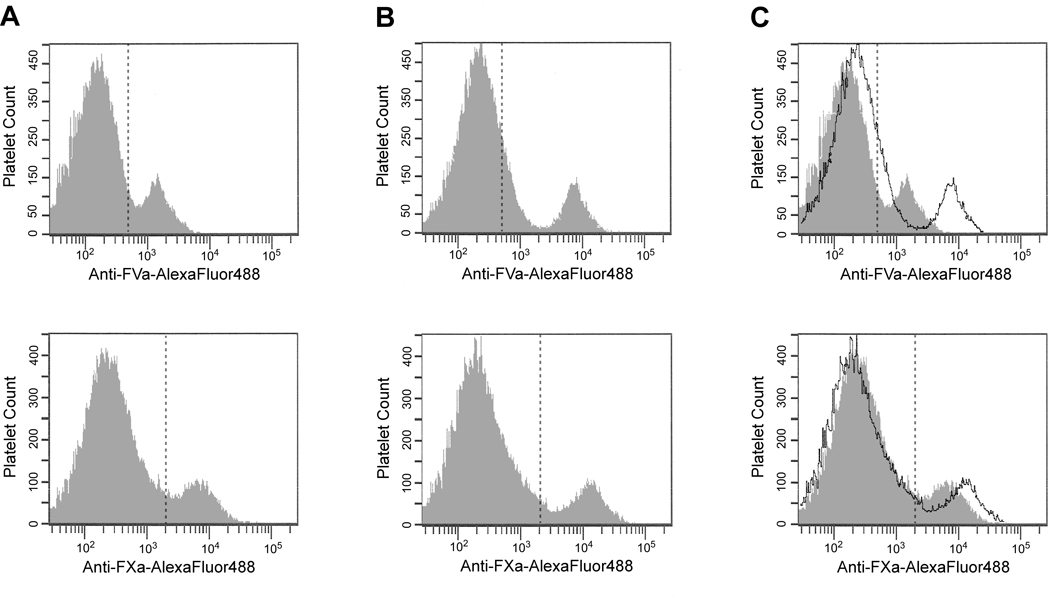
Consequently, experiments were done to determine if, and how, this non-dissociable pool of platelet-derived FVa contributed to the formation of the procoagulant platelet subpopulation. Flow cytometric analyses were used to quantify FVa and FXa binding to thrombin-activated platelets in the presence and absence of plasma-derived FVa. In the absence of added FVa, only the platelet-derived cofactor is expressed/bound to the activated platelet membrane, and is defined by both non-dissociable and dissociable cofactor pools, each of which is capable of binding FXa as determined by functional asssays. A representative experiment (n=6) is shown in Figure 4 where FVa binding is depicted in the upper histograms and FXa binding in the lower histograms. In the absence of added FVa, platelets intensely positive for FVa represented approximately 16% of the entire activated platelet population. FXa binding under identical conditions likewise represented 16% of the activated platelet population. When FVa was added to ensure saturation of all available binding sites, the percentage of platelets intensively positive for both factors Va and Xa remained virtually the same (18% vs 17%), whereas the amount of bound proteins increased as indicated by the increased fluorescence associated with this population of activated platelets. These data are consistent with the formation of a single platelet subpopulation responsible for regulating functional prothrombinase assembly.
Figure 4. Procoagulant platelet subpopulation formation is defined by a single population capable of binding/expressing both dissociable and non-dissociable pools of platelet-derived FVa.
FVa (top row) and FXa (bottom row) binding to thrombin-activated human platelets in the absence of exogenous FVa (A), or in the presence of saturating concentrations of plasma-derived FVa (B) were determined by flow cytometric analyses as in Figure 1. Panel (C) depicts a merged image of A & B with the transparent histogram indicating the additional FVa or FXa binding obtained in the presence of the added plasma-derived cofactor.
In all six experiments, the percentage of activated platelets binding both FVa and FXa was not significantly affected by the presence or absence of additional plasma-derived FVa, though the amount of FXa bound increased by 15% to 59% in the presence of saturating plasma-derived FVa. Differences were also observed between experiments, as the percentage of platelets representing the procoagulant subpopulation varied from as little as 8% to as much as 54% depending upon the platelet donor.
DISCUSSION
In this study, the subpopulation of activated platelets capable of regulating the functional interactions of factors Va and Xa on their membrane surface was defined and characterized, under conditions ensuring maximal prothrombinase binding. Thus, the binding characteristics of FVa and FXa to thrombin-activated platelets could be unequivocally characterized, thereby extending previous studies in which it was assumed that activated platelets are homogeneous regarding their procoagulant potential.10 The ability of thrombin-activated platelets to subsequently generate additional thrombin via a functional prothrombinase was defined by a subpopulation of predominantly “young” platelets expressing significantly increased adhesive receptor density, as well as a non-dissociable pool of platelet-derived FVa in which the heavy chain of the functional cofactor is “tethered” to the platelet membrane, most likely through a GPI anchor23. This procoagulant platelet subpopulation was also capable of binding additional platelet- and plasma-derived FVa in a freely dissociable manner to facilitate the saturation of all available prothrombinase binding sites on the activated platelet membrane. This observation is consistent with earlier studies defining prothrombinase assembly and function in the presence and absence of added plasma-derived FVa which indicated that the amount of platelet-derived cofactor expressed or released by the activated platelet membrane is not sufficient to saturate all available prothrombinase sites. In those studies, addition of plasma-derived FVa consistently increased rates of thrombin generation by 1.2 – 3-fold, consistent with an increase in FXa binding.24
In those same studies, saturation of the activated platelet membrane with FVa and FXa resulted in the binding of approximately 6000 molecules of FVa/platelet, whereas only 3000 molecules of bound FXa were observed,24 suggesting that only approximately one-half of the platelet-bound FVa would support a FXa binding interaction. Those data are now confirmed by the identification of a subpopulation of platelets which binds FVa but does not bind FXa, and allow us to conclude that FVa and FXa form a 1:1 complex on the procoagulant platelet membrane capable of assembling prothrombinase. Taken together, these data also confirm that the binding of FVa and FXa to the activated platelet surface is independently regulated, and add to a growing body of literature that argues for the existence of activation-dependent “receptors” for FVa and/or FXa at the human platelet surface.10, 25
This procoagulant platelet subpopulation demonstrated a 4- to 6-fold increase in P-selectin, integrin β3, integrin αIIb, and GPIbα expression, thereby placing these platelets in a unique position to contribute to both hemostatic and thrombotic events, with increased adhesive receptor density guaranteeing the adhesion of these procoagulant platelets to subendothelial vWF and/or vWF released by activated endothelial cells in a number of pathological processes. Following shear-induced platelet activation, platelet-derived FVa is released and expressed on the activated membrane surface. FXa, formed via the tissue factor/factor VIIa complex assembles with platelet-bound FVa to form prothrombinase and generate small amounts of thrombin which subsequently activate more platelets. Recruitment and incorporation of procoagulant platelet subpopulations into the growing thrombus via their increased expression of αIIbβ3 will follow. These activated platelets also express a non-dissociable, functional pool of platelet-derived FVa comprising approximately 35–50% of the total membrane-bound cofactor contributed by the activated platelet. Thus, this series of events will be as likely to promote thrombosis as it is to prevent hemorrhage. Likewise, the role of these platelets in the processes of wound healing, atherosclerosis, and inflammation will be favored by their increased expression of P-selectin which will facilitate not only platelet binding to the activated endothelium but also the recruitment of monocytes/macrophages and neutrophils via their expression of PSGL-1.26
Our data support the concept that all platelets do not express the same procoagulant potential, a notion that is supported by several studies,13–19 yet studies regarding prothrombinase are limited to two laboratories.13, 16 Among these, the work of Kempton et al16 indicate that platelet activation with low concentrations of thrombin and convulxin leads to the development of a platelet subpopulation capable of binding increased amounts of factors V, VIII, IX, and X, and that increased binding of these coagulation factors correlated with increased production of FXa and thrombin, suggesting that these platelet subpopulations describe a single procoagulant population capable of supporting the assembly of a functional tenase complex which will provide the FXa necessary to interact with platelet-bound FVa in prothrombinase.
Work from Dale’s laboratory was the first to demonstrate that a subset of platelets activated simultaneously with low concentrations of thrombin and convulxin/collagen express high levels of platelet-derived FV/Va at their membrane surface.13 The formation of these “COAT-platelets” is hypothesized to occur when serotonin, released by dense granules, crosslinks platelet-derived coagulation proteins, such as FV, to fibrinogen or thrombospondin bound to their receptors (αIIbβ3 or CD36, respectively). Based on the ability of dansylcadaverine to inhibit COAT-platelet formation, this cross-linking appeared to be mediated by platelet-derived factor XIIIa.15, 27 In contrast to COAT-platelets, the presence of dansylcadaverine had no effect on the formation of the procoagulant platelet subpopulation described here. This is not surprising for the following reason: Even though factor V is a substrate for factor XIIIa,28 the glutamine residues required for the transamidation reaction are all located within the activation peptide that is released when FV is activated to FVa.
The idea that platelets are not all created equally is not restricted to the human system.22, 29, 30 For example, Jagadeeswaran and colleagues identified two populations of zebrafish thrombocytes distinguishable by age and lipophilicity.22 Consistent with our data, the younger, more lipophilic thrombocytes, which appear to be responsible for the initiation of arterial thrombus formation, constitutively express a higher density of adhesive receptors and, following activation, express more P-selectin and anionic phosphoplipid than their more mature counterparts.22
Considering their complexity, perhaps the heterogeneous nature of activated platelets should be expected. Platelets participate in a wide variety of physiological and pathophysiological processes. In some, thrombin generation may not be as important as the release of granular contents, which appear to be heterogenous as well.31, 32 Differential packaging of the α-granule proteins vWF and fibrinogen allows their differential release.32 In addition, pro- and anti-angiogenic proteins appear to be organized into separate platelet granules which are also differentially released.31 Thus, platelets appear to express very different phenotypes, and therefore are most likely under distinct hematopoietic regulation via megakaryocyte differentiation.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to acknowledge Nicole Maille and John Malcolm for their technical assistance and Jay R. Silveira, Ph.D. for critical reading of the manuscript.
SOURCES OF FUNDING
This work was supported by HL46703 (Project 3) (to P.B.T). A.M.F. and J.P.W. were supported by T32 HL007594, Hemostasis and Thrombosis Program for Academic Trainees. B.A.B. was supported by an American Heart Association Scientist Development Grant (National Affiliate) and K02 HL091111.
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Kalafatis M, Egan JO, van't Veer C, Cawthern KM, Mann KG. The regulation of clotting factors. Crit Rev Eukaryot Gene Expr. 1997;7:241–280. doi: 10.1615/critreveukargeneexpr.v7.i3.40. [DOI] [PubMed] [Google Scholar]
- 2.Strukova SM. Thrombin as a regulator of inflammation and reparative processes in tissues. Biochemistry (Mosc) 2001;66:8–18. doi: 10.1023/a:1002869310180. [DOI] [PubMed] [Google Scholar]
- 3.Martorell L, Martinez-Gonzalez J, Rodriguez C, Gentile M, Calvayrac O, Badimon L. Thrombin and protease-activated receptors (PARs) in atherothrombosis. Thromb Haemost. 2008;99:305–315. doi: 10.1160/TH07-08-0481. [DOI] [PubMed] [Google Scholar]
- 4.Osterud B, Rapaport SI, Lavine KK. Factor V activity of platelets: evidence for an activated factor V molecule and for a platelet activator. Blood. 1977;49:819–834. [PubMed] [Google Scholar]
- 5.Bouchard BA, Williams JL, Meisler NT, Long MW, Tracy PB. Endocytosis of plasma-derived factor V by megakaryocytes occurs via a clathrin-dependent, specific membrane binding event. J Thromb Haemost. 2005;3:541–551. doi: 10.1111/j.1538-7836.2005.01190.x. [DOI] [PubMed] [Google Scholar]
- 6.Gould WR, Simioni P, Silveira JR, Tormene D, Kalafatis M, Tracy PB. Megakaryocytes endocytose and subsequently modify human factor V in vivo to form the entire pool of a unique platelet-derived cofactor. J Thromb Haemost. 2005;3:450–456. doi: 10.1111/j.1538-7836.2005.01157.x. [DOI] [PubMed] [Google Scholar]
- 7.Gould WR, Silveira JR, Tracy PB. Unique in vivo modifications of coagulation factor V produce a physically and functionally distinct platelet-derived cofactor: characterization of purified platelet-derived factor V/Va. J Biol Chem. 2004;279:2383–2393. doi: 10.1074/jbc.M308600200. [DOI] [PubMed] [Google Scholar]
- 8.Conlon SJ, Camire RM, Kalafatis M, Tracy PB. Cleavage of platelet-derived factor Va by plasmin results in increased and sustained cofactor activity on the thrombin-activated platelet surface. Thromb Haemost. 1997;77 Supplement:616. (Abstract PS-2507) [Google Scholar]
- 9.Camire RM, Kalafatis M, Simioni P, Girolami A, Tracy PB. Platelet-derived factor Va/Va Leiden cofactor activities are sustained on the surface of activated platelets despite the presence of activated protein C. Blood. 1998;91:2818–2829. [PubMed] [Google Scholar]
- 10.Bouchard BA, Catcher CS, Thrash BR, Adida C, Tracy PB. Effector cell protease receptor-1, a platelet activation-dependent membrane protein, regulates prothrombinase-catalyzed thrombin generation. J Biol Chem. 1997;272:9244–9251. doi: 10.1074/jbc.272.14.9244. [DOI] [PubMed] [Google Scholar]
- 11.Karpatkin S. Heterogeneity of human platelets. II. Functional evidence suggestive of young and old platelets. J Clin Invest. 1969;48:1083–1087. doi: 10.1172/JCI106064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karpatkin S. Heterogeneity of human platelets. I. Metabolic and kinetic evidence suggestive of young and old platelets. J Clin Invest. 1969;48:1073–1082. doi: 10.1172/JCI106063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alberio L, Safa O, Clemetson KJ, Esmon CT, Dale GL. Surface expression and functional characterization of alpha-granule factor V in human platelets: effects of ionophore A23187, thrombin, collagen, and convulxin. Blood. 2000;95:1694–1702. [PubMed] [Google Scholar]
- 14.Batar P, Dale GL. Simultaneous engagement of thrombin and Fc gamma RIIA receptors results in platelets expressing high levels of procoagulant proteins. J Lab Clin Med. 2001;138:393–402. doi: 10.1067/mlc.2001.120049. [DOI] [PubMed] [Google Scholar]
- 15.Dale GL, Friese P, Batar P, Hamilton SF, Reed GL, Jackson KW, Clemetson KJ, Alberio L. Stimulated platelets use serotonin to enhance their retention of procoagulant proteins on the cell surface. Nature. 2002;415:175–179. doi: 10.1038/415175a. [DOI] [PubMed] [Google Scholar]
- 16.Kempton CL, Hoffman M, Roberts HR, Monroe DM. Platelet heterogeneity: variation in coagulation complexes on platelet subpopulations. Arterioscler Thromb Vasc Biol. 2005;25:861–866. doi: 10.1161/01.ATV.0000155987.26583.9b. [DOI] [PubMed] [Google Scholar]
- 17.Panteleev MA, Ananyeva NM, Greco NJ, Ataullakhanov FI, Saenko EL. Two subpopulations of thrombin-activated platelets differ in their binding of the components of the intrinsic factor X-activating complex. J Thromb Haemost. 2005;3:2545–2553. doi: 10.1111/j.1538-7836.2005.01616.x. [DOI] [PubMed] [Google Scholar]
- 18.London FS, Marcinkiewicz M, Walsh PN. A subpopulation of platelets responds to thrombin- or SFLLRN-stimulation with binding sites for factor IXa. J Biol Chem. 2004;279:19854–19859. doi: 10.1074/jbc.M310624200. [DOI] [PubMed] [Google Scholar]
- 19.Munnix IC, Kuijpers MJ, Auger J, Thomassen CM, Panizzi P, van Zandvoort MA, Rosing J, Bock PE, Watson SP, Heemskerk JW. Segregation of platelet aggregatory and procoagulant microdomains in thrombus formation: regulation by transient integrin activation. Arterioscler Thromb Vasc Biol. 2007;27:2484–2490. doi: 10.1161/ATVBAHA.107.151100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tracy PB, Nesheim ME, Mann KG. Platelet factor Xa receptor. Methods Enzymol. 1992;215:329–360. doi: 10.1016/0076-6879(92)15075-n. [DOI] [PubMed] [Google Scholar]
- 21.Hayes KL, Leong L, Henriksen RA, Bouchard BA, Ouellette L, Church WR, Tracy PB. alpha-Thrombin-induced human platelet activation results solely from formation of a specific enzyme-substrate complex. J Biol Chem. 1994;269:28606–28612. [PubMed] [Google Scholar]
- 22.Thattaliyath B, Cykowski M, Jagadeeswaran P. Young thrombocytes initiate the formation of arterial thrombi in zebrafish. Blood. 2005;106:118–124. doi: 10.1182/blood-2004-10-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood JP, Fager AM, Silveira JR, Tracy PB. Platelet-derived factor Va expressed on the surface of the activated platelet is GPI-anchored. Blood. 2008;112:219a. [Google Scholar]
- 24.Tracy PB, Eide LL, Mann KG. Human prothrombinase complex assembly and function on isolated peripheral blood cell populations. J Biol Chem. 1985;260:2119–2124. [PubMed] [Google Scholar]
- 25.Nesheim ME, Furmaniak-Kazmierczak E, Henin C, Cote G. On the existence of platelet receptors for factor V(a) and factor VIII(a) Thromb Haemost. 1993;70:80–86. [PubMed] [Google Scholar]
- 26.van Gils JM, Zwaginga JJ, Hordijk PL. Molecular and functional interactions among monocytes, platelets, and endothelial cells and their relevance for cardiovascular diseases. J Leukoc Biol. 2008 doi: 10.1189/jlb.0708400. [DOI] [PubMed] [Google Scholar]
- 27.Szasz R, Dale GL. Thrombospondin and fibrinogen bind serotonin-derivatized proteins on COAT-platelets. Blood. 2002;100:2827–2831. doi: 10.1182/blood-2002-02-0354. [DOI] [PubMed] [Google Scholar]
- 28.Francis RT, McDonagh J, Mann KG. Factor V is a substrate for the transamidase factor XIIIa. J Biol Chem. 1986;261:9787–9792. [PubMed] [Google Scholar]
- 29.Jobe SM, Leo L, Eastvold JS, Dickneite G, Ratliff TL, Lentz SR, Di Paola J. Role of FcRgamma and factor XIIIA in coated platelet formation. Blood. 2005;106:4146–4151. doi: 10.1182/blood-2005-03-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dale GL. Coated-platelets: an emerging component of the procoagulant response. J Thromb Haemost. 2005;3:2185–2192. doi: 10.1111/j.1538-7836.2005.01274.x. [DOI] [PubMed] [Google Scholar]
- 31.Italiano JE, Jr., Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sehgal S, Storrie B. Evidence that differential packaging of the major platelet granule proteins von Willebrand factor and fibrinogen can support their differential release. J Thromb Haemost. 2007;5:2009–2016. doi: 10.1111/j.1538-7836.2007.02698.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.