Abstract
Extracellular ATP has been proposed to act as a danger signal to alert the immune system of cell damage. Release of high local concentrations of ATP activates the nucleotide receptor P2RX7 on monocytic cells, which promotes the processing/release of pro-inflammatory mediators. Although the pro-inflammatory actions of P2RX7 are well recognized, little is known regarding the potential function of P2RX7 in repair responses. Because the resolution of inflammation is characterized by monocytic cell-dependent production of pro-angiogenic factors, we evaluated the contribution of P2RX7 to this process. We observed that both transient and long-term P2RX7 activation promotes the robust release of VEGF from primary human monocytes. This VEGF release is calcium-dependent and associated with reactive oxygen species production. This previously unrecognized action of P2RX7 suggests that it may not only participate in inflammation and cell death, but that it is also likely to be important in the control of angiogenesis and wound repair.
Keywords: P2RX7, VEGF, monocytes, LPS, inflammation, angiogenesis
Introduction
Damage-associated molecular patterns (DAMPs) are associated with molecules that are produced and/or released by cells undergoing stress or death, These agents often act as co-stimulatory signals that initiate a highly regulated immune response (1). Extracellular ATP can act as a DAMP, given that it is normally confined to intracellular sites but can be released at high local levels following cell lysis, infection, or via regulated efflux (2, 3).
ATP released into the extracellular space can modulate the immune response through their capacity to bind and activate multiple nucleotide receptor family members. Of relevance to the present report, the nucleotide receptor P2RX7 can function as a ligand-gated ion channel and has been implicated in the progression of several inflammatory disorders including sepsis, arthritis, and tuberculosis (4–6). The activation of P2RX7 on monocytic cells, which play key roles in all phases of inflammation, is known to initiate or enhance the production and release of several pro-inflammatory mediators, including IL-1β, tumor necrosis factor alpha (TNF-α), inducible nitric oxide synthase, and reactive oxygen species (ROS) (7–11).
In addition to the production of pro-inflammatory mediators that promote the progression of inflammation, monocytic cells also have a major role in inflammatory wound repair via their ability to scavenge cellular debris and to produce pro-angiogenic factors. A key angiogenic factor produced by activated monocytic cells is vascular endothelial growth factor (VEGF) (12, 13). VEGF promotes new blood vessel formation, induces endothelial cell proliferation, and initiates immune cell migration (14, 15). Interestingly, VEGF release in monocytic cells is often induced by shear stress and hypoxia (15, 16), which are previously-recognized mechanisms of ATP release in several cell types (17, 18). VEGF is important in many pathological processes, including tumor progression, arthritis and ischemia (15, 19, 20). Although the pro-inflammatory role of P2RX7 in monocytic cell function is well documented, little is known about P2RX7 action in monocytic-dependent wound repair, and no reports have linked P2RX7 to the production of angiogenic proteins in these cells.
Because P2RX7 action is known to potentiate inflammatory mechanisms that can promote tissue damage, it would be a potentially important homeostatic mechanism if this receptor system also initiated effects that would ultimately facilitate the repair of damage induced during the inflammatory response. In this regard, activated monocytic cells serve a key action by initiating angiogenesis and tissue repair, and thus we tested the hypothesis that activation of P2RX7 on monocytes would result in the production and release of the pro-angiogenic and wound repair-associated factor VEGF. In the present study, we report a previously unrecognized action of P2RX7 agonists, namely the stimulation of the robust, concentration- and time-dependent expression and release of VEGF from primary human monocytes. The expression and release of VEGF was found to be P2RX7-dependent, and the release of this pro-angiogenic factor is linked to both intracellular calcium influxes and ROS production. This process reveals that P2RX7 may not only function in inflammation and cell death, but that it may also participate in the control of angiogenesis and wound repair.
Materials and Methods
Materials
ATP, 2'(3')-O-(4-benzoylbenzoyl)-ATP (BzATP), 2-Methylthioadenosine 5′-monophosphate (MeSAMP), PMA, LPS (E. coli, serotype 0111:B4), EGTA, and N-acetyl cysteine (NAC) were purchased from Sigma Chemical Co. (St Louis, MO). BAPTA-AM was purchased from Invitrogen (Carlsbad, CA). The P2RX7 antagonist A438079 and P2Y11 antagonist NF-157 was acquired from Tocris (Ellisville, MO).
Isolation of Human Blood Monocytes
Human blood-derived monocytes were purified as described (21). Heparinized blood was drawn from healthy donor volunteers at the University of Wisconsin Hospital in compliance with an approved Human Subjects protocol, and cells were separated using a Percoll density gradient. CD14+ cells were identified by flow cytometry and comprised 90–95% of the population. Cells were maintained at 37 °C in a humidified atmosphere with 5% CO2 in RPMI supplemented with 10% fetal bovine serum (HyClone, Logan, UT), 2 mM sodium pyruvate, 2 mM L-glutamine, and 100 U/ml penicillin/streptomycin.
ELISA
A sandwich ELISA for human VEGF was performed using antibodies obtained from R&D Systems (Minneapolis, MN) according to the manufacturer’s protocol. VEGF concentrations were calculated via interpolation from a standard curve, and all determinations were performed in duplicate.
Quantitative RT-PCR
Primers used were directed toward human VEGF or 18S (loading control) and are as follows: VEGF primer: F-ATCTTCAAGCCATCCTGTGTGC, R-GCTCACCGCCTCGGCTTGT; 18S primer: F-GGACACGGACAGGATTGACAG, R-ATCGCTCCACCAACTAAGAACG. PCR reactions were detected by Sybr green iQ supermix dye (Bio-Rad) and were performed on an a I-cycler real time thermal cycler (Bio-Rad) (54°C annealing temp, 50 cycles).
Cell Cytotoxicity Assays
Human monocytes were plated in 96-well plates at a density of 1×106 cells/ml. In certain cases, the cells were pretreated with either BAPTA-AM or P2RX7 antagonist A438079 followed by stimulation with BzATP or ATP for 4 h at 37°C. Following these incubations, cell viability was assessed by either using the Non-Radioactive Cell Proliferation Assay (an MTS-based assay) (Promega, Madison, WI) according to the manufacturer’s protocol, or by assessing Trypan blue exclusion wherein a portion of the treated cells were removed, and the percentage of live cells (excluding trypan blue) were compared to total cells (live + dead cells).
Statistical Analysis
The Student’s two-tailed, paired t-test was used to assess potential statistical differences between samples. Significance levels were set at P < 0.05.
Results
VEGF release following stimulation of human monocytes with P2RX7 ligands
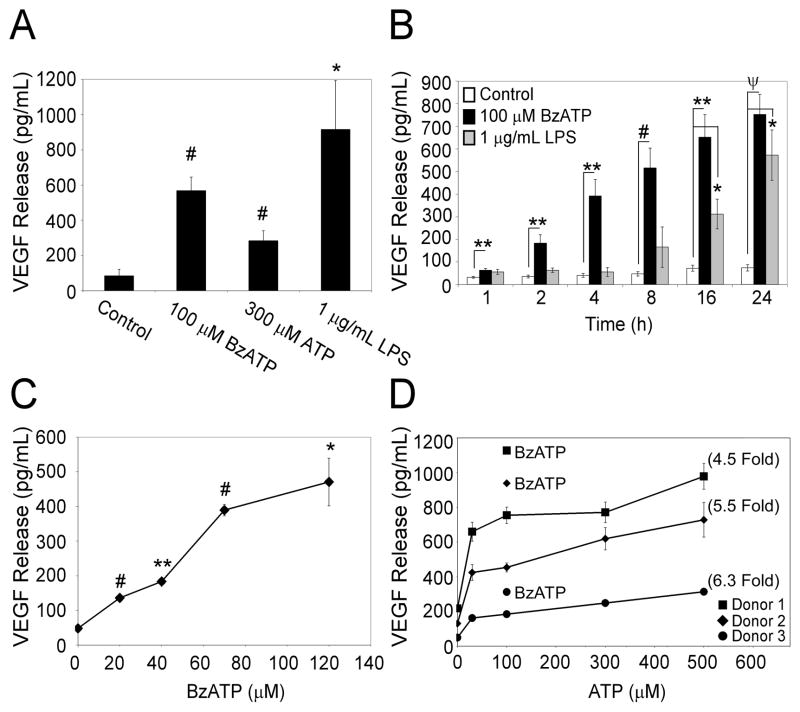
Primary human monocytes were stimulated for 24 h with the P2RX7 agonists 100 μM BzATP or 300 μM ATP, as well as with a known stimulus for VEGF release, i.e., LPS (1 μg/mL)(22). The cell supernatants were then assayed for VEGF release. When compared to vehicle control, the data in Fig. 1A demonstrate an over 6 fold increase in the release of VEGF from monocytic cells after stimulation with BzATP for 24 h and an over 3 fold increase in VEGF release after ATP stimulation for 24 h. A detailed evaluation of P2RX7 agonist-induced effects revealed that the VEGF release is both time- and concentration-dependent. When cells were treated with 100 μM BzATP or 1 μg/mL LPS for 1 to 24 h, we observed that the P2RX7 agonist BzATP promotes a statistically significant release of VEGF in as little as 1 h (Fig. 1B). Conversely, the levels of VEGF release induced by high concentrations of LPS are not significant until 16 h post-treatment. The results presented in Fig. 1C illustrate that significant VEGF release (p = 0.002) is observed after a 4 hr treatment with as little as 20 μM BzATP. The data in Fig. 1D demonstrate that ATP-induced VEGF release is also concentration-dependent, with high concentrations of ATP (>300 μM) leading to the highest release of VEGF protein. Collectively, these results indicate that comparatively low levels of P2RX7 agonists are able to promote the rapid, concentration-dependent release of VEGF, a potent pro-angiogenic cytokine, from primary human monocytes.
Fig. 1. P2RX7 agonists stimulate the time- and concentration-dependent release of VEGF from primary human monocytes.
(A) Primary human monocytes purified from healthy volunteer donors were treated with control (HEPES), 100 μM BzATP, 300 μM ATP, or 1 μg/mL LPS for 24 hr. (B) Cells were treated with either control, 100 μM BzATP, or 1 μg/mL LPS for 1–24 hr. (C) Cells were treated with control or varying amounts of the P2RX7 agonist BzATP for 4 h. The results depicted in panels A-C each represent at least 3 independent experiments with error bars representing mean +/- SEM. (D) Cells were treated with control or varying amounts of the P2RX7 agonist ATP for 4 h. Single gray data points represent VEGF release (pg/mL) from each donor after 100 μM BzATP treatment. The results depicted in panel D are from 3 independent donors with error bars representing mean +/- range, and numbers in parenthesis indicating fold increase in VEGF release after treatment with 500 μM ATP as compared to control for each donor. *p<0.05, **p<0.01, #p<0.005, ψp<0.001
Short-term stimulation by P2RX7 agonists induces VEGF release from human monocytes
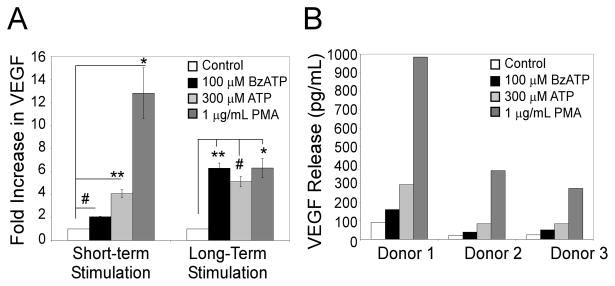
Recent work has shown that short-term activation of P2RX7 (< 1 h) can result in altered gene expression (8). To evaluate whether short-term stimulation with P2RX7 agonists can also induce VEGF release, primary cells were treated for 5 min with 100 μM BzATP, 300 μM ATP or 1 μg/mL PMA (a known inducer of VEGF release (23)). The media was then removed and replaced with fresh media containing no agonists. Supernatants were harvested after 4 h and assayed for VEGF release. As shown in Fig. 2A, short-term exposure to BzATP and ATP results in significant VEGF release (2 and 4 fold respectively). The data in Fig. 2B depict the results of BzATP-induced VEGF release as pg/mL for each individual patient.
Figure 2. Short-term stimulation by P2RX7 agonists induces VEGF release from primary human monocytes.
Cells were treated with control, 100 μM BzATP, 300 μM ATP, or 1 μg/mL PMA. The media was either removed after 5 min and replaced with fresh, agonist-free media (short-term stimulation), or not removed (long-term stimulation), and the cells were incubated for 4 h. Supernatants were assayed for VEGF as described under Materials and Methods. (A) The results are depicted as fold increase in VEGF compared to vehicle control and are represent of 3 independent experiments (mean +/- SEM). (B) Results are depicted for each individual donor as VEGF release in pg/mL. *p<0.05, **p<0.01, #p<0.005, ψp<0.001
A P2RX7-selective antagonist attenuates BzATP and ATP-induced VEGF release
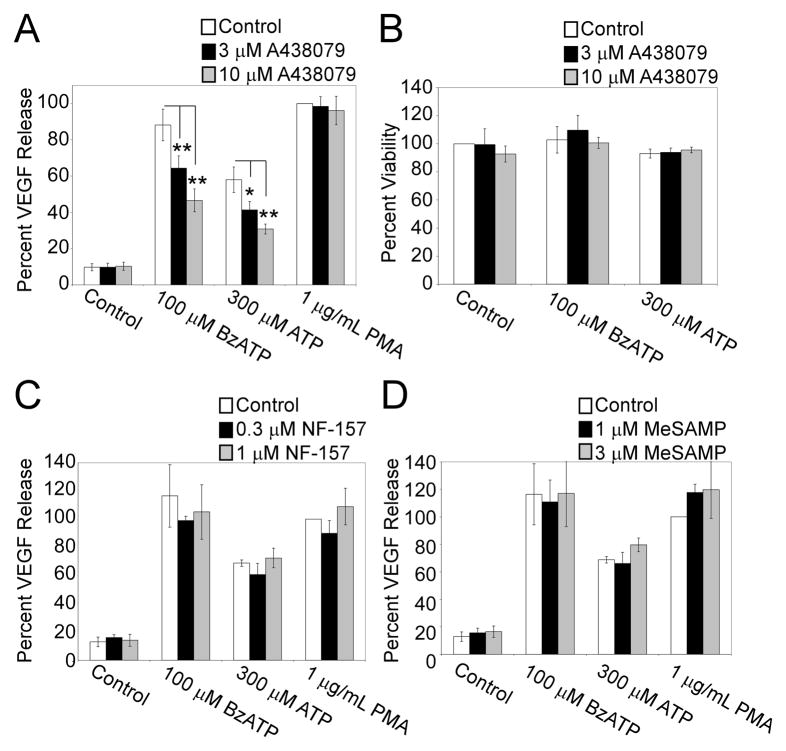
Although BzATP is a potent agonist of P2RX7, it can stimulate a few other P2 receptors (e.g., P2RY11-13) at high concentrations. To examine the specific involvement of P2RX7 in regulating VEGF release, we evaluated the sensitivity of nucleotide-induced VEGF release to the addition of the selective P2RX7 antagonist A438079. This antagonist has been previously shown to have no detectable effects on other P2X and P2Y receptors (24). The results in Fig. 3A reveal that the release of VEGF by primary cells treated for 4 h with either 100 μM BzATP or 300 μM ATP, but not with PMA, is concentration-dependently inhibited by the P2RX7 antagonist A438079. Data in Supplemental Figure 1 demonstrates VEGF Release (pg/mL) following pretreatment with A438079 for each individual donor. The percent inhibition of BzATP-induced VEGF after preincubation with the P2RX7 antagonist was observed to be similar following 24 h treatment (unpublished observation). To ensure that A438079 pretreatment was not attenuating P2RX7 agonist-induced VEGF release because of cytotoxicity, MTS viability assays were performed in parallel with the VEGF release experiments. As illustrated in Fig. 3B, pretreatment with A438079 had no significant effect on the metabolic activity of the cells.
Fig. 3. P2RX7 antagonist A438079 attenuates BzATP and ATP-induced VEGF production.
(A) Primary human monocytes were pre-incubated with vehicle or A438079 (3 μM or 10 μM) for 30 min at 37°C. The cells were then treated with control (HEPES), 100 μM BzATP, or 300 μM ATP, or 1 μg/ml PMA for 4 h and supernatants were assayed for VEGF. (B) Cells were pretreated and stimulated in parallel to “A”. After 4 h, cell viability was assessed as detailed in Materials and Methods. Primary human monocytes were pre-incubated with vehicle or either (C) P2Y11 antagonist NF-157 (0.3 μM or 1 μM) or (D) P2Y12/13 antagonist 2-Methylthioadenosine 5′-monophosphate (MeSAMP) (1 μM or 3 μM) for 30 min at 37°C. The cells were then treated with control (HEPES), 100 μM BzATP, or 300 μM ATP, or 1 μg/ml PMA for 4 h and supernatants were assayed for VEGF. The results shown in panels A and B include at least 3 independent experiments (mean +/- SEM), whereas the results presented in panels C and D include at least 2 independent experiments (mean +/- Range). *p<0.05, **p<0.01, #p<0.005, ψp<0.001
To asses the contribution of other P2 receptors to VEGF release, selective antagonists for P2RY11 and P2RY12/13 were utilized. The results in Fig. 3C and 3D show that preincubation of primary human monocytic cells with the P2RY11 antagonist NF-157, or the P2RY12/13 antagonist MeSAMP, had no effect on BzATP, ATP, or PMA-induced VEGF release. Also, the co-addition of NF-157 or MeSAMP with the P2RX7 antagonist A438079 resulted in a similar degree of attenuation of BzATP-induced VEGF as the addition of the P2RX7 antagonist alone (unpublished observation). However, as seen previously, we found that NF-157 and MeSAMP could attenuate the P2Y11-dependent IL-8 release (25) and the P2Y12/13-dependent stimulation of ERK phosphorylation (26), respectively (Supplemental Figs. 2 and 3). These data support the hypothesis that BzATP and ATP-induced VEGF release is primarily associated with P2RX7 activation in human monocytic cells.
BzATP and ATP induce P2RX7-dependent VEGF expression
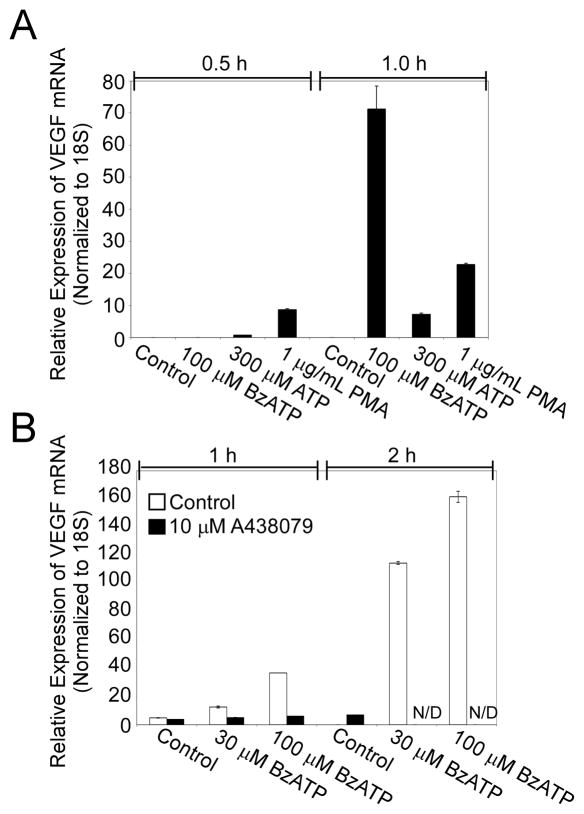
To determine whether P2RX7 agonists induce VEGF expression as well as release, we evaluated the effect of BzATP and ATP on relative mRNA levels of VEGF via qRT-PCR. Primary human monocytes were stimulated with the P2RX7 agonists 100 μM BzATP, 300 μM ATP, or 1 μg/mL PMA for 0.5 and 1 h, and cell lysates were subsequently analyzed for VEGF mRNA by RT-PCR as outlined in Materials and Methods. The results presented in Fig. 4A indicate that P2RX7 agonists can induce a robust, time-dependent increase in VEGF expression in as little as 1 h of treatment time. To evaluate whether induction of VEGF expression by BzATP and ATP is dependent on P2RX7 activation, we determined the sensitivity of nucleotide-induced VEGF expression to the addition of the selective P2RX7 antagonist A438079. As demonstrated in Fig. 4B, preincubation with the P2RX7 antagonist A438079 attenuates BzATP-induced VEGF expression in a concentration- and time-dependent manner. These results further support the idea that nucleotide-induced VEGF expression is dependent upon P2RX7 activation.
Fig. 4. P2RX7 agonists stimulate P2RX7-dependent induction of VEGF mRNA from primary human monocytes.
(A) Primary human monocytes were treated with control (HEPES), 100 μM BzATP, 300 μM ATP, or 1 μg/mL LPS for 0.5 and 1 hr. Cells were then lysed in Trizol and the VEGF mRNA levels were measured using quantitative RT-PCR as detailed in Materials and Methods. Data from a representative experiment are shown as averages with error bars representing mean +/- SD. Results are representative of at least 2 analogous experiments. (B) Primary human monocytes were pre-incubated with vehicle or A438079 (3 μM or 10 μM) for 30 min at 37°C. The cells were then treated with control (HEPES), 30 μM BzATP, or 100 μM BzATP for 1 or 1.5 h, lysed in Trizol, and VEGF mRNA levels were measured using quantitative RT-PCR as detailed in Materials and Methods. Data from a representative experiment are shown as averages with error bars representing mean +/- SD. The results are representative of at least 2 analogous experiments.
LPS priming or co-stimulation does not enhance P2RX7 agonist-induced VEGF release
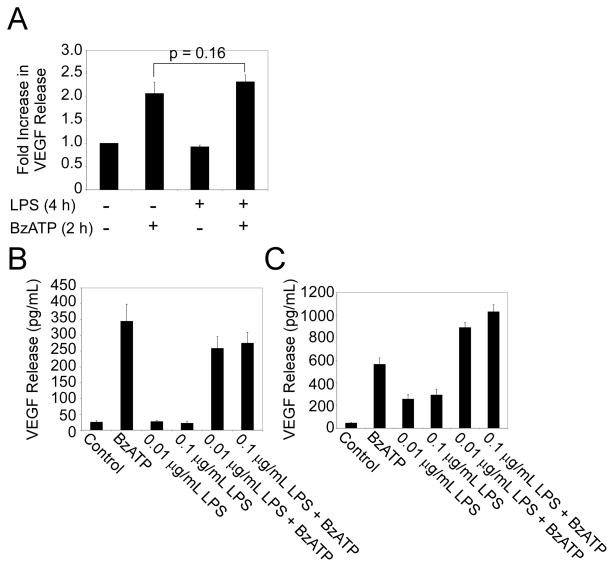
Several P2RX7 signaling events are enhanced by priming or the co-administration of various immune stimulants such as LPS [7, 8]. To determine whether LPS-priming could modulate nucleotide-induced VEGF release, primary human monocytes were first treated with 100 ng/mL LPS for 4 h and then stimulated with BzATP for 2 h. Fig. 5A provides data demonstrating that LPS priming has no detectable effect on BzATP-induced VEGF release. To examine whether LPS co-administration can alter BzATP-induced VEGF release, monocytes were stimulated with 100 μM BzATP and various concentrations of LPS for 4 and 24 h. The results shown in Figs. 5B and 5C indicate that co-administration with LPS does not significantly enhance BzATP-induced VEGF release at 4 or 24 h. These results indicate that P2RX7 agonist-induced VEGF release is not dependent on, or enhanced by, priming or co-administration with LPS.
Fig. 5. LPS-priming or co-stimulation does not synergistically enhance P2RX7 agonist-induced VEGF production.
(A) Primary human monocytes were primed with vehicle control (HEPES) or 100 ng/mL LPS for 4 h and stimulated with either vehicle control (HEPES) or 100 μM BzATP for 2 h. (B-C) Primary human monocytes were stimulated with vehicle control (HEPES), 100 μM BzATP, 0.01 μg/mL LPS, 0.1 μg/mL LPS or co-stimulated with 100 μM BzATP/0.01 μg/mL LPS or 100 μM BzATP/0.1 μg/mL LPS for 4 h (B) or 24 h (C). Supernatants were collected and analyzed for VEGF release. The results depicted in panels A-C each represent three independent experiments (mean +/- SEM).
P2RX7 agonist-induced VEGF release is Ca2+-dependent
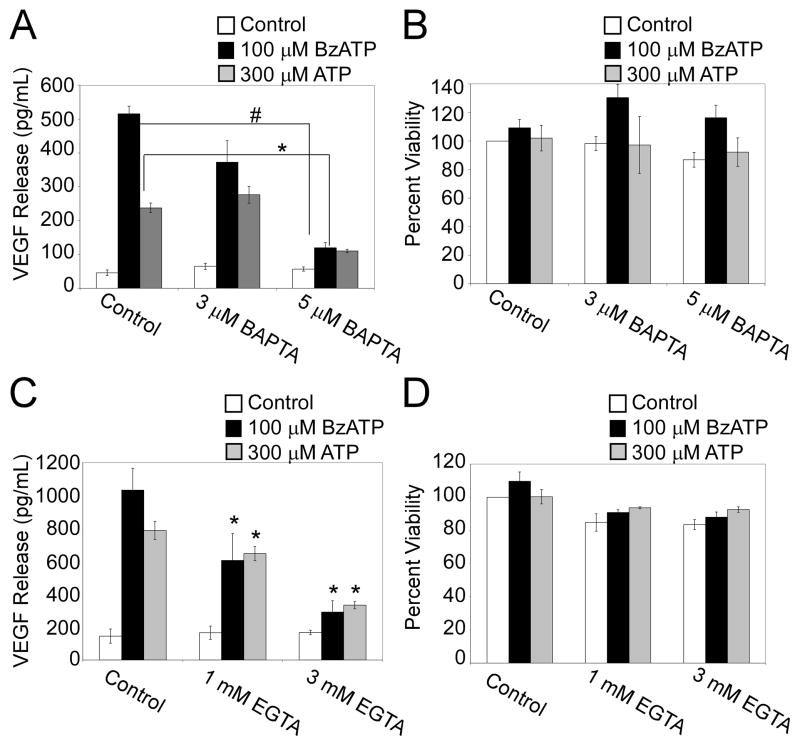
To determine if the P2RX7 agonist-stimulated release of VEGF by primary human monocytes is linked to the rapid increase in intracellular Ca2+ observed after P2RX7 ligation, we examined the influence of treatment with EGTA (an extracellular Ca2+ chelator) or a cell permeable Ca2+ chelator BAPTA-AM on this process. Cells were incubated with BAPTA-AM (3–5 μM or EGTA (1–3 mM) for 20 min prior to stimulation with 100 μM BzATP, 300 μM ATP or the Ca2+ ionophore ionomycin (1 μg/ml). As shown in Fig. 6A, pretreatment of monocytic cells with BAPTA-AM significantly attenuated P2RX7 agonist-induced VEGF release. Conversely, ionomycin treatment alone was unable to stimulate robust VEGF release from these cells (VEGF levels: 131 ± 76 pg/mL (ionomycin-treated) compared to 153 ± 72 pg/mL (vehicle-treated)). Similarly, as shown in Fig. 6C, pretreatment with EGTA concentration-dependently attenuated P2RX7 agonist-induced VEGF release. To ensure that BAPTA-AM or EGTA pretreatment were not attenuating P2RX7 agonist-induced VEGF release because of their possible cytotoxic effects, we performed MTS viability assays in parallel with the VEGF release experiments. As shown in Fig. 6B and D respectively, pretreatment with BAPTA-AM or EGTA has no significant effect on the metabolic activity of the cells. Collectively, these results provide evidence that Ca2+ fluxes are necessary but not sufficient for P2RX7 agonist-induced VEGF release.
Fig. 6. P2RX7 agonist-induced VEGF production is calcium-dependent.
(A) Primary human monocytes were loaded with either vehicle control (DMSO) or BAPTA-AM (3 μM or 5 μM) for 20 min at 37°C. The cells were then treated with control (HEPES), 100 μM BzATP, or 300 μM ATP for 4 h and supernatants were assayed for VEGF. (B) Cells were treated in parallel to panel “A”. Cell viability was measured as noted under Materials and Methods. (C) Primary human monocytes were loaded with either vehicle control (water) or EGTA (1 mM or 3 mM) for 20 min at 37°C. The cells were then treated with control (HEPES), 100 μM BzATP, or 300 μM ATP for 4 h and supernatants were assayed for VEGF. (D) Cells were treated in parallel to panel “C”. Cell viability was measured as noted under Materials and Methods. The results shown in panels A-D include 3 independent experiments (mean +/- SEM). *p<0.05, **p<0.01, #p<0.005, ψp<0.001
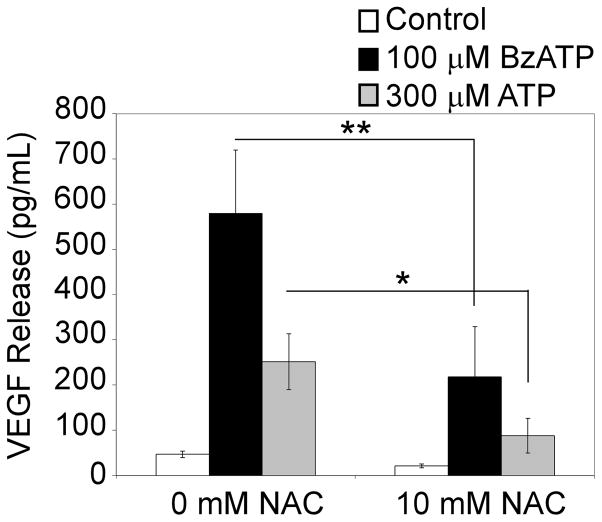
P2RX7 agonist-induced VEGF release is sensitive to the antioxidant N-Acetyl Cysteine (NAC)
Although P2RX7 agonist-induced VEGF release appears dependent on an increase in intracellular Ca2+ levels, the failure of ionomycin to trigger VEGF release suggests that an elevation of cytoplasmic Ca2+ is not sufficient for this process. Because VEGF is known to be induced by shear stress and hypoxia, and given that activation of P2RX7 can induce the rapid production of ROS in a Ca2+-independent manner (8, 27), we tested the hypothesis that P2RX7 agonist-induced VEGF release in primary human monocytes is linked, at least in part, to ROS production. In these experiments, cells were pretreated with the antioxidant NAC (10 mM) for 20 min prior to stimulation with 100 μM BzATP or 300 μM ATP. Fig 7 illustrates that NAC pretreatment attenuates P2RX7 agonist-induced VEGF release. Conversely, hydrogen peroxide (H2O2) treatment alone (169 ± 70 pg/mL, compared to control 153 ± 72 pg/mL, or in combination with ionomycin treatment (170 ± 85 pg/mL, compared to control 153 ± 72 pg/mL) is unable to stimulate robust VEGF release from these cells. Taken together, these results indicate that P2RX7-mediated VEGF release is dependent on both Ca2+ and ROS production, but that additional processes are likely to be critical.
Fig. 7. P2RX7 agonist-induced VEGF production is sensitive to NAC.
Primary human monocytes were pretreated with either vehicle control (HEPES) or 10 mM NAC for 20 min at 37°C. Following pre-incubation, cells were treated with control, 100 μM BzATP, or 300 μM ATP for 4 h. Supernatants were collected and assayed for VEGF. The results represent 3 independent experiments (mean +/- SEM). *π<0.05, **π<0.01, #π<0.005, ψp<0.001
Discussion
Monocytic cells play an integral role in the resolution of inflammation via the production of angiogenic factors that stimulate new blood vessel formation and allow for immune cell trafficking, tissue remodeling and repair. Relatively little is understood regarding the role of P2RX7 in monocytic cell-dependent wound repair, and there are no previous reports demonstrating a link between P2RX7 activation and the production of angiogenic proteins. In the present report, we provide evidence supporting a previously unrecognized action of P2RX7 signaling, namely the capacity to induce rapid and robust expression and release of the pro-angiogenic factor VEGF from primary human monocytes. The magnitude of VEGF release after stimulation with P2RX7 agonists appears biologically relevant in that it is comparable to the concentrations of this factor found in the serum of patients after injury or infection (28, 29) and comparable to that induced by LPS.
Although BzATP is a selective agonist for P2RX7, it can activate other P2 receptors at high concentrations. The data presented here indicate that VEGF release stimulated by P2RX7 agonists is concentration-dependently attenuated by the selective P2RX7 antagonist A438079, whereas PMA-stimulated VEGF release is unaltered by the addition of the P2RX7 antagonist. This attenuation does not appear to arise from possible non-selective cytotoxic effects of this antagonist as two independent viability assays were preformed in parallel and both indicated that the P2RX7 antagonist had no discernible effect on cell viability (Fig 3B and Supplemental Fig. 4). This antagonist can block several BzATP-induced P2RX7 events and appears devoid of activity towards other P2 receptors and a wide array of other ion channels (24). To asses the contribution of other P2 receptors to VEGF release, we utilized several selective agonists and antagonists. We found that uridine nucleotides UTP and UDP were unable to stimulate substantial VEGF release (79 ± 3 pg/mL and 84 ± 2 pg/mL respectively compared to control 60 ± 5 pg/mL) suggesting that P2 receptors sensitive to these nucleotides, such as P2RY2, P2RY4, and P2RY6, are not likely to be significantly involved in nucleotide-induced VEGF release (unpublished observation). Also, selective inhibition of P2RY11, P2RY12, or P2RY13, which are receptors that can be activated by high concentrations of BzATP, had no detectable effect on nucleotide-induced VEGF release. Together, these data are consistent with the idea that BzATP-and ATP-induced VEGF release is largely dependent on P2RX7 signaling.
As discussed above, the rapid production and release of VEGF by primary monocytes is selectively observed with P2RX7 agonists, whereas LPS-stimulated VEGF release requires a considerably longer period of time to become detectable by ELISA. This difference in the time course of action suggests several possibilities. For example, differing signaling mechanisms of VEGF induction may exist between P2RX7- and LPS-dependent systems. In this regard, VEGF production can be regulated at the level of transcription, mRNA stability, and translation, thereby allowing for variations in the kinetics of VEGF production in response to distinct stimuli (30). We were able to determine that P2RX7 agonists induce robust, A438079-sensitive expression of VEGF mRNA (40–70 fold after 1 h) in primary human monocytes, in contrast to the modest levels seen after nucleotide stimulation of a glioma cell line (0.6 fold after 8 h) (31). These observations support the idea that P2RX7 nucleotides are able to affect VEGF expression at the transcriptional level and provide further evidence for the role of P2RX7 in this modulation. In addition, because several cell types have been reported to release ATP following LPS administration (33, 34), the differing time course of action between P2RX7 ligands and LPS may be attributable to a delayed autocrine effect initiated by LPS that partly involves P2RX7 signaling events. However, this possibility appears less likely because we observed that the P2RX7 antagonist A438079 did not attenuate LPS-stimulated VEGF release (unpublished observation). Interestingly, although P2RX7 activation is known to enhance several signaling processes initiated by other immune stimulants, such as LPS (7, 8), it is noteworthy that LPS-priming of primary human monocytes did not enhance P2RX7 agonist-induced VEGF, and co-stimulation with P2RX7 agonists and LPS did not result in synergistic enhancement of VEGF release. Thus, P2RX7 agonist-induced VEGF appears unique in that its release is not dependent on co-administration with other immune stimulants.
Another consideration with respect to the dynamics of VEGF release is that short-term activation of P2RX7 may more accurately recapitulate the transient availability of ATP after events such as cell lysis at sites of injury or infection. In this respect, data presented herein indicate that activation of P2RX7 for as little as 5 minutes results in significant VEGF release from primary human monocytes. Therefore, the release of VEGF after short-term stimulation of P2RX7 may be relevant in multiple injury models including ischemic stroke where the presence of ATP in the extracellular environment is markedly but transiently elevated.
The activation of P2RX7 by high concentrations of ATP observed during inflammation is known to result in cell death in as little as 4–5 h. Interestingly, our results reveal significant VEGF release after cell stimulation with very low levels of P2RX7 agonists. Few P2RX7-dependent signaling pathways are known to be sensitive to such small concentrations of agonist, suggesting that VEGF release is an exquisitely sensitive endpoint for P2RX7 action. It is of note that these low concentrations of BzATP and ATP do not result in cell death even 24 h post treatment (Supplemental Fig. 5), suggesting a role for P2RX7-dependent VEGF release in the absence of nucleotide-induced apoptosis or necrosis. Recently, several papers have identified P2RX7-dependent protein expression in the absence of cell death (8, 21, 32, 33), supporting the idea that P2RX7 may be capable of participating in more long term responses such as wound repair and angiogenesis. Because VEGF can stimulate several transcription factors recently linked to P2RX7 activation, namely Egr-1 and AP-1 (34, 35), it is conceivable that P2RX7-induced VEGF may also play a role in modulating P2RX7-dependent gene expression.
P2RX7 activation results in a rapid influx of Ca2+ ions, and our data support a role for an elevation of intracellular free Ca2+ in P2RX7-induced VEGF release. However, ionomycin alone is unable to stimulate VEGF release, suggesting that an elevation of cytoplasmic Ca2+ is necessary but not sufficient for VEGF release. Although P2RX7 is a ligand-gated ion channel, it does signal via several Ca2+-independent mechanisms, including ROS production (8, 27), which in turn can stimulate the MAP kinases p38 and JNK (7). We observed that P2RX7 agonist-induced VEGF release is sensitive to the antioxidant NAC, supporting a role for ROS in VEGF release. However, H2O2 stimulation alone, or in concert with ionomycin, is insufficient to produce VEGF release from primary cells. These data suggest that multiple P2RX7-dependent signaling events must co-occur to enable robust VEGF release in these cells, and that Ca2+ influxes together with ROS production can not solely recapitulate this P2RX7-dependent event. It is noteworthy that inhibition of the MAP kinases ERK, p38 and JNK, which are Ca2+-and/or ROS-sensitive P2RX7 endpoints, has no discernable effect on P2RX7 agonist-induced VEGF release (unpublished observation). Altogether, our data suggest that a Ca2+- and ROS-independent effector(s) upstream of VEGF release can contribute to P2RX7 signaling.
Nucleotide release can occur by processes other than infection and cytolysis, including platelet degranulation and release through certain membrane channels (36). Endothelial cells are known to release ATP using non-lytic pathways in response to shear stress or hypoxia (37, 38). Therefore, endothelial cells may activate P2RX7 on circulating monocytes and trigger VEGF production as a means to combat hypoxic environments resulting from normal endothelial turnover. ATP release and subsequent P2RX7 activation has also been implicated in cell transformation, and P2RX7 has been linked to numerous cancers including chronic lymphocytic leukemia, neuroblastomas, and cervix squamous carcinoma (for review see (8)). Dysregulated VEGF production is often a hallmark of tumor development and linked to tumor angiogenesis (19). Thus, ATP-activated monocytic cells within a tumor environment may contribute to tumor vascularization and growth through P2RX7-dependent VEGF release. The targeting of P2RX7 or P2RX7-dependent VEGF release may prove beneficial in the treatment of tumor growth.
In sum, P2RX7 activation can promote the rapid and robust release of VEGF from primary human monocytes, suggesting a previously unrecognized role for P2RX7 in angiogenesis and wound repair.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants HL56396 and AI50500
Abbreviations used are
- P2RX7
purinergic receptor X7
- VEGF
vascular endothelial growth factor
- BzATP
2′(3′)-O-(4-benzoylbenzoyl)-ATP
- ROS
reactive oxygen species
- LPS
lipopolysaccharide
- DAMP
damage associated molecular pattern
- NAC
N-acetyl cysteine
References
- 1.Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429–436. doi: 10.1016/j.it.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 2.la Sala A, Ferrari D, Di Virgilio F, Idzko M, Norgauer J, Girolomoni G. Alerting and tuning the immune response by extracellular nucleotides. J Leukoc Biol. 2003;73:339–343. doi: 10.1189/jlb.0802418. [DOI] [PubMed] [Google Scholar]
- 3.Gordon JL. Extracellular ATP: effects, sources and fate. Biochem J. 1986;233:309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proctor RA, Denlinger LC, Leventhal PS, Daugherty SK, van de Loo JW, Tanke T, Firestein GS, Bertics PJ. Protection of mice from endotoxic death by 2-methylthio-ATP. Proc Natl Acad Sci U S A. 1994;91:6017–6020. doi: 10.1073/pnas.91.13.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 6.Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Goldberg H, Marks GB, Wiley JS, Britton WJ. A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. Am J Respir Crit Care Med. 2007;175:360–366. doi: 10.1164/rccm.200607-970OC. [DOI] [PubMed] [Google Scholar]
- 7.Pfeiffer ZA, Guerra AN, Hill LM, Gavala ML, Prabhu U, Aga M, Hall DJ, Bertics PJ. Nucleotide receptor signaling in murine macrophages is linked to reactive oxygen species generation. Free Radic Biol Med. 2007;42:1506–1516. doi: 10.1016/j.freeradbiomed.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenertz LY, Gavala ML, Hill LM, Bertics PJ. Cell signaling via the P2X(7) nucleotide receptor: linkage to ROS production, gene transcription, and receptor trafficking. Purinergic Signal. 2009;5:175–187. doi: 10.1007/s11302-009-9133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aga M, Johnson CJ, Hart AP, Guadarrama AG, Suresh M, Svaren J, Bertics PJ, Darien BJ. Modulation of monocyte signaling and pore formation in response to agonists of the nucleotide receptor P2X(7) J Leukoc Biol. 2002;72:222–232. [PubMed] [Google Scholar]
- 10.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. Journal of Immunology. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 12.Eubank TD, Galloway M, Montague CM, Waldman WJ, Marsh CB. M-CSF induces vascular endothelial growth factor production and angiogenic activity from human monocytes. J Immunol. 2003;171:2637–2643. doi: 10.4049/jimmunol.171.5.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiriakidis S, Andreakos E, Monaco C, Foxwell B, Feldmann M, Paleolog E. VEGF expression in human macrophages is NF-kappaB-dependent: studies using adenoviruses expressing the endogenous NF-kappaB inhibitor IkappaBalpha and a kinase-defective form of the IkappaB kinase 2. J Cell Sci. 2003;116:665–674. doi: 10.1242/jcs.00286. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol. 2001;280:C1358–1366. doi: 10.1152/ajpcell.2001.280.6.C1358. [DOI] [PubMed] [Google Scholar]
- 15.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 16.Harmey JH, Dimitriadis E, Kay E, Redmond HP, Bouchier-Hayes D. Regulation of macrophage production of vascular endothelial growth factor (VEGF) by hypoxia and transforming growth factor beta-1. Ann Surg Oncol. 1998;5:271–278. doi: 10.1007/BF02303785. [DOI] [PubMed] [Google Scholar]
- 17.Qin KR, Xiang C, Xu Z, Cao LL, Ge SS, Jiang ZL. Dynamic modeling for shear stress induced ATP release from vascular endothelial cells. Biomech Model Mechanobiol. 2008;7:345–353. doi: 10.1007/s10237-007-0088-8. [DOI] [PubMed] [Google Scholar]
- 18.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- 19.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 20.Afuwape AO, Kiriakidis S, Paleolog EM. The role of the angiogenic molecule VEGF in the pathogenesis of rheumatoid arthritis. Histol Histopathol. 2002;17:961–972. doi: 10.14670/HH-17.961. [DOI] [PubMed] [Google Scholar]
- 21.Gavala ML, Pfeiffer ZA, Bertics PJ. The nucleotide receptor P2RX7 mediates ATP-induced CREB activation in human and murine monocytic cells. J Leukoc Biol. 2008;84:1159–1171. doi: 10.1189/jlb.0907612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itaya H, Imaizumi T, Yoshida H, Koyama M, Suzuki S, Satoh K. Expression of vascular endothelial growth factor in human monocyte/macrophages stimulated with lipopolysaccharide. Thromb Haemost. 2001;85:171–176. [PubMed] [Google Scholar]
- 23.Shih SC, Mullen A, Abrams K, Mukhopadhyay D, Claffey KP. Role of protein kinase C isoforms in phorbol ester-induced vascular endothelial growth factor expression in human glioblastoma cells. J Biol Chem. 2000;275:29178. [PubMed] [Google Scholar]
- 24.Nelson DW, Gregg RJ, Kort ME, Perez-Medrano A, Voight EA, Wang Y, Grayson G, Namovic MT, Donnelly-Roberts DL, Niforatos W, Honore P, Jarvis MF, Faltynek CR, Carroll WA. Structure-activity relationship studies on a series of novel, substituted 1-benzyl-5-phenyltetrazole P2X7 antagonists. J Med Chem. 2006;49:3659–3666. doi: 10.1021/jm051202e. [DOI] [PubMed] [Google Scholar]
- 25.Meis S, Hamacher A, Hongwiset D, Marzian C, Wiese M, Eckstein N, Royer HD, Communi D, Boeynaems JM, Hausmann R, Schmalzing G, Kassack MU. NF546 [4,4'-(carbonylbis(imino-3,1-phenylene-carbonylimino-3,1-(4-methyl-phenyle ne)-carbonylimino))-bis(1,3-xylene-alpha,alpha'-diphosphonic acid) tetrasodium salt] is a non-nucleotide P2Y11 agonist and stimulates release of interleukin-8 from human monocyte-derived dendritic cells. J Pharmacol Exp Ther. 332:238–247. doi: 10.1124/jpet.109.157750. [DOI] [PubMed] [Google Scholar]
- 26.Feng C, Mery AG, Beller EM, Favot C, Boyce JA. Adenine nucleotides inhibit cytokine generation by human mast cells through a Gs-coupled receptor. J Immunol. 2004;173:7539–7547. doi: 10.4049/jimmunol.173.12.7539. [DOI] [PubMed] [Google Scholar]
- 27.Seil M, Fontanils U, Etxebarria IG, Pochet S, Garcia-Marcos M, Marino A, Dehaye J. Pharmacological evidence for the stimulation of NADPH oxidase by P2X7 receptors in mouse submandibular glands. Purinergic Signal. 2008;4:347. doi: 10.1007/s11302-008-9118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seko Y, Fukuda S, Nagai R. Serum levels of endostatin, vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) in patients with acute myocardial infarction undergoing early reperfusion therapy. Clin Sci (Lond) 2004;106:439–442. doi: 10.1042/CS20030365. [DOI] [PubMed] [Google Scholar]
- 29.Choi SH, Park EY, Jung HL, Shim JW, Kim DS, Park MS, Shim JY. Serum vascular endothelial growth factor in pediatric patients with community- acquired pneumonia and pleural effusion. J Korean Med Sci. 2006;21:608–613. doi: 10.3346/jkms.2006.21.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Claffey KP, Shih SC, Mullen A, Dziennis S, Cusick JL, Abrams KR, Lee SW, Detmar M. Identification of a human VPF/VEGF 3' untranslated region mediating hypoxia-induced mRNA stability. Mol Biol Cell. 1998;9:469–481. doi: 10.1091/mbc.9.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei W, Ryu JK, Choi HB, McLarnon JG. Expression and function of the P2X(7) receptor in rat C6 glioma cells. Cancer Lett. 2008;260:79–87. doi: 10.1016/j.canlet.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 32.Hu Y, Fisette PL, Denlinger LC, Guadarrama AG, Sommer JA, Proctor RA, Bertics PJ. Purinergic receptor modulation of lipopolysaccharide signaling and inducible nitric-oxide synthase expression in RAW 264.7 macrophages. J Biol Chem. 1998;273:27170–27175. doi: 10.1074/jbc.273.42.27170. [DOI] [PubMed] [Google Scholar]
- 33.Stefano L, Rossler OG, Griesemer D, Hoth M, Thiel G. P2X(7) receptor stimulation upregulates Egr-1 biosynthesis involving a cytosolic Ca(2+) rise, transactivation of the EGF receptor and phosphorylation of ERK and Elk-1. J Cell Physiol. 2007;213:36–44. doi: 10.1002/jcp.21085. [DOI] [PubMed] [Google Scholar]
- 34.Pufe T, Harde V, Petersen W, Goldring MB, Tillmann B, Mentlein R. Vascular endothelial growth factor (VEGF) induces matrix metalloproteinase expression in immortalized chondrocytes. J Pathol. 2004;202:367–374. doi: 10.1002/path.1527. [DOI] [PubMed] [Google Scholar]
- 35.Sassa Y, Hata Y, Murata T, Yamanaka I, Honda M, Hisatomi T, Fujisawa K, Sakamoto T, Kubota T, Nakagawa K, Sueishi K, Ishibashi T. Functional role of Egr-1 mediating VEGF-induced tissue factor expression in the retinal capillary endothelium. Graefes Arch Clin Exp Ophthalmol. 2002;240:1003–1010. doi: 10.1007/s00417-002-0576-6. [DOI] [PubMed] [Google Scholar]
- 36.Detwiler TC, Feinman RD. Kinetics of the thrombin-induced release of adenosine triphosphate by platelets. Comparison with release of calcium. Biochemistry. 1973;12:2462–2468. doi: 10.1021/bi00737a015. [DOI] [PubMed] [Google Scholar]
- 37.Pearson JD, Gordon JL. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature. 1979;281:384–386. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]
- 38.Bodin P, Bailey D, Burnstock G. Increased flow-induced ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br J Pharmacol. 1991;103:1203–1205. doi: 10.1111/j.1476-5381.1991.tb12324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.