Abstract
Human blood eosinophils exhibit a hyper-active phenotype in response to chemotactic factors following cell “priming” with IL-5 family cytokines. Earlier work has identified ERK1/2 as molecular markers for IL-5 priming, and herein we show that IL-3, a member of IL-5 family, also augments fMLP-stimulated ERK1/2 phosphorylation in primary eosinophils. Besides ERK1/2, we also observed an enhancement of chemotactic factor-induced Akt phosphorylation following IL-5 priming of human blood eosinophils. Administration of a peptide antagonist that targets the Src family member Lyn prior to cytokine (IL-5/IL-3) priming of blood eosinophils inhibited the synergistic increase of fMLP-induced activation of Ras, ERK1/2 and Akt, as well as the release of the proinflammatory factor leukotriene-C4. In the current study, we also examined a human eosinophil-like cell line HL-60 clone-15, and observed that these cells exhibited significant surface expression of IL-3 and GM-CSF receptors, as well as ERK1/2 phosphorylation in response to the addition of IL-5 family cytokines or the chemotactic factors fMLP, CCL5 and CCL11. Consistent with the surface profile of IL-5 family receptors, HL-60 clone-15 recapitulated the enhanced fMLP-induced ERK1/2 phosphorylation observed in primary blood eosinophils following priming with IL-3/GM-CSF, and siRNA-mediated knockdown of Lyn expression completely abolished the synergistic effects of IL-3 priming on fMLP-induced ERK1/2 phosphorylation. Altogether, our data reveal a central role for Lyn in the mechanisms of IL-5 family priming, and suggest that Lyn contributes to the up-regulation of the Ras-ERK1/2 and PI3K-Akt cascades as well as the increased leukotriene-C4 release observed in response to fMLP in “primed” eosinophils.
Keywords: Eosinophils, Signal Transduction, Inflammation, Cytokines, Chemokines
Introduction
Asthma is an inflammatory airway disease that has experienced an increase in global incidence in recent years (1, 2). Eosinophils are a multifaceted granulocyte and prominent effector cell in asthma, and the infiltration of these cells into the airways is a hallmark of asthma exacerbation (3–6). The function of human eosinophils is largely regulated by IL-5 family cytokines, including IL-5, IL-3 and GM-CSF (7–10). Recently, several lines of evidence demonstrate that IL-5 family members can enhance blood eosinophil responsiveness to a second stimulus, such as the chemotactic factors CCL5 and formyl-Met-Leu-Phe (fMLP), resulting in a synergistic response known as “priming” (10–15). The capacity of IL-5 family cytokines to prime blood eosinophils for altered chemoattractant responsiveness has been associated with changes in intracellular signaling events, e.g. an increase in chemoattractant-induced phosphorylation of the mitogen-activated protein kinases ERK1 and 2 (11), and biological responses, such as chemotaxis, degranulation and the release of proinflammatory mediators (7, 9, 10, 12, 14, 15). Furthermore, the priming of blood eosinophils with IL-5 family members allows for these cells to exhibit a similar phenotype and hyper-responsiveness as that observed with airway eosinophils (9, 16–19). Therefore, understanding the molecular bases of priming is key for delineating the function of eosinophils in asthma.
The capacity of IL-5 family cytokines to prime the action of a wide variety of chemotactic factors that signal via G-protein-coupled receptors (GPCRs) (9, 11, 14) suggests the presence of a fundamental mechanism by which IL-5 family members serve to enhance the responses induced by GPCR ligands. In addition, the effects of cytokine priming on chemoattractant-induced intracellular signaling (ERK1/2 phosphorylation) are rapid and relatively long lasting (11). Therefore, we hypothesized that the crosstalk between IL-5 family- and GPCR- mediated pathways is a key characteristic of priming, and given the rapid nature of cytokine priming on chemoattractant-induced ERK1/2 phosphorylation, it is likely that it entails the modification and/or reorganization of signaling molecules via pre-assembly of adaptor proteins and localization of downstream effectors.
With respect to the molecular mechanisms of eosinophil priming by IL-5 family cytokines, little is known, although it has been reported that the Src family member Lyn can physically associate with the IL-5 receptor β chain (which is shared with the IL-3 and GM-CSF receptors) upon receptor ligation. This association appears critical for the subsequent activation of the Ras-Raf-MEK-ERK pathway (20–25). Previous research focusing on the role of Lyn in human eosinophil function has also revealed that this tyrosine kinase is important for IL-5-induced ERK1/2 phosphorylation as well as cell survival and differentiation (20–27). Additionally, Src family members, including Lyn, have been suggested to initiate events leading to ERK1/2 activation in numerous GPCR systems (28–31). Accordingly, in the present study we tested the idea that Lyn is central to IL-5 family cytokine-mediated eosinophil priming, and we show that Lyn is not only important for IL-5 induced signaling and cellular responses, but that it also serves as a key component in the crosstalk between IL-5 family cytokines and the chemotactic factor fMLP. We demonstrate that the synergistic increase of fMLP-induced activation of the Ras-ERK1/2 and PI3K-Akt cascades following IL-5/IL-3 priming is dependent on Lyn activity in human primary blood eosinophils and the human eosinophil-like cell line HL-60 clone-15. Furthermore, we observed that the enhanced release of the proinflammatory mediator leukotriene-C4 (LTC4) in response to fMLP treatment of IL-5- or IL-3-primed human blood eosinophils is also dependent upon Lyn, supporting the concept that Lyn is involved in the enhanced inflammatory capacity of “primed” eosinophils. These results advanced our understanding of the molecular basis of priming in human blood eosinophils and provide a potential therapeutic target for suppressing the appearance of the hyper-responsive phenotype of airway eosinophils in individuals with asthma.
Materials and Methods
Reagents and Cells
The human promyelocytic cell line HL-60 clone-15 (catalog number CRL-1964) was purchased from ATCC and maintained in the growth medium as instructed. All experiments were performed on cells within the first ten passages to minimize phenotypic reversion. The HL-60 clone-15 cells were differentiated by treatment with freshly prepared 0.5 mM sodium butyrate for 5 days. Recombinant human IL-5, IL-3, GM-CSF and CCL11 were obtained from R&D Systems (Minneapolis, MN), and CCL5 from Peprotech (Rocky Hill, NJ). Sodium butyrate, fMLP and human serum albumin (HSA) were purchased from Sigma-Aldrich (St. Louis, MO). Chemical PP2 and PP3 were obtained from EMD (Gibbstown, NJ). The following antibodies were used in immunoblotting: anti-phosphoERK1/2 (Invitrogen-Biosource, Carlsbad, CA), anti-phosphoAkt (Cell Signaling Technology, Danvers, MA), anti-actin (BC Bioscience, San Jose, CA), anti-Grb2 (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-β-tubulin (Sigma-Aldrich, Saint Louis, MO). To assess receptor expression by flow cytometry, we used phycoerythrin (PE)-conjugated antibodies against IL-5 receptor α, IL-3 receptor α, as well as fluorescein isothiocyanate (FITC)-conjugated antibodies for GM-CSF receptor α and the corresponding IgG controls that were obtained from BD Bioscience (San Jose, CA).
Isolation of Human Eosinophils from Peripheral Blood
Human subjects recruited to this study were between 18 and 55 years old and included non-allergic, atopic and physician-diagnosed allergic asthmatic individuals. Human blood eosinophils were purified from heparinized peripheral blood as described previously (11). Briefly, peripheral blood was subjected to hypotonic shock to remove erythrocytes and followed by centrifugation through a Percoll monolayer (1.090 g/ml) to obtain a granulocyte mixture. Subsequently, neutrophils were removed by negative selection using anti-CD16 paramagnetic microbeads and Automacs machine obtained from Miltenyi Biotechnology (Auburn, CA). The recovered mixture was at least 96% eosinophils and greater or equal to 95% viability by Giemsa's-based Diff-Quik stain (Baxter Scientific Products, McGaw Park, IL) and trypan blue exclusion respectively. The purified eosinophils were then resuspended in Hanks' Balanced Salt Solution supplemented with 2% newborn calf serum. This study has been reviewed and approved by The Health Science Institutional Review Board at University of Wisconsin-Madison.
Peptide Inhibitor of Lyn Recruitment (PILR) and siRNA of Lyn
Custom synthesized peptides PILR (IL-5 receptor β chain 450–465: stearyl-YGYRLRRKWEEKIPNP-NH2) and control peptide (stearyl-YEKRWNPKGEPRLIRY-NH2) were purchased from AnaSpec with >95% purity as assessed by HPLC. A validated siRNA for human Lyn (Hs_LYN_12) and Allstar Negative Control siRNA were purchased from Qiagen (San Jose, CA).
Cell Stimulation and Immunoblotting
Cells were suspended in incubation medium (RPMI1640, 25 mM HEPES, 0.1% HSA) and rested at 37°C for 1 hr and 2 hr for human blood eosinophils and HL-60 clone-15 respectively. Subsequently, the cells were stimulated with IL-5 family cytokines and/or the chemotactic factors fMLP and CCL5 for the indicated time periods. In the priming experiments, cells were treated for 1 hr with IL-5 family cytokines, followed by stimulation with the specified chemotactic factors for 2 min. When indicated, the Lyn peptide PILR and the control peptide were added 30 min prior to IL-5 family cytokine treatment. Cell lysates were prepared using RIPA buffer (0.1% triton x-100, 0.25% deoxycholate, 0.1% SDS) and were subjected to SDS-PAGE and immunoblotting as described previously (11). To ensure that equal protein levels were being evaluated, loading controls (Grb2, actin, β-tubulin) were also blotted on the same membranes. Chemiluminescense was captured using an EpiChemi II Darkroom (UVP, Upland, CA) equipped with a 12-bit cooled CCD camera and quantified using Image J (NIH). Band intensities of the proteins of interest were normalized to the corresponding loading controls.
Flow Cytometry
Cells (105) were suspended in 150 ul FACS buffer and incubated on ice with 5 ul of antibodies of PE-IL-5Rα, PE-IL-3Rα FITC-GM-CSFRα or corresponding mouse IgG control for 30 min in the dark. The reactions were stopped by the addition of 2 ml ice-cold PBS and centrifugation. Cell pellets were resuspended in 250 μl of ice-cold FACS buffer and were analyzed by flow cytometry.
Nucleofection
HL-60 clone-15 cells (2×106) were suspended in 100 μl of nucleofection solution V (Lonza, Swizerland) and 5 μl of negative siRNA or si-Lyn (Qiagen, San Jose). The transfection was carried out according to the manufacturer's manual (Lonza) using program T-19. Cells were then allowed to recover in growth medium for 48 hr in 37°C incubator with 5% CO2 before stimulation.
Ras Pull-down Assay
An active-Ras pull-down assay was performed as described previously (32). Briefly, after stimulation, cell lysates were prepared by sonication and insoluble components were removed by centrifugation. The soluble mixtures were then incubated with GST-beads preconjugated with the Ras-binding domain of Raf (RBD) for 2.5 hr at 4°C. After several washes, the beads were resuspended in 2× sample buffer. Protein analysis was carried out by SDS-PAGE and immunoblotting for Ras.
Eosinophil LTC4 Release
Human blood eosinophils (3.25×106 cells/ml) were suspended in 125 ul RPMI+0.1%HSA and preincubated with 10 μM control peptide or PILR for 30 min. Cells then were primed with control, 100 pM IL-5, or 1 nM IL-3 for 1 hr followed by a 20-minute stimulation with 100 nM fMLP. Supernatants were collected by centrifugation and snap frozen in a mixture of methanol and dry ice and stored at −80°C. The LTC4 content in the supernatants was measured using an enzyme-linked immunosorbent assay (Cayman Chemical Co., Ann Arbor, MI). All supernatants were assayed in duplicate.
Statistical Analysis
Statistical analysis was carried out using a paired two-tailed t test unless stated otherwise. A p value of <0.05 was considered significant.
Results
Effects of IL-5 family cytokines on chemotactic factor-induced ERK1/2 and Akt phosphorylation in human primary blood eosinophils
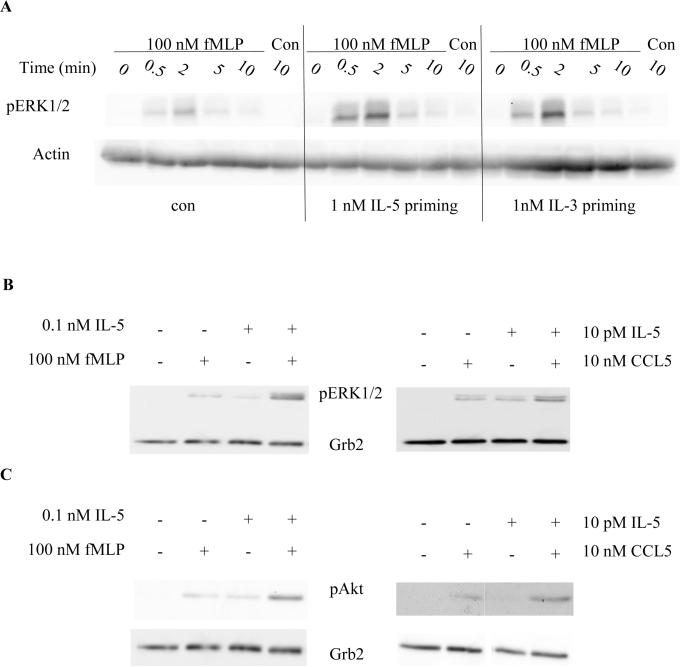
Previous studies have established that ERK1/2 phosphorylation can be used as a molecular marker for IL-5 and GM-CSF priming of chemotactic factor-induced responses in human blood eosinophils (11). However, another IL-5 family member, IL-3, is also detected in the peripheral blood and the airway (33–36), and numerous reports have shown that IL-3 and GM-CSF can elicit similar effects as IL-5, such as prolonged cell survival and the activation of ERK1/2 and PI3K in human blood eosinophils (21, 37, 38). Given the biological relevance of IL-3 to eosinophil function, we first tested whether IL-3 can also induce a similar priming response as IL-5 with respect to chemoattractant-induced ERK activation. In these experiments, purified human blood eosinophils were primed with IL-5 or IL-3 for 1 h. Subsequently, the cells were stimulated with 100 nM fMLP for 2 min. Consistent with the previous findings, IL-5 priming increased fMLP-induced ERK1/2 phosphorylation in human blood eosinophils (Fig. 1A). Likewise, IL-3 also induced the synergistic enhancement of ERK1/2 phosphorylation in fMLP-stimulated human blood eosinophils (Fig. 1A) to a level comparable to that observed with IL-5. These data suggest that the molecular mechanisms of priming are conserved between IL-5 family members, and therefore most likely involve at least in part the common β chain shared by the IL-5, IL-3 and GM-CSF receptors.
Figure 1. IL-5 and IL-3 prime chemotactic factor-induced phosphorylation of ERK1/2 and Akt.
A. Purified human blood eosinophils were primed with control buffer, IL-5 or IL-3 for 1 hr, followed by stimulation with fMLP or control for the indicated time points. Cell lysates were processed by SDS-PAGE and immunoblotted for pERK1/2 and actin (A, N=3) as detailed under Materials and Methods. B and C. Eosinophils were primed with control buffer or IL-5 for 1 hr followed by a two-min stimulation with fMLP or CCL5. Cell lysates were subjected to SDS-PAGE and immunoblotted for pERK1/2 (B, N=5), pAkt (C, N=4) and Grb2.
It has been reported that IL-5 family members and selected chemotactic factors can activate the PI3K-Akt pathway in human blood eosinophils (38–42). Akt is a known downstream effector of the PI3K pathway and activation of PI3K-Akt pathway regulates survival and migration in many cell systems, including murine eosinophils (39, 40, 42, 43). In addition, it has been reported that, in eosinophils, PI3K can be found in a complex with the Src family member Lyn, which is known to regulate IL-5-induced ERK1/2 phosphorylation (39). Therefore, in addition to ERK1/2, we hypothesized that the PI3K-Akt pathway is also up-regulated in response to chemotactic factors fMLP and CCL5 in IL-5-primed eosinophils. Our results demonstrate that, following IL-5 priming, both fMLP and CCL5 were able to induce an enhanced phosphorylation of ERK1/2 (Fig. 1B) and Akt (Fig. 1C). However, IL-5 priming alone or the addition of chemotactic factors (fMLP and CCL5) alone had little to no effect on the levels of ERK1/2 and Akt phosphorylation. These results suggest that IL-5 priming is able to enhance the activation of both ERK1/2 and Akt in response to fMLP or CCL5, which supports the idea that the ERK1/2 and PI3K-Akt activation are enhanced by common upstream cascades following IL-5 priming.
Peptide inhibitor of Lyn attenuates ERK1/2 and Akt phosphorylation in fMLP-stimulated human blood eosinophils following priming with IL-5 family cytokines
The tyrosine kinase Lyn is a Src family member and has been reported to be recruited to the common β chain of IL-5 family receptors upon ligand activation. The activation of Lyn is thought to be important for IL-5-induced activation of ERK1/2 and PI3K-Akt pathways (20, 21, 23, 26, 27, 39). To assess the involvement of Lyn in eosinophil priming, we utilized a peptide inhibitor of Lyn recruitment (PILR). PILR is an oligo-peptide corresponding to the intracellular domain of the common β chain of IL-5 family receptors that has been shown to bind to the N-terminal domain of Lyn, which contains the lipid modification sites for membrane targeting (44). This peptide has been used to block Lyn recruitment to the common β chain and the subsequent activation of Lyn and ERK1/2 following IL-5 stimulation of human blood eosinophils (22, 44).
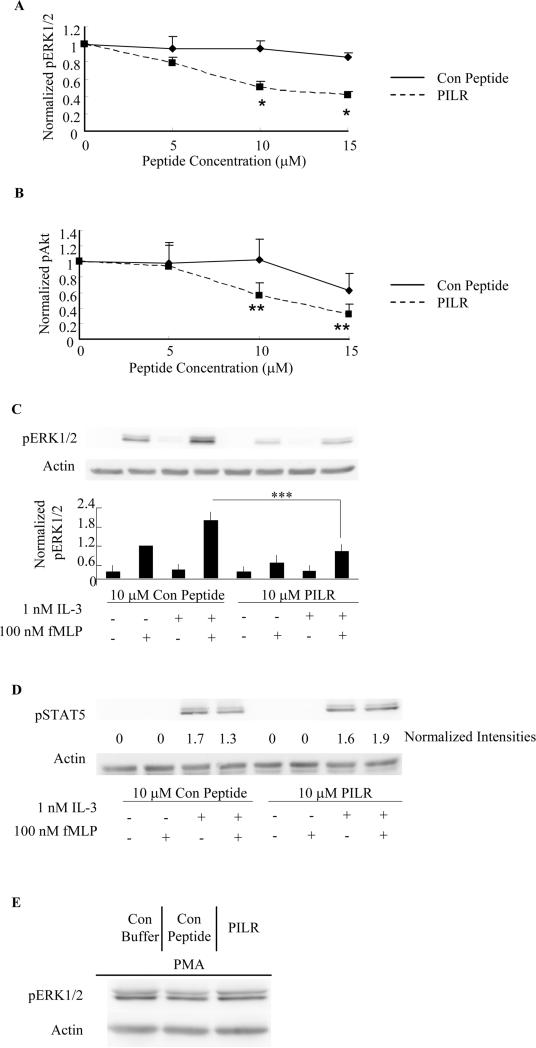
To assess the capacity of PILR to antagonize eosinophil priming by IL-5, we performed experiments similar to those described in Fig. 1 but with the addition of a step involving the pre-incubation of eosinophils with PILR or control peptide for 30 min. As shown in Fig. 2A, fMLP-induced ERK1/2 phosphorylation in IL-5 primed eosinophils was significantly reduced (~ 50%) by PILR at doses of 10 μM (p<0.0003, N=6, Fig. 2A) and 15 μM (p<0.002, N=6, Fig. 2A). The control peptide had no significant effects on fMLP-induced ERK1/2 phosphorylation following IL-5 priming even at the highest concentration (15 μM) tested. Similarly, 10 μM and 15 μM PILR was able to significantly decrease fMLP-induced Akt phosphorylation in IL-5 primed eosinophils by approximately 50% (p<0.05, N=4, Fig. 2B), although the control peptide also promoted a small level of inhibition (not significant) at a peptide concentration of 15 μM. Overall, the phosphorylation of ERK1/2 and Akt in response to fMLP in IL-5-primed blood eosinophils exhibited a dose-dependent decrease in response to PILR administration. To minimize potential non-specific inhibitory effects, we chose 10 μM PILR as the inhibitor concentration for all subsequent experiments. In addition, cell viability was not affected by incubation with 10 μM PILR or control peptide as determined using an MTS assay (data not shown).
Figure 2. PILR inhibits fMLP-stimulated ERK1/2 and Akt phosphorylation in human blood eosinophils primed with IL-5 family members.
Blood eosinophils were preincubated with the Lyn recruitment antagonist PILR or control peptide for 30 min prior to priming with 100 pM IL-5 (A, B. D) or 1 nM IL-3(C) for 1 hr, followed by stimulation with 100 nM fMLP for 2 min. Phosphorylation of ERK1/2, Akt and STAT5 was assessed by SDS-PAGE and immunoblotting as detailed under Materials and Methods. Levels of actin were used as loading controls in all blots. Band intensities of pERK1/2 (A, *p<0.002, N=6, PILR vs. Con Peptide) and pAkt (B, **p<0.05, N=4, PILR vs. Con Peptide) were quantified by densitometry analysis and normalized to that of actin on the same blot. Data are summarized as the mean ± (SEM). C. Representative immunoblots are shown for five independent experiments. Data are summarized in the lower panel as the mean ± (SEM). ***p<0.05, N=5. D. Immunoblots of pSTAT5 and actin are shown as representatives of three independent experiments. Normalized band intensities expressed as fold induction are shown under the pSTAT5 immunoblot. E. Eosinophils were pretreated with control buffer, 10 μM control peptide or PILR for 30 min prior to the stimulation with 10 ng/ml PMA for 10 min. Representative immunoblots of pERK1/2 and actin of three independent experiments are shown.
Because the common β chain is a component of both the IL-5 and IL-3 receptors, and because Lyn is associated with the common β chain, it is conceivable that Lyn is also central to the mechanism of IL-3 priming. To begin to address this issue, we tested the effects of PILR on fMLP-induced ERK1/2 phosphorylation in IL-3-primed human blood eosinophils. At a 10 μM concentration, PILR was able to suppress ERK1/2 phosphorylation in response to fMLP stimulation following IL-3 priming in blood eosinophils (p<0.05, N=5, Fig. 2C). It was also noted that PILR exhibited a slight inhibitory effect on ERK1/2 phosphorylation (not significant) stimulated with fMLP alone (Fig. 2C). The inhibitory effects of PILR on ERK1/2 phosphorylation appeared to be Lyn-selective, as PILR had no detectable effect on IL-3-induced STAT5 phosphorylation or PMA-induced ERK1/2 phosphorylation in human blood eosinophils (Figs. 2 D and E). Altogether, these data support an important role of Lyn in the enhanced ERK1/2 and Akt phosphorylation in response to fMLP following priming with IL-5 family members in human blood eosinophils, and implicate Lyn involvement in the early steps of the crosstalk between IL-5/IL-3- and fMLP-stimulated events.
Differential responses of the naïve and differentiated HL-60 clone-15 to cytokines and the chemotactic factors fMLP and CCL5
Investigations on the cell signaling events that are operative in human eosinophils have been hampered by the limited supply of these cells as well as their resistance to molecular manipulation. Although murine models of airway inflammation have been very useful for studying the contribution of murine eosinophils to airway inflammation, it is recognized that murine eosinophils exhibit a number of differences from their human counterparts (45, 46). Thus, to overcome these limitations, we chose to establish an in vitro cell line model for the study of cytokine priming of human eosinophils. Such a model cell line would facilitate our ability to modify the expression of key signaling molecules and thus allow for us to more thoroughly test for the importance of discrete molecules in eosinophil priming and function. Although several cell lines (such as: EoL-1, EoL-3 and AML14.3D10) have been used to study selected aspects of eosinophil biology, there is no recognized cell line model for the study of eosinophil priming. To this end, we first examined the responses of the human eosinophil-like cell line, HL-60 clone-15 (HC15) in terms of its responsiveness to the coordinate action of IL-5 family cytokines and chemotactic factors.
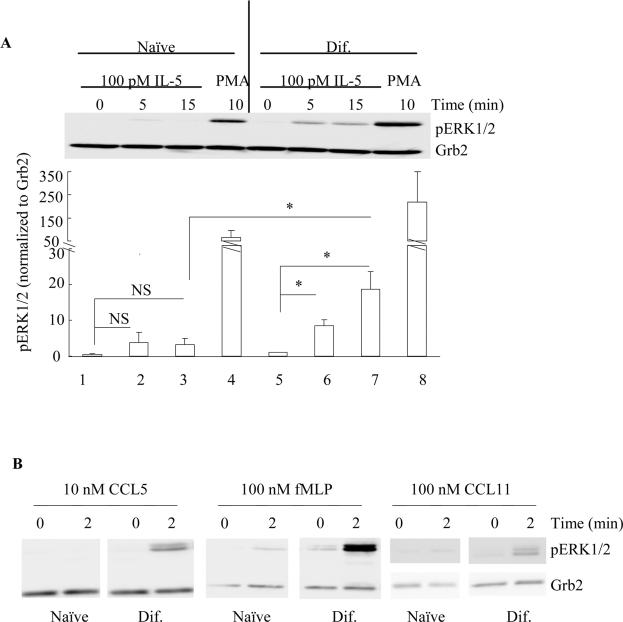
The HC15 cell line has long been recognized for its ability to differentiate into eosinophil-like cells that express eosinophil granule proteins and mRNA for several cytokine and chemokine receptors (47–50). Furthermore, HC15 has been reported to undergo chemotaxis, to release granule proteins, and to produce reactive oxygen species when stimulated with various chemotactic factors (50–55). Using previously reported protocols, we differentiated HC15 by incubating with 0.5 mM sodium butyrate for 5 days (dif-HC15) (47, 48). Although the dif-HC15 cells show an eosinophil-like phenotype, the intracellular signaling events that occur in response to the addition of cytokines and chemotactic factors have not been studied. In this regard, we first stimulated naïve and dif-HC15 cells with IL-5 or the chemotactic factors CCL5, fMLP and CCL11 for the indicated times. With the naïve HC15 cells, little or no ERK1/2 phosphorylation was detected following treatment with IL-5, CCL5 or fMLP (Fig. 3A). In contrast, the cells were considerably more responsive to IL-5 and chemotactic factors after differentiation with sodium butyrate for 5 days. The dif-HC15 cells exhibited a significant increase in ERK1/2 phosphorylation in response to 100 pM IL-5 at 5 min and 15 min (Fig. 3A). Stimulation of dif-HC15 cells with either 10 nM CCL5, 100 nM fMLP or another CCR3 ligand, i.e., 100 nM CCL11, all induced a robust ERK1/2 phosphorylation, although fMLP was also able to promote a low level of ERK1/2 phosphorylation in naïve HC15 cells as well (Fig. 3B). These data suggest that the dif-HC15 cells are, in general, more responsive to various stimuli than the naïve HC15 and this observation is consistent with previous reports on the phenotype of the dif-HC15.
Figure 3. Differential responses of ERK1/2 phosphorylation in the naïve and dif-HC15 in response to IL-5 and chemotactic factors fMLP and CCL5.
A. The naïve (lane 1–4) and dif-HC15 (lane 5–8) cells were stimulated with 100 pM IL-5 for 0 min (lane 1, 5), 5 min (lane 2, 6), 15 min (lane 3, 7) or 10 ng/ml PMA for 10 min (lane 4, 8). Band intensities of pERK1/2 were normalized to that of Grb2, and then normalized to that of lane 1. Data are summarized as the mean ± (SEM) from three independent experiments. NS=Not Significant. * p<0.05, N=3. B. Naïve and dif-HC15 cells were treated with 10 nM CCL5, 100 nM fMLP or 100 nM CCL11 for 0 or 2 min. Cell extracts were then prepared and immunoblotted as detailed under Materials and Methods. Representative immunoblots of pERK1/2 and Grb2 from three independent experiments are shown.
Kinetics of ERK1/2 phosphorylation in the dif-HC15 in response to IL-5 family cytokines and the chemotactic factors fMLP and CCL5
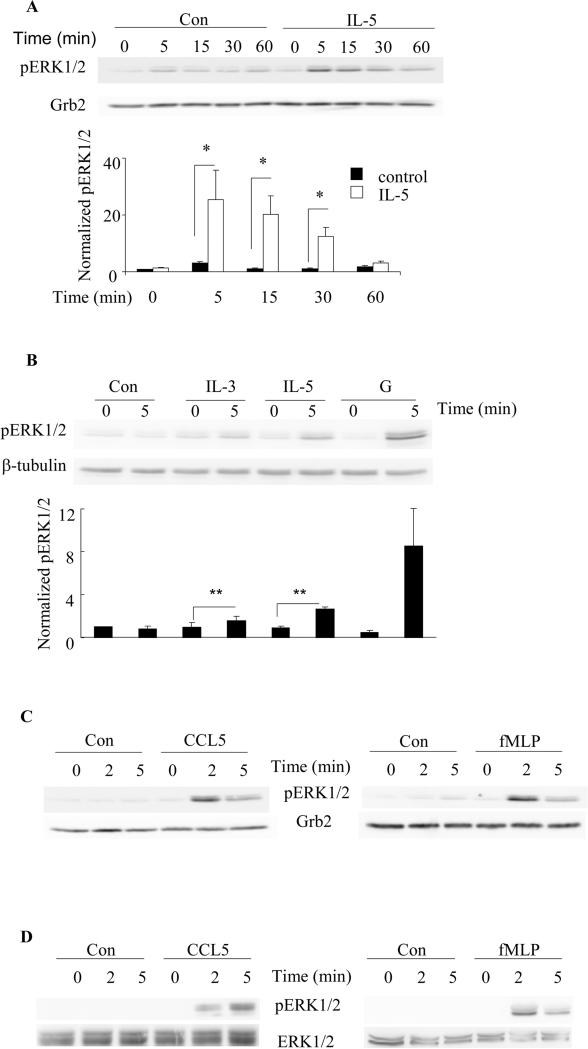
The dif-HC15 were stimulated with 100 pM IL-5 for up to 60 min, or with chemotactic factors (100 nM fMLP or 10 nM CCL5) for up to 5 min. Our results reveal that IL-5-induced ERK1/2 phosphorylation in the dif-HC15 is time-dependent with the maximum level being observed within the first 15 min (p<0.04, N=6 Fig. 4A) and returning to baseline by approximately 60 min. The other IL-5 family members IL-3 and GM-CSF also induced a rapid (within 5 min) ERK1/2 phosphorylation in the dif-HC15 as shown in Fig. 4B (p<0.05, N=3). Of note, at the doses tested, GM-CSF appeared to promote the greatest level of ERK1/2 phosphorylation among the IL-5 family members (Fig. 4B). The time-dependent phosphorylation of ERK1/2 observed in the dif-HC15 in response to IL-5 family members appears to recapitulate the kinetics of ERK1/2 phosphorylation in human primary blood eosinophils in response to IL-5 family members, as shown by our lab and others previously (11, 39). Similarly, comparable profiles of ERK1/2 phosphorylation in response to fMLP or CCL5 were detected in the dif-HC15 cells and human primary blood eosinophils (Figs. 4 C and D, respectively) with maximal phosphorylation being observed within 2 min of stimulation. Altogether, these data indicate that IL-5 family cytokines and selected chemotactic factors stimulate equivalent responses of ERK1/2 phosphorylation in the dif-HC15 and human primary blood eosinophils, further supporting the idea that HC15 may be used to pilot studies relevant to an understanding of the intracellular signaling pathways associated with human primary blood eosinophils.
Figure 4. Kinetics of ERK1/2 phosphorylation in the dif-HC15 cells in response to IL-5 family cytokines and chemotactic factors fMLP and CCL5.
A. The dif-HC15 were stimulated with 100 pM IL-5 or control vehicle for the indicated times. Cell lysates were subject to SDS-PAGE and immunoblotting for pERK1/2 and Grb2. The upper panel shows representative immunoblots of pERK1/2 and Grb2 of six independent experiments. ERK1/2 phosphorylation levels were normalized to that of Grb2, and IL-5-treated at time 0 was set to be 1. Data are summarized as the mean ± (SEM) of six experiments in the lower panel. *p<0.05, N=6. A two-way ANOVA was used for the statistical analysis. B. The dif-HC15 cells were stimulated with control (Con), 1nM IL-3, IL-5 or GM-CSF (G) for 0 or 5 min. The upper panel shows representative immunoblots of pERK1/2 and β-tubulin of three independent experiments. The lower panel is the summary of the mean ± (SEM). The control-treated cells at time 0 was set to be 1. **p<0.05 N=3. The dif-HC15 cells (C) and human blood eosinophils (D) were stimulated with 10 nM CCL5 or 100 nM fMLP for 0, 2 or 5 min. Representative immunoblots of pERK1/2 and the corresponding loading controls of three independent experiments are shown.
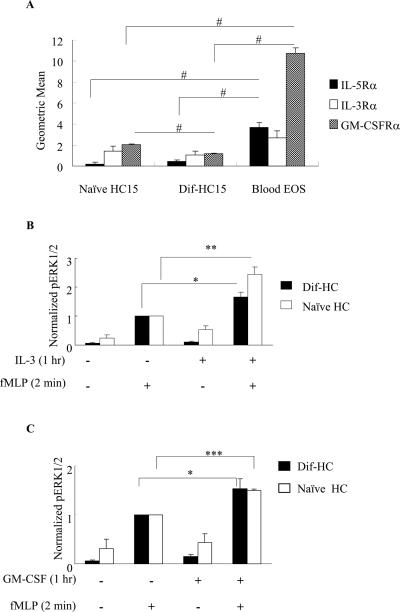
Expression profile of IL-5 family member receptors and priming of fMLP-induced ERK1/2 phosphorylation in HC15
Although the IL-5 receptor has been documented to be expressed in dif-HC15 as determined by mRNA analyses and by ligand binding experiments, the surface expression of the receptors for IL-5 family members has not been reported (56–58). Because we are interested in the priming mechanisms involved with IL-5 family members, we evaluated the expression profile of the receptors for all three family members in the naïve and dif-HC15 cells in comparison to that in human primary blood eosinophils utilizing PE/FITC-conjugated antibodies targeting the specific α subunit of each IL-5 family receptor. Overall, both the naïve and dif-HC15 expressed lower levels of the ligand-specific α subunit of each IL-5 family receptor compared to human blood eosinophils (Fig. 5A). IL-5Rα appeared to be marginally up-regulated upon HC-15 differentiation. Interestingly, the level of IL-3Rα was readily detectable and did not appear to be affected by differentiation (Fig. 5A). Similarly, GM-CSFRα was detected in both the naïve and dif-HC15 cells; however, the dif-HC15 showed a significant decrease in GM-CSFRα levels when compared to the naïve HC15 cells (p<0.006, N=4, Fig. 5A). Among all three receptors, IL-3Rα was the only subunit that exhibited a comparable level of expression in the cell line and in primary human blood eosinophils. Furthermore, our data reveal differential regulation of the α subunits of IL-5 family receptors during differentiation in HC15.
Figure 5. Surface expression profile of IL-5 family receptors and priming in HC15 cells.
A. Naïve HC15, dif-HC15 and human blood eosinophils (Blood EOS) were examined for surface expression of IL-5Rα, IL-3Rα and GM-CSFRα using flow cytometry as described under Materials and Methods. Geometric means of the α subunits were normalized by subtracting the geometric means of the corresponding IgG control and are presented as the mean ± (SEM); similar results were obtained when the data were normalized to a positive control (primary eosinophils) The data are summarized from four independent experiments for each receptor. #p<0.006, N=4. HC15 cells were primed with 1 nM of IL-3 (B) or GM-CSF (C) for 1 hr, and stimulated with 100 nM fMLP for 2 min. Protein levels of pERK1/2 and actin were detected by immunoblotting. Band intensities of pERK1/2 were normalized to that of actin. Cells treated with fMLP alone were set to be 1 in both panels. The data are presented as the mean ± (SEM). *p<0.02 **p<0.003 N=6, ***p<0.004 N=3.
To further investigate whether HC15 can be used as a model to study intracellular signaling as it pertains to cytokine priming, we examined fMLP-induced ERK1/2 phosphorylation with and without priming by IL-5 family members. Cells were primed with 1 nM IL-5 family members for 1 hr followed by 100 nM fMLP for 2 min. IL-3 was able to potentiate the responses of both the naïve and dif-HC15 cells, resulting in an enhancement (~ 2 fold) of fMLP-induced ERK1/2 phosphorylation. However, the naïve HC15 appeared to respond more to IL-3 priming than dif-HC15 (Fig. 5B). Furthermore, GM-CSF also induced a modest increase (~1.5 fold) in fMLP-induced ERK1/2 phosphorylation in the naïve and dif-HC15 (Fig. 5C). In contrast, IL-5 failed to induce the synergistic increase of ERK1/2 phosphorylation followed by fMLP in both the naïve and dif-HC15 cells (data not shown). Overall, we detected the greatest priming responses using IL-3 in both the naïve and dif-HC15 cells, followed by GM-CSF, which is a pattern that is consistent with the receptor profile shown in Fig. 5A. Because of the comparable expression level of IL-3 receptor α in the cell line and primary eosinophils, and given that IL-3 can prime chemoattractant responses in both primary eosinophils (Figs. 1 and 2) and naïve or differentiated HC15 cells (Fig. 5), we chose to use IL-3 as the priming agent for subsequent experiments performed in the cell line.
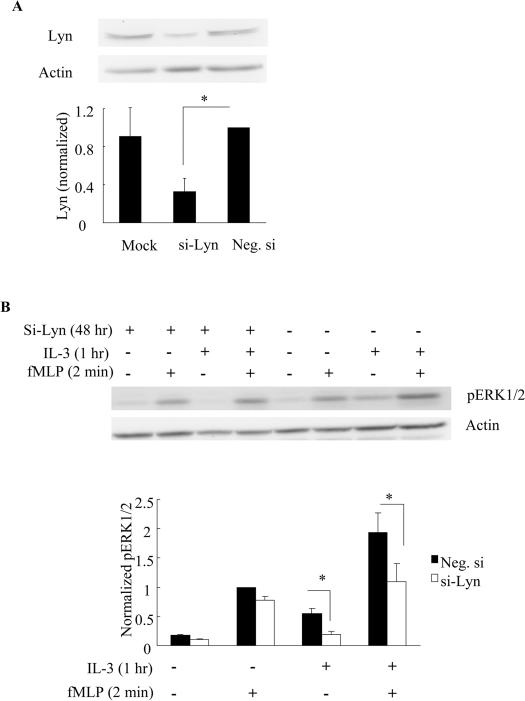
Effects of siRNA-mediated knockdown of Lyn on fMLP-induced ERK1/2 phosphorylation in IL-3-primed naïve HC15 cells
To further investigate the role of Lyn in the intracellular mechanisms of priming, we utilized siRNA to knock down Lyn in the naïve HC15 cells. As shown in Fig. 7A, siRNA directed against Lyn decreased Lyn expression by approximately 67% when compared to the negative siRNA-treated and mock transfection-treated cells (p<0.02, N=4, Fig. 6A). Cell viability was assessed by trypan blue staining 48 hr post transfection and was found to be not significantly different between the mock, si-Lyn and negative siRNA transfectants (data not shown). To test the influence of Lyn knockdown on cytokine-mediated priming of chemoattractant responses, cells were treated 48 hrs post nucleofection with IL-3 or control vehicle for 1 hr (priming) followed by a 2-min stimulation with fMLP. As shown in Fig. 6B, the effect of priming was largely retained in the cells transfected with the control siRNA, whereas the synergistic enhancement of fMLP-induced ERK1/2 phosphorylation following IL-3 treatment was diminished when Lyn expression was reduced by approximately 67% (p<0.02. N=4, Fig. 6B). In addition, we observed a significant decrease in ERK1/2 phosphorylation in IL-3-treated cells after Lyn knockdown, consistent with a role of Lyn in IL-5 family-induced ERK1/2 phosphorylation (p<0.02, N=4, Fig. 6B). Conversely, fMLP-stimulated ERK1/2 phosphorylation in the absence of IL-3 priming appeared to be minimally affected in Lyn-knockdown cells, suggesting that Lyn is not critical for this activity. Altogether, these data are consistent with the observed effects of PILR on human primary blood eosinophils, and demonstrate that suppressing the expression, localization or activity of Lyn can disrupt the synergy between IL-5 family cytokine receptors and fMLP receptors. These observations further support the idea that Lyn is critical for priming to occur between IL-5 family cytokine- and fMLP-induced events.
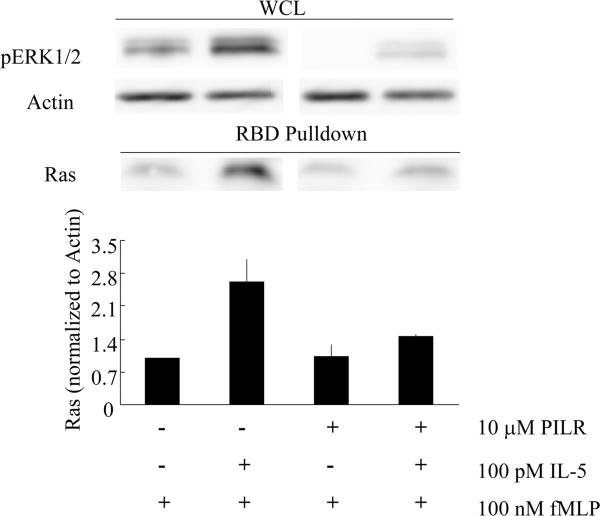
Figure 7. PILR inhibits fMLP-stimulated Ras activation following IL-5 priming in human blood eosinophils.
Human primary eosinophils were preincubated with 10 μM control peptide or PILR for 30 min. The cells were then primed with 100 pM IL-5 or buffer control for 1 hr followed by 100 nM fMLP for 2 min. Cells lysates were subjected to the RBD pull-down assay as detailed under Materials and Methods. An aliquot of the whole cell lysate (WCL) from each treatment was removed to assess levels of pERK1/2 and actin (upper panel). The levels of active Ras were detected in the pull-down samples via immunobloting (upper panel) and normalized to that of actin in the whole cell lysates. The mean ± (SEM) from four independent experiments are summarized in the lower panel.
Figure 6. Suppressing Lyn expression using siRNA inhibits the enhanced ERK1/2 phosphorylation in response to fMLP following IL-3 priming in the naïve HC15.
Naïve HC15 cells were transfected with no siRNA (Mock), siRNA against Lyn (si-Lyn) or negative control siRNA (Neg. si) for 48 hr as detailed under Materials and Methods. The expression levels of Lyn were examined by immunoblotting (A, upper panel) and summarized as the mean ± (SEM) from four independent experiments (A, lower panel). Following Lyn knockdown for 48 hr, the cells were treated with 1 nM IL-3 for 1 hr and then stimulated with 100 nM fMLP for 2 min. Representative immunoblots of pERK1/2 and actin (B, upper panel), and the summary of the mean ± (SEM) from four independent experiments (B, lower panel) are shown. *p<0.02, N=4.
Effects of PILR on Ras activation in fMLP-stimulated human blood eosinophils following IL-5 family priming
The small MW G protein Ras has been previously shown to be critical for IL-5-induced ERK1/2 phosphorylation in human blood eosinophils (32) and is a known upstream effector of both the ERK1/2 and PI3K-Akt cascades (59–61). A previous report from our lab has showed that Ras activity is up-regulated in concert with the enhanced ERK1/2 phosphorylation in fMLP-stimulated human blood eosinophils upon IL-5 priming (62), and the data in the present report (Figs. 1 and 2) reveal that a Ras-associated pathway, namely chemoattractant-induced Akt phosphorylation/activation, is also sensitive to cytokine priming. Thus, we hypothesized that Lyn can participate in regulating Ras activity in response to IL-5 family cytokines, thereby modulating ERK1/2 phosphorylation/activation following fMLP-stimulation of human blood eosinophils. Accordingly, we employed an active-Ras pull-down assay using cytokine and/or chemoattractant-stimulated primary blood eosinophils. In these studies, pre-incubation of the cells with the Lyn recruitment antagonist PILR, but not with the control peptide, diminished the enhanced Ras activation in response to fMLP in IL-5-primed eosinophils (Fig. 7), indicating that Lyn appears critical for fMLP-stimulated Ras activation following IL-5 priming. Similar inhibitory effects of PILR on Ras activation were also observed with IL-3 priming in human eosinophils (data not shown). On the contrary, analogous to the lack of effect of siRNA knockdown of Lyn on fMLP-induced ERK1/2 phosphorylation in HC15 cells, treatment with PILR appeared to have no effect on fMLP-stimulated Ras activation in primary eosinophils. Overall, these data support a model wherein Lyn regulates and amplifies fMLP-stimulated ERK1/2 activation following priming with IL-5/IL-3 via Ras-dependent pathways.
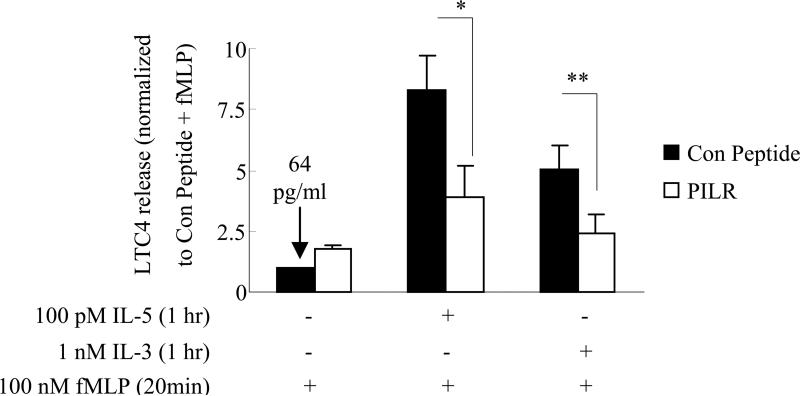
PILR decreases LTC4 release in response to fMLP in IL-5/IL-3-primed human blood eosinophils
Although the above studies are consistent with a role for Lyn in IL-5 family cytokine signaling in primary human blood eosinophils and a human cell line (HC15), it is important to assess whether this effector plays a role in the biological action of these cytokines. In this regard, LTC4 is a member of the cysteinyl-leukotrienes, which are potent mediators of airway inflammation and hypersensitivity (63–67), and major sources of LTC4 include mast cells and eosinophils. In the case of eosinophils, the enhanced release of LTC4 in response to fMLP following IL-5/IL-3 priming has been reported (11, 68). Furthermore, the fMLP-stimulated LTC4 release following IL-5 priming has been shown to be an ERK1/2-dependent process (11). These data, along with the present report, lead us to propose a model wherein the Lyn-Ras-ERK axis is important for the enhanced LTC4 release observed following fMLP treatment of IL-5/IL-3-primed eosinophils. To test this model, we examined the effect of the Lyn recruitment inhibitor PILR on fMLP-induced LTC4 release following priming of human blood eosinophils with IL-3 or IL-5. As shown in Fig. 8, treatment with PILR significantly decreased fMLP-induced LTC4 release following IL-5 or IL-3 priming compared to the control peptide (p<0.04 and p<0.02 respectively, N=7, Fig. 8). However, PILR had little effect on LTC4 release in response to fMLP alone when compared to the control peptide (N=7, Fig. 8). Furthermore, pretreatment with PILR or control peptide did not alter the production of LTC4 (~ 60 pg/ml) in response to IL-5/IL-3 priming alone (data not shown). These results are consistent with the effects of PILR on fMLP-stimulated Ras and ERK1/2 activation following IL-5/IL-3 priming, and suggest that Lyn activity is critical for the enhanced release of LTC4 from “primed” eosinophils in response to fMLP stimulation.
Figure 8. PILR inhibits LTC4 release in response to fMLP stimulation in IL-5/IL-3-primed human blood eosinophils.
Human blood eosinophils were preincubated with 10 μM control peptide or PILR for 30 min. Cells were then primed with control buffer, 100 pM IL-5, or 1 nM IL-3 for 1 hr followed by a 20 min stimulation with 100 nM fMLP. Supernatants were then collected and assayed for immunoreactive LTC4 as described under Materials and Methods. The data are normalized to the LTC4 released from the leftmost bar and presented as fold increases. The dark bars and open bars represent the pretreatment with control peptide and PILR, respectively. Seven independent experiments are summarized and presented as the mean ± (SEM). * p<0.04 **p<0.02, N=7.
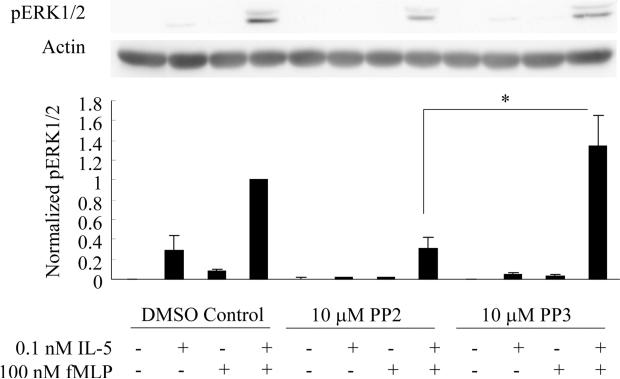
The Src family inhibitor PP2 attenuates fMLP-induced ERK1/2 phosphorylation in IL-5-primed human blood eosinophils
Although the above data support an important role of Src family member Lyn in potentiating eosinophils responsiveness following IL-5/IL-3 priming, it is not clear whether the kinase activity and/or the structural function of Src family member is essential for this process. To investigate the function of the kinase activity of Src family members in the priming mechanisms, we pretreated human blood eosinophils with a Src inhibitor PP2, its inactive analog PP3, or vehicle (DMSO) alone for 30 min. The cells were then primed with IL-5 for 1 hr and stimulated with fMLP for 2 min. With the pretreatment of DMSO or the inactive analog PP3, the priming effect of IL-5 on ERK1/2 phosphorylation was observed (Fig. 9). However, the Src inhibitor PP2, as opposed to the inactive analog PP3, was able to significantly inhibit fMLP-induced ERK1/2 phosphorylation following IL-5 priming by approximately 80% (p<0.04, N=6, Fig. 9). These data suggest that the kinase activity of Src family members is an integral part of the priming mechanisms, and support the possible involvement of other Src family members in the mechanisms of IL-5 priming of human blood eosinophils.
Figure 9. Src inhibitor PP2 attenuates fMLP-induced ERK1/2 phosphorylation in IL-5-primed human blood eosinophils.
Human blood eosinophils were pretreated with 10 μM PP2, PP3 or vehicle (DMSO) control for 30 min. Subsequent to this treatment, the cells were primed with 100 pM IL-5 for 1 hr followed by the addition of 100 nM fMLP for 2 min. Cell lysates were collected and subjected to SDS-PAGE. The phosphorylation status of ERK1/2 (pERK1/2) was detected by immunoblotting and actin was used as a loading control. Representative immunoblots of pERK1/2 and actin are shown in the upper panel. Fold increases in pERK1/2 were ascertained by normalizing the band intensity of pERK1/2 to the corresponding actin control. Six independent experiments are summarized as the mean ± (SEM) in the lower panel. *p<0.04, N=6.
Discussion
The observation of synergistic activation of downstream pathways in the presence of two factors, so called “priming”, has been reported in various cell types, including neurons and immune cells (69–71). In eosinophils, priming with IL-5 family members is known to increase cellular responsiveness to chemoattractants and to render a hypersensitive phenotype. The goal of the present study was to test the importance of the Src family member Lyn in the fMLP-induced activation of Ras-ERK1/2 and PI3K-Akt pathways as well as the release of LTC4 in human blood eosinophils following priming with IL-5 family members. In particular, we focused on Ras-ERK pathway that has been previously shown by our lab to be a molecular marker for IL-5 priming in human blood eosinophils.
As one of the “primed” pathways, chemoattractant-stimulated ERK1/2 phosphorylation is synergistically increased following IL-5 priming of blood eosinophils. In the present study, we extend this observation to IL-3-primed eosinophils, supporting the idea that the mechanisms underlying priming are conserved between IL-5 family members. In addition, we provide evidence that fMLP-induced Akt phosphorylation is enhanced upon IL-5 priming of human blood eosinophils, which is also a Ras associated process and is consistent with the prolonged survival of airway eosinophils and IL-5 family-primed blood eosinophils in vitro. We further delineate that the enhanced activation of Ras-ERK1/2 and PI3K-Akt pathways in response to fMLP following priming with IL-5/IL-3 is, at least in part, dependent on the activity of the Src family kinase member Lyn, which physically associates with the common β chain of the IL-5, IL-3 and GM-CSF receptors (23, 44, 72). Lyn is also critical for the enhanced release of LTC4 in response to fMLP following priming with IL-5/IL-3. Our data are consistent with previous reports that LTC4 generation is an ERK1/2-dependent event and suggest the existence of a Lyn-Ras-ERK1/2-LTC4 cascade in fMLP-stimulated human blood eosinophils following IL-5/IL-3 priming. These results support a model wherein the Lyn-regulated crosstalk between IL-5 family- and fMLP-mediated pathways leads to activation of the Ras-ERK1/2 and PI3K-Akt cascades, which in turn contribute to the increased inflammatory capacity and survival of “primed” eosinophils.
The processes by which Lyn contributes to the crosstalk between IL-5 cytokines and chemoattractant-regulated events such as activation of the Ras-ERK1/2 and PI3K-Akt networks are not fully understood. Association of Lyn with the common β chain of IL-5 family receptors has been reported (23, 44, 72) and there is evidence for complex formation between several Src kinases and CCR3 as well as the activation of Src family members, including Lyn, in response to eotaxin (28–31). Our investigations show that Lyn is not critical for fMLP-mediated activation of Ras and ERK1/2 as well as LTC4 release in the absence of IL-5 family priming, suggesting the involvement of other Src family members in fMLP-mediated pathways. Although attenuation of Lyn activity or its level of expression substantially reduced Ras activation, ERK1/2 phosphorylation, and LTC4 release in response to fMLP following IL-5 or IL-3 priming, this manipulation of Lyn status did not completely abolish the effects of IL-5/IL-3 priming. Interestingly, the Src inhibitor PP2 induced a greater attenuation of fMLP-induced ERK1/2 phosphorylation following IL-5 priming compared to that observed with the peptide antagonist PILR, which is consistent with the idea that other Src members may be involved in the mechanisms of priming. Among the Src kinase family, Fyn has been reported to be activated by IL-3 or IL-5 in other cell systems (73, 74). In addition, Fyn knock-out mice demonstrated exacerbation of pulmonary inflammation as well as an increase in airway eosinophils upon antigen challenge (75), suggesting a negative regulatory role of Fyn in airway inflammation in murine systems. Therefore, as opposed to the proposed role of Lyn, Fyn may function in the negative regulation of eosinophil activation.
Given our data, we propose that IL-5 family cytokines prime/modulate the Src kinase Lyn to allow for its further activation, which in turn facilitates the enhanced signal propagation of Src family-regulated pathways upon stimulation with chemotactic factors. Analogous to other Src family members, membrane targeting is known to be critical for controlling Lyn activity. Thus, priming with IL-5 family cytokines may lead to the recruitment of Lyn into membrane domains (e.g., lipid rafts), which have been shown to protect the phosphorylation at tyrosine 397 and to increase the basal activity of Lyn (76, 77). Additionally, fMLP receptors are known to cluster into lipid rafts upon ligand stimulation, which could bring the receptors into close proximity with Lyn (76, 78, 79), and thereby lead to the enhanced activation of downstream pathways. Furthermore, adaptor proteins, such as Shc and Grb2, may also be involved by forming a complex with Lyn and protecting it from inactivation by phosphatases (20, 80). In this regard, it is noteworthy that two modes of action have been proposed for Src family members, i.e., they may function as a kinase and/or as an adaptor protein. Our studies using the Src inhibitor PP2 provide evidence to support the concept that the kinase activity of Src family members is critical for the priming process. Although these data do not exclude a possible adaptor function of these proteins in IL-5 priming, it appears that the kinase activity of Src family members is a key factor in the crosstalk between IL-5- and fMLP-mediated signaling pathways in human blood eosinophils.
Because of the difficulty in transfecting/infecting primary human eosinophils, the present study also entailed an analysis of a suitable cell line for initially assessing the role of specific signaling molecules in the priming actions of IL-5 cytokine family members. To evaluate the relevance of an in vitro cell line model for studying the role of Lyn in the mechanism of priming in human blood eosinophils, we tested several human eosinophil-like cell lines including HC15, EoL-3, and AML14.3D10 (data not shown for EoL-3 and AML14.3D10). Only HC15 recapitulated the GM-CSF/IL-3 priming of chemoattractant-induced Ras-ERK1/2 activation observed in human primary eosinophils. Although analysis of the activation of Ras-ERK1/2 pathway in both the naïve and dif-HC15 cells using IL-5 revealed that differentiation increased ERK1/2 phosphorylation in response to IL-5, the α subunit of IL-5 receptor was detected at very low level in both the naïve and dif-HC15 by flow cytometry. In contrast, IL-3 receptor α subunit was detected at comparable levels as human blood eosinophils in both the naïve and dif-HC15 cells. Furthermore, the modest priming responses to IL-5, which may be partly a result of the low level of IL-5 receptor α suggest that IL-5 is not an ideal candidate to study priming in this cell line. However, both IL-3 and GM-CSF were able to induce priming responses in fMLP-simulated Ras-ERK1/2 activation in naïve and dif-HC15 cells, which reflects the greater levels of receptor for these cytokines. Because IL-3 and GM-CSF were both able to prime chemoattractant-induced ERK1/2 phosphorylation, it appears that the common β chain is not a limiting factor.
During the process of characterizing IL-3 priming of chemoattractant-induced effects in HC 15 cells, we observed that attenuation of Lyn expression using siRNA abolished IL-3 mediated priming of fMLP-induced ERK1/2 phosphorylation. This observation reveals that the Lyn-Ras-ERK1/2 pathway is intact in this cell line and parallels the observation that Lyn recruitment inhibitor PILR antagonizes fMLP-induced ERK1/2 phosphorylation in IL-5-primed eosinophils. However, in these cells, Akt phosphorylation levels were high under basal conditions and were thus less sensitive to IL-5 family cytokine stimulation (data not shown). This high Akt phosphorylation status may arise from likely alternations in survival signaling in this immortalized cell line. Together with the previous findings, we conclude that the naïve HC15 can be used as an in vitro cell line model to study selected signaling pathways in human blood eosinophils, and that the dif-HC15 exhibit a greater range of sensitivity to various IL-5 family cytokines and chemotactic factors. The advantage of using undifferentiated cells is that they are more amendable to molecular manipulations because they are continuously dividing. This system permits the establishment of stable cell lines with heterologous expression of desired proteins including the α subunit of IL-5, IL-3 or GM-CSF receptors. In sum, given the limited number and lifetime of human eosinophils, together with their limited capacity for use with various molecular approaches, HL-60 clone-15 cells should facilitate the more rapid development of methods and hypotheses that can then be taken back for the study of selected signaling mechanisms of priming in human primary eosinophils.
The present work reveals that the mechanisms by which IL-5 family members prime chemoattractant-induced Ras-ERK1/2 and PI3K-Akt pathways in human eosinophils involves a role for the Src kinase family member Lyn. This mechanism also contributes to the enhanced release of LTC4 in response to fMLP following IL-5/IL-3 priming. Therefore, our data support Lyn as one of the key players in the mechanisms underlying the hyper-active phenotype of primed blood eosinophils, as well as airway eosinophils, and may contribute to airway inflammation and exacerbation. This knowledge regarding the molecular mechanisms of priming in eosinophils also suggests that Lyn could be a potential therapeutic target for suppressing/modulating eosinophil hyper-activity in the airway or at other sites of eosinophilic inflammation.
Acknowledgement
We thank Elizabeth Schwantes and Paul Fichtinger for preparation of eosinophils, and Dr. Emery Bresnick for generously lending us the nucleofector. We are grateful to Dr. Gregory Wiepz, Dr. Monica Gavala and Mandy Burnham for editorial input during the preparation of this manuscript.
This work was supported by NIH grants HL0885940, HL56396, AI070503 and HL069116 to P.J.B.
References
- 1.Moore WC, Peters SP. Severe asthma: an overview. J Allergy Clin Immunol. 2006;117 doi: 10.1016/j.jaci.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 2.Sims JM. An overview of asthma. Dimens Crit Care Nurs. 2006;25 doi: 10.1097/00003465-200611000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Balkissoon R. Asthma overview. Prim Care. 2008;35 doi: 10.1016/j.pop.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Arm JP, Lee TH. The pathobiology of bronchial asthma. Adv Immunol. 1992;51 doi: 10.1016/s0065-2776(08)60491-5. [DOI] [PubMed] [Google Scholar]
- 5.Frigas E, Gleich GJ. The eosinophil and the pathophysiology of asthma. J Allergy Clin Immunol. 1986;77 doi: 10.1016/0091-6749(86)90341-6. [DOI] [PubMed] [Google Scholar]
- 6.Wegmann M. Th2 cells as targets for therapeutic intervention in allergic bronchial asthma. Expert Rev Mol Diagn. 2009;9 doi: 10.1586/14737159.9.1.85. [DOI] [PubMed] [Google Scholar]
- 7.Blom M, Tool AT, Kok PT, Koenderman L, Roos D, Verhoeven AJ. Granulocyte-macrophage colony-stimulating factor, interleukin-3 (IL-3), and IL-5 greatly enhance the interaction of human eosinophils with opsonized particles by changing the affinity of complement receptor type 3. Blood. 1994;83 [PubMed] [Google Scholar]
- 8.Kariyawasam HH, Robinson DS. The eosinophil: the cell and its weapons, the cytokines, its locations. Semin Respir Crit Care Med. 2006;27 doi: 10.1055/s-2006-939514. [DOI] [PubMed] [Google Scholar]
- 9.Sampson AP. IL-5 priming of eosinophil function in asthma. Clin Exp Allergy. 2001;31 doi: 10.1046/j.1365-2222.2001.01046.x. [DOI] [PubMed] [Google Scholar]
- 10.Takafuji S, Tadokoro K, Ito K. Effects of interleukin (IL)-3 and IL-5 on human eosinophil degranulation induced by complement components C3a and C5a. Allergy. 1996;51 doi: 10.1111/j.1398-9995.1996.tb04669.x. [DOI] [PubMed] [Google Scholar]
- 11.Bates ME, Green VL, Bertics PJ. ERK1 and ERK2 activation by chemotactic factors in human eosinophils is interleukin 5-dependent and contributes to leukotriene C(4) biosynthesis. J Biol Chem. 2000;275 doi: 10.1074/jbc.275.15.10968. [DOI] [PubMed] [Google Scholar]
- 12.Hakansson L, Venge P. Priming of eosinophil and neutrophil migratory responses by interleukin 3 and interleukin 5. Apmis. 1994;102 doi: 10.1111/j.1699-0463.1994.tb04880.x. [DOI] [PubMed] [Google Scholar]
- 13.Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2008;38 doi: 10.1165/rcmb.2007-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schweizer RC, Welmers BA, Raaijmakers JA, Zanen P, Lammers JW, Koenderman L. RANTES- and interleukin-8-induced responses in normal human eosinophils: effects of priming with interleukin-5. Blood. 1994;83 [PubMed] [Google Scholar]
- 15.Woschnagg C, Garcia R, Rak S, Venge P. IL-5 priming of the PMA-induced oxidative metabolism of human eosinophils from allergic and normal subjects during a pollen season. Clin Exp Allergy. 2001;31 doi: 10.1046/j.1365-2222.2001.00995.x. [DOI] [PubMed] [Google Scholar]
- 16.Busse WW, Nagata M, Sedgwick JB. Characteristics of airway eosinophils. Eur Respir J Suppl. 1996;22 [PubMed] [Google Scholar]
- 17.Luijk B, Lindemans CA, Kanters D, van der Heijde R, Bertics P, Lammers JW, Bates ME, Koenderman L. Gradual increase in priming of human eosinophils during extravasation from peripheral blood to the airways in response to allergen challenge. J Allergy Clin Immunol. 2005;115 doi: 10.1016/j.jaci.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Sedgwick JB, Busse WW. Adhesion proteins on airway eosinophils in allergy and asthma. Agents Actions Suppl. 1993;43 doi: 10.1007/978-3-0348-7324-6_14. [DOI] [PubMed] [Google Scholar]
- 19.Warringa RA, Mengelers HJ, Raaijmakers JA, Bruijnzeel PL, Koenderman L. Upregulation of formyl-peptide and interleukin-8-induced eosinophil chemotaxis in patients with allergic asthma. J Allergy Clin Immunol. 1993;91 doi: 10.1016/0091-6749(93)90323-8. [DOI] [PubMed] [Google Scholar]
- 20.Pazdrak K, Schreiber D, Forsythe P, Justement L, Alam R. The intracellular signal transduction mechanism of interleukin 5 in eosinophils: the involvement of lyn tyrosine kinase and the Ras-Raf-1-MEK-microtubule-associated protein kinase pathway. J Exp Med. 1995;181 doi: 10.1084/jem.181.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adachi T, Alam R. The mechanism of IL-5 signal transduction. Am J Physiol. 1998;275 doi: 10.1152/ajpcell.1998.275.3.C623. [DOI] [PubMed] [Google Scholar]
- 22.Adachi T, Stafford S, Sur S, Alam R. A novel Lyn-binding peptide inhibitor blocks eosinophil differentiation, survival, and airway eosinophilic inflammation. J Immunol. 1999;163 [PubMed] [Google Scholar]
- 23.Alam R, Pazdrak K, Stafford S, Forsythe P. The interleukin-5/receptor interaction activates Lyn and Jak2 tyrosine kinases and propagates signals via the Ras-Raf-1-MAP kinase and the Jak-STAT pathways in eosinophils. Int Arch Allergy Immunol. 1995;107 doi: 10.1159/000236985. [DOI] [PubMed] [Google Scholar]
- 24.Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21 doi: 10.1093/intimm/dxp102. [DOI] [PubMed] [Google Scholar]
- 25.Pazdrak K, Olszewska-Pazdrak B, Stafford S, Garofalo RP, Alam R. Lyn, Jak2, and Raf-1 kinases are critical for the antiapoptotic effect of interleukin 5, whereas only Raf-1 kinase is essential for eosinophil activation and degranulation. J Exp Med. 1998;188 doi: 10.1084/jem.188.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stafford S, Lowell C, Sur S, Alam R. Lyn tyrosine kinase is important for IL-5-stimulated eosinophil differentiation. J Immunol. 2002;168 doi: 10.4049/jimmunol.168.4.1978. [DOI] [PubMed] [Google Scholar]
- 27.Yousefi S, Hoessli DC, Blaser K, Mills GB, Simon HU. Requirement of Lyn and Syk tyrosine kinases for the prevention of apoptosis by cytokines in human eosinophils. J Exp Med. 1996;183 doi: 10.1084/jem.183.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonacchi A, Romagnani P, Romanelli RG, Efsen E, Annunziato F, Lasagni L, Francalanci M, Serio M, Laffi G, Pinzani M, Gentilini P, Marra F. Signal transduction by the chemokine receptor CXCR3: activation of Ras/ERK, Src, and phosphatidylinositol 3-kinase/Akt controls cell migration and proliferation in human vascular pericytes. J Biol Chem. 2001;276 doi: 10.1074/jbc.M010303200. [DOI] [PubMed] [Google Scholar]
- 29.El-Shazly A, Yamaguchi N, Masuyama K, Suda T, Ishikawa T. Novel association of the Src family kinases, Hck and c-Fgr, with CCR3 receptor stimulation: A possible mechanism for eotaxin-induced human eosinophil chemotaxis. Biochemical and Biophysical Research Communications. 1999;264 doi: 10.1006/bbrc.1999.1379. [DOI] [PubMed] [Google Scholar]
- 30.Tomkowicz B, Lee C, Ravyn V, Cheung R, Ptasznik A, Collman RG. The Src kinase Lyn is required for CCR5 signaling in response to MIP-1beta and R5 HIV-1 gp120 in human macrophages. Blood. 2006;108 doi: 10.1182/blood-2005-12-012815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welch H, Maridonneau-Parini I. Lyn and Fgr are activated in distinct membrane fractions of human granulocytic cells. Oncogene. 1997;15 doi: 10.1038/sj.onc.1201356. [DOI] [PubMed] [Google Scholar]
- 32.Hall DJ, Cui J, Bates ME, Stout BA, Koenderman L, Coffer PJ, Bertics PJ. Transduction of a dominant-negative H-Ras into human eosinophils attenuates extracellular signal-regulated kinase activation and interleukin-5-mediated cell viability. Blood. 2001;98 doi: 10.1182/blood.v98.7.2014. [DOI] [PubMed] [Google Scholar]
- 33.O'Byrne PM. Airway inflammation and asthma. Aliment Pharmacol Ther. 1996;10(Suppl 2) doi: 10.1046/j.1365-2036.1996.22164016.x. [DOI] [PubMed] [Google Scholar]
- 34.Ip WK, Wong CK, Wang CB, Tian YP, Lam CW. Interleukin-3, -5, and granulocyte macrophage colony-stimulating factor induce adhesion and chemotaxis of human eosinophils via p38 mitogen-activated protein kinase and nuclear factor kappaB. Immunopharmacol Immunotoxicol. 2005;27 doi: 10.1080/08923970500240925. [DOI] [PubMed] [Google Scholar]
- 35.Daffern PJ, Jagels MA, Saad JJ, Fischer W, Hugli TE. Upper airway epithelial cells support eosinophil survival in vitro through production of GM-CSF and prostaglandin E2: regulation by glucocorticoids and TNF-alpha. Allergy Asthma Proc. 1999;20 doi: 10.2500/108854199778339008. [DOI] [PubMed] [Google Scholar]
- 36.Bates ME, Liu LY, Esnault S, Stout BA, Fonkem E, Kung V, Sedgwick JB, Kelly EA, Bates DM, Malter JS, Busse WW, Bertics PJ. Expression of interleukin-5- and granulocyte macrophage-colony-stimulating factor-responsive genes in blood and airway eosinophils. Am J Respir Cell Mol Biol. 2004;30 doi: 10.1165/rcmb.2003-0234OC. [DOI] [PubMed] [Google Scholar]
- 37.Yousefi S, Blaser K, Simon HU. Activation of signaling pathways and prevention of apoptosis by cytokines in eosinophils. Int Arch Allergy Immunol. 1997;112 doi: 10.1159/000237424. [DOI] [PubMed] [Google Scholar]
- 38.Coffer PJ, Schweizer RC, Dubois GR, Maikoe T, Lammers JW, Koenderman L. Analysis of signal transduction pathways in human eosinophils activated by chemoattractants and the T-helper 2-derived cytokines interleukin-4 and interleukin-5. Blood. 1998;91 [PubMed] [Google Scholar]
- 39.Zhu X, Jacobs B, Boetticher E, Myou S, Meliton A, Sano H, Lambertino AT, Munoz NM, Leff AR. IL-5-induced integrin adhesion of human eosinophils caused by ERK1/2-mediated activation of cPLA2. J Leukoc Biol. 2002;72 [PubMed] [Google Scholar]
- 40.Sano M, Leff AR, Myou S, Boetticher E, Meliton AY, Learoyd J, Lambertino AT, Munoz NM, Zhu X. Regulation of interleukin-5-induced beta2-integrin adhesion of human eosinophils by phosphoinositide 3-kinase. Am J Respir Cell Mol Biol. 2005;33 doi: 10.1165/rcmb.2005-0076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myou S, Leff AR, Myo S, Boetticher E, Meliton AY, Lambertino AT, Liu J, Xu C, Munoz NM, Zhu X. Activation of group IV cytosolic phospholipase A2 in human eosinophils by phosphoinositide 3-kinase through a mitogen-activated protein kinase-independent pathway. J Immunol. 2003;171 doi: 10.4049/jimmunol.171.8.4399. [DOI] [PubMed] [Google Scholar]
- 42.Mishra RK, Scaife JE, Harb Z, Gray BC, Djukanovic R, Dent G. Differential dependence of eosinophil chemotactic responses on phosphoinositide 3-kinase (PI3K) Allergy. 2005;60 doi: 10.1111/j.1398-9995.2005.00845.x. [DOI] [PubMed] [Google Scholar]
- 43.Pinho V, Souza DG, Barsante MM, Hamer FP, De Freitas MS, Rossi AG, Teixeira MM. Phosphoinositide-3 kinases critically regulate the recruitment and survival of eosinophils in vivo: importance for the resolution of allergic inflammation. J Leukoc Biol. 2005;77 doi: 10.1189/jlb.0704386. [DOI] [PubMed] [Google Scholar]
- 44.Adachi T, Pazdrak K, Stafford S, Alam R. The mapping of the Lyn kinase binding site of the common beta subunit of IL-3/granulocyte-macrophage colony-stimulating factor/IL-5 receptor. J Immunol. 1999;162 [PubMed] [Google Scholar]
- 45.Gleich GJ, Kita H. Bronchial asthma: lessons from murine models. Proc Natl Acad Sci USA. 1997;94 doi: 10.1073/pnas.94.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Epstein MM. Do mouse models of allergic asthma mimic clinical disease? Int Arch Allergy Immunol. 2004;133 doi: 10.1159/000076131. [DOI] [PubMed] [Google Scholar]
- 47.Ishihara K, Hong J, Zee O, Ohuchi K. Mechanism of the eosinophilic differentiation of HL-60 clone 15 cells induced by n-butyrate. Int Arch Allergy Immunol. 2005;137(Suppl 1) doi: 10.1159/000085436. [DOI] [PubMed] [Google Scholar]
- 48.Ishihara K, Hong J, Zee O, Ohuchi K. Possible mechanism of action of the histone deacetylase inhibitors for the induction of differentiation of HL-60 clone 15 cells into eosinophils. Br J Pharmacol. 2004;142 doi: 10.1038/sj.bjp.0705869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tiffany HL, Alkhatib G, Combadiere C, Berger EA, Murphy PM. CC chemokine receptors 1 and 3 are differentially regulated by IL-5 during maturation of eosinophilic HL-60 cells. J Immunol. 1998;160 [PubMed] [Google Scholar]
- 50.Lopez JA, Newburger PE, Condino-Neto A. The effect of IFN-gamma and TNF-alpha on the eosinophilic differentiation and NADPH oxidase activation of human HL-60 clone 15 cells. J Interferon Cytokine Res. 2003;23 doi: 10.1089/107999003772084851. [DOI] [PubMed] [Google Scholar]
- 51.Badewa AP, Hudson CE, Heiman AS. Regulatory effects of eotaxin, eotaxin-2, and eotaxin-3 on eosinophil degranulation and superoxide anion generation. Exp Biol Med (Maywood) 2002;227 doi: 10.1177/153537020222700814. [DOI] [PubMed] [Google Scholar]
- 52.Hirota R, Akimaru K, Nakamura H. In vitro toxicity evaluation of diesel exhaust particles on human eosinophilic cell. Toxicol In Vitro. 2008;22 doi: 10.1016/j.tiv.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 53.Hua J, Hasebe T, Someya A, Nakamura S, Sugimoto K, Nagaoka I. Evaluation of the expression of NADPH oxidase components during maturation of HL-60 clone 15 cells to eosinophilic lineage. Inflamm Res. 2001;50 doi: 10.1007/s000110050740. [DOI] [PubMed] [Google Scholar]
- 54.Michail S, Abernathy F. A new model for studying eosinophil migration across cultured intestinal epithelial monolayers. J Pediatr Gastroenterol Nutr. 2004;39 doi: 10.1097/00005176-200407000-00012. [DOI] [PubMed] [Google Scholar]
- 55.Takashi S, Park J, Fang S, Koyama S, Parikh I, Adler KB. A peptide against the N-terminus of myristoylated alanine-rich C kinase substrate inhibits degranulation of human leukocytes in vitro. Am J Respir Cell Mol Biol. 2006;34 doi: 10.1165/rcmb.2006-0030RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tagari P, Pecheur EI, Scheid M, Brown P, Ford-Hutchinson AW, Nicholson D. Activation of human eosinophils and differentiated HL-60 cells by interleukin-5. Int Arch Allergy Immunol. 1993;101 doi: 10.1159/000236450. [DOI] [PubMed] [Google Scholar]
- 57.Ingley E, Young IG. Characterization of a receptor for interleukin-5 on human eosinophils and the myeloid leukemia line HL-60. Blood. 1991;78 [PubMed] [Google Scholar]
- 58.Baltus B, van Dijk TB, Caldenhoven E, Zanders E, Raaijmakers JA, Lammers JW, Koenderman L, de Groot RP. An AP-1 site in the promoter of the human IL-5R alpha gene is necessary for promoter activity in eosinophilic HL60 cells. FEBS Lett. 1998;434 doi: 10.1016/s0014-5793(98)00991-0. [DOI] [PubMed] [Google Scholar]
- 59.Yang JY, Widmann C. The RasGAP N-terminal fragment generated by caspase cleavage protects cells in a Ras/PI3K/Akt-dependent manner that does not rely on NFkappa B activation. J Biol Chem. 2002;277 doi: 10.1074/jbc.M111540200. [DOI] [PubMed] [Google Scholar]
- 60.Menges CW, McCance DJ. Constitutive activation of the Raf-MAPK pathway causes negative feedback inhibition of Ras-PI3K-AKT and cellular arrest through the EphA2 receptor. Oncogene. 2008;27 doi: 10.1038/sj.onc.1210957. [DOI] [PubMed] [Google Scholar]
- 61.Freilinger A, Rosner M, Hanneder M, Hengstschlager M. Ras mediates cell survival by regulating tuberin. Oncogene. 2008;27 doi: 10.1038/sj.onc.1210844. [DOI] [PubMed] [Google Scholar]
- 62.Bates ME, Sedgwick JB, Zhu Y, Liu LY, Heuser RG, Jarjour NN, Kita H, Bertics PJ. Human airway eosinophils respond to chemoattractants with greater eosinophil-derived neurotoxin release, adherence to fibronectin, and activation of the Ras-ERK pathway when compared with blood eosinophils. J Immunol. 184 doi: 10.4049/jimmunol.0900634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Munoz NM, Hamann KJ, Rabe KF, Sano H, Zhu X, Leff AR. Augmentation of eosinophil degranulation and LTC(4) secretion by integrin-mediated endothelial cell adhesion. Am J Physiol. 1999;277 doi: 10.1152/ajplung.1999.277.4.L802. [DOI] [PubMed] [Google Scholar]
- 64.Laviolette M, Ferland C, Comtois JF, Champagne K, Bosse M, Boulet LP. Blood eosinophil leukotriene C4 production in asthma of different severities. Eur Respir J. 1995;8 [PubMed] [Google Scholar]
- 65.Kim DC, Hsu FI, Barrett NA, Friend DS, Grenningloh R, Ho IC, Al-Garawi A, Lora JM, Lam BK, Austen KF, Kanaoka Y. Cysteinyl leukotrienes regulate Th2 cell-dependent pulmonary inflammation. J Immunol. 2006;176 doi: 10.4049/jimmunol.176.7.4440. [DOI] [PubMed] [Google Scholar]
- 66.Faith A, Fernandez MH, Caulfield J, Loke TK, Corrigan C, O'Connor B, Lee TH, Hawrylowicz CM. Role of cysteinyl leukotrienes in human allergen-specific Th2 responses induced by granulocyte macrophage-colony stimulating factor. Allergy. 2008;63 doi: 10.1111/j.1398-9995.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- 67.Ogawa Y, Calhoun WJ. The role of leukotrienes in airway inflammation. J Allergy Clin Immunol. 2006;118 doi: 10.1016/j.jaci.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 68.Iikura M, Yamaguchi M, Fujisawa T, Miyamasu M, Takaishi T, Morita Y, Iwase T, Moro I, Yamamoto K, Hirai K. Secretory IgA induces degranulation of IL-3-primed basophils. J Immunol. 1998;161 [PubMed] [Google Scholar]
- 69.Kostrzewa RM, Kostrzewa JP, Brown RW, Nowak P, Brus R. Dopamine receptor supersensitivity: development, mechanisms, presentation, and clinical applicability. Neurotox Res. 2008;14 doi: 10.1007/BF03033804. [DOI] [PubMed] [Google Scholar]
- 70.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32 doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mecklenburgh K, Murray J, Brazil T, Ward C, Rossi AG, Chilvers ER. Role of neutrophil apoptosis in the resolution of pulmonary inflammation. Monaldi Arch Chest Dis. 1999;54 [PubMed] [Google Scholar]
- 72.Dahl ME, Arai KI, Watanabe S. Association of Lyn tyrosine kinase to the GM-CSF and IL-3 receptor common betac subunit and role of Src tyrosine kinases in DNA synthesis and anti-apoptosis. Genes Cells. 2000;5 doi: 10.1046/j.1365-2443.2000.00312.x. [DOI] [PubMed] [Google Scholar]
- 73.Anderson SM, Jorgensen B. Activation of src-related tyrosine kinases by IL-3. J Immunol. 1995;155 [PubMed] [Google Scholar]
- 74.Appleby MW, Kerner JD, Chien S, Maliszewski CR, Bondada S, Perlmutter RM, Bondadaa S. Involvement of p59fynT in interleukin-5 receptor signaling. J Exp Med. 1995;182 doi: 10.1084/jem.182.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kudlacz EM, Andresen CJ, Salafia M, Whitney CA, Naclerio B, Changelian PS. Genetic ablation of the src kinase p59fynT exacerbates pulmonary inflammation in an allergic mouse model. Am J Respir Cell Mol Biol. 2001;24 doi: 10.1165/ajrcmb.24.4.4266. [DOI] [PubMed] [Google Scholar]
- 76.Fuhler GM, Blom NR, Coffer PJ, Drayer AL, Vellenga E. The reduced GM-CSF priming of ROS production in granulocytes from patients with myelodysplasia is associated with an impaired lipid raft formation. J Leukoc Biol. 2007;81 doi: 10.1189/jlb.0506311. [DOI] [PubMed] [Google Scholar]
- 77.Young RM, Holowka D, Baird B. A lipid raft environment enhances Lyn kinase activity by protecting the active site tyrosine from dephosphorylation. J Biol Chem. 2003;278 doi: 10.1074/jbc.M211402200. [DOI] [PubMed] [Google Scholar]
- 78.Kannan KB, Barlos D, Hauser CJ. Free cholesterol alters lipid raft structure and function regulating neutrophil Ca2+ entry and respiratory burst: correlations with calcium channel raft trafficking. J Immunol. 2007;178 doi: 10.4049/jimmunol.178.8.5253. [DOI] [PubMed] [Google Scholar]
- 79.Xue M, Vines CM, Buranda T, Cimino DF, Bennett TA, Prossnitz ER. N-formyl peptide receptors cluster in an active raft-associated state prior to phosphorylation. J Biol Chem. 2004;279 doi: 10.1074/jbc.M407053200. [DOI] [PubMed] [Google Scholar]
- 80.Bates ME, Busse WW, Bertics PJ. Interleukin 5 signals through Shc and Grb2 in human eosinophils. Am J Respir Cell Mol Biol. 1998;18 doi: 10.1165/ajrcmb.18.1.2766. [DOI] [PubMed] [Google Scholar]