Abstract
Objective To examine the relationship of paternal involvement in diabetes care with adherence and glycemic control. Methods One hundred and thirty-six mothers and fathers of preadolescents (aged 9–12 years) with type 1 diabetes reported on paternal involvement. Adherence was measured by interview and blood glucose meter downloads. Mothers’ and fathers’ ratings of paternal involvement in diabetes care were compared. We evaluated three structural equation models linking paternal involvement with adherence and glycemic control. Results Mothers and fathers reported similar amounts of paternal involvement, yet mothers rated paternal involvement as more helpful. The data supported a model indicating links between more paternal involvement and higher HbA1c and between lower adherence and higher HbA1c. Mediation and moderation models were not supported. Discussion Although paternal involvement was not directly associated with treatment adherence, it was associated with poorer glycemic control. Some fathers may increase their involvement in response to suboptimal glycemic outcomes.
Keywords: adherence, children, diabetes, fatherhood, structural equation modeling
Fathers are an important but understudied influence on the management of children and adolescents with chronic illness (Dashiff, Morrison, & Rowe, 2008; Phares, Lopez, Fields, Kamboukos, & Duhig, 2005). Paternal influences vary widely in quantity and quality, as some fathers are primary caregivers and report high levels of involvement in their children's care, while others have only peripheral involvement and little participation in or knowledge of their children's chronic illness management (Leonard, Garwick, & Adwan, 2005). For children with asthma, cystic fibrosis, type 1 diabetes, phenylketonuria, inflammatory bowel disease, or spina bifida, greater paternal involvement in care has been associated with children having better medical regimen adherence and health-related quality of life, and with family-level resilience factors, including more positive ratings of the marital relationship and family environment, fewer symptoms of maternal psychiatric distress and a lesser impact of chronic illness on the family's everyday functioning (Gavin & Wysocki, 2006; Wysocki & Gavin, 2006). Although youth with type 1 diabetes were included in these previous studies, the associations between mothers’ and fathers’ perceptions of paternal involvement and diabetes outcomes in particular were not reported.
Fathers’ Involvement in Diabetes Care
The vast majority of research on parental involvement in diabetes management has been with mothers, and has indicated that greater maternal support, monitoring, and responsibility for care are associated with better adherence and glycemic control (Berg et al., 2008; Wiebe et al., 2005). Although less is known about the role of fathers, paternal influences have been researched more extensively in type 1 diabetes than in other pediatric conditions (Dashiff et al., 2008; Phares et al., 2005). Yet, much remains to be understood about the quantity, quality, and impact of fathers in diabetes care. Fathers’ contributions appear to primarily demonstrate indirect relations with children’s diabetes outcomes by bolstering the impact of maternal caregiving (Dashiff et al., 2008; Wysocki et al., 2009). In addition, while mothers tend to be more involved in their children’s diabetes self-care than fathers, when fathers participate or when both parents are involved, their children tend to have lower HbA1c values (Berg et al., 2008; Horton, Berg, Butner, & Wiebe, 2009; Palmer et al., 2010; Wysocki et al., 2009). However, the direct associations between paternal involvement and diabetes outcomes have been inconsistent, and the nature of how much and in what ways fathers impact youth outcomes remains unclear (Dashiff, 2003; Dashiff et al., 2008). These discrepant findings heighten the need for additional research concerning the roles of both mothers’ and fathers’ involvement in adherence and glycemic control (Dashiff et al., 2008; Phares et al., 2005).
Paternal involvement in diabetes care is an emerging area of study, and the existing literature in this area has been limited. The focus has primarily been on quantifying paternal contributions to care (e.g., Berg et al., 2008) or understanding fathers’ perceptions about the family or styles of interacting with their children (Seiffge-Krenke, 1998, 2002),while the quality (i.e., helpfulness) of fathers’ involvement on glycemic control has received less empirical attention in a type 1 diabetes population. Previous research across several illness groups suggests that mothers may rate fathers’ involvement as more helpful than fathers do (Wysocki & Gavin, 2006). However, mothers’ and fathers’ ratings of the amount and helpfulness of paternal involvement have not been compared in depth in a diabetes sample. Differences in parents’ perceptions about fathers’ contributions to diabetes care are important to understand and may inform family-based clinical interventions to enhance illness management (Wysocki & Gavin, 2006). Further, the mechanisms by which fathers might influence HbA1c values (e.g., by promoting or assisting with adherence) have yet to be determined. These are critically important relationships to understand, as they may have direct implications for clinical care and improving diabetes outcomes.
The broad age ranges and developmental stages of the children and adolescents in many prior studies related to paternal involvement in diabetes care have limited the clinical and scientific significance of research in this area (Palmer et al., 2009). Paternal involvement during the early teen years in particular may be important for maintaining treatment adherence during the normative developmental transition to increasing autonomy in self-care (Palmer et al., 2004). Parents tend to be less involved in diabetes care during adolescence, which can interfere with effective family management of the illness, and both adherence and glycemic control tend to deteriorate across the teen years (Anderson, Ho, Brackett, Finkelstein, & Laffel, 1997; Helgeson, Honcharuk, Becker, Escobar, & Siminerio, 2011). On the other hand, when parents are more collaboratively involved in diabetes management, their children tend to engage in more consistent self-care, exhibit less deterioration in glycemic control, and have better diabetes outcomes (Berg et al., 2008; Nansel et al., 2009; Wiebe et al., 2005). Unfortunately, as mothers vastly outnumber fathers in research participation (Phares et al., 2005), these data almost exclusively speak about the important role of mothers in diabetes management, while the role of fathers in diabetes regimen adherence and glycemic control for children about to enter adolescence is not well understood.
The current study expands on the nascent literature in the area of fathers and diabetes care by examining the amount and helpfulness of paternal involvement in preadolescent diabetes management. We compared ratings from mothers and fathers of preadolescents with type 1 diabetes, and we examined associations with critical diabetes outcomes including treatment adherence and glycemic control. In contrast to earlier research, our study provides a focused examination of children between the ages of 9 and 12 years, given the changes that occur in diabetes self-management and glycemic control starting at the entry to adolescence. This approach provided a focused look at the nature of fathers’ involvement and relations with key health outcomes within the context of a common childhood chronic illness during an important transitional developmental period.
Methodological Issues Related to Studies of Paternal Involvement
Across populations, the analysis of data from fathers has been limited by small sample sizes, low statistical power, and inadequate collection, utilization, and treatment of family-level data (Holmbeck, Li, Schurman, Friedman, & Coakley, 2002; Hoyle, Georgesen, & Webster, 2001; Kenny, Kashy, & Cook, 2005), and this has indeed been the case for research in diabetes as well (Phares et al., 2005). For example, while commonly implemented and relatively easy to conduct, running separate parallel analyses for mothers and fathers has not allowed for research questions regarding family systems issues to be addressed. Combining the data without sufficiently measuring the degree of similarity or dissimilarity between theoretically nonindependent reports (i.e., individuals with family members, household, or lifestyle characteristics in common) has also been used but can result in potentially biased or misleading findings (Holmbeck et al., 2002; Hoyle et al., 2001; Kenny et al., 2005; Phares et al., 2005).
To address these limitations, the present study utilized a relatively large sample of mothers, fathers, and preadolescent children with type 1 diabetes from a multisite study. The similarities and dissimilarities between maternal and paternal ratings were compared, and structural equation modeling was used to analyze family-level data, which allowed for the estimation and management of nonindependence of same-family respondents (Kenny, 1995). Structural equation modeling using latent variables (i.e., indirectly observed variables) constructed from multiple indicators allowed us to measure the constructs of interest and incorporate multiple family members’ perspectives more accurately.
The Current Study
The aims and methods of this study are built upon previous literature in two ways. First, we examined the degree of agreement between mothers’ and fathers’ ratings of the amount and helpfulness of paternal involvement in their children’s diabetes care. We expected that scores would be significantly correlated between reporters, indicating intrafamilial nonindependence of the data, and we constructed latent variables to appropriately use all respondents’ data.
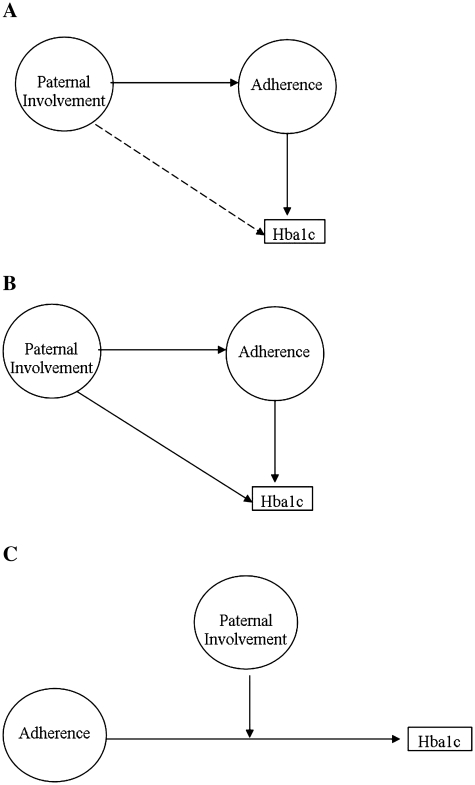
Second, we evaluated three alternative models of associations between the amount and perceived helpfulness of father’s involvement in their children’s diabetes care with diabetes regimen adherence and glycemic control. See Figure 1 for a diagram of the hypothesized models. The first model was a mediation model testing the potential role of fathers in preadolescents’ diabetes adherence and ultimately glycemic control. Based on findings linking paternal involvement with adherence in adolescents with chronic illness (Palmer et al., 2010; Wysocki & Gavin, 2006), we hypothesized that more frequent and helpful paternal involvement in diabetes care would be significantly associated with better glycemic control (i.e., lower HbA1c), and that this relationship would be mediated by more frequent blood glucose monitoring (BGM). Evidence for this model would support interventions with fathers that target adherence, with the aim of improving glycemic control.
Figure 1.
Hypothesized models. (A) Hypothesized Mediation Model, (B) Alternative, Direct and Indirect Model, and (C) Alternative, Moderation Model.
The second model examined a broader set of factors associated with glycemic control, and included both direct and indirect paternal influences. Based on similar findings in other illness groups (Wysocki & Gavin, 2006), the indirect pathway hypothesized that greater paternal involvement would be associated with better adherence, which would be associated with lower HbA1c values. The additional direct pathway hypothesized that more paternal involvement would also be directly linked with better glycemic control, likely through associations with other individual or family variables unmeasured in this dataset (e.g., support for maternal caregiving, youth autonomy in self-management; Wysocki & Gavin, 2006). Support for this model would indicate that promoting fathers’ global involvement in diabetes management should be a focus of intervention.
Given the benefits of having two parents collaborating in diabetes management (Wysocki et al., 2009), we also examined a third model testing whether the degree of paternal involvement was linked with individual differences in adherence and glycemic control. In this model, paternal involvement was hypothesized to moderate the association between adherence and glycemic control. Specifically, greater adherence would be more strongly related to HbA1c for preadolescents with fathers who were more involved than for those with fathers less involved in diabetes care. Data supporting this model would suggest targeting adherence-promotion interventions to those preadolescents at greater risk based on having less paternal engagement in care, focusing on increasing and enhancing the role of the father in diabetes management.
Method
Participants
Participants included a subset of families from an ongoing multisite prospective study of type 1 diabetes care and adherence trajectories during the transition to adolescence. We used baseline data from those families with a participating father in order to focus on dyadic and family-level analyses of paternal contributions to adherence and glycemic control. Of the 361 families approached for the larger study, 240 (66.5%) completed baseline data. Of those families, 151 (63.2%) included a secondary caregiver (e.g., father, grandmother, sister, etc.). The majority of those families (n = 146) included both a male and female caregiver. Thirty-six male secondary caregivers were available but did not participate. In addition, 10 families were excluded for not completing at least one of the primary measures. The resulting sample size was 136. The differences between the families in this sample, those with male caregivers that did not participate (N = 36), and those without a male caregiver (N = 47) were that the children participating in this study had lower HbA1c values [F (2, 218) = 11.08], had higher maternal-report of adherence [F (2, 216) = 5.08], were more likely to use a pump or pod versus a basal/bolus regimen (χ2 = 21.01, df = 6), and had higher income (χ2 = 66.25, df = 10), all with p’s < .05. Twenty participants were not included in these comparisons due to small cell sizes (6 female–female caregiver dyads, 4 male caregivers without a female caregiver available, and 10 with missing data). Following enrollment, one participant was identified as ineligible, as she was diagnosed with monogenic diabetes of the young (MODY) (Hattersley, Bruining, Shield, Niolstad, & Donaghue, 2006) and no longer treated with insulin. This participant’s data were therefore removed from the study and all analyses.
Adolescents in this subsample were 54% female, 91% Caucasian, and their ages at baseline ranged from 9.0 to 12.0 years (M = 10.5 years, SD = 0.9 years). The majority received insulin via insulin pump or pod (66%), and the mean illness duration was 4.1 years (SD = 2.4 years). The mean HbA1c closest to baseline was 7.9% (SD = 1.2%). Most parents completed a high school education (97%), and the modal income level was $73,000–126,500 (35%). The majority of self-identified primary caregivers were mothers (98.3%).
Procedure
Baseline data were used from an ongoing longitudinal study conducted at four children’s hospitals across the United States. Potential participant families were identified from the hospitals’ diabetes clinic rosters. Families from ethnic minority groups were oversampled to maximize the likelihood of adequate representation. Information about the study was provided to eligible families by their diabetes physician or study coordinators in the diabetes clinic. Eligibility required: (1) duration of type 1 diabetes of at least 1 year, (2) age 9 to 11 years at recruitment, (3) absence of comorbid chronic physical condition, and (4) fluency in English. After screening for eligibility by age and type 1 diabetes diagnosis, 85 youth across the 3 sites did not meet criteria due to the presence of one or more of the following exclusions: illness duration of less than 1 year (47%), secondary cause for diabetes diagnosis (5%), serious comorbid medical or psychological condition or intellectual disability (26%), no identified caregiver who could participate (12%), in foster care (13%), non-English speaking (29%), and anticipated moving away from the study catchment area during the study observation period (3 years, 56%). Parents and children aged 11 years or older provided written consent and assent to research assistants in clinic. Children under age 11 years provided verbal assent. Self- and parent-report measures were completed independently at regular medical visits whenever possible. Families received a modest incentive for completion of baseline data ($20 to parents and $35 to children). An additional $5 was provided to the child as an incentive to bring their blood glucose meter to the visit. The institutional review boards for the participating hospitals approved this study.
Measures
Paternal Involvement in Diabetes Management
Father’s involvement in diabetes care was measured with the Dads’ Active Disease Support scale (DADS; Wysocki & Gavin, 2004). The DADS asks mothers and fathers to rate the frequency with which the male caregiver in their family completed 24 diabetes care tasks when needed (amount scale), and the degree to which his contribution made the family’s coping with diabetes easier or harder (helpfulness scale). Respondents indicated (yes/no) whether each task was needed over the past 6 months. For those items that were needed, respondents used a 5-point Likert scale to rate the frequency of paternal involvement in the task (‘never’ to ‘always’) and the degree to which the contribution made family coping with the disease harder or easier (‘harder’ to ‘much easier’). A total score was calculated for each respondent on each scale by dividing the sum for each scale by the number of items endorsed as needed. Possible scores range from 24 to 120, and higher scores indicate greater frequency and helpfulness of paternal involvement, respectively. The DADS has demonstrated excellent psychometric properties (Wysocki & Gavin, 2004). In the current sample, internal consistency coefficients were excellent: α = 0.96 for mother-reported frequency and helpfulness scales, α = 0.90, 0.95 for father-reported frequency and helpfulness scales, respectively. Unfortunately, no parallel measure of maternal involvement was available for use.
Treatment Adherence
Child adherence was assessed through mother-, father- and child-report on the Diabetes Self-Management Profile (DSMP; Harris et al., 2000). The DSMP is a semi-structured interview designed to assess completion of a number of diabetes management tasks in the domains of exercise, hypoglycemia management, nutrition, BGM, and insulin administration and adjustment over the previous 3 months. Questions were re-worded as needed to assess the tasks unique to intensive therapy regimens. Scores on this measure range from 0 to 88, and higher scores indicate greater levels of diabetes adherence. Psychometric properties of the DSMP have been deemed adequate to excellent (DirecNet Study Group, 2005). The internal consistency coefficients for the current sample were moderate, consistent with alphas reported elsewhere (DirecNet Study Group, 2005): α = 0.62 for mother report, α = 0.63 for father report, α = 0.61 for child report.
BGM frequency was used as a behavioral indicator of adherence. Two weeks’ worth of blood glucose meter data were downloaded at the time of data collection, and the frequency of daily meter readings was averaged over the number of days collected. In this sample, daily frequency of BGM ranged from 1 to 10 (M = 5.35, SD = 1.7).
Medical and Background Information
Parents completed a background information form regarding family demographic and medical information (e.g., child age, ethnicity, and SES). Glycosylated hemoglobin A1c (HbA1c) was used to measure glycemic control. Blood samples for HbA1c were obtained during the study visit and shipped to a central laboratory for standardization purposes. Samples were analyzed using the TOSOH-G7 method (reference range 4.0–6.0%). Medical information (e.g., date of diagnosis, insulin delivery method, etc.) was confirmed through medical chart review.
Data Analysis
For the first aim, we compared mothers’ and fathers’ reports on the measures and constructed latent variables to account for nonindependence. Descriptive analyses were conducted with SPSS software (version 16: SPSS, Inc., 2007). We used Pearson correlations and ANOVAs to determine the degree of nonindependence between respondents and the relationships between the constructs and relevant demographic and medical variables. We used Student’s t-tests to compare maternal and paternal ratings of the amount and helpfulness of fathers’ involvement. Given the statistically significant correlations between the two reporters’ scores on the DADS subscales and between BGM frequency and DSMP total scores, we created two latent variables to represent (1) the broad construct of paternal involvement and (2) a composite measure of adherence for use in the structural equation models.
We tested aims 1 and 2 using structural equation modeling and Mplus software (version 6.1: Muthén & Muthén, 2008–2010). We used mathematical integration and robust maximum likelihood estimation for all Mplus analyses. We used a stepwise process (Bollen, 1989) and empirically established fit indices (Hu & Bentler, 1998, 1999) to evaluate the models in our analyses. Given our use of robust maximum likelihood estimation, we calculated the chi-square test of fit and other fit indices based upon Satorra and Bentler’s (2001) scaled chi-square approach. Due to the chi-square’s known sensitivity to trivial misfit, we focused on the root mean square error (RMSEA), comparative fit index (CFI), and standardized root mean square (SRMR) to guide model evaluation (Hu & Bentler, 1998, 1999). We adopted RMSEA values less than 0.05 as ideal and values less than 0.08 as acceptable, SRMR values less than 0.10, and CFI values greater than 0.90 as indicating good fit, where fit refers to the ability of the model to reproduce the observed covariance.
Aim 2 included an interaction between two latent variables. Nonlinear structural equation modeling is a relatively new field, especially with regard to evaluating model fit (Marsh, Wen, & Hau, 2004; Mooijaart & Satorra, 2009). As Mooijaart and Satorra (2009) recently showed, traditional fit indices are not sensitive to the presence of latent variable interactions and nonlinear terms. To date, no standard method for evaluating these models exists and traditional fit indices are inappropriate when evaluating models with a latent interaction (Mooijaart & Satorra, 2009; Mooijart & Bentler, 2010). However, this research suggests that although indices may miss the presence of a nonlinear term, a statistically significant interaction presumably should not lead to poorer fit. Thus, to evaluate our model that included an interaction, we first evaluated the fit of a model that included all terms except the latent interaction. If this model fit well, we then included the interaction between the two latent variables. If the interaction term was statistically significant, we took this as evidence that (1) an interaction between latent variables existed and (2) given that the previous model had fit well, the model with the latent interaction also fit well.
In our models, we expected some degree of shared variance across measures completed by the same reporter and within latent variables that were constructed using subscales of the same measure. MPlus modification indices indicated that one correlated error (between mother-reported DADS Amount and DSMP total) was needed.
Given the number of comparisons we made in our analyses, we adopted a more conservative p-value of 0.01 as the cutoff for statistical significance. We used standardized path loadings and critical ratios (CR, path coefficient/standard error ≥2.57) to determine significance (Kline, 2005; MacCallum & Austin, 2002; Muthén & Muthén, 2008–2010) and Cohen’s (1988) recommendations (0.1 = small, 0.3 = medium, 0.5 = large) regarding effect size.
Results
Descriptives
Mean scores, SDs, and zero-order correlations between the DADS and DSMP scores for all reporters, BGM frequency, and HbA1c values are displayed in Table I. All inter-rater correlations on the DADS and DSMP measures were significantly correlated. Higher DSMP total scores (all three reporters) were significantly correlated with more frequent BGM (r = 0.35–0.38, p’s < .01) and lower HbA1c (r = −0.23 to −0.37, p’s < .01). Demographic variables were uncorrelated with HbA1c, paternal involvement, and adherence scores, and were consequently not included in subsequent analyses.
Table I.
Means, SDs, and Bivariate Correlations Between Study Variables
| M (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| 1 DADS-amount (M) | 68.5 (22.2) | ||||||||
| 2 DADS-amount (F) | 68.9 (16.6) | .57** | |||||||
| 3 DADS-helpful (M) | 71.4 (18.0) | .65** | .37** | ||||||
| 4 DADS-helpful (F) | 63.2 (14.0) | .22* | .22* | .26** | |||||
| 5 DSMP total (M) | 66.0 (8.4) | .21* | .12 | .26** | .18* | ||||
| 6 DSMP total (F) | 65.2 (8.8) | .14 | .18* | .31** | .12 | .68** | |||
| 7 DSMP total (C) | 61.5 (8.1) | −.06 | .02 | .02 | −.02 | .41** | .52** | ||
| 8 BGM frequency | 5.35 (1.7) | −.01 | −.05 | .11 | .05 | .38** | .35** | .36** | |
| 9 A1c | 7.9 (1.2) | .11 | .17* | .02 | −.08 | −.39** | −.27** | −.23** | −.37** |
Note. DADS = Dads Active Disease Support Scale; DSMP = Diabetes Self-Management Profile; BGM = Blood Glucose Monitoring, A1c = glycemic control; M = Mother report; F = Father report; C = Child report. *p < .05, **p < .01.
Aim 1: Comparison between Reporters and Construction of Latent Variables
All inter-rater correlations were significant (p < .01), indicating statistical nonindependence between reporters on the DADS and DSMP measures. Mothers’ and fathers’ ratings of the amount of father involvement were significantly correlated and were not significantly different. However, mothers rated fathers’ contributions as significantly more helpful than did fathers themselves [t = 4.8 (131), p < .01].
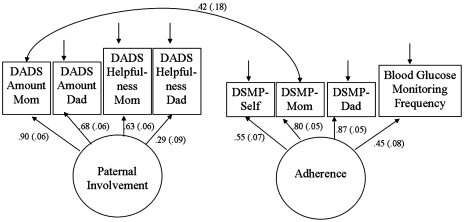
To capitalize on the fact that multiple indicators of a construct provide more accurate measurement than do single indicators, we used structural equation modeling and specified two latent variables. We chose to label them as paternal involvement and adherence because the indicators were well-developed measures of these constructs. The paternal involvement latent variable included maternal and paternal report on both subscales of the DADS measure. The adherence latent variable utilized maternal, paternal, and child reports for the total score on the DSMP interview, as well as meter-downloaded BGM frequency. The measurement model provided adequate fit [χ2 = 32.78 (18), p = .02, RMSEA = 0.08 (CI = 0.03–0.12), CFI = 0.947, SRMR = 0.07]. All indicators loaded significantly onto the hypothesized latent variables. The measurement model with latent variable factor loadings and standard errors is presented in Figure 2.
Figure 2.
Measurement model with standardized factor loadings. Note. Factor loadings (standard errors), all loadings are significant.
Aim 2: Investigation of Mediation, Direct and Indirect Effects, and Moderation Model Hypotheses
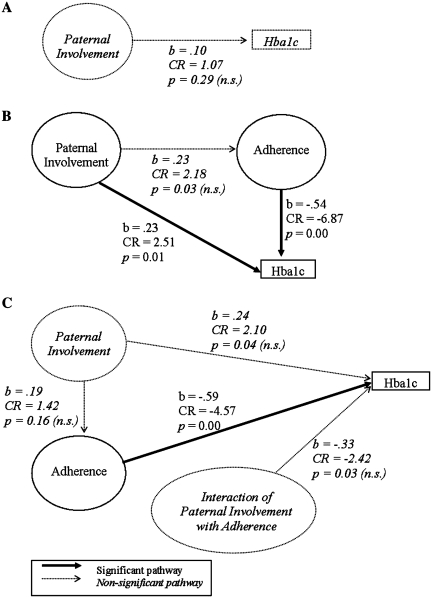
The results of the three alternative models are presented in Figure 3. First, we tested the mediation hypothesis with a series of three path models (MacKinnon, 2008). In the first step, we hypothesized that the proposed independent variable (here, paternal involvement) would have a significant pathway with the proposed outcome (here, HbA1c). In the second step, we hypothesized that paternal involvement would have a significant pathway with the proposed mediator (here, adherence). Finally, we hypothesized that the path between paternal involvement and HbA1c would become not significant with the inclusion of adherence in the model (Step 3). The first step was tested and had good overall model fit [χ2 = 9.32 (5), p = .10, RMSEA = 0.08 (CI = 0.00–0.16), CFI = 0.965, SRMR = 0.05]. However, the pathway between paternal involvement and HbA1c was not significant (b = .10, CR = 1.07, p = .42). As the requisite steps for mediation were not met, the data did not support the mediation hypothesis.
Figure 3.
Model results, standardized path loadings, critical ratios, and p-values. (A) Mediation Model, (B) Direct and Indirect Model, and (C) Moderation Model.
Next, we tested the indirect and direct effects model by entering all constructs and pathways into one model and examining overall model fit. The model provided adequate fit to the data [χ2 = 49.36 (25), p = .00, RMSEA = 0.09 (CI = 0.05–0.12), CFI = 0.922, SRMR = 0.06]. We hypothesized that more paternal involvement would be associated with higher adherence, which would be associated with lower HbA1c. We also hypothesized that more paternal involvement would have a small direct association with lower HbA1c. We examined the effect sizes of standardized path loadings (Cohen, 1988) to interpret the associations between variables. In this model, the association between more paternal involvement and higher adherence had a small effect that was not significant (b = 0.23, CR = 2.18, p = .03), although the association between higher adherence and lower HbA1c was significant with a large effect (b = − 0.54, CR = −6.87, p = .000). There was also a significant direct association between more paternal involvement and higher HbA1c, although this was a small effect (b = 0.23, CR = 2.51, p = .01). The direct and indirect effects model was largely consistent with the data. However, the direct pathway had a positive association rather than the hypothesized negative association.
Finally, we tested the moderation model by entering each of the predictor latent variables (paternal involvement and adherence), the interaction term between the two latent variables, and the outcome variable (HbA1c) into the model at once. We hypothesized that better adherence would have a stronger association with lower HbA1c for adolescents from families in which the father was more involved when compared to families where the father was less involved. As noted above, traditional fit indices are not appropriate for models with an interaction between latent variables. Therefore, we first examined the fit of a model with all terms except the interaction term. This model provided adequate fit [χ2 = 49.36 (25), p = .00, RMSEA = 0.09, CFI = 0.922], indicating we could examine a model with the interaction. However, the interaction term was not significant. Paternal involvement was not significantly associated with adherence (b = 0.19, CR = 1.42, p = .16). The association between higher adherence and lower HbA1c was significant with a large effect size (b = −0.59, CR = −4.57, p < .01). On the other hand, the associations between more paternal involvement and higher HbA1c (b = 0.24, CR = 2.10, p = .04) and between the moderator term and HbA1c (b = −0.33, CR = −2.42, p = .02) had small to medium effects that were not significant. The moderation hypothesis was thus not supported by the data.
Discussion
By concentrating on the role of fathers in diabetes management, this work expands on the existing body of research that has emphasized the importance of mother–child collaboration—and, to a lesser extent, the involvement of two parents—in self-care with relation to mitigating the risk for deteriorating adherence and glycemic control during this period (Berg et al., 2008; Wysocki et al., 2009). This study extends the initial work of Wysocki and Gavin (2004, 2006) in that it provides a focused examination of alternative relationships between paternal involvement and youth outcomes among preadolescents with type 1 diabetes.
The methodological and statistical advances employed in this study contribute to the growing literature on the quantity, quality, and impact of paternal contributions to children’s health care (e.g., Dashiff et al., 2008; Phares et al., 2005; Wysocki & Gavin, 2006). A recently developed, standardized measure of fathers’ contributions to children’s health care was used, lending validity to the measurement of the central construct. The sample size was larger than previous father-focused studies and drew from multiple sites across the country, thus contributing to increased power and generalizability. Moreover, we analyzed family data appropriately by employing statistical methods that allowed us to account for nonindependence among family member’s responses as necessary. Structural equation modeling effectively aggregated these nonindependent data from multiple reporters and partialled out random measurement error, which strengthened the measurement of the primary constructs of interest in the study. The measurement models fit the data well, thereby supporting our use of this modeling strategy. The examination of three feasible and clinically relevant models was an additional strength of this study, as it allowed us to explore a series of alternative relationships between the constructs of interest.
While the central role of mothers during the transition to adolescence has been well-documented (e.g., Berg et al., 2008; Nansel et al., 2009; Wiebe et al., 2005), the current study provides evidence that fathers may play an active role in family diabetes management in this age range as well. These data demonstrate that the association between preadolescents’ diabetes outcomes is linked to a small degree with the level of paternal involvement in diabetes care. In contrast to previous studies (Gavin & Wysocki, 2006; Wysocki & Gavin, 2006), in this study with youth with type 1 diabetes at the entry to adolescence, the amount and helpfulness of paternal involvement did not demonstrate a direct association with regimen adherence. The data indicated small and nonsignificant associations between paternal involvement and better adherence. The indirect and direct effects model was supported by the data. However, contrary to hypotheses, greater paternal involvement was associated with higher HbA1c values, which were also linked with poorer adherence. It may be that some fathers become more involved in diabetes care as a result of their escalating concerns about poor glycemic control. Fathers may also engage with their children with diabetes differently than mothers (Povey, Hallas, White, Clarke, & Samuel, 2005) or play unique roles in their children’s lives (e.g., engagement in leisure, athletics, and other activities) that may interfere with glycemic control (Seiffge-Krenke, 2002). Each of the findings in this study may be specific to the transitional preadolescent age range in this study, and paternal engagement during later adolescence may demonstrate stronger links with adherence and ultimately impact glycemic control (Wysocki & Gavin, 2006). If fathers indeed become more directly involved in care as HbA1c values begin to rise, their ongoing contributions throughout adolescence might cumulatively result in improvements in adherence and glycemic control that were not detected in the age range included in this cross sectional study (Palmer et al., 2010). For these reasons, longitudinal research during adolescence is needed to determine the reasons for and point at which fathers increase their involvement in diabetes management and the impact on adherence and HbA1c over time.
Data suggest that fathers may undervalue their impact on their children’s health relative to mothers. Mothers and fathers in this sample agreed on the amount of paternal involvement. This is comparable with findings from the developmental literature, which indicate that mothers generally report similar or slightly lower amounts of time fathers spend with children than do fathers (Coley & Morris, 2004; Wical & Doherty, 2005). The interparental agreement in this study is likely related to behavioral nature of the measurement and its narrow focus on illness-related involvement behaviors. Further, overall time spent with a child does not necessarily have a direct correlation with involvement in diabetes management. Consistent with previous research (Wysocki & Gavin, 2004), fathers in this study provided lower helpfulness ratings of their contributions than did mothers. It may be that mothers, who tend to take on primary parental responsibility for diabetes management, appreciate any helpful contributions from fathers and as such more highly rate the benefit of the assistance fathers provide. Fathers, on the other hand, may compare the quantity and quality of their contributions to what mothers do and judge their own additions to care as having relatively less impact. If fathers do become more involved as their children achieve higher HbA1c values, mothers may be particularly appreciative of their contributions or efforts to assist during a time of worsening diabetes control. However, fathers may feel ineffective if HbA1c values do not quickly improve when they become involved.
The study had limitations that need to be considered in interpreting our findings. The measures and data analysis in this study did not assess potential influences on glycemic control outside of paternal involvement and adherence. While mothers and fathers rated the amount and helpfulness of paternal involvement, valuable subjective ratings from children regarding their perceptions of their father’s role in diabetes management (Povey et al., 2005; Seiffge-Krenke, 1998) were not collected due to a lack of available measures. There was also no measure of the amount and helpfulness of maternal involvement to parallel that of paternal involvement. In addition, the participants had a relatively low mean HbA1c value and were primarily from well-educated, middle- to upper-middle class families with two parents present, and regularly attended diabetes clinics and consented to participate in research. This convenience sample likely overrepresents families with greater resources and may not adequately represent the medical or socio-economic status or family structure of many patients with type 1 diabetes. Those fathers who were unable or opted not to participate in this study likely have distinct patterns of involvement in diabetes and differential impact on their children’s adherence and glycemic control outcomes. Similarly, those families who were screened out of the study due to no father involvement may also demonstrate different diabetes management and glycemic control (Hanson, Henggeler, Rodrigue, Burghen, & Murphy, 1988). The primary caregivers in this study were overwhelmingly mothers, which is consistent with previous research yet limits our understanding of families in which fathers take a primary or equally shared caregiving role. For example, these results do not speak about what may be unique roles for fathers in single-parent households, where they may have greater or sole responsibility for diabetes care. Finally, these cross sectional data represent a single time-point and cannot be used to infer causality between the constructs assessed.
Nevertheless, the results of this study are meaningful for future research and clinical care of children and families with type 1 diabetes. Clinically, the direct and indirect effects model results suggest that both paternal involvement and youth’s adherence behaviors may be useful targets of intervention to improve HbA1c. A number of empirically supported family-based treatments exist that target adherence promotion with the ultimate goal of improving glycemic control (e.g., Anderson, Brackett, Ho, & Laffel, 1999; Ellis et al., 2005; Wysocki et al., 2008). The results of this study indicate that modifications to these intervention programs focusing on the involvement of not only mothers, but also fathers, could be valuable. As noted by Phares and colleagues (2005), fathers are disproportionately absent from treatment outcome studies in pediatric psychology, and these interventions should be tested in samples that include more fathers. In addition, as fathers may not perceive themselves to be as helpful as mothers do, it is likely important to reinforce their roles in diabetes care, as this may enhance family management of diabetes among preadolescents with poorer glycemic control.
In order to answer empirical family-level questions, such as how maternal perceptions about fathers’ help with diabetes predict fathers’ subsequent involvement and vice versa, future researchers should consider using available analytic methods designed for dyadic data in longitudinal samples. For example, the actor–partner interdependence model (APIM) can be used to analyze the influences of two individuals’ past behavior on their own and the other individuals’ future behavior (i.e., actor effects and partner effects) (Cook & Kenny, 2006). This would be an important step for understanding the reciprocal mechanisms by which each parent can impact the other’s contributions to family illness management, treatment adherence, and glycemic control. Dyadic and family-level analytic approaches (e.g., structural equation modeling and combined path models for mothers and fathers) can also be used by researchers to compare the similarities and differences between mothers’, fathers’, and children’s contributions to diabetes care, thereby providing greater specificity in our understanding of families’ collaborative illness management. Resulting data will likely inform family-based interventions to encourage helpful parent engagement and promote better adolescent diabetes outcomes, as particular family interaction patterns could be identified. For example, specific communication styles (e.g., explicitly requesting fathers’ assistance and expressing appreciation) could be used to increase the amount and helpfulness of fathers’ involvement in their children’s diabetes care.
Funding
The National Institute of Diabetes and Digestive and Kidney Diseases (grant 1R01-DK-069486).
Conflicts of interest: None declared.
Acknowledgments
The HbA1c data were analyzed by the Diabetes Diagnostic Laboratory at the University of Missouri Columbia Health Sciences Center. The efforts of study participants who gave their time and energy to this work are gratefully acknowledged. We appreciate the support of our physician colleagues, Larry Dolan and Grafton Reeves. Data collection and management of this study were facilitated by a talented group of research assistants, including Claire Peterson, Michelle Eakin, Danielle Rosnov, Daniela Fernandez, Jennifer Hernandez, Katie Wetterau, and Megan Miller.
Footnotes
*Methods of study based on study first reported in McNally, K., Rohan, J., Pendley, J. S., Delamater, A., & Drotar, D. (2010). Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care, 33, 1159–1162.
References
- Anderson B J, Brackett J, Ho J, Laffel L M B. An office-based intervention to maintain parent–adolescent teamwork in diabetes management. Diabetes Care. 1999;22:713–721. doi: 10.2337/diacare.22.5.713. [DOI] [PubMed] [Google Scholar]
- Anderson B, Ho J, Brackett J, Finkelstein D, Laffel L. Parental involvement in diabetes management tasks: Relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. The Journal of Pediatrics. 1997;130:257–265. doi: 10.1016/s0022-3476(97)70352-4. [DOI] [PubMed] [Google Scholar]
- Berg C A, Butler J M, Osborn P, King G, Palmer D L, Butner J, Murray M, Lindsay R, Donaldson D, Foster C, Swinyard M, Wiebe D J. Role of parental monitoring in understanding the benefits of parental acceptance on adolescent adherence and metabolic control of type 1 diabetes. Diabetes Care. 2008;31:678–683. doi: 10.2337/dc07-1678. [DOI] [PubMed] [Google Scholar]
- Bollen K. Structural equations with latent variables. New York: Wiley; 1989. [Google Scholar]
- Cohen J. Statistical power for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Coley R L, Morris J E. Comparing father and mother reports of father involvement among low income minority families. Journal of Marriage and Family. 2004;64:982–997. [Google Scholar]
- Cook W L, Kenny D A. The actor-partner interdependence model: A model of bidirectional effects in developmental studies. International Journal of Behavioral Development. 2006;29:101–109. [Google Scholar]
- Dashiff C J. Self- and dependent-care responsibility of adolescents with IDDM and their parents. Journal of Family Nursing. 2003;9:166–183. [Google Scholar]
- Dashiff C, Morrison S, Rowe J. Fathers of children and adolescents with diabetes: What do we know? Journal of Pediatric Nursing. 2008;23:101–119. doi: 10.1016/j.pedn.2007.08.007. [DOI] [PubMed] [Google Scholar]
- DirecNet Study Group. Diabetes self-management profile for flexible insulin regimens: Cross-sectional and longitudinal analysis of psychometric properties in a pediatric sample. Diabetes Care. 2005;28:2034–2035. doi: 10.2337/diacare.28.8.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D A, Frey M A, Naar-King S, Templin T, Cunningham P, Cakan N. Use of multisystemic therapy to improve regimen adherence among adolescents with type 1 diabetes in chronic poor metabolic control. Diabetes Care. 2005;28:1604–1610. doi: 10.2337/diacare.28.7.1604. [DOI] [PubMed] [Google Scholar]
- Gavin L, Wysocki T. Associations of paternal involvement in disease management with maternal and family outcomes in families with children with chronic illness. Journal of Pediatric Psychology. 2006;31:481–489. doi: 10.1093/jpepsy/jsj043. [DOI] [PubMed] [Google Scholar]
- Hanson C L, Henggeler S W, Rodrigue J R, Burghen G A, Murphy W D. Father-absent adolescents with insulin-dependent diabetes mellitus: A population at risk? Journal of Applied Developmental Psychology. 1988;9:243–252. [Google Scholar]
- Harris M A, Wysocki T, Sadler M, Wilkinson K, Harvey L M, Buckloh L M, Mauras N, White N H. Validation of a structured interview for the assessment of diabetes self-management. Diabetes Care. 2000;23:1301–1304. doi: 10.2337/diacare.23.9.1301. [DOI] [PubMed] [Google Scholar]
- Hattersley A, Bruining J, Shield J, Njolstad P, Donaghue K. International Society for Pediatric and Adolescent Diabetes. ISPAD Clinical Practice Consensus Guidelines 2006–2007: The diagnosis and management of monogenic diabetes in children. Pediatric Diabetes. 2006;7:352–360. doi: 10.1111/j.1399-5448.2006.00217.x. [DOI] [PubMed] [Google Scholar]
- Helgeson V S, Honcharuk E, Becker D, Escobar O, Siminerio L. A focus on blood glucose monitoring: Relation to glycemic control and determinants of frequency. Pediatric Diabetes. 2011;12:25–30. doi: 10.1111/j.1399-5448.2010.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck G N, Li S T, Schurman J V, Friedman D, Coakley R M. Collecting and managing multisource and multimethod data in studies of pediatric populations. Journal of Pediatric Psychology. 2002;27:5–18. doi: 10.1093/jpepsy/27.1.5. [DOI] [PubMed] [Google Scholar]
- Horton D, Berg C A, Butner J, Wiebe D J. The role of parental monitoring in glycemic control: Effect on adherence and externalizing behaviors during adolescence. Journal of Pediatric Psychology. 2009;34:1008–1018. doi: 10.1093/jpepsy/jsp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle R H, Georgesen J C, Webster J M. Analyzing data from individuals in groups: The past, the present, and the future. Group Dynamics: Theory, Research, and Practice. 2001;5:41–47. [Google Scholar]
- Hu L, Bentler P. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods. 1998;3:424–453. [Google Scholar]
- Hu L, Bentler P. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:1–55. [Google Scholar]
- Kenny D A. The effect of nonindependence on significance testing in dyadic research. Personal Relationships. 1995;2:67–75. [Google Scholar]
- Kenny D A, Kashy D A, Cook W L. Dyadic Data Analysis. New York: The Guilford Press; 2005. [Google Scholar]
- Kline R B. Principles and Practice of Structural Equation Modeling. 2nd ed. New York, NY: Guilford Press; 2005. [Google Scholar]
- Leonard B J, Garwick A, Adwan J Z. Adolescents’ perceptions of parental roles and involvement in diabetes management. Journal of Pediatric Nursing. 2005;20:405–414. doi: 10.1016/j.pedn.2005.03.010. [DOI] [PubMed] [Google Scholar]
- MacCallum R C, Austin J T. Applications of structural equation modeling in psychological research. Annual Review of Psychology. 2000;51:201–226. doi: 10.1146/annurev.psych.51.1.201. [DOI] [PubMed] [Google Scholar]
- MacKinnon D P. Introduction to statistical mediation analysis. New York: Lawrence Erlbaum Associates; 2008. [Google Scholar]
- Marsh H, Wen Z, Hau K. Structural equation models of latent interactions: Evaluation of alternative estimation strategies and indicator construction. Psychological Methods. 2004;9:275. doi: 10.1037/1082-989X.9.3.275. [DOI] [PubMed] [Google Scholar]
- Mooijaart A, Bentler P M. An alternative approach for nonlinear latent variable models. Structural Equation Modeling. 2010;17:357–373. [Google Scholar]
- Mooijaart A, Satorra A. On insensitivity of the chi-square model test to nonlinear misspecification in structural equation models. Psychometrika. 2009;74:443–455. [Google Scholar]
- Muthén B O, Muthén L K. Mplus User’s Guide (6th ed.) Los Angeles, CA: Muthén and Muthén; 1998–2010. Retrieved from http://www.statmodel.com/download/users guide/Mplus%20Users%20Guide%20v6.pdf. [Google Scholar]
- Nansel T R, Rovner A J, Haynie D, Iannotti R J, Simons-Morton B, Wysocki T, Anderson B, Weissberg-Benchell J, Laffel L. Development and validation of the Collaborative Parent Involvement Scale for youths with Type 1 diabetes. Journal of Pediatric Psychology. 2009;34:30–40. doi: 10.1093/jpepsy/jsn058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D L, Berg C A, Butler J, Fortenberry K, Murray M, Lindsay R, Donaldson D, Swinyard M, Foster C, Wiebe D J. Mothers’, fathers’, and children’s perceptions of parental diabetes responsibility in adolescence: Examining the roles of age, pubertal status, and efficacy. Journal of Pediatric Psychology. 2009;34:195–204. doi: 10.1093/jpepsy/jsn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D L, Berg C A, Wiebe D J, Beveridge R M, Korbel C D, Upchurch R, Swinyard M T, Lindsay R, Donaldson D L. The role of autonomy and pubertal status in understanding age differences in maternal involvement in diabetes responsibility across adolescence. Journal of Pediatric Psychology. 2004;29:35–46. doi: 10.1093/jpepsy/jsh005. [DOI] [PubMed] [Google Scholar]
- Palmer D L, Osborn P, King P S, Berg C A, Butler J, Butner J, Horton D, Wiebe D J. The structure of parental involvement and relations to disease management for youth with type 1 diabetes. Journal of Pediatric Psychology. 2010 doi: 10.1093/jpepsy/jsq019. Advance online publication. doi:10.1093/jpepsy/jsq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phares V, Lopez E, Fields S, Kamboukos D, Duhig A M. Are fathers involved in pediatric psychology research and treatment? Journal of Pediatric Psychology. 2005;30:631–643. doi: 10.1093/jpepsy/jsi050. [DOI] [PubMed] [Google Scholar]
- Povey R C, Hallas C N, White D G, Clarke T, Samuel T J. Children’s beliefs about the impact of their type 1 diabetes on their family and peers: An exploratory study. Practical Diabetes International. 2005;22:333–338. [Google Scholar]
- Satorra A, Bentler P M. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika. 2001;66:507–514. doi: 10.1007/s11336-009-9135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiffge-Krenke I. The highly structured climate in families of adolescents with diabetes: Functional or dysfunctional for metabolic control? Journal of Pediatric Psychology. 1998;23:313–322. doi: 10.1093/jpepsy/23.5.313. [DOI] [PubMed] [Google Scholar]
- Seiffge-Krenke I. “Come on, say something, Dad!”: Communication and coping in fathers of diabetic adolescents. Journal of Pediatric Psychology. 2002;27:439–450. doi: 10.1093/jpepsy/27.5.439. [DOI] [PubMed] [Google Scholar]
- SPSS, Inc. SPSS (Computer Program), Version 16.0. Chicago, IL, USA: SPSS, Inc; 2007. [Google Scholar]
- Wical K A, Doherty W J. How reliable are fathers’ reports of involvement with their children? A methodological report. Fathering. 2005;3:81–91. [Google Scholar]
- Wiebe D J, Berg C A, Korbel C D, Palmer D L, Beveridge R M, Upchurch R, Lindsay R, Swinyard M T, Donaldson D L. Children's appraisals of maternal involvement in coping with diabetes: Enhancing our understanding of adherence, glycemic control, and quality of life across adolescence. Journal of Pediatric Psychology. 2005;30:167–178. doi: 10.1093/jpepsy/jsi004. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Gavin L. Psychometric properties of a new measure of fathers' involvement in the management of pediatric chronic diseases. Journal of Pediatric Psychology. 2004;29:231–240. doi: 10.1093/jpepsy/jsh024. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Gavin L. Paternal involvement in the management of pediatric chronic diseases: Associations with adherence, quality of life, and health status. Journal of Pediatric Psychology. 2006;31:501–511. doi: 10.1093/jpepsy/jsj042. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Harris M A, Buckloh L M, Mertlich D, Lochrie A S, Taylor A, Sadler M, White N H. Randomized, controlled trial of behavioral family systems therapy for diabetes: Maintenance and generalization of effects on parent–adolescent communication. Behavior Therapy. 2008;39:33–46. doi: 10.1016/j.beth.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Nansel T R, Holmbeck G N, Chen R, Laffel L, Anderson B J, Weissberg-Benchell J, Steering Committee of the Family Management of Childhood Diabetes Study Collaborative involvement of primary and secondary caregivers: Associations with youths' diabetes outcomes. Journal of Pediatric Psychology. 2009;34:869–881. doi: 10.1093/jpepsy/jsn136. [DOI] [PMC free article] [PubMed] [Google Scholar]