Abstract
Two studies tested the hypothesis that certain positive emotions speed recovery from the cardiovascular sequelae of negative emotions. In Study 1, 60 subjects (Ss) viewed an initial fear-eliciting film, and were randomly assigned to view a secondary film that elicited: (a) contentment; (b) amusement; (c) neutrality; or (d) sadness. Compared to Ss who viewed the neutral and sad secondary films, those who viewed the positive films exhibited more rapid returns to pre-film levels of cardiovascular activation. In Study 2, 72 Ss viewed a film known to elicit sadness. Fifty Ss spontaneously smiled at least once while viewing this film. Compared to Ss who did not smile, those who smiled exhibited more rapid returns to pre-film levels of cardiovascular activation. We discuss these findings in terms of emotion theory and possible health-promoting functions of positive emotions.
INTRODUCTION
Despite the currently burgeoning state of research on emotions, a review of this literature reveals an overwhelming focus on negative emotions and a relative neglect of positive emotions. Why is this?
The Problem of Positive Emotions
Perhaps one reason empirical research on positive emotions has been slow to accumulate is that, by and large, positive emotions have been a thorn for most emotion theorists. Take, for instance, the frequent assertion that emotions are—by definition—associated with specific action tendencies (Frijda, 1986; Frijda, Kuipers, & Schure, 1989; Lazarus, 1991; Levenson, 1988, 1994; Tooby & Cosmides, 1990); anger calls to mind the urge to attack, fear the urge to escape, disgust the urge to expel, and so on. Key to this assertion is that specific action tendencies and organised physiological change go hand in hand—emotions not only prepare the mind to act in specific ways, but they prepare the body as well. As Lazarus (1991, p. 285) put it, “an action tendency is what makes an emotion embodied”. An evolutionary note is often sounded among these theorists, with emotions seen as promoting specific adaptive actions in life-threatening situations that have been ancestrally recurrent.
Linking specific emotions with specific action tendencies seems easy enough when working within the subset of negative emotions. Positive emotions, however, pose theoretical stumbling blocks. Frijda’s (1986) descriptions of action tendencies, for instance, grow vague when emotions are positive: He pairs contentment with inactivity, and joy with (p. 89), “free activation”, which he describes as an “aimless, unasked-for readiness to engage in whatever interaction presents itself”. Likewise, Lazarus (1991) concedes that the action tendency for happiness/joy is “hard to pin down”, and that for pride “is difficult to specify with confidence”. And although affection is linked with approach (Frijda, 1986) and relief with ceasing to be vigilant (Lazarus, 1991), one question to ask is approach and do what?—cease vigilance and do what? It appears that the specific action tendencies named for positive emotions are not nearly as specific as those named for negative emotions. At best they resemble generic orientations toward action or inaction, rather than urges to do something quite specific, like attack, flee, or spit. Moreover, in research on autonomic responding associated with discrete emotions, except for situations where extreme respiratory or somatic activity is involved (e.g. outright laughter or yawning), positive emotions seem to be characterised by a relative lack of autonomic activation (e.g. Levenson, Carstensen, Friesen, & Ekman, 1991; Levenson, Ekman, & Friesen, 1990).
In short, action-oriented explanatory models, which have served the negative emotions well, may not do as well for describing the functions of positive emotions. Although we are not the first to note problems that positive emotions pose for emotion theorists (e.g. Ekman, 1992; Lazarus, 1991), seldom has this acknowledgment spurred revision of, or qualifications to, purportedly general models of emotion.
An “Undoing” Effect of Positive Emotions
The question remains: How might models of emotion better accommodate the positive emotions? We propose that one major obstacle to understanding positive emotions is the predilection toward adopting a single general purpose model of emotion. Why not, as Ekman (1994) has suggested, allow different theories for distinct emotions (e.g. a theory of anger, a theory of sadness)? Still another multiple-model landscape would allow one model to describe a subset of distinct negative emotions (e.g. anger, fear, disgust, sadness), and a separate model to describe a subset of distinct positive emotions (e.g. contentment, amusement). Traditional action-oriented models, then, could be maintained as suitable descriptions for these negative emotions, whereas alternative models could be developed for positive emotions.
In building a more suitable model for this subset of positive emotions, we suggest leaving behind the presumption that all emotions must necessarily yield specific action tendencies. In its place, we offer an alternative prediction regarding the effects of these positive emotions on physiological change and provide two empirical tests of its viability. This work expands on the speculation offered by Levenson (1988, p. 25) in a discussion of psychophysiological research on emotion:
… the evolutionary meaning of positive emotions such as happiness might be to function as efficient “undoers” of states of ANS [autonomic nervous system] arousal produced by certain negative emotions. To test this hypothesis a reasonable baseline condition for the investigation of ANS concomitants of happiness would be one that produces a prior state of fear, anger or sadness.
Extrapolating from these ideas, we suggest that perhaps one function of certain positive emotions may not be to spark specific action (as do many negative emotions), but instead may be to loosen the hold that these negative emotions gain on an individual’s mind and body by dismantling, or undoing this psychological and physiologic al preparation for specific action. In particular, it may be that the positive emotions of contentment and amusement act in the service of homeostasis, restoring quiescence; as such, a switch from a negative emotion to one of these positive emotions may in effect rid individuals of the psychological and physiological sequelae of action readiness, allowing them to pursue a wider variety of actions or experiences. Although this “undoing” hypothesis builds clear linkages between certain positive and negative emotions, the tie is more one of complementarity than of isomorphism.
Physiologic ally, the undoing hypothes is does not propose that these positive emotions lead to an inert bodily state, one characterised by extremely low levels of autonomic activation. Rather, it suggests that certain positive emotions serve to restore autonomic activity to more mid range levels.
It bears underscoring that this undoing hypothesis is intended to describe one particular psychophysiological effect of certain positive emotions, and does not claim that this is their sole function. Additionally, just as all discrete negative emotions are not necessarily associated with high-action motor programmes (e.g. boredom and envy may be exceptions), we do not hold that all discrete positive emotions should necessarily show the undoing effect (e.g. pride may be an exception). Also, the undoing hypothesis is limited to those contexts in which certain positive emotions follow certain negative emotions in close temporal sequence; it does not speak to the effects these positive emotions may have when experienced by themselves. Moreover, our hypothesis and the experiments we describe here focus on cardiovascular concomitants of emotions (which are tightly linked to the metabolic demands produced by somatic action; Obrist, 1981) and not on other physiological systems.
Overview of Empirical Strategy
The undoing hypothesis predicts that certain positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Representative of this class of positive emotions are contentment and amusement, neither of which is associated with a high-activity action tendency. Our strategy for testing this hypothesis is based on Levenson’s (1988) suggestion of examining transitions between negative and positive emotions. In Study 1, we begin from a starting state of fear, and in Study 2 we begin from a starting state of sadness. In each study, we use emotionally evocative film clips to generate these initial negative emotions. We then assess the effects of positive emotions that are either experimentally induced (Study 1) or that occur naturally (Study 2). Specifically, we examine the duration of cardiovascular reactivity as a function of the experience or expression of positive emotions. In Study 1, after exposing subjects to a fear-inducing film, we experimentally manipulate the presence of positive emotions by showing subjects one of four different secondary films designed to elicit either (a) contentment, (b) amusement, (c) neutrality, or (d) sadness [ a negative emotion that, like the two positive emotions, has no obvious association with a high-activity action tendency (Lazarus, 1991; Levenson, 1992)]. In Study 2, we make use of a naturally occurring temporal union of negative and positive affect, examining whether people who spontaneously smiled during a sad film exhibited faster recovery from the cardiovascular activation occasioned by that film than those who did not smile.
STUDY 1
Method
Participants
Sixty female undergraduate students enrolled in introductory psychology courses at the University of California, Berkeley served as voluntary participants in this study: 37% of these participants identified themselves as Asian, 30% as Hispanic, 25% as Caucasian, and 8% as Black, which approximates the demographics of the student population at the university. Each received course credit in exchange for their participation. Participants were randomly assigned to one of four experimental conditions defined by the emotional content of the secondary film stimulus (i.e. contentment, amusement, neutral, or sadness) that followed the initial fear-eliciting film stimulus.
Visual Materials
Selection
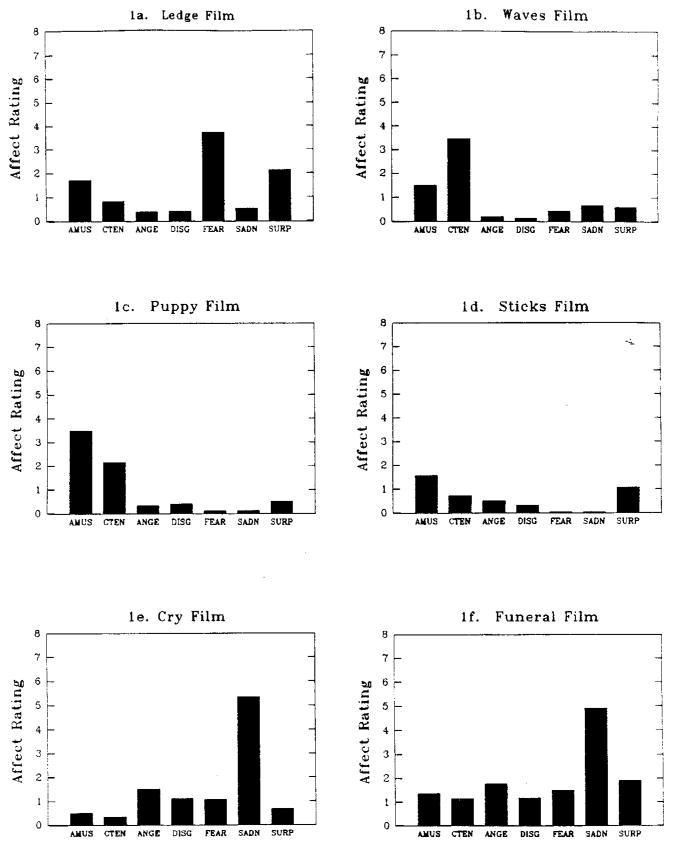
Five short film clips were used in this study (the initial fear-eliciting film and the four secondary films). Film selection was based in part on prior work in Levenson’s laboratory to compile a library of emotion-eliciting films that have been found in group screenings to elicit self-reports of relatively specific emotional states (e.g. Gross & Levenson, 1995). In these screening sessions, respondents viewed a film, and then immediately rated it in terms of each of seven emotion terms (i.e. amusement, anger, contentment, disgust, fear, sadness, surprise) on 9-point Likert scales (0 = none, 8 = most in my life; adapted from Ekman, Friesen, & Ancoli, 1980). Figure 1 presents the emotional ratings for the five film clips used in Study 1 (along with the one sadness-eliciting film clip used in Study 2). These ratings were obtained from independent samples in group viewings.
FIG. 1.
Mean self-reports of emotion from independent samples who viewed stimulus films used in Studies 1 and 2: (1a) displays self-reports of emotion in response to the Ledge film (n = 75), used in Study 1; (1b–1e) display self-reports of emotion in response to each of the secondary films used in Study 1: The Puppy film (n = 50); the Waves film (n = 42); the Sticks film (n = 32); and the Cry film (n = 32); (1f ) displays self-reports of emotion in response to the Funeral film (n = 46), used in Study 2. (AMUS, Amusement; CTEN, Contentment; ANGE, Anger; DISG, disgust; FEAR, Fear; SADN, Sadness; SURP, Surprise.)
Film Content and Emotions Elicited
The film clip used to induce the initial negative emotion [“Ledge”, drawn from the feature film Cat’s Eye (DeLaurentiis, Schumacher, Subotsk, & Teague, 1985)]; shows a man inching along the ledge of a high-rise building, hugging the side of the building; at one point he loses his grip, dangles high above traffic, and struggles to keep from dropping. We chose this clip because it seems a face valid elicitor of a (perhaps innate) fear of falling. Examination of Fig. 1a reveals that this film primarily elicits self-reports of fear, with lesser reports of other emotions.
Reports of emotion elicited by the four secondary films are displayed in Figs 1b–1e: (b) “Waves” shows waves breaking on a beach and primarily elicits self-reports of contentment; (c) “Puppy “ shows a small dog playing with a flower and primarily elicits self-reports of amusement; (d) “Sticks” shows an abstract dynamic display of coloured sticks piling up and produces minimal report of any of the seven rated emotions (modal reports were “0” across all emotion terms); and (e) “Cry” depicts a young boy crying as he watches his father die and primarily elicits self-reports of sadness [drawn from the feature film The Champ (Lovell & Zeffirelli, 1979)].
The initial film, “Ledge”, was 83 seconds long and was presented with sound. For comparability between experimental conditions, all four secondary films were 100 seconds long and presented without sound.
Apparatus
Rating Dial
A positive-negative affective measure, developed by Levenson and Gottman (1983), was used to obtain continuous reports of affect during the study. Participants manipulated a dial whose pointer moved on a 180-degree scale divided into nine divisions ranging from “very negative” to “neutral” to “very positive”. These anchors were selected so that respondents could rate the intensities of multiple affects in real time. Specifically, participants were told that “negative and positive can mean a lot of different things. In this context, we’d like you to consider ‘Positive’ as referring to any positive emotions such as amusement, contentment, happiness, or calmness, and ‘Negative’ as referring to any negative emotions such as sadness, anger, disgust, frustration, irritation, fear, or contempt”. The dial was attached to a potentiometer in a voltage dividing circuit that was monitored by the same computer that monitored the physiological data. Participants were instructed to adjust the dial position as often as necessary so that it always reflected how positive or negative they felt. Validity data for this rating dial procedure can be found in Gottman and Levenson, 1985 (see Fredrickson & Kahneman, 1993, for a similar real-time rating procedure).
Audiovisual
A remote-controlled high resolution colour video camera placed behind darkened glass behind a bookshelf was used to record participants’ (unobtrusively) facial behaviour and upper body movement.
Cardiovascular
Continuous recordings were made using a 12-channel Grass Model 7 polygraph connected to a Digital Electronics Corporation LSI-11/73 microcomputer. The resolution of the computer system was one millisecond for measures of time and one millivolt for measures of amplitude. Computer software developed in Levenson’s laboratory controlled the experiment and provided second-by-second averages of four cardiovascular measures. (1) Heart period (HP), Beckman miniature electrodes with Beckman electrolyte were placed in a bipolar configuration on opposite sides of the participant’s chest. The interbeat interval was calculated as the time in milliseconds between successive R waves in the electrocardiogram (ECG). (2) Pulse transmission time to the ear (PTE), a UFI photoplethysmograph was attached to the right ear. The interval was timed between the R wave of the ECG and the upstroke of the pulse wave at the ear. (3) Pulse transmission time to the finger (PTF), a UFI photoplethysmograph was attached to the distal phalange of the second finger of the nondominant hand. The interval was timed between the R wave of the ECG and the upstroke of the pulse wave at the finger. (4) Finger pulse amplitude (FPA), the trough-to-peak amplitude of each finger pulse was measured to assess the amount of blood in the tip of the finger.
This set of measures was selected to allow for continuous measurement, to be as unobtrusive as possible, and to sample broadly from the cardiovascular system. Whereas heart period is under both sympathetic and parasympathetic control, both finger pulse amplitude (an index of peripheral vasoconstriction), and pulse transmission times (indices of contractile force of the heart along with distensibility of the blood vessels; Newlin & Levenson, 1979) track processes mediated by the sympathetic nervous system. Pulse transmission times have also been shown to correlate with changes in blood pressure (Steptoe, Smylyan, & Gribbin, 1976).
Procedure
On arrival, participants were seated in a comfortable chair in a small well-lit room. Participants were told that the study was about people’s emotional reactions to various film clips, that they would be videotaped, that their bodily reactions would be monitored using physiologic al sensors, and that they would use a rating dial to indicate how they felt during the study. After participants signed a consent form, the experimenter attached the physiological sensors.
After a five-minute adaptation period, the experimenter returned to introduce the study in more detail. Participants were told that they would be watching film clips that would depict either positive, negative, or neutral events and that they should watch the video monitor at all times. They were also instructed in the use of the rating dial. Specifically, they were told that their task was to move the dial as often as necessary so it always reflected how positive or negative they were feeling moment-by-moment during the entire experimental session. Participants were given an opportunity to practise manipulating the dial without looking down at their hand. During the actual data collection, participants were alone in the room.
Following an additional three-minute adaptation period, participants were instructed to “relax, and empty your mind of all thoughts, feelings and memories”. This commenced a two-minute resting baseline period, the second minute of which was used as the pre-film rest period. Immediately following this resting baseline phase, all participants viewed the fear-eliciting Ledge film. Depending on experimental condition, this was followed one second later by either: (a) the Waves film (contentment), (b) the Puppy film (amusement), (c) the Sticks film (neutral), or (d) the Cry film (sad). The second film was followed by a 150-second post-film period during which the video monitor was blank.
At the end of the study, participants completed a number of questionnaire measures. Among these was an item asking them to describe how “interested” they were in each of the films that they viewed. Participants responded on a 9-point Likert scale ranging from 0 to 8.
Results
Overview of Analytic Strategy
We first confirmed that the initial fear film successfully induced negative emotion by comparing subjective and cardiovascular data obtained during the film to those obtained during the pre-film baseline. Next, we conducted a manipulation check to confirm that the secondary films altered subjective emotional experience as intended. We then used planned contrasts to test the hypothesis that attention to positive emotional stimuli speeds recovery from the cardiovascular activation generated by the initial negative emotion. Specifically, we compared the durations of cardiovascular reactivity for participants who viewed each of the two positive secondary films to the durations of cardiovascular reactivity for participants who viewed neutral or negative secondary films. This resulted in four pairwise comparisons. To correct for familywise Type I error, we used modified Bonferroni tests, setting alpha to .0375 for each planned comparison (Keppel, 1991).
Measuring the Duration of Cardiovascular Reactivity
To quantify the duration of cardiovascular reactivity to the initial fear film, we measured the time that it took for each participant’s cardiovascular indices to return to her own pre-film resting levels. Our aim was to create an individualised, time-based index of cardiovascular recovery sensitive to the temporal dynamics of the targeted cardiovascular variable s. To do this, for each participant, and for each measure of cardiovascular activity, we first calculated a baseline confidence interval to represent pre-film baseline levels, defined by that participant’s own 60-second pre-film mean, plus and minus one standard deviation of that mean. Cardiovascular reactivity is evidenced when cardiovascular levels escaped the baseline confidence interval (in either direction) during emotion-elicitation. The duration of cardiovascular reactivity is defined as the time elapsed after the offset of the initial fear film until cardiovascular levels returned to within an individual’s own baseline confidence interval and remained within this confidence interval for at least five of six consecutive seconds. Calculating duration as a return to (and remaining within) the baseline confidence interval helped assure that biphasic responses and responses in the opposite direction of the initial emotional response were not misclassified as recovery. In addition, this computational strategy allowed us to calculate the duration of cardiovascular reactivity for all participants who exhibited cardiovascular reactivity, regardless of the direction or magnitude of this reactivity. For those few participants who did not return to within their baseline confidence interval during the data collection period, duration values were considered missing.1
As in other studies using this time-based measure of the duration of cardiovascular reactivity (Fredrickson et al., submitted), duration measures were largely uncorrelated with measures of the peak magnitude of reactivity (r s = .28, .00, −.01, and −.06 for HP, PTE, PTF, and FPA, respectively; all n.s., except HP, for which P < .05), indicating that recovery time was not a simple reflection of initial response magnitude.
Finally, to create an aggregate index to reflect the overall duration of cardiovascular reactivity, for each participant, we calculated the mean duration score across those cardiovascular indices that exhibited significant change during the emotion-eliciting stimulus. (All nonmissing duration scores contributed to the aggregate index.)
Baseline Cardiovascular Activity
For each participant, we calculated mean levels (and standard deviations) for heart period (HP), pulse transmission times to the ear (PTE) and to the finger (PTF), and finger pulse amplitude (FPA) across the last 60 seconds of the resting baseline phase. The first two columns of Table 1 report the means across participants for these individualised baseline means and standard deviations. Baseline levels did not differ across the four experimental conditions (all F-values < 1, all n.s.).
TABLE 1.
Mean Subjective and Cardiovascular Levels during Pre-film Baseline and Fear Film across Participants (N = 60)
| Variable | Pre-film Baseline
|
Fear Film (83 sec)
|
|||
|---|---|---|---|---|---|
| Mean | SD | Mean | Peak | Latency to Peak (sec) | |
| RATE | 4.86 (0.85) | – | 3.31*** (0.98) | 2.57*** (1.28) | 54.40 (23.40) |
| HP | 784.00 (107.87) | 56.13 (25.80) | 804.44** (118.81) | 927.83*** (150.82) | 38.13 (27.35) |
| PTE | 186.32 (18.21) | 8.64 (4.08) | 183.63** (18.77) | 159.52*** (30.09) | 34.37 (26.11) |
| PTF | 260.31 (21.43) | 9.58 (5.20) | 260.60 (22.30) | – | – |
| FPA | 12.38 (5.87) | 1.36 (0.73) | 11.56** (5.95) | 8.37*** (4.76) | 40.92 (29.39) |
Note: RATE, rating dial; HP, heart period; PTE, pulse transmission time to the ear; PTF, pulse transmission time to the finger; FPA, finger pulse amplitude. Standard deviations (across subjects) are reported in parentheses. Results of paired t-tests (df = 59) comparing mean and maximum levels during fear film to pre-film baseline means are indicated with asterisks.
P < .05;
P < .01;
P < .001.
Subjective and Cardiovascular Responses to the Fear Film
For each participant, we calculated mean rating dial reports and cardiovascular activity averaged across the entire 83 seconds of the initial fear film. These mean values are presented in the third column of Table 1. We conducted within-subject t-tests to examine whether these means represented significant changes from pre-film baseline (see Table 1). Next, for those variables that showed reliable change (in either direction), we also determined peak responses during the fear film. These peak values and the mean latencies to achieve them (presented in the last columns of Table 1) provide a sharper picture of the mean magnitude of participants’ responses to the fear film.
As Table 1 shows, participants reported feeling significantly more negative during the fear film than during the pre-film baseline period, with rating dial reports dropping an average mean of 1.55 points, and an average maximum of 2.29 points (on a 0–9 scale). Participants also exhibited significant changes on three of the four cardiovascular indices during the fear film: (a) heart rate decreased, as indicated by an average mean increase in heart period of 20.44 m sec, and an average maximum increase of 143.83 m sec; (b) pulse transmission time to the ear decreased by an average mean of 2.70 m sec and an average maximum of 26.80 m sec; and (c) finger pulse amplitude decreased by an average mean of 0.82 millivolts (mV), and an average maximum of 4.01 mV. On average, participants showed no reliable mean changes on pulse transmission time to the finger. Further descriptive analyses confirmed that all participants exhibited cardiovascular reactivity (defined by escaping the baseline confidence interval) on heart period, pulse transmission time to the ear, and finger pulse amplitude during the fear film, and that no responses differed across the four experimental conditions (all F-values < 1.13, all n.s.).
Two of the cardiovascular changes evident during the fear film represent a pattern indicative of sympathetic arousal: Decreased finger pulse amplitude (peripheral vasoconstriction), and decreased pulse transmission time to the ear (increased contractility and/or decreased vascular distensibility; Newlin & Levenson, 1979). The deceleration in heart rate may be consistent with an orienting response (Obrist, 1981) and may be the result of watching films in general, or perhaps of watching suspenseful films in particular.
In sum, the fear film produced significant reports of negative subjective experience and significant changes on three of the four cardiovascular measures. These data, together with the pre-test data reported in Fig. 1a, suggest that the Ledge film was effective in inducing a negative emotional subjective state along with attendant cardiovascular activation.
Manipulation Check
We randomly assigned participants to view different secondary films in order to manipulate experimentally the subjective emotional experience introduced into the context of negative emotional arousal. To confirm that this manipulation worked, we examined group differences in rating dial reports averaged over the 100 seconds of the secondary films. Across all participants, the mean rating dial position during the secondary film was 4.42 on the 0–9 scale (SD = 1.70). An omnibus ANOVA confirmed that the four experimental groups differed on these subjective reports [F(3,56) = 23.54, P < .0001]. Inspection of Fig. 2 shows that group means for the two positive films are in the positive range of the dial scale, whereas those for the neutral and negative films fall in the negative range. One-sample z-tests confirmed that mean ratings for each group differ significantly from the midpoint of the rating dial scale (4.5, labelled “Neutral” ), and planned comparisons confirmed that the two positive films were rated as significantly more positive than both the negative and the neutral films (t-values, with df = 56, ranged from 4.02 to 7.30, all Ps < .001), and that ratings for the neutral and negative films differed significantly from each other [t(56) = 2.43, P < .05].
FIG. 2.
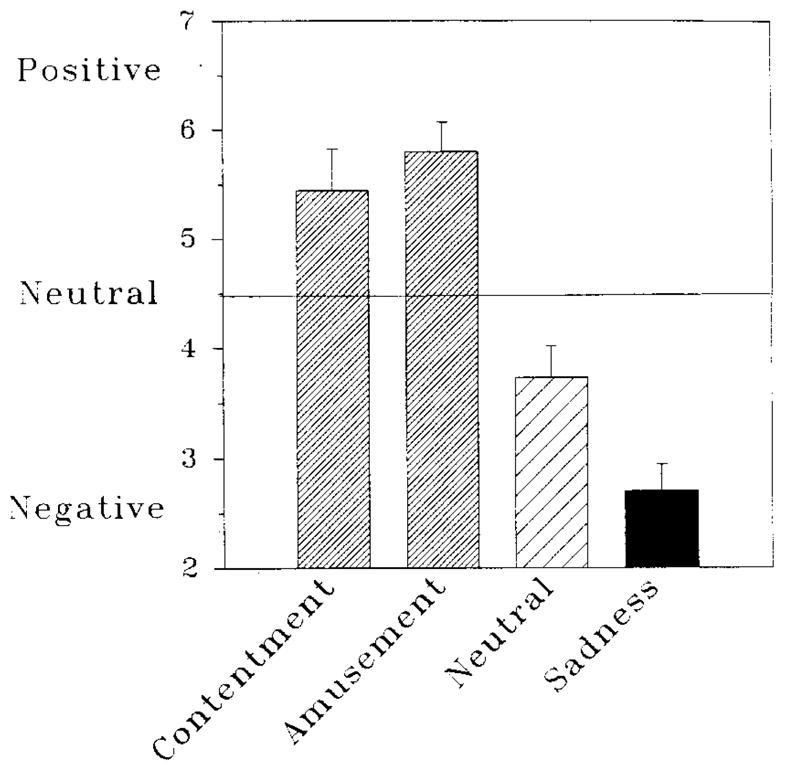
Mean reports of negative affect during each of the secondary films shown in Study 1. Groups are identified by the emotion featured in the second film viewed. Error bars represent standard errors of the means.
Duration of Cardiovascular Reactivity
Having confirmed both that the initial fear film induced negative emotion reports with attendant cardiovascular activation, and that the secondary films altered emotion reports as expected, we next tested our hypothesis that positive emotions would speed recovery from cardiovascular sequelae of a negative emotion. To do this we looked at the time elapsed after the offset of the fear film until the cardiovascular changes induced by that film subsided. We calculated the duration of cardiovascular reactivity for each participant individually using the cardiovascular aggregate described previously. Across all participants, the mean time to achieve recovery from cardiovascular reactivity was 33.59 seconds (SD = 27.29, N = 60).
Before conducting the specific planned comparisons that derive from our hypothesis, we first conducted an omnibus one-way ANOVA to protect against Type I error. This ANOVA yielded a significant main effect for group [ F(3,56) = 8.37, P < .001], with a relatively large effect size (omega-squared = .27; Keppel, 1991), indicating that the four experimental groups differed in their times to achieve cardiovascular recovery.
Planned Comparisons
Figure 3 illustrates the group differences in duration of cardiovascular response. The four planned comparisons confirmed our predictions about recovery times:2 Participants who viewed the contentment-inducing Waves film exhibited (1) faster returns to pre-film levels of cardiovascular activity than those who viewed the neutral Sticks film [t(26.8) = 2.29, P = .030] ; and (2) faster recovery than those who viewed the sad Cry film [t(26.0) = 3.78, P = .001]. Likewise, those who viewed the amusing Puppy film exhibited (3) faster returns to pre-film levels of cardiovascular activity than did those who viewed the neutral film [t(27.9) = 2.28, P = .030], and (4) faster recovery than those who viewed the sad film [t(22.3) = 3.83, P = .001].
FIG. 3.
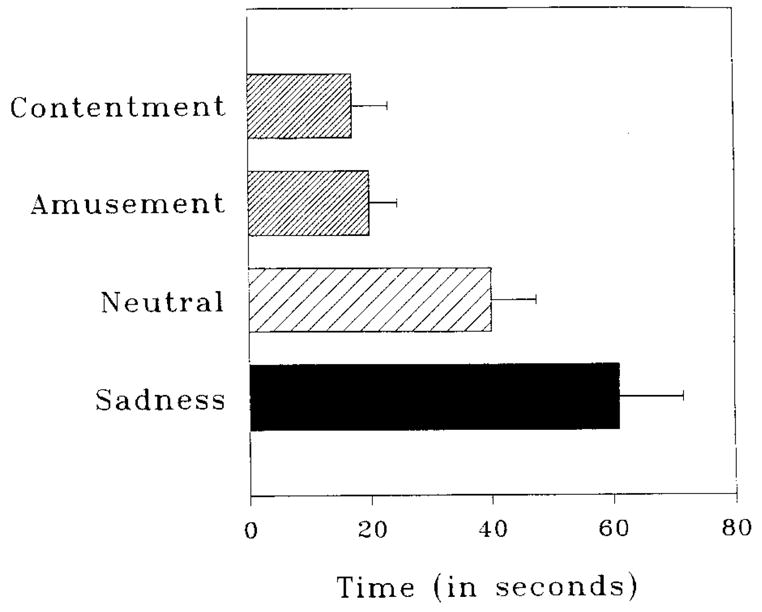
Mean time to achieve cardiovascular recovery in Study 1. Groups are identified by the emotion featured in the second film viewed. Error bars represent standard errors of the means.
As is typical with time-based data, the duration scores exhibited a positive skew, with standard deviations increasing with increasing means. To explore whether outliers accounted for the observed pattern of results, we repeated the analyses of duration scores using nonparametric tests on ranked data. The omnibus test and each planned pairwise comparison remained significant. We chose to present the analyses of raw duration scores to preserve the meaning inherent in time-based units.
Are Positive Films More Interesting than Negative or Neutral Films?
Given the indications that the Puppy and Waves films sped cardiovascular recovery, we considered the possibility that this might have resulted, not from their positive affective qualities, but simply because they were more interesting, and thus possibly more distracting, than the other secondary films. To explore this issue, we utilised the interest ratings participants provided at the end of the experiment. In terms of the 0–8 rating scale, across all secondary films, interest ratings were relatively low (M = 2.15, SD = 2.00). Importantly, the four experimental groups did not differ on these ratings [F(3,56) = 1.54, n.s.], arguing against this alternative explanation of the findings. Moreover, when we repeated the analyses reported earlier using interest ratings as a covariate, an identical pattern of results emerged.
Discussion
Data from Study 1 support the undoing hypothesis: Participants who viewed positive secondary films exhibited faster recovery from their cardiovascular arousal than those who viewed neutral or negative secondary films. Although these findings bolster our confidence in the undoing effect of positive emotions, they also raise a number of intriguing issues.
Recovery vs. Replacement
We have interpreted our data as indicating that positive stimuli speed recovery from negative emotional arousal. Another construal, however, is that participants have not recovered, but rather the initial negative emotion has been replaced by the subsequent positive emotion. The replacement view implies that the secondary film quickly takes over the reigns of the cardiovascular system, substituting its own pattern of activation for that produced by the initial fear film. The recovery view, in contrast, suggests that the initial film produces a pattern of activation that lingers on even after the secondary film has begun and then diminishes gradually. The distinction between these two interpretations is subtle, and not subject to definitive resolution solely on the basis of the data reported here. Although we prefer to interpret the data in terms of recovery rather than replacement, we recognise the viability of the alternative interpretation.
An example might convey the complexities of settling this matter. Recall that our definition of cardiovascular recovery required that activation on the indices of heart rate, pulse transmission time, and peripheral vasoconstriction return to within a confidence interval surrounding the pre-film resting level, and remain within that interval for at least five of six consecutive seconds. Using this analytic al strategy, we found that those who viewed positive films, on average, achieved a pattern of cardiovascular activation akin to their resting levels within the first 20 seconds of watching the positive film. At first consideration, this would clearly seem to point to “recovery”. If positive affect had “replaced” negative affect, we would expect to see the pattern of cardiovascular activation associated with positive affect to appear. If, however, the pattern of cardiovascular activation associated with positive affect is indistinguishable from resting levels, as is suggested by previous work on autonomic patterning in emotion (e.g. Levenson et al., 1990, 1991), then recovery and replacement would result in the same final cardiovascular state, thus rendering the distinction between the two explanations moot. It should be noted, however, that recovery and replacement are not mutually exclusive, and that some middle ground between the two models might best characterise the phenomenon. For instance, the transition moments between films might reflect a composite of both negative and positive physiological states.
Do Positive Emotions facilitate Recovery More Efficiently than “Nothing”?
In designing this study, we felt that the ideal control condition for evaluating the effects of positive emotions was to have participants engaged in a task that was perceptually comparable, with equivalent interest value, and yet bereft of emotion. Providing this isomorphism of perceptual demand seemed particularly important given the sensitivity of the autonomic nervous system to perceptual and attentional processes. The condition in the present study in which participants watched the affectively neutral Sticks film represented our best approx imation to this ideal control condition.
We considered at great length, and experimented with having a condition in which participants watched the initial fear-inducing film and then simply did “nothing” (e.g. watched a blank screen). In theory, such a condition might reveal the “natural” course of recovery. Yet, fortunately or unfortunately, people rarely (if ever) do nothing, perhaps especially when they are experiencing an emotion. Careful debriefing revealed that, absent a secondary film, pilot participants engaged in a number of different activities, many of which were explicitly designed to reduce the negative emotion produced by the film that just ended. For example, some participants tried to get their minds off the film, others continued to think about the film and their emotional reactions to it, and still others spontaneously created an analogue of our positive affect conditions, smiling or even laughing. Self-chosen coping strategies such as these have been shown to have profound influences on the duration of emotional experience (e.g. Cioffi & Hollow ay, 1993; Lazarus, Speisman, Mordkoff, & Davison, 1962; Nolen-Hoeksema & Morrow, 1991; Nolen-Hoeksema, Morrow, & Fredrickson, 1993).
For each of these reasons, we see the neutral film as providing the most appropriate experimental control condition. The question of whether positive emotions facilitate recovery more efficiently than “nothing” may be unanswerable. Even so, we should be able to learn more about whether variation in the ways people respond to negative emotion can alter the duration of the cardiovascular after-effects of negative emotions. Study 2 in this series moves in this direction.
Generality of Findings
Our second study was also motivated by a number of methodological features of this first experiment that limit the generality of the findings. First, Study 1 tested recovery from only one type of negative emotion. Second, the sample in Study 1, although ethnically diverse, was limited to college-aged women, and by consequence, generalisation of these findings to males and to different age groups has not yet been established. And third, Study 1 tests the restorative capacities of positive emotions using only films to elicit emotions. We chose films in effort to standardise, as much as possible, the modality and duration of the emotion eliciting stimulus. We would want to know whether similar results could be found with positive emotions elicited in other ways.
STUDY 2
People use multiple strategies of varying effectiveness to regulate their own moods and emotions (Nolen-Hoeksema et al., 1993; Stone, Kennedy-Moore, & Neale, 1995; Thayer, Newman, & McClain, 1994). Evidence that positive emotions speed recovery from the cardiovascular sequelae of negative emotions suggests that intermixing positive and negative emotions might be a particularly effective strategy for reducing the impact of negative emotions.
For instance, it is not unusual to see people smile during or following negative emotional experiences (Ekman, 1989). Such smiles have often been interpreted as social signals, ways that people regulate emotions interpersonally. Yet, these smiles might also offer people a means to modulate their own inner experiences of emotions. As William James put it over a century ago (1884/1983, p. 178, emphasis in original): “If we wish to conquer undesirable emotional tendencies in ourselves, we must assiduously, and in the first instant cold-bloodedly, go through the outward motions of those contrary dispositions we prefer to cultivate.”
Recent empirical evidence has demonstrated that facial expressions of emotion—and smiles in particular—do, in fact, alter inner physiological states: Using a procedure called the directed facial action task, Ekman and Davidson (1993; Ekman, Davidson, & Friesen, 1990) instructed individuals to contract certain facial muscles to produce smiles both with and without Duchenne’s marker of enjoyment (muscle contraction around the eyes) and observed that each elicited a distinct pattern of regional brain activity. Previous work has also demonstrated the capacity of directed facial actions to produce emotion-specific autonomic nervous system activity and, under certain conditions and with certain populations, subjective emotional experience (Ekman, Levenson, & Friesen, 1983; Levenson et al., 1990, 1991; Levenson, Ekman, Heider, & Friesen, 1992).
The aim of Study 2 was to provide a conceptual replication and extension of Study 1, this time using a more naturalistic union between negative and positive affects. We tested the hypothesis that people who, for whatever reasons, spontaneously smiled while viewing a sad film would recover more rapidly from the cardiovascular activation induced by that film than those who did not smile.
One thing to wonder is whether we can presume that when a person smiles, especially in a negative emotional context, that that person is experiencing a positive emotion. We think that this is an unnecessary presumption. Given the known connections between facial action and autonomic nervous system activation, it seemed reasonable to predict that facial configurations indicative of positive emotions, namely, smiles, might have the ability to speed recovery from the cardiovascular sequelae of a negative emotion, with or without accompanying changes in subjective experience.
Method
Participants
Seventy-two individuals between the ages of 20 and 35 (50% female) were recruited by a San Francisco-based survey research firm to serve as participants in this study. Although not ethnically diverse (all participants were Caucasian), this sample was constructed to represent the socioeconomic distribution of the San Francisco Bay area. Participants were paid $25 for participating in a one-hour study.
Visual Materials
A short film clip known to elicit sadness was used in this study. Emotional ratings for the clip, obtained using the same group-screening procedures as described in Study 1, are presented in Fig. 1f. The film clip [“Funeral”, drawn from the feature film Steel Magnolias (Stark, Stone, White, & Ross, 1989)], shows a woman at her adult daughter’s funeral, surrounded by a group of her women friends. She describes what it was like for her to be with her daughter as she died, and then begins to cry in outrage that her daughter’s life has ended (the subsequent humorous scenes were omitted). Examination of Fig. 1f reveals that this film primarily elicits self-reports of sadness, with lesser report of other emotions. The film clip is 205 seconds long and was presented with sound.
Apparatus
The rating dial, audiovisual, and cardiovascular measures were the same as in Study 1.
Procedure
The procedure for Study 2 was virtually identical to that used in Study 1. The single difference was that, after the pre-film rest period, all participants viewed only one film stimulus, the sad Funeral film. This film was follow ed by a 180-second post-film period during which the video monitor was blank. The entire session was videotaped.
Results
Overview of Analytic Strategy
As in Study 1, we first confirmed that the sad film successfully induced negative emotion by comparing subjective and cardiovascular data obtained during the film to those obtained prior to the film. From the video records, we coded the occurrence of smiles. Next, we tested the hypothesis that spontaneous smiling speeds recovery from the cardiovascular after-effects of negative emotion: Using the same strategies for quantifying the duration of cardiovascular reactivity as used in Study 1, we compared the durations of cardiovascular responding for participants who smiled to those for participants who did not smile.
Baseline Cardiovascular Activity
For each participant, we calculated mean levels (and standard deviations) for heart period (HP), pulse transmission times to the ear (PTE) and to the finger (PTF), and finger pulse amplitude (FPA) across the last 60 seconds of the resting baseline phase. The first two columns of Table 2 report the means across participants for these individualised baseline means and standard deviations. (Comparison across Tables 1 and 2 suggests that baseline values were generally comparable across the two studies.)
TABLE 2.
Mean Subjective and Cardiovascular Levels during Pre-film Baseline and Sad Film across Participants (N = 72)
| Variable | Pre-film Baseline
|
Sad Film (205 sec)
|
|||
|---|---|---|---|---|---|
| Mean | SD | Mean | Peak | Latency to Peak (sec) | |
| RATE | 5.13 (0.79) | – | 4.56*** (1.12) | 3.26*** (1.26) | 120.40 (78.32) |
| HP | 826.89 (115.50) | 50.82 (22.47) | 823.87 (107.54) | – | – |
| PTE | 186.84 (20.14) | 7.56 (4.78) | 189.04** (19.03) | 215.70*** (33.98) | 106.35 (63.35) |
| PTF | 260.93 (18.67) | 9.50 (3.27) | 266.52*** (17.45) | 303.31*** (25.91) | 98.40 (72.32) |
| FPA | 13.92 (5.14) | 1.72 (0.89) | 11.70*** (5.29) | 6.91*** (4.58) | 113.89 (69.14) |
Note: RATE, rating dial; HP, heart period; PTE, pulse transmission time to the ear; PTF, pulse transmission time to the finger; FPA, finger pulse amplitude. Standard deviations (across subjects) are reported in parentheses. Results of paired t-tests (df = 71) comparing mean and maximum levels during sad film to pre-film baseline means are indicated with asterisks.
P < .01;
P < .001.
Subjective and Cardiovascular Responses to the Sad Film
For each participant, we calculated mean rating dial reports and cardiovascular activity averaged across the entire 205 seconds of the sad film. These mean values are presented in the third column of Table 2. We conducted within-subject t-tests to examine whether these means represented significant changes from baseline levels (see Table 2). Next, for those variables that showed significant change, we also determined peak responses during the sad film. These peak values, presented in the fourth column of Table 2, provide a sharper picture of the mean magnitude of participants’ responses to the sad film.
As Table 2 shows, participants reported feeling reliably more negative during the sad film than during the pre-film baseline period, with rating dial reports dropping an average mean of 0.57 points, and an average maximum of 1.87 points. Participants also exhibited significant changes on three of the four cardiovascular indices during the sad film: (1) pulse transmission time to the ear increased by an average mean of 2.20 m sec, and an average maximum of 28.86 msec; (2) pulse transmission time to the finger also increased by an average mean of 5.56 m sec, and an average maximum of 42.38 m sec; and (3) finger pulse amplitude dropped by an average mean of 2.22 mV, and an average maximum of 7.01 mV. On average, participants showed no reliable mean changes in heart period. Further descriptive analyses confirmed that all participants exhibited cardiovascular reactivity on pulse transmission times to the ear and finger and on finger pulse amplitude, and that no sex differences were evident in either emotion ratings or cardiovascular responses during the sad film.
The pattern of cardiovascular change produced by the sad film reveals a rather complex response, with evidence of increased sympathetic nervous system arousal (decreased finger pulse amplitude) along side evidence of decreased sympathetic arousal (lengthened pulse transmission suggesting decreased cardiac contractility and/or increased vascular distensibility). Comparison across Tables 1 and 2 reveals that the pattern of cardiovascular change from baseline produced by the sad film is notably different from the pattern of change from baseline produced by the fear film in Study 1.3
In sum, the sad film produced reports of negative subjective experience and changes on three of the four cardiovascular measures. These data, together with the pretest data reported in Fig. 1f, suggest that the Funeral film was effective in inducing negative emotion and attendant cardiovascular activation.
Measuring Voluntary Smiles
To classify participants as “smilers” or “nonsmilers”, two trained coders (both female) examined the videotape recordings made of each participant. Reliability was established by having the two coders independently score the videotapes for 25 randomly selected participants. Coders tallied the number of times during the sad film that a participant’s lip corners turned up. Not surprisingly, participants differed widely in smile frequency, ranging from 0 to 19 smiles (mean 3.2, mode = 0). Fifty participants (46% female) smiled at least once, whereas 22 (59% female) never smiled. Inter-coder agreement on the dichotomous classification of participants as smilers or nonsmilers was 100%.
Descriptive analyses suggested that smilers and nonsmilers did not differ in their rating dial reports or cardiovascular activity during the pre-film baseline (t-values, with df = 70, ranged from 0.15 to 1.61, all n.s.). Nor did smilers and nonsmilers differ in their mean or maximum subjective and cardiovascular responses to the sad film (t-values, with df = 70, ranged from 0.37 to 1.28, all n.s.). The single exception was that smilers, on average, showed a larger maximum drop in finger pulse amplitude compared to nonsmilers [7.81 vs. 5.18 mV, respectively, t(70) = 2.59, P = .012]. Thus, on the whole, during the sad film, those who smiled did not differ substantially from those who did not smile on these aspects of emotional responding.
Duration of Cardiovascular Reactivity
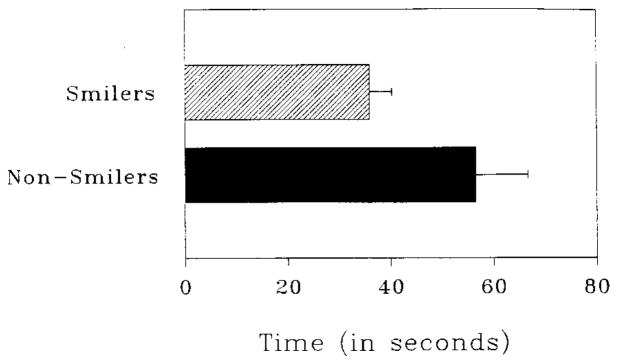
To test our hypothesis that spontaneous smiling would speed recovery from the cardiovascular after-effects of negative emotion, we looked at the time elapsed until the cardiovascular changes induced by the initial sad film subsided. Duration of cardiovascular reactivity was calculated for each participant individually using the methods described in Study 1. Across all participants, the mean time to achieve recovery from cardiovascular arousal was 42.19 seconds (SD = 37.57, N = 72).
To determine whether smilers and nonsmilers differed in the duration of cardiovascular reactivity, we conducted an omnibus ANOVA, using both smile group and sex of participant as between-subjects variables. This ANOVA yielded a main effect for smile group [F(1, 68) = 5.03, P = .028] of medium effect size (omega-squared = .05; Keppel, 1991) as well as for sex of participant [F(1, 68) = 4.86, P = .031, omega-squared = .05]. Figure 4 portrays the differences between smilers and nonsmilers in duration of cardiovascular response. Smilers recovered about 20 seconds faster than nonsmilers (35.9 vs. 56.4 sec, respectively); and men recovered about 20 seconds faster than women (31.8 vs. 52.6 sec, respectively). The interaction between smile group and sex of participant was not significant [F(1,68) < 1, n.s.], indicating that smiling predicted faster recovery equally well for women and men. Again, as for Study 1, to explore whether outliers might have accounted for this pattern of results, we repeated the analysis of duration scores with nonparametric tests on ranked data. The effects for smile groups and sex remained significant.
FIG. 4.
Mean time to achieve cardiovascular recovery in Study 2. Error bars represent standard errors of the means.
In sum, although smilers and nonsmilers were virtually indistinguishable in their reports of negative affect and cardiovascular activation during the sad film, once the sad film ended, those who had smiled reliably returned their own baseline levels of cardiovascular activation faster than those who had never smiled.
Discussion
Smiling during a sad film was associated with faster recovery from the cardiovascular changes occasioned by that sad film. Perhaps, then (to paraphrase William James), putting on a happy face can alleviate unhappiness, at least at the cardiovascular level. It is important to note, however, that the data reported here cannot tell us whether smiling per se was the critical ingredient that sped cardiovascular recovery. Our empirical strategy was to track naturally occurring facial actions to classify participants into one of two groups: smilers or nonsmilers. Other person variables could certainly covary with the tendency to smile in this type of context. For instance, high scores on measures of trait optimism, or trait cheerfulness, or low scores on measures of depression or trait hostility might predict the likelihood of smiling in this context. Further, smiling may be epiphenomenal, a marker of the process that is actually responsible for the restoration of emotional equilibrium (e.g. reappraisal of the sad event). Future experimental tests are needed to confirm whether the facial actions inherent in smiling per se promote speedier cardiovascular recovery.
Although not the intended focus of Study 2, the unanticipated finding that the duration of cardiovascular arousal was longer for women than for men warrants some discussion. First, it should be noted that the Funeral clip is unquestionably women-centred, and by consequence may have sparked more film-related thinking in female participants. Relatedly, considering that the emotion elicited was sadness, it is conceivable that the divergent recovery times reflect women being more likely than men to ruminate about the causes and consequences of their own sad moods (e.g. Nolen-Hoeksema et al., 1993). This ruminative style has been shown to prolong both naturally occurring and laboratory-induced sad moods (Morrow & Nolen-Hoeksema, 1990; Nolen-Hoeksema et al., 1993). Men, in contrast, have generally been found to withdraw more quickly than women from situations that produce negative emotion (e.g. Gottman & Levenson, 1988), possibly reflecting men’s greater sensitivity to physiological activation (e.g. Katkin, Blascovich, & Goldband, 1981; Pennebaker & Roberts, 1992; Roberts & Pennebaker, 1995) and their greater likelihood to report feeling emotionally negative when physiologically aroused (Levenson, Carstensen, & Gottman, 1994). Considering that affective disorders are more prevalent in women than in men, issues of sex differences in responses to negative emotion deserve further empirical attention.
GENERAL DISCUSSION
The research reported here was designed to provide initial tests of an alternative view of the effects that positive emotions might have on physiological systems. Basic to this view is an effort to examine the effects of positive emotions within the context of negative emotional arousal. Whereas certain negative emotions, through their association with specific action tendencies, reliably spark cardiovascular activation, certain positive emotions may function to quell this cardiovascular activation. The two studies represent two quite different contextual unions of negative and positive emotions and each has provided evidence consistent with the hypothesised undoing function of positive emotions.
Differences between Studies 1 and 2 add to the strength and generality of the empirical support. One key difference was that in Study 1 we tested recovery from fear, whereas in Study 2 we tested recovery from sadness. Not only are these two negative emotions experienced as qualitatively different (see Fig. 1) but they are also accompanied by somewhat different patterns of cardiovascular activation (cf. Tables 1 and 2; see Footnote 3). Nonetheless, in both emotional contexts, participants who experienced or expressed positive affect showed quickest recovery from whatever pattern of cardiovascular activation they had exhibited. Another key difference between the two studies is that Study 1 manipulated positive emotion experimentally, enabling inferences of causality, whereas Study 2 used an index of spontaneously occurring positive emotions, offering greater ecological validity. Further, in Study 1, we used carefully selected films to focus on two particular positive emotions, contentment and amusement, whereas in Study 2, we focused on the smile, which can mark any of a number of positive emotions. Finally, Study 2 revealed consistency in the undoing effect for both men and women. These and other variations across the two studies, demonstrate that the undoing effect of positive emotions shows some replicability across different emotional contexts and in different samples.
Implications for Emotion Theories
As noted in the Introduction to this article, emotion theories have generally had difficulty incorporating positive emotions within their general action-oriented models of the functions of emotions. The undoing hypothesis may point to a more fitting way to understand the adaptive value of positive emotions. If negative emotions promote the activation of a limited number of well-rehearsed, time-tested, adaptive actions along with their attendant physiological support, certain positive emotions can be seen as assuming a complementary role, efficiently restoring equilibrium to the organism both in terms of returning physiological activation to prior levels, and restoring psychological openness to a wide range of action possibilities.
This undoing effect of positive emotions connects to several lines of thinking about positive affect, albeit in indirect ways. The prediction that positive emotions speed internal homeostatic processes links to Cabanac’s research on the physiological role of sensory pleasure (1971 (1979), as well as to Solomon’s opponent-process theory of affect (1980). Cabanac (1971 Cabanac (1979) proposed that any external stimulus that corrects an “internal trouble” is experienced as pleasurable; a cool bath, for instance, is quite pleasant to someone who is hyperthermic, but quite unpleasant to someone who is hypothermic. Relatedly, Solomon’s (1980) opponent-process theory of motivation envisions a reflexive relation between emotional states, with every valanced affective state automatically evoking an affective state of opposite valence that serves to return the organism to a state of affective and biologic al neutrality (Solomon, 1980; Solomon & Corbit, 1974). Moreover, studies in behavioural medicine document the effectiveness of relaxation therapies for treating cardiovascular disorders (Blumenthal, 1985). Relaxation techniques vary greatly; although not typically discussed in terms of positive emotion, some techniques explicitly direct people to conjure up positive images (e.g. sunbathing at the beach), perhaps thereby capitalising on the undoing effects of contentment. Finally, at the level of behaviour, the idea that positive emotions can expand an individual’s options for action connects to Isen’s research on creativity. Isen and colleagues have suggested that “positive affect … facilitate(s) unusual responding rather than typical responding” (Isen, Daubman, & Nowicki, 1987, p. 1129), a finding that they use to relate positive affect to creative problem solving.
Implications for Health
We opened this paper by noting that research on negative emotions has far outpaced research on positive emotions. Although this may in part reflect the important role that negative events play in grabbing people’s attention (Pratto & John, 1991) and mobilising them for action (Taylor, 1991), it may also reflect our growing understanding of the ways that negative emotions can adversely affect physical and psychological health.
One particularly active arena of empirical work concerns the role that cardiovascular reactivity, occasioned by the negative emotional states of hostility, anger, and anxiety, plays in the aetiology of cardiovascular diseases such as coronary heart disease and essential hypertension (for reviews see Anderson, 1989; Blascovich & Katkin, 1993; Krantz & Manuck, 1984; Williams, 1991). Although precise mechanisms are only beginning to be pinpointed in humans, compelling research with nonhuman primates suggests that recurrent chronic activation of the sympathetic nervous system speeds atherosclerosis and impairs vascular responsiveness, thereby contributing to the development of cardiovascular disease (Kaplan, Manuck, Williams, & Strawn, 1993; see also Spense, Barnett, Manuck, & Jennings, 1996, for recent evidence with humans). Negative emotions are further implicated by striking parallels between group and individual differences in risk for cardiovascular disease and group and individual differences in cardiovascular reactivity to negative emotional stressors. Specifically, men (Matthews & Stoney, 1988), African-Americans (Anderson, McNeilly, & Myers, 1993), and individuals identified as hostile (Suarez & Williams, 1989) all have higher incidence of cardiovascular disease and all exhibit greater cardiovascular reactivity to laboratory stressors. Taken together, these findings provide the basis for asserting that negative emotions may have health-damaging consequences.
And what of positive emotions and health? It is important to note that data linking negative emotions and illness do not necessarily establish that positive emotions are associated with health. Nonetheless, the notion that positive emotions may be good for health dates back at least to biblical times—Proverbs 17: 22 advises that “a cheerful heart is a good medicine”. More recently, Norman Cousins’ (1979) chronicle of his battle with a serious collagen illness using humour and laughter kindled popular interest in this idea. Perhaps uncovering empirical footing for Cousins’ claim, an intriguing line of studies shows within-subject correlations between positive mood and secretory immune system functioning (Stone, Cox, Valdimarsdottir, & Jandorf, 1987; Stone, Neale, Cox, & Napoli, 1994). Nonetheless, empirical support for the health-promoting effects of positive emotions has been slow to accumulate. The mere possibility that individuals might increase control over their own physical health by cultivating experiences of positive emotion establishes a clear need for additional research to document the relationship between positive emotions and health and to explore possible mediating links.
Given the nature of the two studies reported here and the fact that no health indices were assessed, we can make no direct claims about the correlation between positive emotions and health outcomes. However, if such a relationship were to be established, the undoing effect of positive emotions would provide one possible mechanism by which it could be mediated. For instance, inherent in most models that have linked the cardiovascular reactivity associated with negative emotions to the development of cardiovascular diseases such as essential hypertension and coronary heart disease is the notion that sustained and chronic cardiovascular activation has a pathophysiological effect on the cardiovascular system. The finding that positive emotions shorten the duration of cardiovascular arousal produced by negative emotions suggests a potential for lessening the exposure of the cardiovascular system to these damaging effects. It also seems likely that any harmful effects of sustained cardiovascular activation associated with negative emotion will be cumulative, building up in small increments over time until some threshold is passed and the functioning of the cardiovascular system is compromised. It may be that positive emotions function to provide a momentary interruption in these purported pathophysiological processes, slowing the incremental progression toward disease, and thus functioning in the service of health.
Acknowledgments
This research was supported by a post-doctoral fellowship (NIMH 18931) awarded to the first author, NIA grant AG07476 and NIMH grant MH50841 awarded to the second author, as well as NIA grant AG08816 awarded to Laura L. Carstensen of Stanford University. We wish to thank Phoebe Ellsworth and Randy Larsen for helpful comments on earlier versions of this article.
Footnotes
For HP, no duration values were missing. For PTE, duration values for 4 of 60 participants (6.7%) were considered missing. For PTF, 2 of 60 (3.3%) were considered missing. For FPA, 4 of 60 (6.7%) were considered missing.
Because Levene’s test for homogeneity of variances was significant [F(3,56) = 3.00, P = .038], separate variance estimates for planned comparisons were used.
Mean change from baseline for HP was +20.44 (SD = 51.40) in Study 1, and −3.02 (SD = 33.25) in Study 2 [t(130) = 3.16, P < .01]. Mean change from baseline for PTE was −2.70 (SD = 7.34) in Study 1, and +2.20 (SD = 6.63) in Study 2 [t(130) = 4.02, P < .001]. Mean change from baseline for PTF was +0.29 (SD = 12.65) in Study 1, and +5.59 (SD = 11.84) in Study 2 [t(130) = 2.48, P < .05]. Finally, mean change from baseline for FPA was −0.82 (SD = 2.38) in Study 1, and −2.22 (SD = 3.54) in Study 2 [t(130) = 2.60, P < .01]. Although these different patterns of cardiovascular change evident during the fear-eliciting and sadness-eliciting films could be taken as evidence for emotion-specific ANS activity, it should be noted that the data reported here are not directly comparable to those previously reported from Levenson’s laboratory (e.g. Ekman et al., 1983; Levenson, 1992; Levenson et al., 1990). In particular, these earlier studies of autonomic differences among emotions found heart rate acceleration both for fear and sadness. However, these earlier studies did not use film clips to elicit emotions, but instead used directed facial actions and relived emotions. Moreover, targeted emotion episodes in this earlier work were only a few seconds long. In contrast, emotions elicited while viewing film clips unfold over a considerably longer time period. Importantly, in both the directed facial action and relived emotions tasks used in the earlier studies, the initiation of emotion came from processes within the subject (voluntarily moving facial muscles, reliving emotional memories). In contrast, films represent an emotional stimulus that originates outside of the subject. Viewing films involves the kinds of attention and intake of external information that reliably occasion heart rate decelerations characteristic of orienting and attentional responses. These decelerations may obscure heart rate accelerative effects found previously. Clearly, additional studies are needed to test these ideas; the two studies reported here were not designed or undertaken to test the autonomic specificity hypothesis. What is needed are studies in which film clips that elicit a full range of emotions are shown to research participants in a within-subject design. This would make it possible to remove the orienting and attentional cardiovascular effects common to all film viewing, thus allowing examination of whether previously found autonomic differences among emotions extend to emotions elicited in this manner.
Contributor Information
Barbara L. Fredrickson, University of Michigan, USA
Robert W. Levenson, University of California, Berkeley, USA
References
- Anderson NB. Racial differences in stress-induced cardiovascular reactivity and hypertension: Current status and substantive issues. Psychological Bulletin. 1989;105:89–105. doi: 10.1037/0033-2909.105.1.89. [DOI] [PubMed] [Google Scholar]
- Anderson NB, McNeilly M, Myers H. A biopsychosocial model of race differences in vascular reactivity. In: Blascovich J, Katkin ES, editors. Cardiovascular reactivity to psychological stress and disease. Washington, DC: American Psychological Association; 1993. pp. 83–108. [Google Scholar]
- Blascovich J, Katkin ES. Cardiovascular reactivity to psychological stress and disease. Washington, DC: American Psychological Association; 1993. [Google Scholar]
- Blumenthal JA. Relaxation therapy, biofeedback and behavioral medicine. Psychotherapy. 1985;22:516–530. [Google Scholar]
- Cabanac M. Physiologic al role of pleasure. Science. 1971;173:1103–1107. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- Cabananc M. Sensory pleasure. Quarterly Review of Biology. 1979;54:1–29. doi: 10.1086/410981. [DOI] [PubMed] [Google Scholar]
- Cioffi D, Holloway J. Delayed costs of suppressed pain. Journal of Personality and Social Psychology. 1993;64:274–282. doi: 10.1037//0022-3514.64.2.274. [DOI] [PubMed] [Google Scholar]
- Cousins N. The anatomy of an illness, as perceived by the patient. New York: Norton; 1979. [DOI] [PubMed] [Google Scholar]
- DeLaurentiis D, Schumacher M, Subots kM, Teague L. Cat’s Eye [Film] MGM/UA; 1985. [Google Scholar]
- Ekman P. The argument and evidence about universals in facial expressions of emotion. In: Wagner H, Manstead A, editors. Handbook of social psychophysiology. London: Wiley; 1989. pp. 143–164. [Google Scholar]
- Ekman P. An argument for basic emotions. Cognition and Emotion. 1992;6:169–200. [Google Scholar]
- Ekman P. Are there basic emotions? In: Ekman P, Davidson RJ, editors. The nature of emotion: Fundamental questions. Oxford: Oxford University Press; 1994. [Google Scholar]
- Ekman P, Davidson RJ. Voluntary smiling changes regional brain activity. Psychological Science. 1993;4:342–345. [Google Scholar]
- Ekman P, Davidson RJ, Friesen WV. The Duchenne smile: Emotional expression and brain physiology: II. Journal of Personality and Social Psychology. 1990;58:342–353. [PubMed] [Google Scholar]
- Ekman P, Friesen WV, Ancoli S. Facial signs of emotional experience. Journal of Personality and Social Psychology. 1980;39:1124–1134. [Google Scholar]
- Ekman P, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221:1208–1210. doi: 10.1126/science.6612338. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Kahneman D. Duration neglect in retrospective evaluations of affective episodes. Journal of Personality and Social Psychology. 1993;65:45–55. doi: 10.1037//0022-3514.65.1.45. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Maynard KE, Helms MJ, Haney TL, Siegler IC, Barefoot JC. Hostility predicts duration of blood pressure response to anger. (submitted) [DOI] [PubMed] [Google Scholar]
- Frijda NH. The emotions. Cambridge, UK: Cambridge University Press; 1986. [Google Scholar]
- Frijda NH, Kuipers P, Schure E. Relations among emotion, appraisal, and emotional action readiness. Journal of Personality and Social Psychology. 1989;57:212–228. [Google Scholar]
- Gottman JM, Levenson RW. A valid measure for obtaining self-report of affect. Journal of Consulting and Clinical Psychology. 1985;53:151–160. doi: 10.1037//0022-006x.53.2.151. [DOI] [PubMed] [Google Scholar]
- Gottman JM, Levenson RW. The social psychophysiology of marriage. In: Nollar P, Fitzpatrick MA, editors. Perspectives on marital interaction. Clevedon, UK: Multilingual Matters; 1988. [Google Scholar]
- Gross JJ, Levenson RW. Emotion elicitation using films. Cognition and Emotion. 1995;9:87–108. [Google Scholar]
- Isen AM, Daubman KA, Nowicki GP. Positive affect facilitates creative problem solving. Journal of Personality and Social Psychology. 1987;52:1122–1131. doi: 10.1037//0022-3514.52.6.1122. [DOI] [PubMed] [Google Scholar]
- James W. Essays in psychology: William James. Cambridge MA: Harvard University Press; 1983. What is an emotion? pp. 168–187. (Reprinted from Mind, 1884, 9, 188–205) [Google Scholar]
- Kaplan JR, Manuck SB, Williams JK, Strawn W. Psychosocial influences on atherosclerosis: Evidence for effects and mechanisms in nonhuman primates. In: Blascovich J, Katkin ES, editors. Cardiovascular reactivity to psychological stress and disease. Washington, DC: American Psychological Association; 1993. pp. 3–26. [Google Scholar]
- Katkin ES, Blascovich J, Goldband S. Empirical assessment of visceral self-perception: Individual and sex differences in the acquisition of heartbeat discrimination. Journal of Personality and Social Psychology. 1981;40:1095–1101. doi: 10.1037//0022-3514.40.6.1095. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis: A researcher’s handbook. 3. Englewood Cliffs, NJ: Prentice Hall; 1991. [Google Scholar]
- Krantz DS, Manuck SB. Acute psychophysiologic reactivity and risk for cardiovascular disease: A review and methodologic al critique. Psychological Bulletin. 1984;96:435–464. [PubMed] [Google Scholar]
- Lazarus RS. Emotion and adaptation. Cambridge, UK: Cambridge University Press; 1991. [Google Scholar]
- Lazarus R, Speisman JC, Mordkoff AM, Davison LA. A laboratory study of psychological stress produced by a motion picture film. Psychological Monographs. 1962;76:1–35. [Google Scholar]
- Levenson RW. Emotion and the autonomic nervous system: A prospectus for research on autonomic specificity. In: Wagner HL, editor. Social psychophysiology and emotion: Theory and clinical applications. London: Wiley; 1988. pp. 17–42. [Google Scholar]
- Levenson RW. Autonomic nervous system differences among emotions. Psychological Science. 1992;3:23–27. [Google Scholar]
- Levenson RW. Human emotion: A functional view. In: Ekman P, Davidson R, editors. The nature of emotion: Fundamental questions. New York: Oxford University Press; 1994. [Google Scholar]
- Levenson RW, Carstensen LL, Friesen WV, Ekman P. Emotion, physiology, and expression in old age. Psychology and Aging. 1991;6:28–35. doi: 10.1037//0882-7974.6.1.28. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Carstensen LL, Gottman JM. The influence of age and gender on affect, physiology, and their interrelations: A study of long-term marriage. Journal of Personality and Social Psychology. 1994;67:56–68. doi: 10.1037//0022-3514.67.1.56. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Ekman P, Friesen WV. Voluntary facial action generates emotion-specific autonomic nervous system activity. Psychophysiology. 1990;27:363–384. doi: 10.1111/j.1469-8986.1990.tb02330.x. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Ekman P, Heider K, Friesen WV. Emotion and autonomic nervous system activity in an Indonesian culture. Journal of Personality and Social Psychology. 1992;62:972–988. doi: 10.1037//0022-3514.62.6.972. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Gottman JM. Marital interaction: Physiological linkage and affective exchange. Journal of Personality and Social Psychology. 1983;45:587–597. doi: 10.1037//0022-3514.45.3.587. [DOI] [PubMed] [Google Scholar]
- Lovell D, Zeffirelli F. The Champ [Film] MGM/UA; 1979. [Google Scholar]
- Matthews KA, Stoney CM. Influences of sex and age on cardiovascular responses during stress. Psychosomatic Medicine. 1988;50:46–56. doi: 10.1097/00006842-198801000-00006. [DOI] [PubMed] [Google Scholar]
- Morrow J, Nolen-Hoeksema S. Effects of responses to depression on the remediation of depressive affect. Journal of Personality and Social Psychology. 1990;58:519–527. doi: 10.1037//0022-3514.58.3.519. [DOI] [PubMed] [Google Scholar]
- Newlin D, Levenson RW. Pre-ejection period: Measuring beta-adrenergic influences upon the heart. Psychophysiology. 1979;16:546–553. doi: 10.1111/j.1469-8986.1979.tb01519.x. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and post-traumatic stress symptoms after a natural disaster: The 1989 Loma Prieta earthquake. Journal of Personality and Social Psychology. 1991;61:115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J, Fredrickson B. Response styles and the duration of episodes of depressed mood. Journal of Abnormal Psychology. 1993;102:20–28. doi: 10.1037//0021-843x.102.1.20. [DOI] [PubMed] [Google Scholar]
- Obrist PA. Cardiovascular psychophysiology: A perspective. New York: Plenum; 1981. [Google Scholar]
- Pennebaker JW, Roberts T. Towards a his and hers theory of emotion: Gender differences in visceral perception. Journal of Social and Clinical Psychology. 1992;11:122–199. [Google Scholar]
- Pratto F, John OP. Automatic vigilance: The attention-g rabbing power of negative social information. Journal of Personality and Social Psychology. 1991;61:380–391. doi: 10.1037//0022-3514.61.3.380. [DOI] [PubMed] [Google Scholar]
- Roberts T, Pennebaker JW. Gender differences in perceiving internal state: Towards a his and hers model of perceptual cue use. In: Berkowitz L, editor. Advances in experimental social psychology. Vol. 27. 1995. pp. 143–175. [Google Scholar]
- Solomon RL. The opponent-process theory of acquired motivation: The costs of pleasure and the benefits of pain. American Psychologist. 1980;35:691–712. doi: 10.1037//0003-066x.35.8.691. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation: I. Temporal dynamics of affect. Psychological Review. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Spence JD, Barnett PA, Manuck SB, Jennings JR. Psychological stress and the progression of carotid atherosclerosis. Stroke. 1996;27:155. doi: 10.1097/00004872-199715010-00004. [DOI] [PubMed] [Google Scholar]
- Stark R, Stone A, White V, Ross H. Steel Magnolias [Film] TriStar Pictures; 1989. [Google Scholar]
- Steptoe A, Smylyan H, Gribbin B. Pulse wave velocity and blood pressure change. Calibration and applications. Psychophysiology. 1976;13:488–492. doi: 10.1111/j.1469-8986.1976.tb00866.x. [DOI] [PubMed] [Google Scholar]
- Stone AA, Cox DS, Valdimarsdottir H, Jandorf L. Evidence that secretory IgA antibody is associated with daily mood. Journal of Personality and Social Psychology. 1987;52:988–993. doi: 10.1037//0022-3514.52.5.988. [DOI] [PubMed] [Google Scholar]
- Stone AA, Kennedy-Moore E, Neale JM. Association between daily coping and end-of-day mood. Health Psychology. 1995;14:341–349. doi: 10.1037//0278-6133.14.4.341. [DOI] [PubMed] [Google Scholar]
- Stone AA, Neale JM, Cox DS, Napoli A. Daily events are associated with a secretory immune response to an oral antigen in men. Health Psychology. 1994;13:440–446. doi: 10.1037//0278-6133.13.5.440. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Williams RB., Jr Situational determinants of cardiovascular and emotional reactivity in high and low hostile men. Psychosomatic Medicine. 1989;51:404–418. doi: 10.1097/00006842-198907000-00004. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Asymmetrical effects of positive and negative events: The mobilization-minimization hypothesis. Psychological Bulletin. 1991;110:67–85. doi: 10.1037/0033-2909.110.1.67. [DOI] [PubMed] [Google Scholar]
- Thayer RE, Newman JR, McClain TM. Self-regulation of mood: Strategies for changing a bad mood, raising energy, and reducing tension. Journal of Personality and Social Psychology. 1994;67:910–925. doi: 10.1037//0022-3514.67.5.910. [DOI] [PubMed] [Google Scholar]
- Tooby J, Cosmides L. The past explains the present: Emotional adaptations and the structure of ancestral environments. Ethology and Sociobiology. 1990;11:375–424. [Google Scholar]
- Williams RB. A relook at personality types and coronary heart disease. In: Zipes D, Rowlands D, editors. Progress in cardiology. Philadelphia, PA: Lea & Febiger; 1991. pp. 91–97. [Google Scholar]