Abstract
In select Helicobacter pylori-infected populations with low gastric cancer, nematode coinfections are common and both helicobacter gastritis and filariasis are modeled in gerbils. We evaluated gastritis, worm counts, tissue cytokine gene expression levels and Th1/Th2-associated antibody responses in H. pylori and Brugia pahangi mono- and coinfected gerbils. H. pylori-associated gastritis indices were significantly lower 21 weeks post-infection in coinfected gerbils (p ≤ 0.05) and were inversely proportional to worm counts (r2 = −0.62, p < 0.003). Additionally, IFN-γ, IL-1β, CXCL1, IL-4 and IL-10 mRNA levels in the gastric antrum reflected a significant host response to gastric H. pylori and as well as systemic filariasis (p ≤ 0.05). Despite increasing worm burden (p < 0.05), gastritis progressed in coinfected gerbils (p < 0.03) becoming equivalent to H. pylori-infected gerbils at 42 weeks (p = 0.7). Pro- and anti-inflammatory mediator mRNA levels were notably downregulated in B. pahangi infected gerbils below uninfected control values, suggesting hyporesponsiveness to B. pahangi. Consistent with an increasing Th1 response to H. pylori, IgG2a (p < 0.01), IL-1β (p = 0.04) and CXCL1 (p = 0.006) responses significantly increased and IL-4 (p = 0.05) and IL-10 (p = 0.04) were decreased in coinfected gerbils at 42 weeks. Initial systemic responses to B. pahangi resulted in attenuated gastritis in coinfected gerbils, but subsequent filarid-associated hyporesponsiveness appears to have promoted H. pylori gastritis.
Keywords: Helicobacter pylori, Brugia pahangi, Gerbils, Gastritis
1. Introduction
Helicobacter pylori (Hp), a gram-negative bacterium that persistently infects the human stomach, is a major cause of gastritis and peptic ulcers, and may lead to cancer in susceptible hosts [1]. The prevalence of Hp infection is 25–50% among the developed world’s population and 70–90% in the developing world [2,3], although the incidence of the infection is decreasing in developed countries and some areas of the developing world primarily due to improved sanitation and the increased use of antibiotics [4]. Epidemiologic data and assessment of genetic polymorphisms of cytokine genes suggest a genetic predisposition to the development of pathologic changes [5]. Furthermore, of those individuals that become infected, only a small percentage develop ulcers or cancer [6].
The term ‘African enigma’ is used to describe the unexplained findings that in select developing populations of Africa, the prevalence of Hp-associated peptic ulcers or gastric cancer is very low despite >90% infection with Hp [7]. A proposed explanation in African human populations with high rates of Hp infection, but low gastric cancer incidence, is the common coinfection with nematodes such as Wuchereria bancrofti, the causative agent of lymphatic filariasis [8–10]. In a mouse model, the Th1 cytokine-mediated gastric inflammatory response to H. felis was attenuated by coinfection with Heligmosomoides polygyrus, a murine enteric nematode [9]. This finding was attributed to an amelioration of the predominantly Th1 response induced by H. felis and a polarization to a Th2 response induced by the parasitic infection. Supporting these experimental findings was a study performed in the developing populations of Colombia, South America where the Hp prevalence is >90%. A very high incidence of gastric cancer is noted in populations in some locales, whereas a low incidence of gastric cancer is noted in those residing in other areas [11]. A study of two distinct populations of Colombian children found that intestinal helminth infections promoted a Th2 immune response to Hp in the gastric cancer low risk population. It was hypothesized that parasitic infection in these children may decrease their risk of gastric cancer as adults by attenuating the progression of Hp gastric lesions [11].
In human populations with endemic filarial nematode infections, many individuals develop a predominant Th2 response accompanied by lack of clinical signs, hyporesponsiveness to the parasite, and chronic microfilaremia. In both human infections and in murine models, it has consistently been shown that the Th2 response to helminth infections is accompanied by a complex network of T regulatory cells, alternatively activated macrophages and other cells [12]. These responses not only regulate host responses to the helminth parasites but also bystander antigens [13], and in many cases, concomitant infections [9]. The Brugia pahangi (Bp)-infected gerbil serves as a model to mimic endemic filarial infection of humans [14–17]. Bp produces a chronic parasitism in the gerbil when injected subcutaneously, characterized by a microfilaremia and a Th2-mediated immune response [16,18,19].
The Mongolian gerbil also has proven to be a valuable model of helicobacter-induced gastric disease [20,21]. Hp induces a Th1 pro-inflammatory response and induces premalignant gastric lesions at 12–30 weeks post-infection [22], and in some studies, gastric cancer in chronically infected male gerbils at 62 weeks [23]. In this study we focused on coinfection of gerbils with Hp and Bp which provided an opportunity to study the interaction between bacterial and parasite-induced host immune responses and whether these interactions affect the severity of disease caused by Hp. We hypothesized that gastric pathology induced by Hp infection in gerbils would be modulated by Bp coinfection resulting in a decrease in the intensity of gastric pathology.
2. Materials and methods
2.1. Gerbils
Outbred male ~ 8-week old Mongolian gerbils (Meriones unguiculatus) were purchased from Charles River Laboratories, Inc (Wilmington, MA). These specific pathogen-free gerbils tested negative for lymphocytic choriomeningitis virus, Clostridium piliforme, Bordetella bronchiseptica, Mycoplasma pulmonis, Salmonella spp., Helicobacter hepaticus, Klebsiella pneumoniae, Klebsiella oxytoca, Pasteurella multocida, Pseudomonas aeruginosa, Streptococcus pneumoniae, beta-hemolytic Streptococcus spp., ectoparasites, endoparasites and enteric protozoa but were positive for Helicobacter bilis and Staphylococcus aureus. Gerbils were housed in microisolator caging in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited animal facility. Food (Prolab® RMH 3000 LabDiet) and water were provided ad libitum. Housing conditions were maintained between 68°–72 °F and 40–50% relative humidity. The Animal Care and Use Committees at both the Massachusetts Institute of Technology (MIT) and the Louisiana State University (LSU) approved the experimental protocols.
2.2. Experimental design
Eighty-five gerbils were randomized into 8 groups (n = 10–15) based on infection status (no infection, Bp only, Hp only, and coinfected HpBp) and duration of Hp infection (21 and 42 weeks post Hp infection (WPHPI)). Forty-five gerbils were infected with Bp and 40 gerbils were sham dosed. On day 56, 5 gerbils dosed with Bp were necropsied to confirm Bp infection. On days 56, 57, and 58 (approximately 8 weeks post-Brugia infection (WPBPI)), 40 gerbils were infected with Hp and 40 gerbils were sham dosed. Gerbils were euthanized and necropsied on days 203, 204, 350, and 351 post-Brugia infection which corresponded to time points of 21 and 42 WPHPI.
2.3. B. pahangi infection
Gerbils were shipped directly to LSU from the vendor for experimental Bp infection. After an acclimatization period of 2 weeks, 45 gerbils were infected with third stage larvae of Bp recovered from infected Aedes aegypti mosquitoes using the Baermann technique as previously described [24]. Fifty L3 larvae in 0.7 ml RPMI medium supplemented with penicillin, streptomycin, and sodium bicarbonate were injected subcutaneously in both sub-inguinal regions using 18 gauge needles for a total of 100 larvae per gerbil. The remaining 40 gerbils in the control and Hp only groups were sham injected with 0.7 ml RPMI harvested from the Baermann apparatus used to collect the L3 larvae, but confirmed to contain no larvae. Gerbils were then shipped to MIT for Hp infection and housing for the remainder of the experiment.
2.4. H. pylori infection
H. pylori SS1 was grown in Brucella broth (BB) containing 5% heat-inactivated fetal calf serum in vented jars containing N2, H2, and CO2 (80:10:10) under microaerobic conditions at 37 °C while shaking for 48 h. Hp was prepared for inoculation by centrifuging at 8000 rpm for 20 min at 4 °C and the bacterial pellet was resuspended in BB at a turbidometric reading of approximately 1.0 (optical density = 660 nm). A gram stain was performed and the bacterial culture was visualized under phase microscopy to ensure a live, uncontaminated culture. The inoculum was then passaged to a new flask of BB and 5% fetal calf serum for preparation of subsequent doses and each time was plated on blood agar and Glaxo plates to verify purity. Hp and HpBp gerbils were fasted approximately 12 h and orally gavaged with 0.2 ml of Hp suspension daily for 3 days. Sham and Bp animals were orally gavaged with 0.2 µl BB daily for 3 days.
2.5. Postmortem examination and tissue collection
At necropsy, gerbils were euthanized by CO2 inhalation, weighed and intracardiac blood was collected for verification of microfilaremia. The glandular portion of the stomach was collected aseptically and submitted for histopathologic analysis as well as flash frozen in liquid nitrogen and stored at −80 °C for RNA and DNA extractions for quantitative PCR (qPCR). Any abnormal gross findings were documented and additional tissues were collected for adult worm counts.
2.6. Confirmation of microfilaremia and adult worm counts
Twenty µl of anticoagulated blood were examined using a hemocytometer and light microscopy at 4X to confirm microfilaremia. Heart, lungs, testes, lymph nodes (inguinal, sub-iliac, renal, popliteal), iliolumbar lymphatics, and spermatic cords were isolated, teased apart and incubated in phosphate buffered saline for 30 min followed by collection and counting of emerging adult worms using a dissecting microscope at low power.
2.7. Histopathologic evaluation
Gastric samples were fixed in 10% neutral buffered formalin, embedded in paraffin and 4 mm sections were stained with hematoxylin and eosin or Alcian blue/PAS at pH 2.5. Samples were scored blind by a veterinary pathologist (SM) on a scale from 0 to 4 for inflammation, hyperplasia, epithelial defects, and dysplasia and scores combined to represent the gastric histological activity index (HAI) (maximum score = 16) using a previously published scoring system [25].
2.8. mRNA gene expression and H. pylori quantification using qPCR
DNA and RNA were extracted from gastric tissue using the Qiagen AllPrep DNA/RNA Mini Kit following the manufacturer’s protocol (Qiagen Inc, Valencia, CA). For cytokine gene expression, RNA was converted to cDNA using the Applied Biosystems High Capacity cDNA RT Kit (Foster City, CA). Each reaction mixture had a total of 5 mg RNA and a final volume of 35.5 µl including 5 µl 10× random primers, 5 µl 10 × RT buffer, 2 µl 100 mM dNTP, 2.5 ml RT enzyme and water. Reaction mixtures were placed in a 37 °C water bath for 2 h 150 µl TE buffer was added to each reaction and then stored at −20 °C until RT-PCR. The following cytokine gene expression levels were evaluated using previously published probe and primer sequences: IFN-γ, IL-1β, CXCL1, IL-4, IL-10, GAPDH [16,26,27]. Lyophilized primers were reconstituted to 100 mM with 100 µl water. A working solution was made of 8 µl forward primer, 8 µl reverse primer, 8 µl probe and 176 µl water. Five µl of cDNA or water as a control were added to duplicate wells in MicroAmp® Fast Optical 96-Well reaction plates (Applied Biosystems). Master mix consisting of 10 µl 2× Universal PCR mix, 1 µl probe/primer mix and 4 µl water per well was added and the plate run in the ABI Prism Sequence Detection System 7700 (Applied Biosystems). Transcript levels of each cytokine were normalized to the endogenous control GAPDH and expressed as a fold change. Hp quantization was performed using a probe and primers generated from the ureB gene of H. pylori SS1 as previously described in Ref. [28]. A standard curve was generated using 10-fold dilutions from 5 × 105 to 5 of H. pylori SS1 genome copies based on the average size of the two completely sequenced Hp genomes. Hp genome copy numbers per mg of gastric DNA were calculated.
2.9. Serum enzyme-linked immunosorbent assay (ELISA)
Serum was tested for Th1-associated IgG2a and Th2-associated IgG1 to outer membrane protein preparations of H. pylori using previously described methods [29]. Ninety-six well plates (Immulon 2, Thermo Fisher Scientific) were coated with 100 µl of outer membrane preps at 10 µg/ml in carbonate buffer (pH 9.6) and incubated overnight at 4 °C. Wells were blocked with 200 µl PBS/2% bovine serum albumen and incubated for 1 h at 37 °C. Serum samples diluted 1:100 were incubated 1 h at 37 °C. Secondary antibodies were polyclonal, biotinylated goat anti-mouse antibodies diluted 1:2000 for detection of gerbil IgG2a and IgG1 (Southern Biotech, Birmingham, AL) as previously reported [30]. Incubation at 37 °C for 1 h with 100 µl per well of secondary antibody was followed by a 30 min incubation with 100 µl per well extravidin-peroxidase (Sigma) at 1:2000. 100 µl ABTS substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD) per well was used for color development. Absorbance was recorded at 405/592 nm using an ELISA plate reader (Dynatech MR7000, Dynatech Laboratories, Inc., Chantilly, VA).
2.10. Statistics
Results were compared using the Mann–Whitney nonparametric test except that the correlation of the gastric HAI with the number of adult worms was evaluated by linear regression. p values ≤0.05 were considered statistically significant.
3. Results
3.1. Gross pathology
At 21 WPHPI, Hp monoinfected gerbils consistently had severe pathology with visible gross changes in gastric tissue seen in all animals, ranging from moderate to severe thickening and pale color of the glandular epithelia. A majority of HpBp infected gerbil stomachs had minimal to moderate thickening, though a minority appeared grossly normal. Sham and Bp only gerbils had gastric tissues with minimal gross changes. By 42 WPHPI, only Hp and HpBp infected gastric tissues had gross changes from moderate to severe thickening and pale color. Stomachs from sham and Bp only infected gerbils were grossly normal.
3.2. Gastric histopathology at 21 WPHPI
The gastric mucosa of all animals at both time points was assessed for histopathological alterations in both the corpus (body) and the antrum. Of note, the gastric corpus of most Hp infected gerbils exhibited an antral-type mucosal phenotype due to the inconsistent ringing of oxyntic glands in the corpus similar to that described in mice [31]. Quantitative histological scoring was therefore restricted to the antrum for inflammation, epithelial defects, hyperplasia and dysplasia whereas the corpus was assessed only qualitatively for pathological changes.
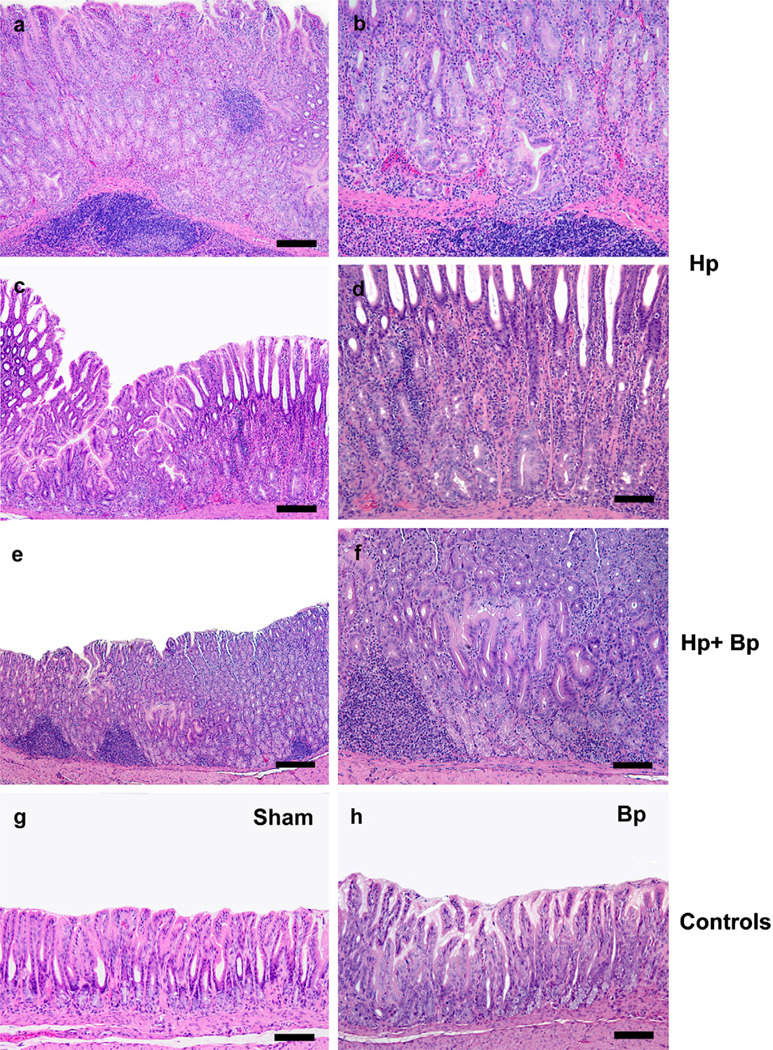
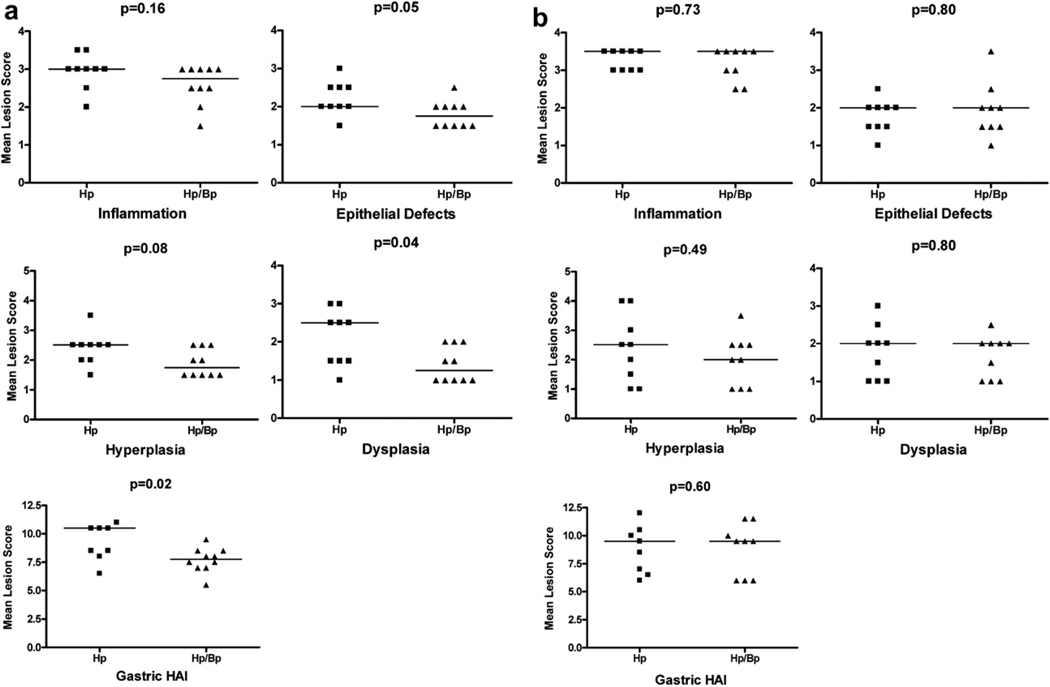
The gastric mucosa of Hp infected animals exhibited a consistent chronic antral predominant gastritis with prominent hyperplasia and variable dysplasia and mild variable extension of the lesions proximally into the adjacent distal corpus. The cellular component of the inflammatory process was composed of an admixture of lymphocytes, macrophages and granulocytes (neutrophils and eosinophils) in varying numbers with lympho-follicular formation (Fig. 1a–d). Of note, Bp coinfection suppressed gastric antral pathology in Hp infected gerbils at 21 WPHPI. The stomach of Hp monoinfected gerbils had significantly higher gastric epithelial defects (p ≤ 0.05), dysplasia (p = 0.04) and overall gastric HAI (p = 0.02) as compared to the HpBp group (Fig. 3a). Occasionally, there was distinct mucosal and submucosal lympho-follicular formation (Fig. 1a) that was frequently seen in the Hp monoinfected group. There was also a trend for increased hyperplasia in the Hp group compared with the HpBp group (p = 0.08) (Fig. 3a). In severely hyperplastic regions in the Hp monoinfected gerbils, the epithelium frequently exhibited villo-papillary transformation (Fig. 1c). Dysplastic changes were more prominent in the Hp monoinfected gerbils and these included multifocal superficial and basal glandular architectural distortion, loss of columnar orientation, closely packed glands, increased cytological atypia and/or focal high grade glandular atypia (Fig. 1a–d). These changes were also observed in the HpBp infected group but usually to a lesser degree and extent of mucosal involvement (Fig. 1e, f). Histological changes observed in sham and Bp only groups were minimal to none (Fig. 1g, h).
Fig. 1.
Representative H&E images of various groups at 21 WPHPI. Hp monoinfected gerbils had moderate to severe mucosal and submucosal inflammation with occasional distinct lympho-follicular formation (1a, c: 160 µM; b, d: 80 µM), severe glandular and foveolar hyperplasia with prominent villo-papillary epithelial transformation (1a, c) and superficial and basal glandular dysplasia (1, µM and 1c, 160 µM). In HbBp infected gerbils at 21 WPHPI, there was mild to moderate inflammation, mild to moderate glandular hyperplasia and mild glandular dysplasia (1e, 400 µM and 1f, 80 µM). In Sham controls and Bp monoinfected gerbils, the mucosa was mostly within normal limits (1g, h, 80 µM).
Fig. 3.
Effect of HpBp coinfection on select features and total histological activity indices (HAI) of the gastric antrum pathology compared with Hp mono-infection. a) 21 WPHPI, b) 42 WPHPI. p ≤ 0.05 defined as statistically significant.
3.3. Gastric histopathology at 42 WPHPI
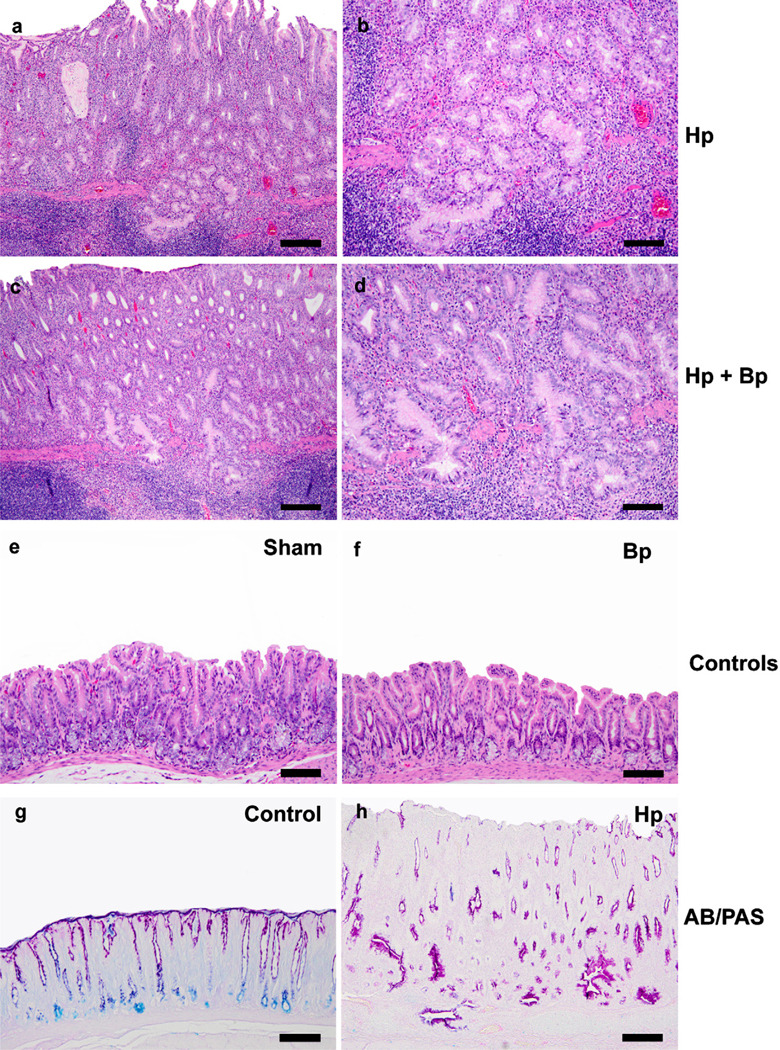
By 42 WPHPI, lesion scores for gastric mucosal inflammation, epithelial defects, hyperplasia, and dysplasia were not significantly different between the Hp and HpBp groups (Figs. 2a–d, 3b). Of note was the frequent herniation of the metaplastic (mucous/globoid change) and dysplastic glands into the prominent submucosal de novo lymphoid follicles in the stomach of both the Hp and HpBp groups (Fig. 2a–d). As observed at 21 WPHPI, histologic changes in the sham and Bp groups were minimal to none (Fig. 2e, f). By Alcian blue/PAS staining, the gastric antrum of sham control animals showed prominent blue (acidic mucin, intestinal) staining in the basal glands and red-purple staining (gastric type, neutral mucins) in the apical brush borders of the foveolar epithelium (Fig. 2g). In the Hp infected groups, the hyper- and metaplastic foveolar epithelium and glands were lined by tall columnar epithelium that showed a predominant expression of neutral (gastric-type) mucins (red/purple) in the apical cytoplasm and along the luminal brush-border-like surfaces. Rarely, glands showed mild intestinal acidic mucin (blue) expression (Fig. 2h). Intestinal-type goblet cells were rare and there was an associated loss of acidic mucin (intestinal) expression that is normally present in the basal antral glands. The hyperplastic and metaplastic changes were primarily of gastric-type foveolar and glandular epithelium. Additionally, the overall gastric HAI was more or less comparable in the Hp mono-infected animals at both time points (21 and 41 weeks) though a significant progression in disease severity was noticed in the HpBp group with higher median gastric HAI scores at 42 weeks vs. 21 weeks (Fig. 3a, b).
Fig. 2.
Representative H&E images of various groups at 42 WPHPI. Both the Hp only (2a, b: 160 µM) and Hp Bp infected animals (2c, d: 80 µM) exhibited severe mucosal inflammation with lympho-follicular formation, glandular hyperplasia, glandular mucinous and/or globoid cytoplasmic change and moderate to severe glandular dysplasia with frequent herniation of the dysplastic glands into the submucosal lymphoid aggregates. In sham and Bp only infected controls, the mucosal changes were none to minimal. On Alcian blue/PAS staining, pH 2.5, the stomach of Hp infected gerbils at WPHPI showed predominant cytoplasmic gastric type (neutral) red mucin expression within the hyperplastic and metaplastic/dysplastic foci with loss of intestinal acidic mucin expression (blue staining) in basal glands as usually seen in the controls (2 g, h, 160 µM). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
3.4. Th1- and Th2-associated cytokine gene expression levels were elevated in the gastric antrum from mono- and coinfected gerbils
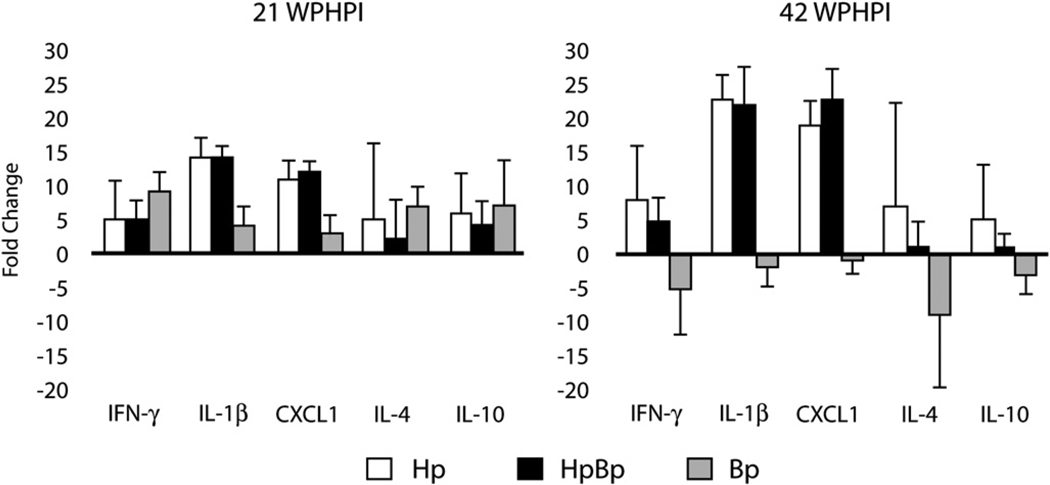
At 21 WPHPI, the Hp and HpBb infected, and notably the Bp infection group, had elevated mRNA expression levels for IFN-γ, IL-1β, CXCL1, IL-4 and IL-10 with a mean change of 2-fold or higher in comparison to gastric antrum from uninfected gerbils (Fig. 4). Comparison of Bp with Hp and HpBp infected gerbils at 21 WPHPI indicated the latter two had statistically higher mRNA levels of pro-inflammatory IL-1β (p = 0.04, p = 0.01, respectively) and CXCL1 (p = 0.04, p = 0.007, respectively).
Fig. 4.
Pro-inflammatory (IFN-γ, IL-1β, CXCL1) and anti-inflammatory (IL-4, IL-10) gene expression measured in gastric antrum by RT-PCR at 21 and 42 WPHPI in gerbils. Values measured from uninfected gerbils (not shown) served as the baseline for fold-change in gene expression levels and were normalized against GAPDH.
At 42 WPHPI, Hp and HpBp infected gerbils had progressively higher, but similar, increases in pro-inflammatory IFN-γ, IL-1β, and CXCL1 and although variable, some Hp infected gerbils had notably elevated anti-inflammatory IL-4 and IL-10 mRNA levels in the gastric antrum compared to control samples. Of considerable interest was that mRNA cytokine levels in the gastric antrum of Bp infected gerbils were downregulated in all inflammatory and anti-inflammatory mediators when compared to control tissues.
While significant differences between mRNA cytokine levels in Hp and HpBp infected gerbils were not seen at 21 or 42 WPHI, there was an increase in pro-inflammatory IL-1β (p = 0.02) and CXCL1 (p = 0.01) in gastric tissue samples from Hp monoinfected gerbils between 21 and 42 WPHPI. Levels from HpBp infected gerbils also progressed significantly higher in IL-1β (p = 0.04) and CXCL1 (p = 0.006) and lower in mRNA expression for the Th2-associated cytokines IL-4 (p = 0.05) and IL-10 (p = 0.04) between 21 and 42 WPHPI. In the Bp infected gerbils there was downregulation compared to control values in IFN-γ (p = 0.005) and IL-10 (p = 0.0001) at 42 WPHPI compared to 21 WPHPI.
3.5. Serum IgG2a and the IgG2a/IgG1 ratio increased at 42 WPHPI in coinfected gerbils
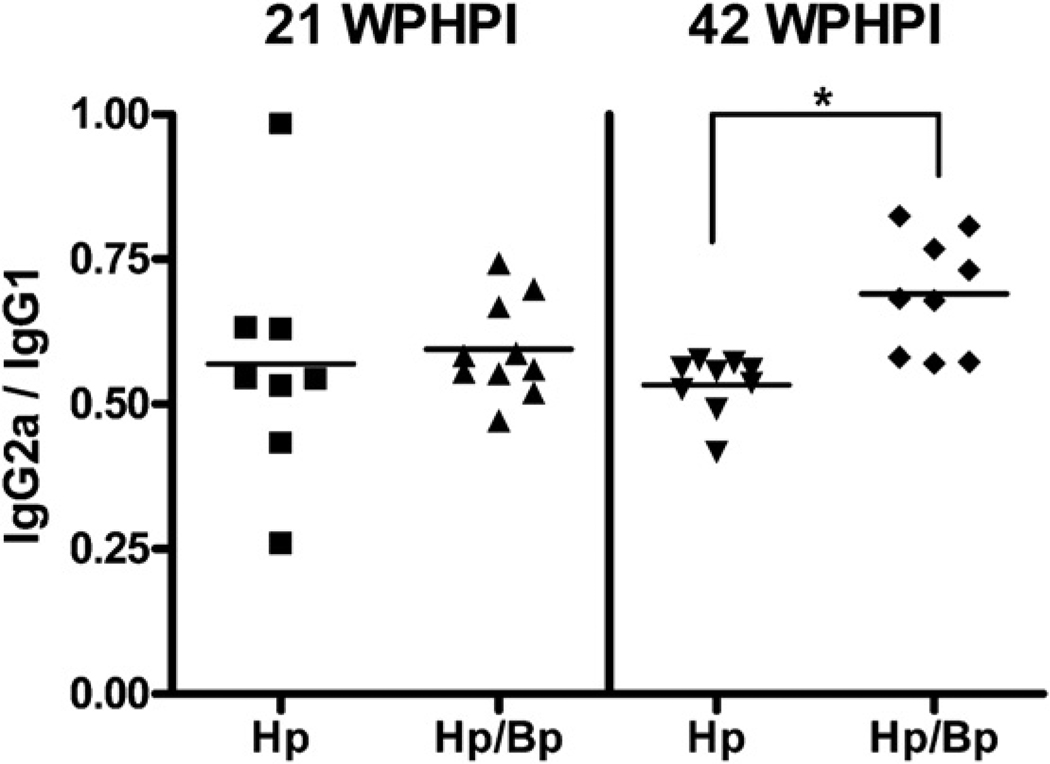
All gerbils dosed with Hp seroconverted to Hp outer membrane antigens by 21 WPHI and IgG2a and IgG1 levels minimally increased through 42 WPHPI (data not shown). The Th1-associated IgG2a response in HpBp infected gerbils was similar to Hp infected gerbils at 21 WPHPI (p = 0.5) but was significantly increased in the HpBp gerbils at 42 WPHI (p < 0.01). The Th2-associated IgG1 responses to Hp were similar amongst both Hp and HpBp infected gerbils at both time points (p = 0.6). When analyzed as ratios of IgG2a to IgG1 responses to Hp, data from Hp and HpBp infected gerbils were similar at 21 WPHPI but significantly higher in HpBp coinfected gerbils at 42 WPHPI (p = 0.0003) (Fig. 7).
Fig. 7.
Serum Th1-associated IgG2a was compared to Th2-associated IgG1 specific for Hp antigens as a ratio. At 42 WPHPI there was a significant increase in the ratio of IgG2a to IgG1 in the HpBp coinfected gerbils. *p = 0.0003.
3.6. Hp colonization and worm counts were both negatively correlated with gastric antral pathology at 21 but not at 42 WPHPI
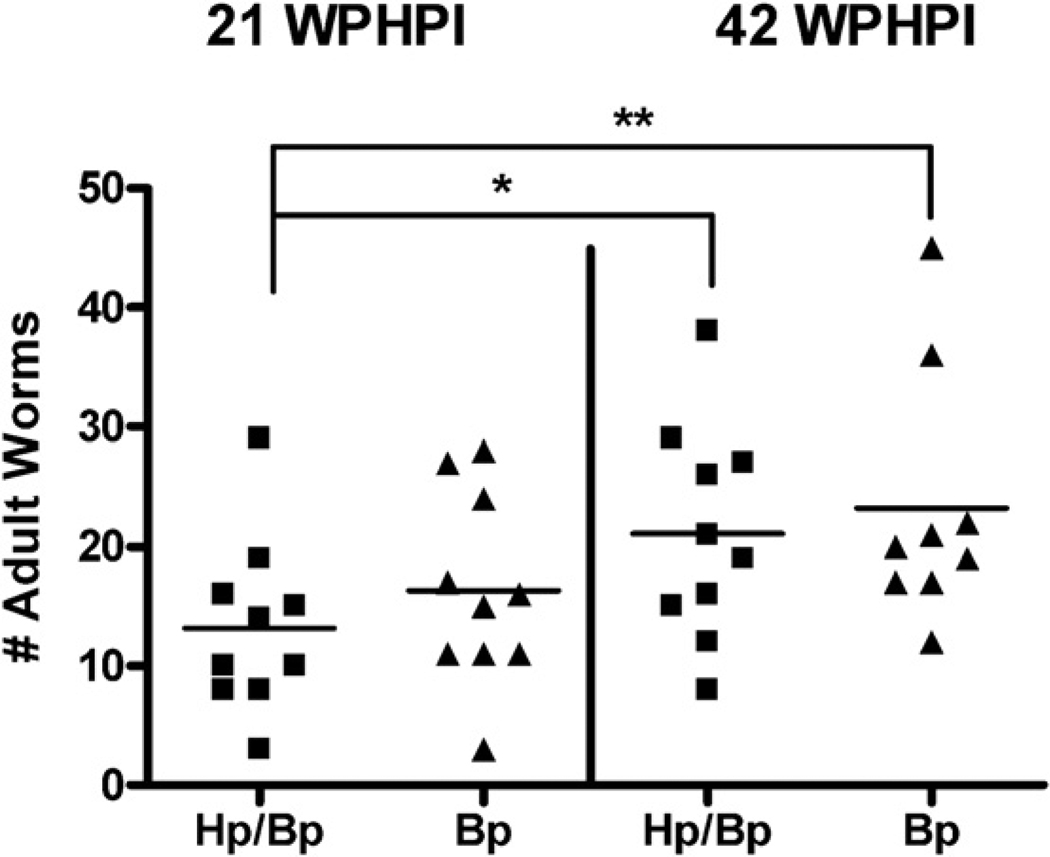
At 21 and 42 WPHPI, microfilaria were noted in blood smears obtained from all gerbils dosed with Bp and were not observed in blood preparations from sham or Hp only gerbils. Adult stage Bp filarids were harvested from all microfilaremic gerbils and were equivalent in number in HpBp and Bp infected gerbils at 21 WPHPI (p = 0.38) and 42 WPHPI (p = 0.64). The number of adult Brugia filarids increased between 21 and 42 WPHPI in HpBp gerbils (p < 0.05) with a trend for increased Bp counts in Bp gerbils (p = 0.12) (Fig. 5).
Fig. 5.
Total adult worm burden per gerbil infected with Bp isolated from several lymphoid and lymphatic tissues by mechanical disruption. Worm counts were similar at 21 and 42 WPHPI but increased between 21 and 42 WPHPI in HpBp infected gerbils. *p < 0.05, **p = 0.12.
Hp colony counts in the antrum were quantified by qPCR and evaluated by regression analysis for a relationship with adult worm burdens and gastric HAI scores. Colonization levels of Hp were similar in Hp and HpBp infected gerbils at 21 WPHPI (9.04 × 105, 9.55 × 105 CFU equivalents, respectively) and at 42 WPHPI (7.12 × 105, 2.28 × 105 CFU equivalents, respectively) with a trend for slightly lower Hp colonization levels between time points that was not significant for either Hp or HpBp infected gerbils (p = 0.07, p = 0.15, respectively).
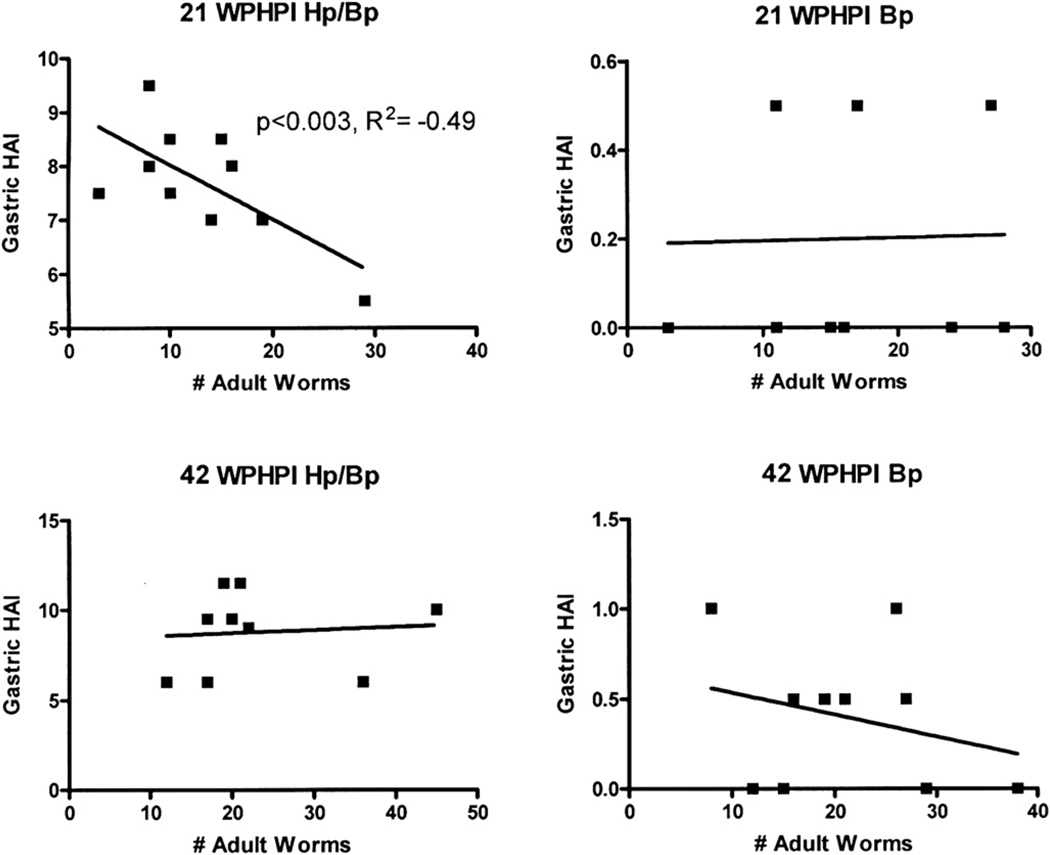
At 21 WPHPI, regression analysis of Hp colony counts from the antrum and Bp counts from multiple tissues indicated significant effects of both infections on gastric HAI scores. There was a negative correlation between Hp colonization levels and HAI scores (r2 = −0.49, p < 0.02) and between Bp counts and HAI scores (r2 = −0.62, p < 0.003) (Fig. 6). There was no significant interaction between Hp and Bp infections on HAI scores. At 42 WPHPI, Hp colonization levels remained negatively correlated with HAI scores but this inverse correlation was no longer significant (r2 = −0.34, p = 0.42). Additionally, although Bp counts were higher in HpBp gerbils at 42 WPHPI compared to 21 WPHPI, the impact of worm burden on gastric antrum HAI scores also was no longer significant (p = 0.82) and had a low positive correlation (r2 = 0.24). Thus, at 21 WPHPI lower pathology scores were associated with higher Hp colonization and higher worm burden, with no similar relationship persisting through 42 WPHPI.
Fig. 6.
Regression analysis of total adult Bp worm counts vs. total gastric histological activity indices (HAI). An inverse linear relationship is evident at 21 WPHPI in the HpBp infected gerbils; as the number of adult worms increased, gastric histopathologic indices decreased in individual coinfected gerbils. This inverse relationship was lost by 42 WPHPI. In Bp monoinfected gerbils, gastric HAI were minimal at 21 and 42 WPHI and thus the data reflects no relationship between Bp worm counts in Bp monoinfected mice and gastric HAI scores.
4. Discussion
The Mongolian gerbil model proved to be useful in assessing the long-term effect of Brugia filariasis on Hp-induced gastritis. Notably, the pathogenesis of Brugia infections in gerbils of this duration has not been previously reported nor have the effects of helminth infections been evaluated in the Hp infected gerbil, one of the few rodent models that progress from gastritis to dysplasia and gastric cancer (22, 23). An antral predominant gastritis was consistently noted in Hp infected gerbils as reported by others [27] and previously by our laboratory [32]. An amelioration of premalignant gastric lesions was noted in the stomach of coinfected gerbils at 21 WPHPI when compared to the Hp mono-infection. The fact that histologic inflammation, i.e. presence of inflammatory cells, did not differ between H.p. infected vs. coinfected gerbils is consistent with our earlier study where the inflammation score did not differ in H. felis infected mice (16 weeks) vs. coinfected mice H. felis/H. polygyrus [8]. Importantly, however, like our coinfected gerbils, coinfected mice had significantly attenuated gastric premalignant lesions at this time point. Interestingly, at 21 WPHPI there was an inverse correlation between the severity of gastric lesions, Hp colonization levels and the number of adult worms present. An inverse relationship between the gastric helicobacter colonization levels and degree of gastritis was also noted in mice coinfected with H. polygyrus and H. felis [8]. In a similar manner, an increased Bp burden in gerbils was associated with a downregulation of pulmonary granulomatous inflammation in response to sepharose bead emboli and decreased splenic lympho-proliferative responses to Brugia antigen [15]. However, the ameliorating effect of helminth coinfection was lost in the present study by 42 WPHPI. Upregulation of mRNA expression levels for anti-inflammatory mediators at 21 WPHPI followed by downregulation at 42 WPHPI in Bp infected gerbils suggests initial systemic anti-inflammatory responses to B. pahangi may have contributed to attenuation of H. pylori gastritis. In contrast, ensuing filarid-associated hyporesponsiveness appears to have subsequently promoted H. pylori gastritis.
The decreased severity of lesions at 21 WPHPI in HpBp infected gerbils followed by equivalent gastric lesions at 42 WPHPI in Hp and HpBp infected gerbils correlated with a shift in the cytokine response in the stomach from a mixed Th1/Th2 profile at 21 WPHPI to a progressively higher Th1-type response and a muted Th2 response by 42 WPHPI. The Th1 profile at 42 WPHPI was characterized particularly by increased mRNA expression for IL-1β and decreased expression for IL-4 and IL-10 in HpBp infected gerbils. The loss of a Th2 immune response, as most noticeably observed in the Bp infection group in which mRNA levels were below control values, has been previously reported to result from prolonged Bp infection in other gerbil studies. Notably, Bp infection for 4 weeks produced a hyper-responsiveness to Bp antigen followed by decreased granulomatous inflammatory and immune proliferative responses to Brugia antigen by 90 days postinfection [33]. Additionally, an upregulated immune response produced by Bp infection was abated by 20 weeks and was associated with a nonspecific downregulation of inflammatory responses [24]. The time points of our study were substantially longer and the downregulated Th2-associated cytokine responses may explain the decreased protective effect against H. pylori gastritis in the coinfected gerbils at 42 WPHPI. Additional evidence for Bp-associated host hyporesponsiveness in our model was the increased adult worm burden at 42 WPHPI in HpBp infected gerbils. Furthermore, Hp colonization levels were similar in Hp and HpBp infected gerbils at 42 WPHPI, consistent with similar levels of chronic gastritis.
At 21 WPHPI, mRNA expression levels for the pro- and anti-inflammatory mediators in the gastric antrum were elevated in the Bp infected gerbils, evidence that host responses to the filarid infection were systemic, impacting not only the lymphoid and lymphatic tissues, but also the stomach. Little is known about systemic inflammatory responses to filariasis in gerbils impacting gastrointestinal tissues, but in spleen, renal and popliteal lymph nodes targeted by Bp [16,32–34] an acute inflammatory response developed within 3 h, characterized by neutrophil infiltration in the dermis and muscle near the injection site. IL-6 and TNF-α gene expression were elevated in lymphoid tissues and concurrently within 1 week of Bp infection, IL-4 expression increased and remained elevated through 4 weeks post-infection [35]. IL-10 gene expression was unchanged but IL-5 was transiently elevated which coincided with a peak in eosinophilia.
In a previous study [27], IL-1β expression in the gastric mucosa of Mongolian gerbils infected with Hp was elevated during early infection, peaked at 4 weeks and then rapidly declined. IFN-γ levels also peaked at 4 weeks post-infection, but remained high through 26 weeks whereas IL-4 and IL-10 peaked later at 8–26 weeks post-infection. Thus, that study documented high Th1 cytokine levels during the acute and chronic phase of infection whereas Th2 cytokine expression levels became elevated only during the chronic phase of Hp infection [27]. A recent report detailed elevated mRNA IL10 in gastric antral tissue in gerbils infected with Hp strain B128 for up to 62 wpi [36]. Our results in Hp infected gerbils demonstrated persistent elevation of IFN-γ, IL-1β and CXCL1 with more variable expression of IL-4 and IL-10. In contrast, the coinfected gerbils developed a Th1/Th2 mixed cytokine response initially followed by a sustained Th1 but decreased Th2 response which also was reflected in an increased ratio of Hp-specific IgG2a to IgG1 responses at 42 WPHPI in the coinfected group. The increased IgG2a/IgG1 ratio at 42 WPHPI was consistent with increased expression of IFN-γ and IL-1β and lower levels of IL-4 and IL-10 expression from 21 to 42 WPHPI in the HpBp infected gerbils. Further, the decreased levels of IL10 and IL-4 at 42 wpi in both Hp and Hp Hb infected gerbils may have accounted in part for the equilibration of gastric lesions in the two groups of gerbils at this time point.
Brugia infection decreased the severity of Hp-associated gastric lesions that developed during the earlier phase of the coinfection. Importantly, an direct inverse correlation of worm burden and severity of Hp gastritis was noted at 21 WPHPI. Based on other studies in murine and human filarial infections, Bp may have induced T regulatory cells and alternatively activated macrophages early in the disease with ameliorating effects on Hp gastritis that could not be sustained during chronic HpBp coinfection [12]. Increased adult worm burdens over time in the HpBp gerbils may have led to host hyporesponsiveness to the parasitic infection, and indirectly enhanced the pathogenic potential of Hp. It was reported that live infective-stage larvae or microfilaria of Brugia malayi impaired Th1 and Th2 cytokine production in infected humans [37]. The impaired Th1/Th2 response was attributed to impaired induction of T-bet and GATA-3 mRNA and increased expression of Foxp3+ regulatory T cells which also have been noted to expand in mice infected with B. malayi [13]. The dysregulation of Th1 and Th2 responses by filariasis in humans was also associated with induction of T cell anergy-inducing factors including cbl-b, c-cbl, Itch, and Nedd4 [37]. B. malayi microfilaria also were recently shown to induce apoptosis in human monocyte-derived dendritic cells and may be a major mechanism underlying antigen-specific T cell hyporesponsiveness in patients with patent filarial infection [38].
Gastric cancer rates may vary due to age at which Hp infection is acquired, host genotype, virulence of specific Hp strains, nutritional status and other environmental factors, including coinfection with other enteric pathogens [1,9,11]. We recently reported in a C57BL/6 mouse model that colonization of H. bilis in the lower bowel attenuated Hp-induced gastric pathology at 6 and 12 months post-infection with Hp [39] and we previously demonstrated that gerbils obtained from commercial sources are commonly naturally infected with H. bilis [40]. Whether H. bilis exerted an immune modulatory role in the HpBp coinfection model is unknown and will require studies in H. bilis-free gerbils, which currently are not widely available. Importantly, our results support that concurrent filariasis in humans may differentially modulate persistent Hp gastritis. Further studies in the gerbil model are warranted using Brugia spp., potentially other helminths and other strains of Hp previously shown to differ in virulence capacity [23,36,41]. As more immunological and molecular reagents become available for the gerbil model, its value for evaluating the host response to concurrent infectious agents will increase.
Acknowledgements
We would like to thank Sharon Coleman, Melissa Mobley, Amanda Potter, and the MIT DCM Diagnostic Laboratory for technical support with this study. This study was supported by NIH grants R01 AI 0337750, P30 ES 02109, P01 CA 028842, T32 RR 07036 to James G. Fox.
References
- 1.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J. Clin. Invest. 2007;117:60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clin. Microbiol. Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tay CY, Mitchell H, Dong Q, Goh KL, Dawes IW, Kan R. Population structure of Helicobacter pylori among ethnic groups in Malaysia: recent acquisition of the bacterium by the Malay population. BMC Microbiol. 2009;19:126. doi: 10.1186/1471-2180-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol. Rev. 2000;22:283–297. doi: 10.1093/oxfordjournals.epirev.a018040. [DOI] [PubMed] [Google Scholar]
- 5.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni JF, Jr, Rabkin CS. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 6.Magnusson PKE, Enroth H, Eriksson I, Held M, Nyrén O, Engstrand L, Hansson LE, Gyllensten UB. Gastric cancer and human leukocyte antigen: distinct DQ and DR alleles are associated with development of gastric cancer and infection by Helicobacter pylori. Cancer Res. 2001;61:2684–2689. [PubMed] [Google Scholar]
- 7.Holcombe C. Helicobacter pylori: the African enigma. Gut. 1992;33:429–431. doi: 10.1136/gut.33.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babu S, Blauvelt CP, Nutman TB. Filarial parasites induce NK cell activation, type 1 and type 2 cytokine secretion, and subsequent apoptotic cell death. J. Immunol. 2007;179:2445–2456. doi: 10.4049/jimmunol.179.4.2445. [DOI] [PubMed] [Google Scholar]
- 9.Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, Nagler-Anderson C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat. Med. 2000;6:536–542. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 10.Gordon D. Solving the African enigma: parasites may have their place. Gastroenterology. 2000;119:611. doi: 10.1016/s0016-5085(00)70104-1. [DOI] [PubMed] [Google Scholar]
- 11.Whary MT, Sundina N, Bravo LE, Correa P, Quinones F, Caro F, Fox JG. Intestinal helminthiasis in Colombian children promotes a Th2 response to Helicobacter pylori: possible implications for gastric carcinogenesis. Cancer Epidemiol. Biomarkers Prev. 2005;14:1464–1469. doi: 10.1158/1055-9965.EPI-05-0095. [DOI] [PubMed] [Google Scholar]
- 12.Maizels RM, Pearce EJ, Artis D, Yazdanbakhsh M, Wynn TA. Regulation of pathogenesis and immunity in helminth infections. J. Exp. Med. 2009;206:2059–2066. doi: 10.1084/jem.20091903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McSorley HJ, Harcus YM, Murray J, Taylor MD, Maizels RM. Expansion of Foxp3+ regulatory T cells in mice infected with the filarial parasite Brugia malayi. J. Immunol. 2008;181:6456–6466. doi: 10.4049/jimmunol.181.9.6456. [DOI] [PubMed] [Google Scholar]
- 14.Ash LR, Riley JM. Development of Brugia pahangi in the jird, Meriones unguiculatus, with notes on infections in other rodents. J. Parasitol. 1970;56:962–968. [PubMed] [Google Scholar]
- 15.Bosshardt SC, Coleman SU, McVay CS, Jones KL, Klei TR. Impact of microfilaremia on maintenance of a hyporesponsive cellular immune response in Brugia-infected gerbils (Meriones unguiculatus) Infect. Immun. 1995;63:940–945. doi: 10.1128/iai.63.3.940-945.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chirgwin SR, Elzer PH, Coleman SU, Nowling JM, Hagius SD, Edmonds MD, Klei TR. Infection outcome and cytokine gene expression in Brugia pahangi-infected gerbils (Meriones unguiculatus) sensitized with Brucella abortus. Infect. Immun. 2002;70:5938–5945. doi: 10.1128/IAI.70.11.5938-5945.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chirgwin SR, Coleman SU, Porthouse KH, Nowling JM, Punkosdy GA, Klei TR. Removal of Wolbachia from Brugia pahangi is closely linked to worm death and fecundity but does not result in altered lymphatic lesion formation in Mongolian gerbils (Meriones unguiculatus. Infect. Immun. 2003;71:6986–6994. doi: 10.1128/IAI.71.12.6986-6994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ah HS, Thompson PE. Brugia pahangi: infections and their effect on the lymphatic system of Mongolian jirds (Meriones unguiculatus) Exp. Parasitol. 1973;34:393–411. doi: 10.1016/0014-4894(73)90099-4. [DOI] [PubMed] [Google Scholar]
- 19.Farrar RG, Klei TR, McVay CS, Coleman SU. Qualitative characterization of antibody responses to single and multiple Brugia pahangi infections in jirds. J. Parasitol. 1991;77:718–726. [PubMed] [Google Scholar]
- 20.Hirayama F, Takagi S, Yokoyama Y, Iwao E, Ikeda Y. Establishment of gastric Helicobacter pylori infection in Mongolian gerbils. J. Gastroenterol. 1996;31 Suppl:24–28. [PubMed] [Google Scholar]
- 21.Matsumoto S, Washizuka Y, Matsumoto Y, Tawara S, Ikeda F, Yokota Y, Karita M. Induction of ulceration and severe gastritis in Mongolian gerbil by Helicobacter pylori infection. J. Med. Microbiol. 1997;46:391–397. doi: 10.1099/00222615-46-5-391. [DOI] [PubMed] [Google Scholar]
- 22.Honda S, Fujioka T, Tokieda M, Gotoh T, Nishizono A, Nasu M. Gastric ulcer, atrophic gastritis, and intestinal metaplasia caused by Helicobacter pylori infection in Mongolian gerbils. Scand. J. Gastroenterol. 1998;33:454–460. doi: 10.1080/00365529850171990. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 24.Klei TR, McVay CS, Dennis VA, Coleman SU, Enright FM, Casey HW. Brugia pahangi: effects of duration of infection and parasite burden on lymphatic lesion severity, granulomatous hypersensitivity, and immune responses in jirds (Meriones unguiculatus. Exp. Parasitol. 1990;71:393–405. doi: 10.1016/0014-4894(90)90065-k. [DOI] [PubMed] [Google Scholar]
- 25.Rogers AB, Taylor NS, Whary MT, Stefanich ED, Wang TC, Fox JG. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res. 2005;65:10709–10715. doi: 10.1158/0008-5472.CAN-05-1846. [DOI] [PubMed] [Google Scholar]
- 26.Rieder G, Merchant JL, Haas R. Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology. 2005;128:1229–1242. doi: 10.1053/j.gastro.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 27.Yamaoka Y, Yamauchi K, Ota H, Sugiyama A, Ishizone S, Graham DY, Maruta F, Murakami M, Katsuyama T. Natural history of gastric mucosal cytokine expression in Helicobacter pylori gastritis in Mongolian gerbils. Infect. Immun. 2005;73:2205–2212. doi: 10.1128/IAI.73.4.2205-2212.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurer KJ, Rogers AB, Ge Z, Wiese AJ, Carey MC, Fox JG. Helicobacter pylori and cholesterol gallstone formation in C57L/J mice: a prospective study. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G175–G182. doi: 10.1152/ajpgi.00272.2005. [DOI] [PubMed] [Google Scholar]
- 29.Pronovost AD, Rose SL, Pawlak JW, Robin H, Schneider R. Evaluation of a new immunodiagnostic assay for H. pylori antibody detection: correlation with histopathological and microbiological results. J. Clin. Microbiol. 1994;32:46–50. doi: 10.1128/jcm.32.1.46-50.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thirugnanam S, Pandiaraja P, Ramaswamy K, Murugan V, Nandakumar K, Reddy MV, Kaliraj P. Brugia malayi: comparison of protective immune responses induced by Bm-alt-2 DNA, recombinant Bm-ALT-2 protein and prime-boost vaccine regimens in a jird model. Exp. Parasitol. 2007;116:483–491. doi: 10.1016/j.exppara.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers AB, Houghton J. Helicobacter-based mouse models of digestive system carcinogenesis. Methods Mol. Biol. 2009;511:267–295. doi: 10.1007/978-1-59745-447-6_11. [DOI] [PubMed] [Google Scholar]
- 32.Jackson CN, Maurer KJ, Taylor N, Xu S, Rogers A, Ge Z, Klei T, Chirgwin S, Coleman S, Fox JG. Brugia pahangi ameliorates Helicobacter pylori-induced gastritis and pre-malignant lesions in the Mongolian gerbil: a model for chronic parasitism and the “African enigma”. Contemp. Top. Lab. Anim. Sci. 2005;44:53–54. [Google Scholar]
- 33.Rao UR, Klei TR. Cytokine profiles of filarial granulomas in jirds infected with Brugia pahangi. Filaria J. 2006;5:3. doi: 10.1186/1475-2883-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chirgwin SR, Rao UR, Mai Z, Coleman SU, Nowling JM, Klei TR. Kinetics of T cell cytokine gene expression in gerbils after a primary subcutaneous Brugia pahangi infection. J. Parasitol. 2005;91:264–268. doi: 10.1645/GE-348R. [DOI] [PubMed] [Google Scholar]
- 35.Porthouse KH, Chirgwin SR, Coleman SU, Taylor HW, Klei TR. Inflammatory responses to migrating Brugia pahangi third-stage larvae. Infect. Immun. 2006;74:2366–2372. doi: 10.1128/IAI.74.4.2366-2372.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiedemann T, Loell E, Mueller S, Stoeckelhuber M, Stolte M, Haas R, Rieder G. Helicobacter pylori cag-Pathogenicity island-dependent early immunological response triggers later precancerous gastric changes in Mongolian gerbils. PLoS One. 2009:e4754. doi: 10.1371/journal.pone.0004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J. Immunol. 2006;176:3248–3256. doi: 10.4049/jimmunol.176.5.3248. [DOI] [PubMed] [Google Scholar]
- 38.Semnani RT, Venugopal PG, Mahapatra L, Skinner JA, Meylan F, Chien D, Dorward DW, Chaussabel D, Siegel RM, Nutman TB. Induction of TRAIL- and TNF-α -dependent apoptosis in human monocyte-derived dendritic cells by microfilariae of Brugia malayi. J. Immunol. 2008;181:7081–7089. doi: 10.4049/jimmunol.181.10.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lemke LB, Ge Z, Whary MT, Feng Y, Rogers AB, Muthupalani S, Fox JG. Concurrent Helicobacter bilis infection in C57BL/6 mice attenuates proinflammatory H. pylori-induced gastric pathology. Infect. Immun. 2009;77:2147–2158. doi: 10.1128/IAI.01395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergin IL, Taylor NS, Nambiar PR, Fox JG. Eradication of enteric helicobacters in Mongolian gerbils is complicated by the occurrence of Clostridium difficile enterotoxemia. Comp. Med. 2005;55:265–268. [PubMed] [Google Scholar]
- 41.Franco AT, Israel DA, Washington MK, Krishna U, Fox JG, Rogers AB, Neish AS, Collier-Hyams L, Perez-Perez GI, Hatakeyama M, Whitehead R, Gaus K, O’Brien DP, Romero-Gallo J, Peek RM., Jr Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc. Natl. Acad. Sci. U.S.A. 2005:10646–10651. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]