Abstract
Objective
Although widely applied as a phenotypic expression of adiposity in population and gene-search studies, body mass index (BMI) is also acknowledged to reflect muscularity even though relevant studies directly measuring skeletal muscle (SM) mass are lacking. The current study aimed to fill this important gap by applying advanced imaging methods to test the hypothesis that, after controlling first for adiposity, SM mass is also a significant determinant of BMI in a population-based sample.
Design
Whole-body magnetic resonance imaging scans were completed in CARDIA Study subjects aged 33-45 years. Physical activity (PA) levels, alcohol intake, and adequacy of food intake were assessed by standardized questionnaires.
Subjects
58 African-American (AA) and 78 Caucasian (C) men; 63 AA and 64 C women.
Measurements
Whole-body AT and SM volumes.
Results
AT was significantly predicted by not only BMI, but PA and alcohol intake with total model R2s of 0.68 (p<0.0001) for men and 0.89 (p<0.0001) for women. Men had more SM than AT at all levels of BMI while SM predominated in women at lower BMIs (C <26 kg/m2; AA <28 kg/m2). Both AT and SM contributed a similar proportion of between-subject variation in BMI in men. In contrast, AT contributed ~30% more than SM to the variation in BMI in women. Developed allometric models indicated SM associations with AT, PA, and race after adjusting for height. There was little association of age, lifestyle factors, or race with BMI after controlling for both AT and SM.
Conclusion
Variation in muscularity provides a mechanistic basis for the previously observed non-specificity of BMI as a phenotypic expression of adiposity. These quantitative observations have important implications when choosing adiposity measures in population and gene-search studies.
Keywords: Body Composition, Ethnicity, Obesity, Body Mass Index
INTRODUCTION
Body mass index (BMI), weight/height2, is now the most widely applied phenotypic expression of human adiposity (1). Quetelet in 1842 (2) was the first to observe that weight scales across adult subjects as height2. Keys et al. (3) largely ended a decades-long debate on the “best” obesity index by showing that among several candidates Quetelet’s index had the highest correlations with percent body fat as measured by underwater weighing. Renamed by Keys et al. (3) as BMI, this simple index serves as an important measure of population and individual adiposity.
Close scrutiny of BMI over the years has led to the consistent observation that correlations with adult adiposity are generally modest and that other factors, such as age, race, and physical activity (PA) levels confound the BMI-adiposity relationship (4-14). Other than adipose tissue, skeletal muscle (SM) mass is the largest adult body compartment, and between-subject variability in muscularity is often cited as a reason for the non-specificity of BMI (7, 10-13), although SM is rarely measured. Total body SM mass has traditionally been difficult to quantify, particularly in large population samples, and surrogate muscularity measures have been used to examine BMI-adiposity relations. Skeletal muscle accounts for about one-half of fat-free mass (FFM) (15) and FFM has been used in previous BMI studies as a marker of muscularity (14). However, the proportion of FFM as SM varies with sex, age, race (16), and physical activity levels and thus is not an ideal measure of SM’s quantitative impact on BMI. More recently the lean-soft tissue compartment of the extremities as measured by dual-energy x-ray absorptiometry (DXA) has been used as a surrogate marker of muscularity (12, 13), but the same concerns apply to this compartment as a measure of SM as for FFM.
The quantitative impact of muscularity on BMI as a phenotypic marker of population adiposity is thus largely unknown. The recent introduction of magnetic resonance imaging (MRI) as a means of quantifying total body SM volume and mass on a relatively large scale (17) affords the important new opportunity to establish specifically and precisely the relationships between adiposity, muscularity, and BMI. The current study applied MRI to test the hypothesis that, after controlling first for adiposity, SM mass is also a significant and independent determinant of BMI in the population-based Coronary Artery Risk Development in Young Adults (CARDIA) sample (18).
METHODS
Protocol and Subjects
The study hypothesis and related questions were examined using a subset of the bi-racial CARDIA cohort that includes African American and Caucasian men and women who underwent whole body MRI as part of the Fat Redistribution and Metabolic Change in HIV Infection (FRAM) study (21). FRAM was designed to evaluate the prevalence and correlates of changes in fat distribution, insulin resistance, and dyslipidemia in a representative sample of HIV-infected subjects and HIV-seronegative controls in the United States. The methods have been described in detail previously (21, 22).
The group analyzed here were HIV-uninfected control men and women enrolled from two CARDIA research sites (18, 21), one located at Kaiser Permanente, Oakland, CA and the other at the University of Alabama, Birmingham that have followed participants longitudinally enrolled in the Visceral Fat and Metabolic Rate in Young Adults (VIM) ancillary study of CARDIA (23).
CARDIA participants were originally recruited as a population-based sample of healthy 18-33 year old Caucasian and African-American men and women from four cities in 1986 for a longitudinal study of cardiovascular risk factors. The VIM ancillary study recruited participants from two of the four CARDIA Centers in 1995-96. VIM enrolled approximately 100 CARDIA participants from each of the race-gender groups with BMI distributed similarly above and below race-gender specific medians of the population-based CARDIA study. Guidelines for human experimentation in accordance with the US Department of Health and Human Services and the Institutional Review Board of each participating institution were followed in the conduct of the study. Appropriate informed consent was obtained from all study participants.
Subjects had a clinic visit that included a comprehensive history, physical examination, and body composition studies. Whole-body MRI was used to partition body volume into AT, SM, and residual (i.e., bone & organ) compartments (24). Percentage of body weight as adipose tissue (i.e., %AT) was used as an alternative measure of adiposity.
Candidate lifestyle factors included level of physical activity (quartiled by gender), smoking status (current, past, or never), alcohol intake (<1/week, 1-7/week, >7/week, or none), adequate food intake, and illicit drug use (current, past, or never) and were assessed by standardized instruments (18, 25-28). Subjects were also asked to rate their physical activity during the past year on a five-point scale ranging from inactive to very active.
Body Composition
Anthropometric Measurements
All staff were centrally trained and certified for measurements. Subjects wore light clothing or a hospital gown and no shoes. Height was measured to the nearest 0.1 cm and weight was measured to the nearest 0.1 kg on calibrated stadiometers and scales, respectively. Waist circumference was measured immediately below the lowest rib. Hip circumference was measured at the maximum extension of the buttocks as viewed from the side.
Magnetic Resonance Imaging
Body composition was measured by MRI with subjects in the supine position with arms extended over their head (24). Using the inter-vertebral space between the fourth and fifth lumbar vertebrae as the origin, 10 mm thick transverse images were obtained every 40 mm from hands to feet. MRI field strength was standardized at 1.5 Tesla. Image analysis software (Tomovision Inc., Montreal, Canada) was used to segment the cross-sectional images for total area and areas of AT and SM; residual (bone & organs) area was calculated as the difference between total image area and the sum of AT and SM areas. The AT compartment was further segmented into total subcutaneous AT, visceral AT, and AT volume in 4 regions [leg, lower trunk (abdomen and back), upper trunk (chest and back), arm].
All MRI scans were read at the Image Reading Center (IRC) located at the Obesity Research Center, St. Luke’s-Roosevelt Hospital, New York, NY. The image acquisition protocol was standardized across sites and visits were conducted by the IRC staff to ensure protocol adherence. Scans were sent to the IRC where AT and SM tissue areas (cm2) were calculated after segmentation by summing appropriate tissue pixels, then multiplying by individual pixel surface area. The volume of AT and SM in each slice was calculated by multiplying tissue area by thickness. The volume of each tissue for the space between two consecutive slices was calculated via a mathematical algorithm (24).
Percent fat was calculated as 100 × 0.92 g/cm3 × the ratio of total AT volume to body weight, since adipose tissue has a density of ~0.92 g/cm3 (24). Percent SM was similarly calculated as 100 × 1.04 g/cm3 × the ratio of SM volume to body weight, as SM has a density of ~1.04 g/cm3.
Statistical Methods
Subject demographic characteristics are reported as median with interquartile range (IQR). Characteristics of African American and Caucasian participants were compared and tested for statistical significance using the Mann-Whitney U test for continuous variables, and Fisher’s exact test for categorical variables. Separate analyses for all measures were conducted for men and women. All analyses were conducted using the SAS system, version 9.1 (SAS Institute, Inc., Cary, NC).
The hypothesis and related questions were evaluated in three stages. We first examined the association of BMI and SM with AT in multivariable linear regression analyses adjusting for age, race, and lifestyle factors, with BMI analyzed linearly, but with potentially different slopes in the ranges of <25, 25-30, and >30 kg/m2. We used a linear spline to model BMI due to the curvilinear relation between BMI and AT. Candidate lifestyle factors included physical activity, smoking, alcohol use, adequate food intake, and illicit drug use. This first series of analyses was aimed at confirming that CARDIA subjects have AT-BMI relations generally similar to those reported in earlier studies (4-14).
In this first set of analyses, we developed AT prediction equations using the simple allometric model (19),
| (1) |
where y is AT volume, α is the proportionality constant, x1 is age, x2 is SM, x3, x4, x5 are BMI linear spline terms, x6 is ethnicity (African American or Caucasian), x7, x8, x9 are levels of physical activity or alcohol use (vs. the lowest level of activity or drinking), βi is the scaling exponent or power, and ε is a multiplicative error term. When converted to logarithmic form, the allometric equation can be solved as:
| (2) |
Allometric model coefficients in equation 2 (α and βi) were derived using least-squares multiple linear regression analysis and log-transformed data.
In the second set of analyses, SM was modeled as:
| (3) |
where y is SM volume, α is the proportionality constant, x1 is height, x2 is age, x3 is %AT, x4 is ethnicity (African American or Caucasian), x5, x6, x7 are quartiles of physical activity (vs. the first quartile or lowest level of activity), βi is the scaling exponent or power, and ε is a multiplicative error term. When converted to logarithmic form, the allometric equation can be solved as:
| (4) |
The third series of analyses, related directly to the study hypothesis, focused on the individual contributions of AT and SM to BMI. Adipose tissue and SM volumes were normalized in this analysis by dividing AT by height-squared, with summaries back-transformed to 1.75 m of height (20) as follows:
where yunadj denotes the unadjusted AT or SM volume, height is in meters, and yt denotes the back-transformed quantity. This adjustment has the effect of normalizing the AT or SM volume to a standard height, which is useful in comparing subjects in populations with differing heights. We investigated the associations of AT and SM with BMI in multivariable linear regression analyses adjusting for age, race, and candidate lifestyle factors. We also considered visceral AT and the sum of bone plus organ volumes in models, but associations of AT and SM were strongest and were not improved by consideration of other body compartments.
Candidate lifestyle factors were tested in all models in a stepwise fashion, with p=0.05 for entry and retention. Interactions of race and age with other factors in the model were also assessed and included if they reached statistical significance. The linearity assumption was tested for continuous measures by adding quadratic terms to the models. We tested for colinearity for body compartments in the model and found that it was not substantial between AT and SM; therefore, both compartments were kept in the final model. Confidence intervals were determined using the bias-corrected accelerated bootstrap method with p-values defined as the one minus the highest confidence level that still excluded zero (29); this procedure was necessary as the error residuals appeared to be non-Gaussian.
RESULTS
Subjects
Body composition studies were available on 263 subjects from the CARDIA study and the characteristics for each sex and race group are presented in Table 1. There were 136 men (58 African Americans; 78 Caucasians) and 127 women (63 African Americans; 64 Caucasians). The Caucasian subjects were slightly older than their African American counterparts by a median of 1-2 years and the IQR was expectedly small (~37-44 yrs) as subjects were enrolled in CARDIA with an age spread of only 12 years.
Table 1.
Subject characteristics.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| African American (n = 58) | Caucasian (n = 78) | P-value | African American (n = 63) | Caucasian (n = 64) | P-value | |
| Age (y) | 39.0 (37.0-42.0) | 41.0 (38.0-43.0) | 0.023 | 41.0 (37.0-43.0) | 42.0 (38.5-44.0) | 0.050 |
| Weight (kg) | 86.1 (75.9-98.5) | 82.4 (77.7-93.0) | 0.43 | 85.6 (72.7-92.1) | 65.9 (58.6-77.7) | <0.0001 |
| Height (cm) | 179 (175-181) | 178 (175-183) | 0.92 | 164 (160-171) | 165 (162-171) | 0.43 |
| BMI (kg/m2) | 27.2 (24.6-30.4) | 26.2 (24.5-28.5) | 0.35 | 31.6 (25.3-33.7) | 23.5 (20.9-28.2) | <0.0001 |
| Phys. Activity | ||||||
| Quartile 1 | 25.9% | 23.1% | 0.58 | 29.0% | 21.9% | 0.20 |
| 2 | 22.4% | 29.5% | 27.4% | 20.3% | ||
| 3 | 24.1% | 28.2% | 25.8% | 23.4% | ||
| 4 | 27.6% | 19.2% | 17.7% | 34.4% | ||
| WC (cm) | 91.3 (86.2-97.8) | 91.3 (86.8-98.2) | 0.74 | 92.3 (82.3-99.2) | 75.9 (69.6-86.6) | <0.0001 |
| HC (cm) | 102 (98-110) | 101 (98-107) | 0.23 | 112 (104-118) | 102 (96-110) | 0.0002 |
| WHR | 0.89 (0.84-0.91) | 0.91 (0.88-0.94) | 0.004 | 0.82 (0.77-0.86) | 0.75 (0.73-0.79) | <0.0001 |
| %AT | 17.4 (14.7-22.8) | 20.6 (15.6-23.4) | 0.080 | 36.4 (32.3-41.1) | 30.7 (22.3-36.8) | 0.004 |
| Total AT (L)* | 15.9 (11.8-22.0) | 17.5 (13.3-21.1) | 0.34 | 37.6 (26.9-45.1) | 23.6 (15.5-35.0) | 0.0001 |
| UTr-AT (L) | 2.9 (2.0-4.1) | 3.1 (2.4-4.0) | 0.40 | 7.0 (4.2-8.6) | 3.3 (2.1-5.4) | <0.0001 |
| LTr-AT (L) | 5.3 (3.9-7.2) | 5.5 (4.2-7.5) | 0.48 | 13.0 (10.0-16.5) | 8.6 (5.7-12.8) | 0.0001 |
| Leg AT (L) | 4.6 (3.7-6.1) | 4.3 (3.7-5.4) | 0.40 | 11.5 (8.8-15.2) | 9.2 (6.5-11.4) | 0.002 |
| Arm AT (L) | 1.1 (0.7-1.4) | 1.1 (0.9-1.3) | 0.17 | 2.5 (1.9-3.1) | 1.8 (1.3-2.6) | 0.004 |
| SAT (L) | 14.6 (10.5-19.4) | 14.5 (11.6-18.0) | 0.68 | 35.7 (25.6-43.3) | 23.2 (15.2-32.7) | <0.0001 |
| VAT (L) | 1.4 (0.9-2.3) | 2.4 (1.6-3.1) | 0.0003 | 1.3 (0.6-2.0) | 0.6 (0.4-1.5) | 0.026 |
| SM (L) | 33.5 (31.3-37.9) | 30.7 (27.7-33.7) | 0.001 | 27.1 (23.2-29.8) | 22.4 (20.8-25.1) | <0.0001 |
| Bone & Organ (L) | 21.4 (19.5-23.1) | 21.6 (19.7-23.1) | 0.57 | 20.9 (19.8-22.8) | 20.8 (19.2-22.1) | 0.27 |
Data are presented as Median (IQR). Test of African American versus Caucasian for men and women (Wilcoxon’s rank-sum test for continuous variables and Fisher’s exact test for categorical variables.
Abbreviations: AT, adipose tissue; BMI, body mass index; HC, hip circumference; IQR, interquartile range; LTr, lower trunk; SM, skeletal muscle; SAT, subcutaneous adipose tissue; AT, adipose tissue; Tr, trunk; UTr, upper trunk; VAT, visceral adipose tissue; WC, waist circumference; WHR, waist to hip circumference ratio.
Note that all MRI measures are height2-normalized and back-transformed.
Both groups of men were, on average, within the overweight range (i.e., BMI ≥25 to < 30 kg/m2) with median BMI in African Americans at 27.2 kg/m2 and Caucasians at 26.2 kg/m2. About two-thirds of the men were overweight or obese (African Americans 69% and Caucasians 67%, p=0.86). Although total and subcutaneous AT were similar between the two groups of men, African Americans had about one-half the amount of visceral AT compared to the Caucasian men (p=0.0003). Of note, African-American men had much higher SM and slightly higher BMI, but lower VAT and slightly lower %AT compared with Caucasian men.
The African American women were, as a group, obese (i.e., BMI, ≥30 kg/m2) while the Caucasian women were normal weight (i.e., BMI, ≤25 kg/m2) with respective median BMIs of 31.6 kg/m2 and 23.5 kg/m2. The proportion of African American women who were overweight or obese (78%) was significantly larger (p<0.001) than the proportion of overweight or obese Caucasian women (42%). However, as in men, African-American women had much higher SM. In women, VAT was higher in African-Americans, while in men, VAT was higher in Caucasians. The racial differences in total and regional adipose tissue compartment volumes paralleled between-race group differences in BMI.
Physical activity levels were similar overall in African American and Caucasian men (p=0.58), although African-American men were somewhat more likely to be in the most active quartile compared with Caucasian men (28% vs. 19%). Among women, although the difference in self-reported physical activity did not reach statistical significance, African Americans had relatively more subjects in the least active physical activity quartile 1 (29% vs. 22% in Caucasians) and Caucasian women more in quartile 4 (34% vs. 18%), suggesting that the Caucasian women tended to be more physically active.
Overall, nearly 80% of the men and women in this analysis self-reported being moderately to very physically active.
Adiposity-BMI Associations
This first series of analyses were designed to characterize the associations of BMI with adiposity.
BMI was strongly associated with height-normalized AT in all four sex and ethnic groups (Figure 1, p<0.0001), although the proportion of BMI variation explained in men (African American, R2=0.71; Caucasian, R2=0.64) was lower than the proportion observed in the women (African American, R2=0.84; Caucasian, R2=0.92).
Figure 1.
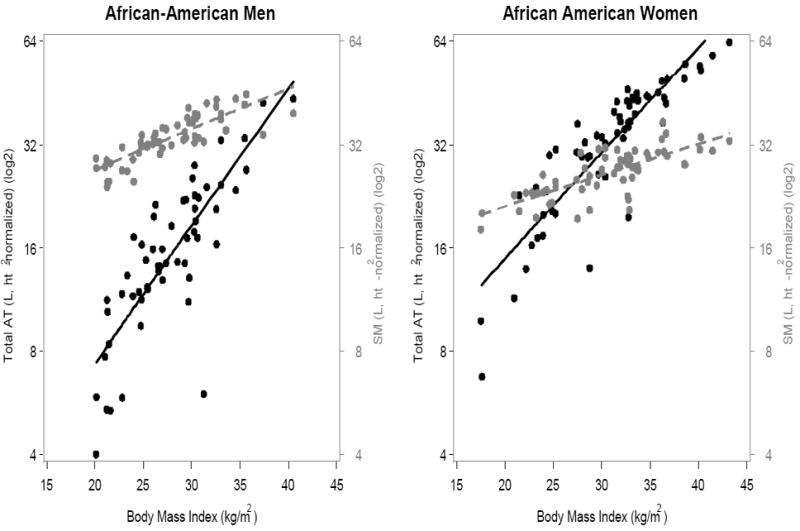
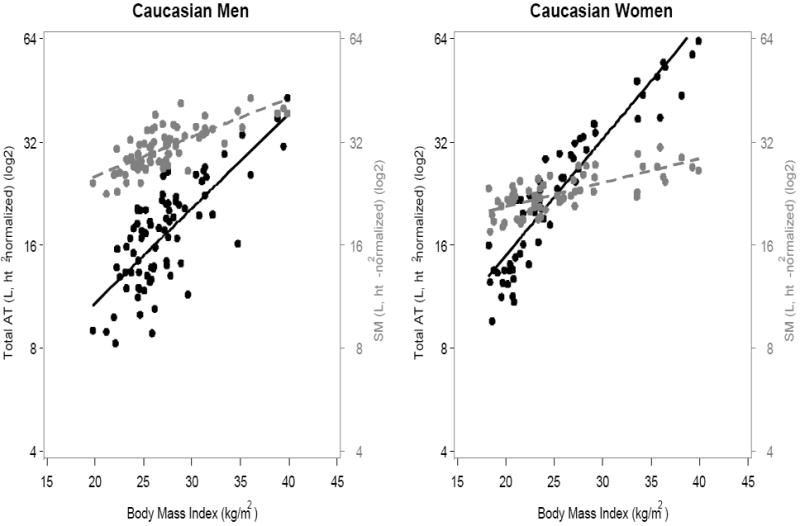
(A) African-Americans; (B) Caucasians. Association of BMI with AT and SM volumes, expressed as ratios to height2, in men (left) and women (right). Black symbols and whole lines denote AT, and grey symbols and dashed lines denote SM. [AT men: African American, y=-26.9+1.6x (R2, 0.71, p<0.0001); Caucasian, y=−17.0+1.3x (R2, 0.64, p<0.0001). AT women: African American, y=−19.8+1.5x+0.011x2 (R2, 0.84, p<0.0001); Caucasian, y=-25.8+1.9x+0.007x2 (R2, 0.92, p<0.0001)]
[SM men: African American, y=8.4+0.92x (R2, 0.68, p<0.0001); Caucasian, y=8.3+0.83x (R2, 0.58, p<0.0001). SM women: African American, y=10.0+0.55x (R2, 0.56, p<0.0001); Caucasian, y=13.0+0.38x (R2, 0.53, p<0.0001)].
The corresponding associations between BMI and %AT are plotted in Figure 2. All four correlations were statistically significant (all p<0.001) in men and women. However, according to these analyses, BMI is only modestly associated with percent adiposity, particularly in men (African American, R2 = 0.46; Caucasian, R2 = 0.24), but also in women (African American, R2 = 0.55; Caucasian, R2 = 0.72).
Figure 2.
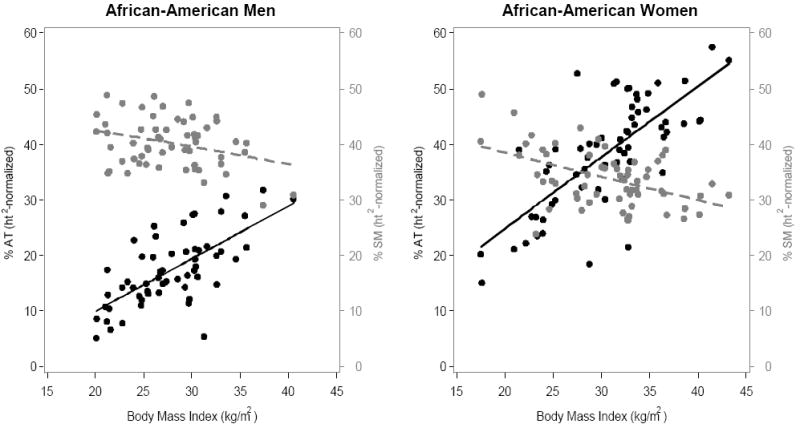
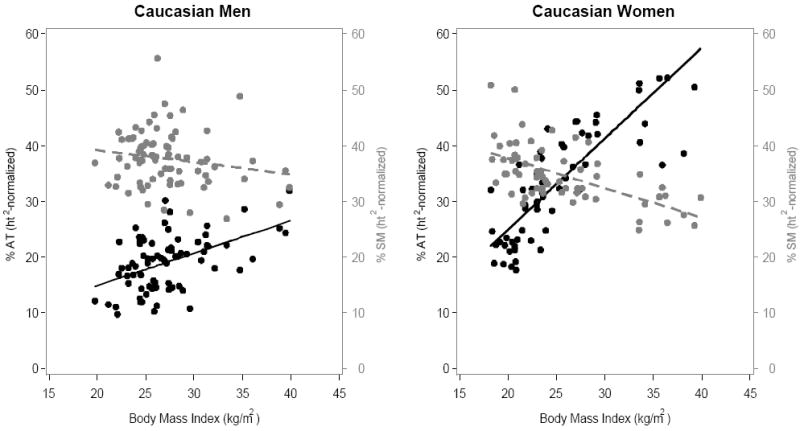
(A) African-Americans; (B) Caucasians. Association of BMI with %AT and %SM , expressed as 100 × 0.92 g/cm3 × the ratio of total height2-normalized AT volume to body weight and 100 × 1.04 g/cm3 × the ratio of total height2-normalized SM volume to body weight in men (left) and women (right). Black symbols and whole lines denote %AT, and grey symbols and dashed lines denote %SM.
[%AT men: African American, y = -9.0 + 0.95x (R2=0.46, p<0.0001); Caucasian, y = 3.1 + 0.59x, (R2=0.24, p<0.0001). %AT women: African American, -0.73 + 1.28x (R2=0.55, p<0.0001); Caucasian, y = -7.6 + 1.63x (R2=0.72, p<0.0001)]
[%SM men: African American, y = 48.5 - 0.30x (R2=0.098, p = 0.018); Caucasian, y =43.5 - 0.22x (R2=0.031, p = 0.13). %SM women: African American, y = 46.9 - 0.43x (R2=0.23, p<0.0001); Caucasian, y = 48.3 - 0.53x (R2=0.35, p<.0001)]
The simple univariate BMI-AT analyses presented in Figure 1 were extended in the multivariable models shown in Table 2 to determine factors associated with AT (i.e., adiposity). Increasing BMI was independently associated with higher AT in both men and women (p<0.0001), with stronger associations seen for those in the normal BMI range (<25 kg/m2). By comparison, increasing SM was associated with lower AT in men (p=0.036) with a weaker negative association in women (p=0.25). In men, the BMI effect was stronger in African Americans compared with Caucasians (p = 0.002, test for interaction). However, we elected to report results from the combined model in men since BMI was positively associated with AT in both African Americans and Caucasians.
Table 2.
Allometric multivariable associations with AT volume *.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Estimate | 95%CI | P-value | Estimate | (95%CI) | P-value | |
| Intercept | 0.68 | (-1.8, 2.8) | 0.58 | 2.06 | (-0.78, 3.7) | 0.11 |
| African-American vs. Caucasian | -0.065 | (-0.19, 0.025) | 0.16 | -0.074 | (-0.18, 0.001) | 0.052 |
| BMI < 25 (m2/kg)** | 0.18 | (0.14, 0.24) | <0.0001 | 0.14 | (0.11, 0.16) | <0.0001 |
| BMI 25-30 (m2/kg) | 0.082 | (0.030, 0.12) | <0.0001 | 0.070 | (0.032, 0.095) | <0.0001 |
| BMI >30 (m2/kg) | 0.085 | (0.067, 0.10) | <0.0001 | 0.054 | (0.043, 0.064) | <0.0001 |
| Skeletal Muscle (ln, L) | -0.90 | (-1.56, -0.076) | 0.036 | -0.35 | (-0.79, 0.37) | 0.25 |
| Age (ln, years) | 0.17 | (-0.22, 0.57) | 0.38 | -0.27 | (-0.66, 0.18) | 0.21 |
| Physical Activity | ||||||
| 2nd Quartile vs. 1st | -0.10 | (-0.22, -0.011) | 0.032 | |||
| 3rd Quartile vs. 1st | -0.073 | (-0.18, 0.019) | 0.12 | |||
| 4th Quartile vs. 1st | -0.16 | (-0.34, -0.035) | 0.012 | |||
| Alcohol use (per week) | ||||||
| <1 vs. none | -0.076 | (-0.17, -0.013) | 0.019 | |||
| 1-7 vs. none | -0.081 | (-0.19, -0.018) | 0.011 | |||
| >7 vs. none | -0.081 | (-0.21, 0.008) | 0.074 | |||
| SEE | 0.24 | 0.17 | ||||
| Adjusted R2 | 0.68 | 0.89 | ||||
Outcome analyzed is natural log (ln) transformed adipose tissue volume (AT, L) from multivariable linear regression analyses. SEE, standard error of the estimate.
Estimates are slopes (βi) from the allometric model. AT and SM (skeletal muscle volume) are height2-normalized.
BMI analyzed using linear spline.
Age showed little association with AT in men or women. Among lifestyle factors, greater physical activity in men and alcohol use in women remained associated with lower AT. The sex-specific multivariable models accounted for 68% of between-individual differences in AT among men and 89% in women. A model of %AT gave similar results.
Skeletal Muscle Associations
This next set of analyses examined the associations between BMI and SM or %SM as well as the determinants of between-individual differences in SM.
The associations of BMI with SM were also significant in both men and women, but the patterns differed. Men had relatively more SM than AT throughout the entire range of evaluated BMIs, but the amount of AT approached SM at larger BMIs (Figure 1). Women had more SM than AT in the normal BMI range, but AT exceeded SM at higher BMIs (Caucasian >26 kg/m2; African-American >28 kg/m2).
The correlation between BMI and %SM in African American and Caucasian men was negative but weak (r=-0.127, p=0.14; Figure 2), although the correlation was somewhat stronger and statistically significant in African American men compared with Caucasian men. The correlation between BMI and %SM was negative and statistically significant (r=-0.52, p<0.0001) in both African American and Caucasian women (Figure 2). Regardless of race, the correlations were much stronger in women than in men.
The multivariable SM prediction models are presented in Table 3. Height was strongly associated with SM (p<0.0001) and height alone explained 13% of the SM variation in men and 16% in women. Skeletal muscle volume in the composite models scaled to height with powers in the range of ~1.3-1.6 (Table 3). Absolute AT volume alone explained 19% of the SM variation in men and 38% in women. Adipose tissue volume in the composite models was independently associated with SM in both men and women. Greater physical activity levels were associated with a larger SM. Age showed little association with SM in men (p=0.41) and contributed to the SM prediction model only weakly in women (p=0.085). After multivariable adjustment for height, age, and lifestyle factors, being African American was associated with approximately 10-11% greater SM (p<0.0001) compared with being Caucasian. The fully adjusted models for men and women accounted for 43% and 60% of between-individual differences in SM volume, respectively. Ethnicity, adiposity, and physical activity levels all thus accounted for between-individual differences in SM even after controlling first for height.
Table 3.
Allometric multivariable associations with SM volume *.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Estimate | 95%CI | P-value | Estimate | (95%CI) | P-value | |
| Intercept | -3.49 | (-6.6, -0.24) | 0.034 | -6.51 | (-9.43, -3.74) | <0.0001 |
| African-American vs. Caucasian | 0.10 | (0.06, 0.14) | <0.0001 | 0.11 | (0.05, 0.14) | <0.0001 |
| Height (ln, cm) | 1.31 | (0.65, 1.91) | <0.0001 | 1.58 | (1.02, 2.11) | <0.0001 |
| Age (ln, yrs) | -0.11 | (-0.35, 0.14) | 0.41 | 0.21 | (-0.03, 0.46) | 0.085 |
| AT (ln, L) | 0.13 | (0.10, 0.16) | <0.0001 | 0.14 | (0.12, 0.17) | <0.0001 |
| Physical Activity | ||||||
| 2nd Quartile vs. 1st | 0.03 | (-0.03, 0.10) | 0.33 | 0.01 | (-0.05, 0.07) | 0.71 |
| 3rd Quartile vs. 1st | 0.06 | (-0.00, 0.12) | 0.070 | 0.04 | (-0.01, 0.10) | 0.11 |
| 4th Quartile vs. 1st | 0.11 | (0.04, 0.17) | 0.001 | 0.07 | (0.02, 0.13) | 0.013 |
| SEE | 0.12 | 0.11 | ||||
| Full Model Adjusted R2 | 0.43 | 0.60 | ||||
| Adjusted R2 (height only) | 0.13 | 0.16 | ||||
| Adjusted R2 (AT only) | 0.19 | 0.38 | ||||
Outcome analyzed is natural log (ln) transformed skeletal muscle (SM, L) from multivariable linear regression analyses. SEE, standard error of the estimate.
Estimates are slopes (βi) from the allometric model. AT, adipose tissue volume.
Contributions of AT and SM to BMI
The third group of analyses examined the determinants of BMI after controlling first for AT. Adipose tissue and SM were both strongly and independently associated with BMI in men and women (each p<0.0001, Table 4) in multivariable models adjusting for demographic and lifestyle factors. Furthermore, a large proportion of the between-subject variation in BMI was now explained in both men (86%) and women (94%).
Table 4.
Multivariable associations with BMI *.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Estimate | 95%CI | P-value | Estimate | (95%CI) | P-value | |
| Intercept | 6.4 | (2.8, 9.2) | <0.0001 | 6.3 | (2.4, 12.5) | <0.00001 |
| African-American vs. Caucasian | -0.35 | (-0.92, 0.25) | 0.26 | 0.51 | (-0.19, 1.75) | 0.15 |
| Age (per decade) | 0.17 | (-0.39, 0.85) | 0.58 | 0.28 | (-0.77, 1.20) | 0.60 |
| AT (L, ht2-normalized) | 0.32 | (0.26, 0.36) | <0.0001 | 0.34 | (0.31, 0.36) | <0.0001 |
| Skeletal Muscle (L, ht2-norm.) | 0.46 | (0.40, 0.53) | <0.0001 | 0.40 | (0.28, 0.48) | <0.0001 |
| SEE | 1.59 | 1.58 | ||||
| Full Model Adjusted R2 | 0.86 | 0.94 | ||||
| Adjusted R2 with SM & without AT | 0.62 | 0.62 | ||||
| Adjusted R2 with AT & without SM | 0.67 | 0.90 | ||||
Outcome analyzed is BMI from multivariable linear regression analyses. SEE, standard error of the estimate.
Estimates are slopes (βi) from the model.
In men, both AT and SM contributed to a similar proportion of the between-subject variation in BMI as established by developing the model with either AT or SM alone along with ethnicity and age as covariates. In contrast, AT in women contributed to nearly 30% more of the variation in BMI compared with SM. In men, a one liter increase in SM was associated with a larger increase in BMI than a one liter increase in AT (0.46 vs. 0.32, p = 0.0042), but in women the SM relationship with BMI was not statistically larger than the AT relationship with BMI (0.40 vs. 0.34, p = 0.34). Furthermore, after controlling for both AT and SM, there was little association of age, race, or lifestyle factors (data not shown) with BMI.
DISCUSSION
The expanding global prevalence of obesity (30) and increasing search for weight-related genetic markers (31) highlight the important need for simple adiposity measures that can be applied to large population samples. A wealth of experimental data supports the use of BMI in this context, but reported BMI-adiposity associations are modest and “muscularity” is often cited as a factor that confounds this relationship (7, 8, 12-14, 32). The present study is the first to report direct SM estimates in a population sample that provides quantitative estimates of the extent to which BMI reflects variation in muscularity.
Our findings in the CARDIA sample show that SM is a major factor determining between-subject differences in BMI, equal to that of AT in men and normal weight women. Additionally, the results of the current study show that factors predicting BMI after controlling for AT, such as age, race, and activity levels, are largely accounted for by their association with SM.
BMI as a Measure of Adiposity
As shown in earlier studies, after controlling for BMI, other factors add to AT prediction, including age (4, 5), race (6, 7), body build (7), and physical activity levels (8). Age had only a small effect in our adiposity prediction models, but the age range of our subjects was relatively narrow (33-45 yrs). Others have found greater %AT with older age even after adjusting first for BMI (5). The relative increase in AT with aging corresponds to a parallel loss of SM (17, 33).
A critical issue for setting universal BMI ranges is the stability of BMI-adiposity relations across different race and ethnic groups. Deurenberg et al. (10, 11) and Rush et al. (12, 13) have reported ethnic variation in BMI-adiposity relationships and have attributed these findings to differences in body build, extremity proportions, and muscularity as defined by DXA estimates of extremity lean soft tissue. Our study extends these earlier observations using advanced whole-body MRI-AT and SM measurements and strongly supports the hypothesis that differences in muscularity account for the observed variation in BMI-AT relationships across African American and Caucasian subjects even after controlling for age and lifestyle factors, including physical activity levels and alcohol intake. The important implication of these findings is that BMI, when applied across race groups, may provide a biased measure of adiposity secondary to race differences in muscularity.
The lower adiposity after controlling first for BMI in physically active subjects is consistent with earlier studies (8) and is in part attributable to exercise-induced effects on body composition, notably SM. At the same BMI, physically active subjects have a lower AT and larger SM mass, an effect that was more evident in men. While young “athletes” may have a high BMI due to lean mass rather than fat, we focus here on subjects with “average” physical activity whose age range and BMI make them important candidates for screening and lifestyle interventions.
Skeletal Muscle as a Source of BMI Variation
A central feature of our study is the presentation of an allometric SM prediction model that provides new insights into BMI-AT associations. We focused our analysis specifically on SM rather than the substantially larger and heterogeneous FFM compartment often considered when critically evaluating BMI as a measure of adiposity.
We developed our SM model a priori by considering factors known to associate with muscle mass including height, mechanical loading conditions (i.e., adiposity, physical activity level)(8), ethnicity (34), and age (17, 33). Other as yet less well defined factors may be involved, but we based our model on established relations. As expected, we observed positive associations between height and SM, although the correlations were generally weak, with only 11-15% of the variation explained in men, and 19-20% in women.
A striking observation emerging as part of our SM model development is that SM represented a larger fraction of body mass than AT in men throughout the entire range of evaluated BMIs. In contrast, SM was larger in women whose BMI on average was ~26-28 kg/m2or lower; above that level AT predominated as a fraction of body mass. These observations in a population-based sample of adults underscore a prominent sex difference in body structure in relation to BMI and adiposity. Accordingly, in men, SM and AT roughly accounted equally for between-individual differences in BMI. This is distinct from the women in whom AT was a predominant determinant of between individual differences in BMI, driven primarily by those who are obese.
A second related observation emerging from our SM models is that all of the entered predictors collectively only accounted for 43% of between individual differences in SM among the men and a somewhat larger percentage (60%) in women. Race remained an independent SM predictor in our models even after controlling for height and physical activity levels. Baumgartner et al. (33) developed similar models for an older 96% non-Hispanic white cohort using extremity lean soft tissue measured by DXA as a proxy for SM mass. Free testosterone and IGF-1 levels added to the SM prediction model for men (total R2, 0.57) in the study by Baumgartner et al, including controlling for knee height and the presence of cardiovascular disease. The corresponding model in women had an R2 of 0.52 and included knee height, fat mass, and physical activity as predictor variables; hormones did not enter the SM prediction model for the women. Thus, a large proportion of the variance in SM remains unexplained, suggesting that there are other important as yet unmeasured factors leading to between-subject differences in muscularity. Our measure of self-reported physical activity was also qualitative and more advanced accurate methods of estimating non-resting energy expenditure are available and should be considered for in future studies.
A limitation of our study is that the age range of CARDIA subjects is relatively small (33-45 years old) compared to the general adult population. Variation in SM relative to BMI may play a smaller role when considered across the entire adult lifespan.
Implications
As in other reports (3-14), our findings support the observation that BMI is a modest surrogate measure of adiposity at the individual level. Some of our subjects evaluated in a population-based sample who were classified as “obese” by BMI, and thus having excess adiposity (35), had actual %AT levels similar to those of subjects within the “normal” BMI range (Figure 2). The potential for diagnosing subjects as obese, a condition defined by “excess” adipose tissue, is particularly present in men who have greater variation in muscularity and related adiposity than women. Another important finding is that when both AT and SM are used to predict BMI, race, age and lifestyle factors are no longer associated with BMI.
Those individuals most at risk for classification as overweight or obese based on BMI in the absence of excess adipose tissue are young males, particularly those who are physically active and African American. Similarly, BMI is also a measure of adiposity with limited predictive value in non-obese women, notably those who are African American. These predictions are consistent with reports examining the question of optimum means of recruiting and retaining military personnel in an environment with clear weight/BMI criteria (36, 37).
Similar concerns apply when considering BMI as a measure of adiposity at the population level. In particular, misinterpretations may prevail when applying BMI as a phenotypic marker of adiposity across populations differing in age, race, and activity levels, factors shown in the present report to associate with variation in muscularity. The search for obesity genes continues to grow with genome wide association studies typically based on BMI as a measure of adiposity. Our findings suggest that BMI can equally represent SM in men and normal weight women.
While BMI has several favourable characteristics as a screening tool for evaluating individual and population adiposity, our findings strongly argue for cautious interpretation in selected contexts. When practical, a second tier evaluation should be considered that provides improved resolution on health risk prediction (e.g., waist circumference estimates or even blood tests) and direct adiposity measures (e.g., DXA estimates of body fat). While additional measures beyond BMI add cost and complexity to evaluations, their improved resolution of health risks and adiposity may add substantial value depending on the context.
Conclusions
Body mass index, growing in worldwide use and promoted as a measure of adiposity, not only represent a subject or group’s adiposity, but their level of muscularity as well. Muscularity at the population level, as defined the current report, is influenced to varying degrees by age, race, and lifestyle factors. In addition to a cautionary note regarding the application and interpretation of BMI, our findings encourage the continued development of other means of quantifying body composition and establishing health risks in individual subjects and populations.
Acknowledgments
Dr. Heymsfield contributed to the manuscript in its conception and design, analysis and interpretation of the data, drafting and critically revising the manuscript. Dr. Scherzer contributed to the manuscript by analysis and interpretation of the data, drafting and critical revising the manuscript. Dr. Pietrobelli contributed to the manuscript by analysis and interpretation of the data and critical review of the manuscript. Dr. Lewis was involved in the acquisition of the data, critical revision, obtaining funding and administrative support. Dr. Grunfeld contributed to the conception and design, acquisition, analysis and interpretation of the data, drafting and critically revising the manuscript.
Supported by NIH grants: K23 - AI 66943, RO1 - DK57508, HL74814, and HL 53359, CFAR - AI50410 and NIH GCRC grants M01- RR00036, RR00046, RR00051, RR00052, RR00054, RR00083, RR0636, and RR0086.
Footnotes
None of the authors report conflicts of interest.
References
- 1.National Institutes of Health, National Heart, Lung, and Blood Institute. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 2.Quetelet LAJ. A Treatise on Man and the Development of his Faculties. Edinburgh: William and Robert Chambers; 1842. [DOI] [PubMed] [Google Scholar]; Comparative statistics in the 19th century. Farnborough: Gregg International Publisher; 1973. [Google Scholar]
- 3.Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chron Dis. 1972;25:329–343. doi: 10.1016/0021-9681(72)90027-6. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, Harris TB, Everhart JE, Schenker N. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89:500–8. doi: 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallagher D, Visser M, Sepúlveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143:228–39. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 7.Gurrici S, Hartriyanti Y, Hautvast JG, Deurenberg P. Differences in the relationship between body fat and body mass index between two different Indonesian ethnic groups: the effect of body build. Eur J Clin Nutr. 1999;53:468–72. doi: 10.1038/sj.ejcn.1600778. [DOI] [PubMed] [Google Scholar]
- 8.Ode JJ, Pivarnik JM, Reeves MJ, Knous JL. Body mass index as a predictor of percent fat in college athletes and nonathletes. Med Sci Sports Exerc. 2007;39:403–9. doi: 10.1249/01.mss.0000247008.19127.3e. [DOI] [PubMed] [Google Scholar]
- 9.Larsson I, Henning B, Lindroos AK, Näslund I, Sjöström CD, Sjöström L. Optimized predictions of absolute and relative amounts of body fat from weight, height, other anthropometric predictors, and age. Am J Clin Nutr. 2006;83:252–9. doi: 10.1093/ajcn/83.2.252. [DOI] [PubMed] [Google Scholar]
- 10.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat percent relationships. Obes Rev. 2002;3:141–6. doi: 10.1046/j.1467-789x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 11.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes. 1998;22:1164–71. doi: 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
- 12.Rush EC, Goedecke JH, Jennings C, Micklesfield L, Dugas L, Lambert EV, Plank LD. BMI, fat and muscle differences in urban women of five ethnicities from two countries. Int J Obes. 2007;31:1232–9. doi: 10.1038/sj.ijo.0803576. [DOI] [PubMed] [Google Scholar]
- 13.Rush E, Plank L, Chandu V, Laulu M, Simmons D, Swinburn B, Yajnik C. Body size, body composition, and fat distribution: a comparison of young New Zealand men of European, Pacific Island, and Asian Indian ethnicities. N Z Med J. 2004;117(1207):U1203. [PubMed] [Google Scholar]
- 14.Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, Allison TG, Batsis JA, Sert-Kuniyoshi FH, Lopez-Jimenez F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes. 2008;32:959–66. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heymsfield SB, Gallagher D, Kotler DP, Wang Z, Allison DB, Heshka S. Body-size dependence of resting energy expenditure can be attributed to non-energetic homogeneity of fat-free mass. Am J Physiol Endocrinol Metab. 2002;282:E132–8. doi: 10.1152/ajpendo.2002.282.1.E132. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Heo M, Lee RC, Kotler DP, Withers RT, Heymsfield SB. Muscularity in adult humans: proportion of adipose tissue-free body mass as skeletal muscle. Am J Hum Biol. 2001;13:612–9. doi: 10.1002/ajhb.1099. [DOI] [PubMed] [Google Scholar]
- 17.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol. 2000;89:81–8. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 18.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–16. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 19.Heymsfield SB, Gallagher D, Mayer L, Beetsch J, Pietrobelli A. Scaling of human body composition to stature: new insights into body mass index. Am J Clin Nutr. 2007;86:82–91. doi: 10.1093/ajcn/86.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scherzer R, Bacchetti P, Shlipak MG, Kotler D, Lewis CE, Shen W, Heymsfield SB, Grunfeld C. Simple Anthropometric Measures Correlate with Metabolic Risk Indicators as well as MRI-Measured Adipose Tissue Depots in both HIV-infected and Control Subjects. Am J Clin Nutr. 2008;87:1809–17. doi: 10.1093/ajcn/87.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tien PC, Benson C, Zolopa AR, Sidney S, Osmond D, Grunfeld C. The study of fat redistribution and metabolic change in HIV infection (FRAM): methods, design, and sample characteristics. Am J Epidemiol. 2006;163:860–9. doi: 10.1093/aje/kwj111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes GH, Cutter G, Donahue R, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (CARDIA) Study. Control Clin Trials. 1987;8:68S–73S. doi: 10.1016/0197-2456(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 23.Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm ER. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Clin Nutr. 1999;69:381–7. doi: 10.1093/ajcn/69.3.381. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi-Sunyer FX, Heymsfield SB. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275:E249–58. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 25.Bacchetti P, Gripshover B, Grunfeld C, Heymsfield S, McCreath H, Osmond D, Saag M, Scherzer R, Shlipak M, Tien P. Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM). Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40:121–31. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bacchetti P, Cofrancesco J, Heymsfield S, Lewis CE, Scherzer R, Shlipak M, Tien PC. Fat distribution in women with HIV infection. J Acquir Immune Defic Syndr. 2006;42:562–71. doi: 10.1097/01.qai.0000229996.75116.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sidney S, Jacobs DR, Jr, Haskell WL, et al. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 1991;133:1231–45. doi: 10.1093/oxfordjournals.aje.a115835. [DOI] [PubMed] [Google Scholar]
- 28.Hoegerman GS, Lewis CE, Flack J, Raczynski JM, Caveny J, Gardin JM. Lack of association of recreational cocaine and alcohol use with left ventricular mass in young adults. The Coronary Artery Risk Development in Young Adults (CARDIA) study. J Am Coll Cardiol. 1995;25:895–900. doi: 10.1016/0735-1097(94)00469-7. [DOI] [PubMed] [Google Scholar]
- 29.Efron B, Tibshirani R. An introduction to the Bootstrap. London: Chapman and Hall; 1993. [Google Scholar]
- 30.Flegal KM. Epidemiologic aspects of overweight and obesity in the United States. Physiol Behav. 2005;86:599–602. doi: 10.1016/j.physbeh.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 31.Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity.Nat Genet 20094118–24. [DOI] [PubMed] [Google Scholar]
- 32.Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes. 2008;32(Suppl 3):S56–9. doi: 10.1038/ijo.2008.87. [DOI] [PubMed] [Google Scholar]
- 33.Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107:123–36. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 34.Jones A, Jr, Shen W, St-Onge MP, Gallagher D, Heshka S, Wang Z, Heymsfield SB. Body-composition differences between African American and white women: relation to resting energy requirements. Am J Hum Biol. 2001;13:612–9. doi: 10.1093/ajcn/79.5.780. [DOI] [PubMed] [Google Scholar]
- 35.Van Itallie TB. Obesity: adverse effects on health and longevity. Am J Clin Nutr. 1979;32(12 Suppl):2723–33. doi: 10.1093/ajcn/32.12.2723. [DOI] [PubMed] [Google Scholar]
- 36.Weight Management State of the Science and Opportunities for Military Programs Institute of Medicine. National Academies Press; Washington, DC: 2004. [PubMed] [Google Scholar]
- 37.Haddock CK, Poston WS, Klesges RC, Talcott GW, Lando H, Dill PL. An examination of body weight standards and the association between weight and health behaviours in the United States Air Force. Milit Med. 1999;164:51–4. [PubMed] [Google Scholar]


