Abstract
Buprenorphine induction poses a barrier for physician adoption of office-based opioid dependence treatment. We conducted a retrospective chart review of the first 41 patients inducted at a newly established outpatient treatment program to examine the induction process and determine strategies associated with greater induction efficiency. Timed withdrawal scales, medication log, and notes enabled reconstruction of the initial day of buprenorphine treatment. To assess change with experience, consecutive patients were divided into three chronological groups for analyses (Phases 1–3). The time required for induction was substantial in Phase 1 (mean 5.5 hours), but temporal efficiency improved to a mean 1.5 hours spent at the program by Phase 3 (p<.001). Phase 2–3 patients arrived to the program after significantly longer opioid abstinence and were in greater withdrawal, with mean Clinical Opioid Withdrawal Scale scores of 6, 10, and 10 for Phases 1–3, respectively (p<.01). Patients in the later phases had less time delay to medication initiation, 5 minutes in Phase 3 compared to 133 minutes in Phase 1 (p<.001). The mean 7 mg buprenorphine dose administered in the office did not differ between groups, but occurred over a smaller time interval for later phases indicating more rapid titration. Patients in the later phases had more rapid withdrawal relief after buprenorphine initiation and were more likely to have used pre-induction ancillary withdrawal medication. The study sheds light on the induction barrier and provides practical procedural information to inform clinical guidelines and hopefully mitigate procedural aspects of the induction barrier.
INTRODUCTION
Office-based buprenorphine maintenance is a safe and effective treatment of opioid dependence1–3 that is supported by a growing body of evidence as an alternative to program-based care to expand treatment access.4 Although buprenorphine treatment availability is increasing, physician adoption has been inadequate to meet national treatment demand, particularly among those lacking specialty addiction treatment experience, and opioid dependence remains largely untreated.5–6 Barriers for physician adoption include factors such as lack of experience, inadequate resources, remuneration, and particularly for novice prescribers, the challenge of induction.7–10
The induction barrier likely stems in part from concern about potential buprenorphine precipitated withdrawal and the logistical process required during initiation. Risk of precipitated withdrawal relates to the amount and duration of action of the abused opioid, as well as time since last use prior to buprenorphine initiation.11 To minimize risk, patients must abstain from the abused opioid and be in early withdrawal prior to buprenorphine initiation. National guidelines recommend 12–24 hours of abstinence from short acting opioids such as heroin and 24 or more hours for long-acting opioids such as methadone.11 Recommended starting doses range from 2–4 mg depending on degree of physical dependence and duration of action of the abused opioid.11 Although guidelines encourage use of a withdrawal scale to quantify withdrawal and monitor treatment response, they do not specify a withdrawal score threshold above which medication initiation is tolerable. Imprecise induction recommendations regarding abstinence length, dosing, and withdrawal threshold is a likely major source of ambiguity for inexperienced physicians; notably, two of the top three clinical support requests from the Physician Clinical Support System, a national buprenorphine mentors network, involve questions about induction procedure dosing (32%) and timing (26%),12 which highlights the induction challenge.
Another procedural factor that may limit physician adoption of buprenorphine involves time required for induction, given that guidelines recommend supervised initiation with up to two hours of monitoring. Physicians consistently rate perceived time constraints as an important barrier to addressing substance abuse.13–14 Certified buprenorphine prescribers with varying levels of treatment experience report that lack of time is a logistical barrier for general implementation.9–10 Following a buprenorphine training program, non-certified physicians reported that time required for induction contributed to hesitancy to adopt treatment in practice.8 Although two community-based primary care programs report allocating as much as 4–8 hours to the process on the initial day of treatment,15–16 limited data exist about the actual amount of time required for office induction in clinical practice.
Addressing barriers that impede buprenorphine diffusion in general practice is a national priority,17 which warrants collection of empirical clinical data to clarify clinical guidelines and mitigate the induction barrier. We conducted a retrospective chart review study to examine the first day of induction at a newly established buprenorphine treatment program. Since a lengthy induction procedure may discourage buprenorphine adoption, we sought to assess the temporal process of induction and identify practices and other factors associated with greater efficiency as experience accrued. The practice-based evidence will inform clinical guideline development and hopefully facilitate greater adoption of buprenorphine treatment in practice.
METHODS
Program Procedures
The Buprenorphine Program is private outpatient clinic in the Department of Psychiatry of Columbia University that began inducting patients September 2003 with practices largely based on national guidelines.11 Staff included a clinical psychologist, a registered nurse, and two buprenorphine-waivered addiction specialist physicians, only one of whom supervised each individual patient’s induction. An initial assessment established an opioid dependence diagnosis18 and determined co-occurring psychiatric disorders, opioid and other illicit substance use, and treatment history with documentation on standardized clinical intake forms. Patients deemed appropriate for buprenorphine treatment were instructed by the physician to abstain from opioids and be in mild withdrawal before returning to the program for the induction visit, which was described to the patient as a general constellation of mild gastrointestinal flu-like symptoms, restlessness, and/or anxiety.11 Non-physician clinical staff also assisted with the patient assessment and provision of induction instructions at the first office visit. Initially, patients were instructed to abstain for 12 hours from short-acting opioids and 24 hours from long-acting opioids. After a case of precipitated withdrawal and as some patients seemed to arrive in inadequate withdrawal, this was later increased to 16 hours for short-acting opioids and 36 hours for patients on methadone (transition from 40mg or less was permitted19).
Timed Clinical Opioid Withdrawal Scales (COWS) were administered by the physician upon arrival to the clinic for induction and serially to monitor treatment response (available in Appendix B of the Treatment Improvement Protocol #40: Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction).11,20 Timing and type of last opioid use was documented along with ancillary withdrawal medication use. Ancillary medication prescription was not a standardized prior to induction. However, some patients reporting concern about achieving adequate abstinence prior to induction due to inability to tolerate withdrawal were prescribed ancillary medication such as an antiemetic, sedative-hypnotic, or clonidine. Patients prescribed a benzodiazepine from an outside physician were permitted to take an extra dose the night before induction for insomnia and/or anxiety.
The COWS was used for all patients to quantify withdrawal prior to induction and to monitor response, with a score between 5–12 considered mild withdrawal.20 After an early case of precipitated withdrawal in which only subjective COWS items were positive, it was required that at least some objective withdrawal signs be present prior to induction (e.g., mydriasis, diaphoresis). Patients arriving in inadequate withdrawal waited in the office for spontaneous withdrawal to increase. After buprenorphine initiation, COWS were performed approximately hourly with the time of COWS and medication administration documented for all patients. Report of increased withdrawal or withdrawal relief might prompt more frequent COWS administration.
Patients received the sublingual buprenorphine/naloxone combination tablet (2mg/0.5mg or 8mg/2mg) with an initial buprenorphine dose of 2–4mg, while those transitioning from methadone received pure buprenorphine.11 Hereafter the term buprenorphine will be used for both preparations unless specified. Additional doses were administered in the office every 1–2 hours until withdrawal subsided. Buprenorphine and ancillary medications were stored at the program in a double locked narcotic cabinet and available for immediate office administration. Ancillary withdrawal medication, such as an antiemetic, anxiolytic, or clonidine, was occasionally administered to patients with persistent symptoms beyond 90–120min after buprenorphine initiation. Patients were discharged from the program after initial withdrawal subsided. They received a small amount of take-home buprenorphine and/or prescription with instructions for additional doses every 2 hours for recurrent withdrawal or cravings to a total maximum dose of 16mg on the initial day, which is higher than the recommended 8mg maximum.11 Patients requesting detoxification or maintenance underwent the same induction procedure. The physician documented phone conversations scheduled with the patient the evening of induction and following day. Patients returned for a follow-up visit in several days.
Study Procedures
Charts were reviewed retrospectively for all of the first consecutive 41 patients inducted by one physician (EWG) who documented the time of all COWS and medication dosing, thus enabling retrospective assessment of temporal outcomes. Data were collected on demographics, co-morbid conditions, recent opioid use pattern, and prior substance use and treatment. COWS scores were collected to determine degree of withdrawal upon program arrival, immediately prior to buprenorphine initiation, and sequentially to document treatment response and initial withdrawal resolution. Buprenorphine administration data included: 1) initial dose; 2) total dose administered while the patient remained at the program; and 3) total dose administered on the first day of induction – a sum of program administered buprenorphine and unobserved doses taken on Day 1 after discharge from the program, which were documented in phone contact notes. Ancillary medication use before and after buprenorphine initiation was recorded, which included prescribed medication and non-medical use of medication prescribed by a physician outside the program (e.g., an extra benzodiazepine dose the night before induction), but did not include use of medication obtained illicitly. All patients returned for a follow-up stabilization appointment, however, data were not collected past induction Day 1 given limited documentation between initial and follow-up visits.
To examine induction process change with experience, patients were divided into three chronologic phases balanced by number and spanning approximately 2–3 months. Phase 1 patients were the first third to be inducted (n=14), Phase 2 the second third (n=13), and Phase 3 the final third (n=14). The decision to divide patients into these phases was made a priori based on the change in induction instructions between Phases 1 and 2 (e.g., more emphatic instructions on opioid abstention, including a change to 16 hours or more for short-acting opioids and 36 hours for methadone) and requirement for objective withdrawal signs. In addition, we expected improved staff confidence and comfort with induction between Phases 2 and 3 would lead to continued improvement in temporal efficiency assessed by the following outcome measures:
Total time spent at the Program on the day of induction: The initial COWS, administered soon after arrival for all patients, estimated program arrival. Hence, the mean time (minutes) at the program was estimated from the elapsed time between 1) initial COWS, and 2) timing of the final COWS, medication administration, or timed note. In most instances, a COWS was the last timed documentation, as this was performed to document withdrawal relief and clinical stability prior to program discharge, which typically occurred soon thereafter.
Time delay prior to initial buprenorphine dosing: As medication was stored and available onsite for immediate administration, the clinical decision to delay buprenorphine initiation was due to physician concern that withdrawal severity on initial COWS was inadequate. In this instance, dosing was delayed and additional COWS were performed prior to starting buprenorphine to document that spontaneous withdrawal had increased. Hence, the occurrence of a time delay was defined dichotomously (Y/N) as having occurred when at least two or more COWS were obtained prior to initial dosing. In addition, the amount of time delay was quantified by the number of minutes that elapsed between initial COWS on arrival to the program and the time of the first buprenorphine dose.
Time until initial withdrawal relief after buprenorphine initiation: The amount of time elapsing between first buprenorphine dose and initial relief of withdrawal was determined by the number of minutes between 1) the first buprenorphine dose and 2) the time of the first COWS score ≤ 4, which was defined as being “without withdrawal,” because mild withdrawal on the COWS has been defined as 5–12.20
Data Analysis
Differences in continuous variables between the three phases were assessed with analysis of variance (ANOVA) and t-tests for post hoc comparisons. As an additional post hoc secondary assessment, we examined differences in induction outcome based on whether or not the patient reported an adequate period of opioid abstinence prior to program arrival. Adequate abstinence was defined dichotomously (Y/N) if the patient abstained a minimum of 16 hours for short-acting opioids, 24 hours for long-acting, sustained-release opioid preparations, and 36 hours for methadone. Independent samples t-tests were used to compare continuous variables between those with or without adequate abstinence. Comparisons of differences between groups across categorical variables were made with Chi-squared or Fisher’s Exact Tests. Descriptive analyses were conducted to evaluate the recent opioid use pattern of the sample. Analyses were performed with SPSS 16.0 (SPSS, Inc., Chicago, IL). Data were collected without a unique identifier. Signed informed consent for retrospective record review was obtained from patients actively receiving maintenance treatment at the program (none declined). For those no longer receiving care at the program (e.g., completed treatment, dropped out, or transferred), records were reviewed with a waiver of informed consent. A Certificate of Confidentiality was obtained from NIDA. The New York State Psychiatric Institute IRB approved the protocol.
RESULTS
Patient Characteristics
Demographic and clinical characteristics of the 41 patients are depicted in Table 1. Data were collapsed given a lack of significant difference between phases by any characteristic except age. The mean (SD) ages for Phases 1, 2, and 3 were 44 (10), 33 (11), and 44 (9) years, respectively (F2,38 = 5.4, p<.01). Two patients (5%) had used buprenorphine purchased illicitly (both Phase 2), but not recently prior to treatment entry. The sample had a heterogeneous current opioid use pattern. For the 17 (41%) patients whose primary opioid was heroin (Table 1, defined as past month daily use), the reported range in bags per day (bpd) was 4–50, with a mean (+/− SD) 11 (11) bpd, and median 7 bpd. Omitting the 50 bpd outlier (Phase 3), mean (SD) usage was 9 (5) bpd. Nine (22%) transitioned from methadone with a range of 2–50 mg/day, mean (SD) 28 (14) mg/day, and median 30 mg/day. Although the program practices permitted methadone transfers from 40 mg or less as described above,19 an exception was made for one patient transitioning from 50 mg daily (Phase 2) who was a rapid methadone metabolizer21 with low trough plasma methadone levels at the opioid treatment program. For the 17 (41%) of patients transitioning from non-methadone prescription opioids, the majority (n=10) transitioned from oxycodone preparations with a dose range of 20–440 mg/day, a mean (+/− SD) of 142 (122) mg/day, and median of 110mg/day. Omitting the 440 mg/d outlier (Phase 3), the range was 20–240 mg, with a mean (SD) of 109 (67) mg/day. Other primary opioids included: hydrocodone (n=3, range 50–500 mg/d, maximum dose in Phase 3); long-acting morphine (n=2, 60–200 mg/d, maximum dose in Phase 2); codeine (n=1, 600 mg/d, Phase 1); and propoxyphine (n=1, 1200 mg/d, Phase 1).
Table 1.
Demographic and Clinical Characteristics
| Characteristic | Total (n=41)* |
|---|---|
| Age, years | 41 (11) |
|
| |
| Sex, male | 24 (59%) |
|
| |
| Race/Ethnicity | |
| White | 32 (78%) |
| Black | 5 (12%) |
| Hispanic | 4 (10%) |
|
| |
| Employed, at least part-time | 23 (56%) |
|
| |
| Insured | 34/39† (83%) |
|
| |
| Education, some college or higher | 29/38† (74%) |
|
| |
| Married | 21 (51%) |
|
| |
| Chronic pain | 11 (27%) |
|
| |
| Psychiatric disorder | 28 (68%) |
|
| |
| Cocaine dependence, lifetime | 9 (22%) |
|
| |
| Cocaine use, past month | 13 (32%) |
|
| |
| Primary Opioid, past month daily use: | |
| Heroin | 17 (41%) |
| Prescription opioids (non-methadone) | 17 (41%) |
| Methadone | 9 (22%) |
|
| |
| Any prior opioid dependence treatment (detoxification, rehabilitation, methadone maintenance) | 30 (73%) |
|
| |
| Prior detoxification | 26 (63%) |
|
| |
| Mean number of prior detoxifications | 1.9 (3.1) |
|
| |
| Prior methadone maintenance treatment | 18 (44%) |
|
| |
| Current methadone maintenance treatment | 7 (17%) |
Data are means (standard deviation) or numbers (percentage)
n<41 due to missing data
Induction Process
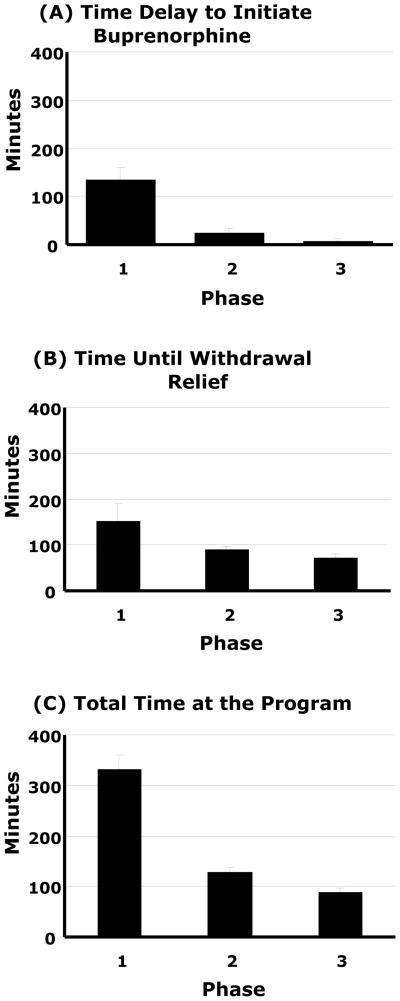
Phase 1 patients arrived to the program in significantly less withdrawal than Phases 2–3 with mean (SD) COWS of 6 (4), 10 (3), and 10 (3), respectively (F2,38 = 5.7, p<.01; Phase 1<2,3, p<.01). Buprenorphine dosing was delayed for 10/14 (71%) patients in Phase 1, 3/13 (23%) in Phase 2, and no patients in Phase 3 (p<.001). The elapsed time delay to medication administration decreased from 133 minutes in Phase 1 to five minutes in Phase 3 (Figure 1a; F2,38 = 18.33, p<.001; Phase 1<2,3, p<.001). Although withdrawal was lower for Phase 1 on arrival, by waiting to initiate treatment, spontaneous withdrawal increased and the COWS obtained immediately prior to dosing did not differ between phases (overall mean 10 (3), range 6–19). Patients in the later phases were more likely to have taken ancillary medication prior to presenting to the clinic for induction: 7%, 31%, and 57% in Phases 1–3, respectively (p<.05). In aggregate, 6 (15%) took clonidine, 5 (12%) a benzodiazepine, 4 (10%) trazodone, 3 (7%) zolpidem, 3 (7%) an antiemetic or antidiarrheal medication, and 2 (5%) ibuprofen.
Figure 1.
Time (minutes) required during the process of office buprenorphine induction assessed across three phases of experience: A) Time delay to buprenorphine initiation after program arrival (1>2,3, p<.05), B) Time until withdrawal relief after buprenorphine initiation (1>3, p<.05), and C) Total time at the program (1>2>3, p<.05). Data are means ± SE.
When buprenorphine was initiated, Phase 1 patients received a significantly smaller mean (SD) first dose than later Phases: 2 (1) mg, 3 (1) mg, and 3 (1) mg (Table 2, F2,38 = 4.3, p<.05). There was no significant difference between phases regarding total dose administered at the program (overall mean 7 (4) mg) or total dose administered on Day 1, which included observed dosing and unobserved dosing after leaving the program (13 (7) mg). Eight (20%) patients took ancillary medication after buprenorphine was initiated, which did not differ between phases. Medications were primarily used among those transitioning from long-acting opioids (5/8, 63%) and included clonidine, 7 (17%), an antiemetic, 6 (15%), and zolpidem or a benzodiazepine, 4 (10%). Most medication was administered due to incomplete response to buprenorphine in which patients had persistent withdrawal beyond 2–3 hours after initiation. One patient experienced precipitated withdrawal (Phase 1, 2% overall incidence), for whom the COWS score doubled after dosing. The patient used heroin just prior to induction and had only subjective findings on the pre-buprenorphine COWS. Withdrawal was relieved after approximately 8 hours using ancillary medication.
Table 2.
Buprenorphine Dosing
| Phase 1 (n=14) | Phase 2 (n=13) | Phase 3 (n=14) | P value | |
|---|---|---|---|---|
| Initial buprenorphine* dose | 2 (1) | 3 (1) | 3 (1) | <.05 (1<2,3) |
| Total buprenorphine* dose at the program | 9 (6) | 7 (3) | 6 (4) | NS |
| Total buprenorphine* dose, Day #1 | 13 (6) | 11 (6) | 14 (9) | NS |
Includes mean (standard deviation) buprenorphine dose in mg for both buprenorphine-naloxone combination and pure buprenorphine formulations.
NS = non-significant
The time until withdrawal was relieved significantly decreased across Phases from 153 minutes in Phase 1 to 71 minutes in Phase 3 (Figure 1b, F2,38 = 3.3, p<.05; Phase 1>3, p<.05). The total length of time at the program on the day of induction significantly decreased as well, from 331 minutes in Phase 1 to 90 minutes in Phase 3 (Figure 1c, F2,38 = 39.1, p<.001; Phase 1>2,3, p<.001; 2>3, p<.05).
Adequacy of Abstinence Prior to Induction
Overall 25/39 (64%) had an adequate period of abstinence prior to program arrival, which improved from 31% in Phase 1, to 83% and 79% in Phases 2–3 (p<.01). Patients with adequate abstinence had significantly higher mean (SD) COWS on arrival than those with inadequate abstinence, 10 (3) vs. 7 (4), p<.05, and less delay to buprenorphine initiation, mean 24 (42) vs. 95 (97) minutes, p<.01. By delaying treatment initiation, spontaneous withdrawal increased and there was no difference between COWS scores immediately preceding buprenorphine initiation: 10 (3) vs. 10 (2) in the adequate vs. inadequate groups, respectively. Despite similar COWS scores at initiation, once medication was started, patients who had arrived after adequate abstinence had a shorter time until withdrawal relief (mean 77 (47) vs. 153 (137) minutes, p<.05) and spent less total time at the program (mean 123 (72) vs. 282 (148) minutes, p<.001).
DISCUSSION
Medical record documentation at a newly established outpatient buprenorphine treatment program was used to reconstruct the process of induction for the first 41 consecutive patients. By dividing the sample into three chronological phases, the findings illustrate the extent of time required for induction early after treatment adoption, and also provide practical procedural information to clarify clinical guidelines and assist physicians challenged by induction. During Phase 1, patients spent a mean 5.5 hours at the program for the initial day of induction, a likely prohibitive burden for many practices with limited resources. The time consuming aspect of induction limits inexperienced physician adoption,8 and a community-based primary care practice allocating up to 4 hours of observation noted the particular challenge of office induction.16 With accrual of experience, the time requirement substantially decreased to 1.5 hours by Phase 3, which is more manageable and hopefully encouraging for hesitant prescribers. The improvement in temporal efficiency parallels physician self-report that induction poses less of a challenge with clinical experience.10
Decreased time at the program during Phases 2–3 resulted from more rapid buprenorphine initiation after patient arrival at the program and faster withdrawal resolution once buprenorphine was started. Unlike Phase 3, where medication initiation occurred approximately 5 minutes after arrival, the mean time delay to start treatment in Phase 1 was over two hours. Phase 1 patients had less adequate abstinence and arrived in significantly less withdrawal, prompting the clinical decision to delay buprenorphine initiation for 71% of patients so that spontaneous withdrawal would increase. By waiting to start treatment, spontaneous withdrawal eventually increased and the COWS score at dosing did not differ between phases (overall mean 10). At this level of withdrawal on the COWS, induction was tolerated well by most patients, consistent with findings from a therapeutic community in which a mean COWS score of 8 preceded buprenorphine dosing.22 These real-world clinical data suggest an appropriate COWS score cutoff of approximately 8–10 prior to induction, which helps clarify current clinical guidelines lacking specific cut-off recommendations.11
After buprenorphine initiation, Phase 2–3 patients experienced more rapid withdrawal relief than Phase 1. Several factors may have contributed to the difference, including lack of precipitated withdrawal, larger initial dose,23 and more rapid office titration,24 in which patients received a similar amount of medication over a shorter time period in the office. In addition, longer opioid abstention prior to induction was associated with more rapid withdrawal relief, further supporting the importance of adequate abstinence11 and suggesting 16 hours for short-acting opioids, 24 hours for sustained-release opioid preparations, and 36 hours for methadone. These findings address common physician questions about buprenorphine dosing and timing.12
Pre-induction ancillary withdrawal medication taken primarily the night before a morning induction was used more often in the later phases and may have enabled longer abstention. In addition, ancillary medication may have masked the degree of spontaneous withdrawal measured by the COWS on arrival to the program and eased the transition. Use of pre-induction ancillary medication to facilitate induction is reported in primary care,25 but is not part of clinical guidelines and requires further study before routine recommendation. Notably in our study, 12% of all patients took an adjunctive benzodiazepine. Most were prescribed a benzodiazepine by a physician outside the program and took an extra dose prior to presenting for induction. However, the potential for synergistic respiratory suppression with opioids, including the partial agonist buprenorphine,26 raises concern about routine recommendation due to overdose risk.27
After buprenorphine initiation, 20% of patients received ancillary medication either at the program or at home. Little is known about the need for ancillary medication in clinical practice once buprenorphine has been started. However, ancillary withdrawal medication prescription was common during the induction phase of a national, community-based, buprenorphine-naloxone detoxification study.28 Over 50% of patients required ancillary medication during all 3 days of induction in which patients received 8mg of buprenorphine on Days 1–2 and 16mg on Day 3.28 Our use of less ancillary medication on the initial day of induction could be related to more flexible and rapid increase in buprenorphine dosing. Amass et al. (2004) prescribed a Day 1 maximum of 8mg, consistent with national guidelines,11 whereas we recommended up to 16mg. Our overall mean dose 13mg (range 2–32) on Day 1 was well tolerated similar to unobserved primary care induction data29,30 and in pregnancy.31 The tolerability of higher Day 1 dosing provides additional support for recent recommendation that a 16mg maximum be permitted on the initial treatment day,32 and will help inform future guideline revision.11
While the findings help elucidate the challenge and process of induction in clinical practice, there are several limitations. Retrospective data collection necessitates that temporal outcome measures remain estimates. However, COWS were systematically performed for all patients quickly after program arrival and serially until withdrawal relief. Standardized use of timed COWS and dosing documentation minimizes the retrospective design limitation in reconstructing temporal measures. Although we were able to evaluate withdrawal alleviation in the office and total buprenorphine dosing on Day #1 after the patients left the office, the lack of standardized follow-up prohibited assessment of recurrent or prolonged withdrawal, or additional dosing during subsequent days. However, the first day of buprenorphine administration is arguably the most crucial, time consuming, and only visit for which direct observation is required in current clinical guidelines.11 Although unobserved initiation is promising alternative,15,16,25,29,30 office induction remains the current standard of care.32
The retrospective methodology precludes assessment of potential impact on patient satisfaction with spending less time in the office during the Phases 2–3. However, all patients during Phases 2–3 returned for a follow-up appointment to continue treatment, had more rapid withdrawal relief and no cases of precipitated withdrawal. Although these findings may suggest tolerability of the more efficient procedures, future studies are needed to examine patient perspectives on induction.
The study documents induction by a single treatment program and physician, limiting generalization. However, the population transitioned from a mix of prescription opioids and heroin, adding to other real world office induction data that has included primarily heroin users.22,28,30 In addition, the physician was certified in addiction medicine and study took place soon after buprenorphine became available in the U.S. Similar challenges with induction were reported by other addiction specialists during the same period,7 with improvement noted thereafter with experience.10 The congruency with national survey data suggests generalizability to other novice physicians and settings.
Overall, the study illustrates an important aspect of the induction barrier and provides practical procedural information to mitigate the induction challenge for hesitant prescribers (Table 3). To facilitate the induction process, physicians should counsel patients up front about the importance of longer periods of abstinence from the abused opioid. Utilization of the COWS with a specific cutoff of approximately 8–10 appears sufficient for buprenorphine initiation. More rapid buprenorphine titration to 16mg on the initial day of treatment is tolerable. Based on our experience, storage of buprenorphine and ancillary withdrawal medications on site may make induction easier. However, the use of ancillary medications during induction warrants further study. By assessing induction in an actual care delivery setting, the study provides practice-based evidence that will help advance clinical guideline development and education, thus enhancing translation into clinical settings. Continued efforts are needed to understand and address the induction barrier to improve physician adoption and ensure availability of effective opioid dependence treatment.
Table 3.
Summary of Procedural Recommendations for Office Buprenorphine Induction
1) Longer abstinence with the following specific cut-offs facilitates induction:
|
| 2) A COWS* score of 8–10, preferably with objective signs, is adequate for initiation |
| 3) After tolerability of initial doses is established, rapid buprenorphine titration if needed to a Day 1 maximum dose of 16mg appears acceptable |
| 4) Ancillary withdrawal medication before or after buprenorphine initiation may facilitate induction but requires further study |
| 5) Inexperienced clinicians may need to allocate several hours for office induction, however the time requirement decreases to 1–2 hours after an initial experience of about a dozen inductions |
COWS = Clinical Opioid Withdrawal Scale
Acknowledgments
This study was supported by grants K23 DA02000 (Dr. Gunderson), K02 DA00465 (Dr. Levin), and K05 DA14284 (Dr. Kleber) from the National Institute on Drug Abuse, Rockville, MD.
We also gratefully acknowledge the Yale-New Haven Hospital Primary Care Center Buprenorphine Program, including David Fiellin, MD, Bonnie Lurie, RN, and Kathleen Gargano, RN, for assistance with program development. We also wish to acknowledge Ashley Williams for assistance with manuscript preparation.
Footnotes
These data were presented in part at the 66th Annual Scientific Meeting for the College of Problems on Drug Dependence, San Juan, PR, June 12–17, 2004.
Declaration on Interests
Dr. Kleber served on the Scientific Advisory Board for Reckitt Benckiser in 2010. The other authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2003;343:1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- 2.Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349:949–958. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- 3.Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355:365–374. doi: 10.1056/NEJMoa055255. [DOI] [PubMed] [Google Scholar]
- 4.Gunderson EW, Fiellin DA. Office-based maintenance treatment of opioid dependence: how does it compare with traditional approaches? CNS Drugs. 2008;2:99–111. doi: 10.2165/00023210-200822020-00002. [DOI] [PubMed] [Google Scholar]
- 5.Fiellin DA. The first three years of buprenorphine in the U.S: experience to date and future directions. J Addict Med. 2007;1:62–67. doi: 10.1097/ADM.0b013e3180473c11. [DOI] [PubMed] [Google Scholar]
- 6.Thomas CP, Reif S, Haq S, Wallack SS, Hoyt A, Ritter GA. Use of buprenorphine for addiction treatment: perspectives of addiction specialists and general psychiatrists. Psychiatr Serv. 2008;59:909–916. doi: 10.1176/ps.2008.59.8.909. [DOI] [PubMed] [Google Scholar]
- 7.Kissin W, McLeod C, Sonnefeld J, Stanton A. Experiences of a national sample of qualified addiction specialists who have and have not prescribed buprenorphine for opioid dependence. J Addict Dis. 2006;25:91–103. doi: 10.1300/J069v25n04_09. [DOI] [PubMed] [Google Scholar]
- 8.Gunderson EW, Fiellin DA, Levin FR, Sullivan LE, Kleber HD. Evaluation of a combined online and in person training on the use of buprenorphine. Subst Abus. 2006;27(3):39–45. doi: 10.1300/J465v27n03_06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walley AY, Alperen JK, Cheng DM, et al. Office-based management of opioid dependence with buprenorphine: clinical practices and barriers. J Gen Int Med. 2008;23:1393–1398. doi: 10.1007/s11606-008-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Netherland J, Botsko M, Egan JE, et al. Factors affecting willingness to provide buprenorphine treatment. J Subst Abuse Treat. 2009;36:244–251. doi: 10.1016/j.jsat.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Center for Substance Abuse Treatment (CSAT) DHHS Publication No. (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Treatment improvement protocol (TIP) series 40. [PubMed] [Google Scholar]
- 12.Egan JE, Casadonte P, Gartenmann T, et al. The physician clinical support system-buprenorphine (PCCS-B): A novel project to expand/improve buprenorphine treatment. J Gen Intern Med. 2010;25:936–941. doi: 10.1007/s11606-010-1377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Center on Addiction and Substance Abuse (CASA) at Columbia University. Missed Opportunity: National Survey of Primary Care Physicians and Patients on Substance Abuse. New York, NY: Center on Addiction and Substance Abuse (CASA) at Columbia University; 2000. [Google Scholar]
- 14.Friedmann PD, McCullough D, Saitz R. Screening and intervention for illicit drug abuse: a national survey of primary care physicians and psychiatrists. Arch Intern Med. 2001;161:248–251. doi: 10.1001/archinte.161.2.248. [DOI] [PubMed] [Google Scholar]
- 15.Alford DP, LaBelle CT, Richardson JM, et al. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. J Gen Intern Med. 2007;22:171–176. doi: 10.1007/s11606-006-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mintzer IL, Eisenberg M, Terra M, MacVane C, Himmelstein DU, Woolhandler S. Treating opioid addiction with buprenorphine-naloxone in community-based primary care settings. Ann Fam Med. 2007;5:146–150. doi: 10.1370/afm.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Center for Substance Abuse Treatment (CSAT) and National Institute on Drug Abuse (NIDA) Executive Summary and Highlights of the Recommendations. Report of the 2005 Buprenorphine Summit. Rockville, MD: Substance Abuse and Mental Health Services Administration (SAMHSA); 2005. Buprenorphine in the Treatment of Opioid Addiction: Review and Progress 2005. [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- 19.Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70(S2):59–77. doi: 10.1016/s0376-8716(03)00060-7. [DOI] [PubMed] [Google Scholar]
- 20.Wesson DR, Ling W. The clinical opiate withdrawal scale (COWS) J Psychoactive Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- 21.Haile CN, Kosten TA, Kosten TR. Pharmacogenetic treatments for drug addiction: alcohol and opiates. Am J Drug Alcohol Abuse. 2008;34:355–381. doi: 10.1080/00952990802122564. [DOI] [PubMed] [Google Scholar]
- 22.Collins ED, Horton T, Reinke K, Amass L, Nunes EV. Using buprenorphine to facilitate entry into residential therapeutic community rehabilitation. J Subst Abuse Treat. 2007;32:167–175. doi: 10.1016/j.jsat.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Soyka M, Zingg C, Koller G, Kuefner H. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11:641–653. doi: 10.1017/S146114570700836X. [DOI] [PubMed] [Google Scholar]
- 24.Doran C, Holmes J, Ladewig D, Ling W. Buprenorphine induction and stabilization in the treatment of opiate dependence. Heroin Addict Rel Clin Problems. 2005;7:7–18. [Google Scholar]
- 25.Sohler NL, Li X, Kunins HV, et al. Home- versus office-based buprenorphine inductions for opioid-dependent patients. J Subst Abuse Treat. 2010;38:153–159. doi: 10.1016/j.jsat.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gueye PN, Borron SW, Risede P, et al. Buprenorphine and midazolam act in combination to depress respiration in rats. Toxicol Sci. 2002;65:107–114. doi: 10.1093/toxsci/65.1.107. [DOI] [PubMed] [Google Scholar]
- 27.Kintz P. Deaths involving buprenorphine: a compendium of French cases. Forensic Sci Int. 2001;121:65–69. doi: 10.1016/s0379-0738(01)00454-6. [DOI] [PubMed] [Google Scholar]
- 28.Amass L, Ling W, Freese W, et al. Bringing buprenorphine-naloxone detoxification to community treatment providers: the NIDA clinical trials network field experience. Am J Addict. 2004;13(S1):S42–S66. doi: 10.1080/10550490490440807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JD, Grossman E, DiRocco D, Gourevitch MN. Home buprenorphine/naloxone induction in primary care. J Gen Internl Med. 2009;24:226–232. doi: 10.1007/s11606-008-0866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunderson EW, Wang XQ, Fiellin DA, Bryan B, Levin FR. Unobserved versus observed buprenorphine/naloxone induction: a pilot randomized clinical trial. Addict Behav. 2010;35:537–540. doi: 10.1016/j.addbeh.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischer G, Ortner R, Rohrmeister K, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101:275–281. doi: 10.1111/j.1360-0443.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- 32.Casadonte P. [Accessed August 26, 2010];PCSS Guidance: Buprenorphine Induction. 2009 Oct 27; Available at: http://www.pcssbuprenorphine.org/pcss/documents2/PCSS_BuprenorphineInduction_050308.pdf.