SUMMARY
BACKGROUND
HIV counseling and testing is the gateway to treatment and care and provides important preventative and personal benefits to recipients. However, in developing countries the majority of HIV infected persons have not been tested for HIV. Combining community mobilization, mobile community-based HIV testing and counseling, and post-test support may increase HIV testing rates.
METHODS
We randomly assigned half of 10 rural communities in Tanzania, 8 in Zimbabwe, and 14 in Thailand to receive a multiple component community-based voluntary counseling and testing (CBVCT) intervention together with access to standard clinic-based voluntary counseling and testing (SVCT). The control communities received only SVCT. The intervention was provided for approximately 3 years. The primary study endpoint is HIV incidence and is pending completion of the post-intervention assessment. This is a descriptive interim analysis examining the percentage of the total population aged 16–32 years tested for HIV across study arms, and differences in client characteristics by study arm.
FINDINGS
A higher percentage of 16–32 year-olds were tested in intervention communities than in control communities (37% vs. 9% in Tanzania; 51% vs. 5% in Zimbabwe; and 69% vs. 23% in Thailand). The mean difference between the percentage of the population tested in CBVCT versus SVCT communities was 40.4% across the 3 country study arm pairs, (95% CI 15.8% – 64.7%, p-value 0.019, df=2). Despite higher prevalence of HIV among those testing at SVCT venues the intervention detected 3.6 times more HIV infected clients in the CBVCT communities than in SVCT communities (952 vs. 264, p< 0.001). Over time the rate of repeat testing grew substantially across all sites to 28% of all those testing for HIV by the end of the intervention period.
INTERPRETATION
This multiple component, community-level intervention is effective at both increasing HIV testing rates and detecting HIV cases in rural settings in developing countries.
INTRODUCTION
HIV counseling and testing can reduce anxiety over infection and assist individuals in making informed reproductive health and breastfeeding decisions. Importantly, HIV counseling and testing has been demonstrated to lower risk behaviors,1–2 especially among those infected with HIV and for couples who test together.3–6 Gaining knowledge of HIV infection status is also the gateway to lifesaving HIV/AIDS treatment,7–9 which also significantly reduces HIV transmission.10 Recent statistical modeling also suggests that treating high proportions of HIV-infected persons in a community may slow or even stop an HIV epidemic.11 However, among the over 33 million persons infected with HIV, most in developing countries,12 less than 30% are aware of their own infection status, and only 10% are aware of their partner’s HIV status.13 Thus, despite the major heralded successes in expanding access to HIV/AIDS treatment, a large proportion of people with HIV remain unaware they are infected, have a high likelihood of transmitting the infection to others, and cannot benefit from potentially lifesaving treatment programs without HIV counseling and testing.
In 2000 the first randomized controlled trial examining the efficacy of HIV counseling and testing in developing countries was published, demonstrating its impact on behavioral risk reduction.2 Since then, and with the advent of expanded AIDS treatment in developing countries, there have been bold efforts to expand HIV testing with major increases in financial support for voluntary counseling and testing (VCT) programs, evolving strategies to increase uptake, and improvements in the linkage between HIV testing and treatment. Strategies include expansion of free standing VCT clinics, home-based testing,14–15 VCT clinics for adolescents,16–17 expansion of HIV testing for pregnant women,18 provider initiated testing in health care settings,19–20 and mass testing campaigns.21 Yet the proportion of persons aware of their HIV infection status has remained well below that which is required to have significant impacts on the epidemic in terms of behavioral risk reduction, linkage to care and treatment, community-level awareness of the scope of the epidemic, and reductions in HIV-related stigma and discrimination. With so few people aware of their HIV infection status and thus unable to access treatment, the potential impact of ARVs in reducing HIV infectivity is also compromised. Mobile VCT has been suggested as a strategy which may help to expand knowledge of personal HIV infection status.22–23 However, rigorous studies examining the comparative benefit of mobile VCT in reaching large proportions of vulnerable populations have to date not been conducted.
This study was designed to test the hypothesis that easily accessible mobile voluntary counseling and testing services coupled with community mobilization programs and post-test psychosocial support will increase HIV testing rates and detection of HIV-infections, reduce individual risk behaviors, enhance reproductive health decision making, improve access to treatment, reduce HIV/AIDS-related stigma and discrimination, and ultimately lower HIV incidence. In this analysis we specifically examine the effect of the intervention on uptake of HIV testing and counseling, and HIV case detection.
METHODS
Study Design
Project Accept is a multisite, community randomized trial being conducted in Tanzania (Kisarawe District), Zimbabwe (Mutoko District), Thailand (Chiang Mai Province), and two sites in South Africa (Kwa Zulu Natal, and Soweto). At each of the South African and Zimbabwean sites there are 8 communities participating in the study, 10 communities in Tanzania, and 14 communities in Thailand. Each community was identified based on ethnographic mapping conducted during the formative phase of the study. Community pairs in each location were matched for similar demographics and environmental characteristics using community mapping results. One community from each pair was randomly selected by the project Statistics Center to receive the standard clinic-based voluntary counseling and testing intervention (SVCT), with the other community selected to receive both the SVCT intervention and an enhanced community-based voluntary counseling and testing intervention (CBVCT). Quality assurance was regularly assessed to maintain intervention fidelity to the protocol and standardization of the CBVCT and SVCT interventions across sites (countries) and venues (testing services).
Here we report only on results from the Tanzanian, Zimbabwean, and Thai sites. In the South African sites standard clinic-based VCT has been available since the inception of the study through a large number of public and private providers. VCT service venues in control communities in South Africa were not affiliated with our study. Thus, we have excluded the South African sites from the analyses herein as we do not have requisite utilization data from control venue service providers.
Persons aged 16 years and older residing in all study communities, whether intervention or control, had access to HIV counseling and testing. The target age group for the Project Accept intervention was 16–32 years based on the high HIV incidence in this age group.12 Here we assess the secondary study endpoint of uptake of HIV testing using intervention utilization data and limit all analyses to persons aged 16–32 years. Collection of post-intervention assessment data is ongoing, and the primary study endpoint is HIV incidence among those aged 18–32 years. HIV incidence is being measured in a post-intervention cross-sectional probability-based sample using an algorithm based on the BED assay, avidity index, and CD4+ T-cell count. Additional information on the study design and assessment of the primary study endpoint can be found in the study protocol, which is registered with ClinicalTrials.Gov (identifier # NCT00203749),24 and is an accepted study protocol with The Lancet (Protocol 05PRT/33).25
Project Accept Intervention
The Project Accept intervention, described elsewhere,26–27 includes: (1) community mobilization activities, (2) easily accessible mobile HIV voluntary counseling and testing, and (3) community-based post-test support services. There are several key principles underlying our intervention strategy. A basic premise of the intervention strategy is that individuals typically benefit from learning whether they are infected with HIV. Clients’ capacity to plan for the future is enhanced, they are able to assuage fears and concerns over whether or not they are infected with HIV, and they are empowered to appropriately adjust behaviors to reduce HIV acquisition and transmission. Project activities were conducted without attempting to conceal HIV testing. This was both to destigmatize HIV testing and encourage a sense of community ownership of the project and collective commitment to addressing HIV. In addition, the intervention strategy was based on the assumption that as large proportions of the population learn their infection status there will be a growing demand for HIV counseling and testing, disclosure and open discussion about HIV will increase, HIV-related stigma will be reduced, and increasing numbers of people will access treatment for HIV/AIDS. Ultimately, we hypothesize that these forces will lead to declines in HIV incidence. To realize these goals, three main intervention components were implemented for the project.
Community Mobilization
The goals of community mobilization activities are to destigmatize and normalize HIV counseling and testing, inculcate a sense of collective commitment to address the HIV epidemic, raise awareness about HIV, and model positive acceptance of those infected and affected by HIV/AIDS. To achieve these goals, project staff worked closely with community members through a series of meetings and consultations to link the intervention to felt needs in the community and to encourage a sense of community ownership of the project. Core intervention elements were adapted to the local environmental conditions and culture, and volunteers recruited from the community were supported as project team members to educate others about the project and to encourage personal participation from their peers and neighbors. Leaders in the community were encouraged to come and be seen receiving HIV counseling and testing and to give testimonials about their experience. This process was ongoing throughout the life of the project. Post-test services, described below, also had community mobilization components including activities to integrate HIV-infected and uninfected people around educational, social, and political goals. Community events were also supported by the project, such as sports teams and presentations in schools and at existing community events. In each site a Community Mobilization coordinator organized and supervised field activities. There were also additional community mobilization outreach workers on staff at the field level – 5 in Tanzania, 2 in Zimbabwe, and 16 in Thailand on average throughout the study. In addition, local volunteers were recruited from each CBVCT community who assisted in mobilizing community members to utilize the intervention (an average at any one time of approximately 74 in Tanzania, 42 in Zimbabwe, and 57 in Thailand).
Mobile Voluntary Counseling and Testing
A core component of the intervention was to provide free HIV testing in easily available venues. We selected high profile venues for our mobile HIV testing services to enhance convenience and the visibility of both the service and of people coming to receive VCT. Uptake of testing and counseling was tracked, and areas and times of day with high utilization were targeted. In all sites community volunteers were mobilized when visits occurred, and together with staff they canvassed the area to alert people of the opportunity to come to intervention venues, ask questions, and receive VCT. Project staff were also positioned at each testing venue to provide educational sessions on HIV and HIV testing, and encourage people to receive HIV VCT. Standards for VCT conformed to the US CDC, WHO, and local national guidelines, and included pre-test counseling, rapid HIV testing while the client waited, and provision of the test result in the context of post-test counseling addressing personalized risk reduction. All counseling and testing was done anonymously, and in private. Free male and female condoms were offered to all clients at the time of HIV testing. HIV-infected clients were referred to treatment services which were available in each of the study sites. In each CBVCT study site, mobile VCT service was provided by teams that rotated through the coverage area. Team size varied, but minimally included a driver, a team leader who also functioned as a VCT counselor, an additional VCT counselor, and a phlebotomist. Over the three year intervention period a mobile VCT team typically visited each community approximately two days a month on average to provide services.
In Tanzania mobile VCT was provided using tents transported to each venue and erected for the day. A community outreach tent was erected, as well as several additional tents that served as testing and counseling stations. In Thailand, the project team traveled to community villages by vehicle, hiking on foot, and by motor scooter. Requisite supplies were carried by staff, and local community centers such as schools and temples were used as service venues. In Zimbabwe, caravans (camper trailers) were converted to mobile HIV testing stations which were stationed in project venues. Tents were also used to provide additional space as needed.
Post-Test Support Services
Post-test support services (PTSS) were made available in all CBVCT study areas. The primary aim of PTSS was to provide psychosocial support to clients after being tested for HIV, regardless of serostatus. This component was organized as a club, with members including persons who previously tested for HIV, although guests were also free to attend. Activities included information-sharing group sessions, psychosocial support groups, crisis counseling, coping effectiveness training workshops, stigma reduction workshops, income generation training, and a referral system to link clients to other available social services. Post-test support services were mobile, and in each country there were at least two PTSS teams. Each team served several communities and was typically comprised of one team leader and two counselors with additional staff dedicated as needed during busy times. In addition there was a PTSS coordinator who supervised the teams. Over the 3 years of intervention provision each CBVCT community was visited by a PTSS team approximately 2–4 times a month on average. Additional details on the PTSS component have been previously published.26
Utilization Data Collection
Data were collected in Tanzania from March 2006 through April 2009, and in Zimbabwe and Thailand from January 2006 through July 2009. Standardized utilization data collection instruments and procedures were used in all study sites, except the Thai SVCT service venues where utilization data was collected retrospectively from clinic records. Upon providing consent to be tested for HIV a client utilization form was completed by staff. Data collected in Tanzania and Zimbabwe included date, time of day, gender, age, whether the client was being testing with a partner, the community in which the client resided, if the client reported a previous HIV test, whether the client had been tested for HIV before by Project Accept, the specific services received (i.e., pre-test counseling, blood collection, post-test counseling, receipt of test results), and the HIV test result. For women, we additionally asked the client if she was pregnant. These same data were collected in Thailand at CBVCT venues, and in SVCT venues all data except for time of day of test, pregnancy status, and repeat testing were available from clinic records. No personal identifying information was collected.
Ethical Review
The study procedures and instruments were approved by the following ethical review committees: The Johns Hopkins University Committee on Human Research (Thailand); Chiang Mai University Research Institute for Health Sciences (Thailand); the Ministry of Public Health (Thailand); The Medical University of South Carolina IRB for Human Research (Tanzania); Muhimbili University of Health and Allied Sciences IRB (Tanzania); The National Institute of Medical Research IRB (Tanzania); The University of California, San Francisco Committee on Human Research (Zimbabwe); and The Medical Research Council of Zimbabwe (Zimbabwe). Project Accept also has an independent Data Safety and Monitoring Board which biannually reviews project benchmarks, outcomes, and adverse events.
Outcome Measures
Client Characteristics and Service Utilization
Details on client characteristics were culled from utilization forms completed for every client receiving HIV testing in both intervention and comparison communities. All data presented are for those aged 16–32 years. Persons reporting previous HIV testing by Project Accept were excluded from analysis, except for Thai SVCT clients where these data were not available. In Tanzania and Zimbabwe Project Accept operated both CBVCT and SVCT intervention services, and we used our standard utilization data collection forms in these sites. In Thailand SVCT (control) service venues were not affiliated with the study. However, there were few such facilities and the study was granted excellent access to well maintained client records. Yet some variables were not originally collected at the Thai SVCT facilities, including pregnancy status, time of day the service was delivered, and whether clients had been previously tested by Project Accept or at the SVCT facility.
Number of HIV Cases Detected
HIV infection status was recorded for each client with their utilization data, and HIV infection was determined using the approved national rapid testing algorithm in each country. Those with equivocal test results are removed from analysis. It is possible that people may have tested for HIV outside our data catchment. Results presented on HIV infection are limited to only those results we had direct access to, and HIV cases detected from testing outside our direct data catchment are not estimated or included in the results shown in Table 1.
Table 1.
Client Characteristics and Service Utilization Among Clients Aged 16 – 32 Years Testing for HIV
| Characteristic Of Clients by Community Type |
Tanzania | Zimbabwe | Thailand | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention N= 2,341 |
Control N= 579 |
P-value* | Intervention N= 5,437 |
Control N= 602 |
P-value* | Intervention§ N= 9,361 |
Control§ N= 2,721 |
P-value* | |
| Age (Years) | |||||||||
| Mean | 22.7 | 23.8 | P<0.001 | 22.2 | 24.2 | P<0.001 | 23.6 | 23.5 | P<0.001 |
| Median | 22.0 | 23.4 | 21.6 | 24.0 | 22.8 | 23.7 | |||
| Age Groups (%) | |||||||||
| 16 to 17 | 15.8 | 8.5 | P<0.001 | 21.7 | 8.2 | P<0.001 | 17.9 | 11.9 | P<0.001 |
| 18 to 32 | 84.2 | 91.5 | 78.3 | 91.8 | 82.1 | 88.1 | |||
| Female (%) | 41.7 | 47.2 | P=0.019 | 48.2 | 46.3 | P=0.386 | 54.5 | 65.9 | P<0.001 |
| Tested as Couple (%) | 2.3 | 4.1 | P=0.011 | 4.1 | 10.2 | P<0.001 | 27.5 | 54.1 | P<0.001 |
|
Pregnant at Time of Test (%) (Among Female Participants) |
2.6 Mis=12 |
10.7 Mis=17 | P<0.001 | 2.9 Mis=30 |
3.5 | P=0.168 | 2.6 Mis=2,187 |
NA | NA |
| Time of Day Tested (%) | P<0.001 | 13.4 | 49.2 | P<0.001 | 12.1 | NA | NA | ||
| Morning | 36.1 | 50.9 | 50.9 | 40.2 | 2.1 | ||||
| Midday | 50.3 | 44.9 | 35.7 | 10.6 | 85.8 | ||||
| Afternoon | 13.6 | 4.1 | Mis=29 | Mis=110 | Mis=3,118 | ||||
| HIV Prevalence (%) | 3.7 | 6.9 | P<0.001 | 12.8 | 22.0 | P<0.001 | 1.9 | 3.4 | P<0.001 |
| Number Testing HIV Positive | 86 | 40 | 693 | 132 | 173 Mis=181 | 92 | |||
Mis = Missing data
NA = Not available, data were not collected
Source data do not include detail required to control for intraclass correlation from such sources as village or household propinquity and thus p-values may be smaller than if adjustment for intraclass correlation had been made.
Repeat testing data were not collected in Thailand in SVCT Venues. Values shown for Thailand include persons who repeat tested in SVCT venues. For results in Table 2 and Figure 1 an adjustment was made to remove these cases from analysis based on estimated frequency of repeat testing at Thai SVCT venues.
Percentage of Individuals Aged 16–32 Years Receiving HIV Counseling and Testing
For this measure the number of persons testing for HIV at least once over the 3 years of intervention provision was divided by the total population size of eligible persons. The total population size was derived from the study baseline evaluation in which we enumerated all household members in a probability-based sample of households. It is possible that testing occurred in other venues outside of our data catchment. However, reports from project staff are that this was rare. All of the study areas were rural, and there are significant transport and opportunity costs to leaving the area for HIV testing. Those reporting that they had previously tested for HIV with Project Accept were removed from analysis. In Thailand the SVCT service venues did not collect data on repeat testing. Based on interviews with project staff in Thailand we assumed that 50% of those residing in CBVCT areas and testing in SVCT venues were repeating their HIV test, and that 15% of those residing in SVCT communities and testing in SVCT venues were repeating their test.
Percentage of CBVCT Residents Aged 16–32 Years Repeating HIV Test at Project Accept CBVCT Service Venues
These values come from project utilization data for residents of CBVCT communities attending CBVCT venue services. Client reporting any previous testing for HIV by Project Accept are counted as repeat testers for analyses.
Statistical Analysis
Analyses were conducted with SPSS for Windows version 17™. Non-parametric analyses used Pearson's chi-square to test for differences in client characteristics across strata. For these tests significance was set at the α < 0.05 level. The statistical analyses do not account for intraclass correlation that may have been present at the community, household, or couple levels. These data come from short questionnaires completed at the time of service utilization. Geographic location of residence was limited to whether clients lived in intervention or control communities, and identifying information, detailed household location, and linking information to sexual partners who may also have tested were not collected. As a result, the P-Values presented may be more likely to show statistically significant associations than if adjustments for interclass correlation had been made. A paired T-test analysis was also conducted to examine the mean difference in percentage of the population tested for HIV across CBVCT and SVCT community pairs (1 pair per country, df=2).
RESULTS
Client Characteristics and Service Utilization (Table 1)
In all sites the number of people testing for HIV was much larger in intervention communities than in comparison communities. In Tanzania approximately 4 times more clients in intervention communities utilized VCT services than in control communities (2341 vs. 579 respectively). In Zimbabwe VCT uptake in intervention communities was 9.0 times greater than control communities (5,437 vs. 602). In Thailand it was 3.4 times greater (9,361 vs. 2,721), although the Thai SVCT venues did not assess previous testing by Project Accept, and thus we are unable to remove cases from analysis who repeated their test at SVCT venues (note that an adjustment for repeat testing at Thai SVCT venues was estimated and is presented in subsequent sections). The mean client age was slightly lower among those testing in intervention communities compared to control communities in all sites. In Tanzania the mean age was 22.7 years for CBVCT clients versus 23.8 years for SVCT clients (p<0.001). In Zimbabwe the mean age was 22.2 years for CBVCT clients versus 24.2 years for SVCT clients (p<0.001). In Thailand the mean age was 23.6 years for CBVCT clients versus 23.5 years for SVCT clients (p<0.001). However, the percentage of those testing for HIV age16–17 years, while still infrequent, was markedly higher in intervention communities as compared to control communities in all sites. In Tanzania 15.8% of those testing for HIV from intervention communities were aged 16–17 years as compared to 8.5% from control communities (P <0.001). Similarly, in Zimbabwe the percentage of HIV testers aged 16–17 years was 21.7% from intervention communities versus 8.2% from control communities (P < 0.001), and in Thailand the percentage was 17.9% and 11.9%, respectively (P < 0.001). In Tanzania and Zimbabwe, a larger percentage of clients in both intervention and control communities were male, while in Thailand the majority of clients were female. The only significant gender effect found across intervention and control communities is in Thailand, where a larger percentage of clients from intervention communities were male as compared to control communities (45.5% versus 34.1%, respectively, P < 0.001). The percentage of clients testing for HIV as a couple was modest in Tanzania and Zimbabwe, and was even less common among those from intervention communities as compared to control communities (Tanzania: 2.3% vs. 4.1%, P = 0.011; Zimbabwe 4.1% vs. 10.2%, P < 0.001). In contrast, the percentage of those testing as a couple in Thailand was much higher than in other sites, especially in control communities. In Thailand 27.5% of those testing for HIV did so as a couple in intervention communities, compared to 54.1% in control communities (P < 0.001). The percentage of female clients reporting pregnancy at the time of HIV testing was modest in all sites (Note: Data on pregnancy were not recorded in Thailand in SVCT community venues). Only in Tanzania were significant differences in pregnancy observed across study arms with 2.6% of clients from intervention communities reporting pregnancy compared to 10.7% from control communities (P < 0.001). In both Tanzania and Zimbabwe the time of day clients were tested differed significantly across intervention and comparison communities, with clients testing later in the day in intervention communities (Note: Data on time of day were not recorded in Thai SVCT venues).
Number of HIV Cases Detected (Table 1)
Prevalence of HIV and the associated number of HIV infections are shown in Table 1. In Tanzania HIV prevalence was lower among intervention community members receiving HIV testing as compared to those in control communities (3.7% vs. 6.9%, p<0.001). However, since many more people were tested in intervention areas, over twice as many HIV cases were detected in intervention as compared to control communities (86 vs. 40, p<0.001). In Zimbabwe, HIV prevalence among those testing from intervention communities was 12.8% compared to 22.0% testing from control communities (p<0.001), and there were 693 HIV cases detected from intervention communities compared to 132 in control communities (p<0.001). A similar finding was seen in Thailand, with HIV prevalence among intervention community testers of 1.9% versus 3.4% among control community testers (p<0.001), yet more HIV cases were detected in intervention communities than in control communities (173 vs. 92, p<0.001).
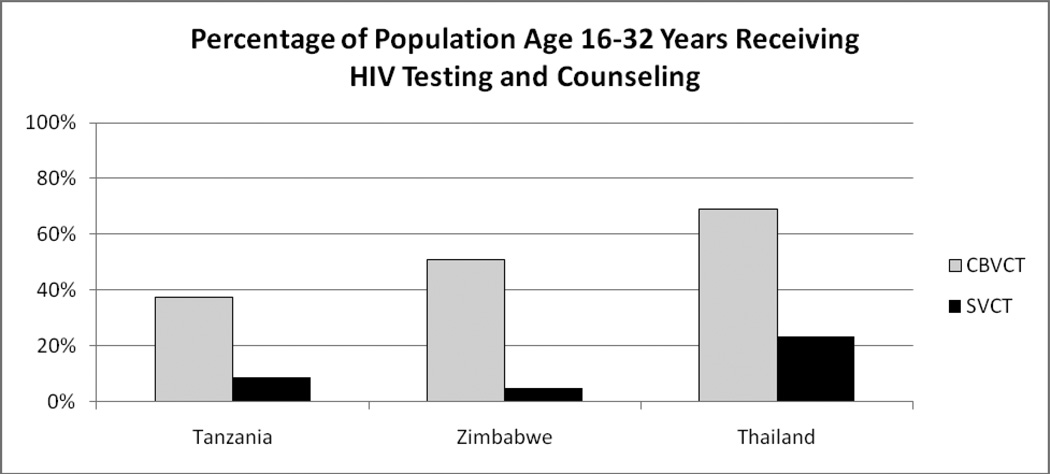
Percentage of Clients Receiving an HIV Test at Project Accept Service Venues (Table 2 / Figure 1)
Table 2.
Patterns of HIV Testing Across Study Arms for Persons Aged 16–32 Years
| Parameter | Tanzania | Zimbabwe | Thailand | |||
|---|---|---|---|---|---|---|
| Among CBVCT Community Residents |
Among SVCT Community Residents |
Among CBVCT Community Residents |
Among SVCT Community Residents |
Among CBVCT Community Residents |
Among SVCT Community Residents |
|
| [A] Population Size | 6,250 | 10,033 | 10,700 | 10,033 | 11,290 | 10,033 |
| [B] Total HIV Tests in CBVCT Venues | 2,810 | 6 | 5,911 | 15 | 7,346 | 41 |
| [C] Number of Repeat Tests in CBVCT Venues | 487 | 1 | 1,106 | 1 | 1,103 | 0 |
| [D] Total HIV Tests in SVCT Venues | 22 | 679 | 668 | 595 | 3,118 | 2,680 |
| [E] Number of Repeat Tests in SVCT Venues | 4 | 105 | 36 | 7 | 1,559 § | 402 § |
| Number of First Time HIV Tests* | 2,341 | 579 | 5,437 | 602 | 7,802 (N= 9,361 without removal of estimated SVCT Repeat Testing) |
2,319 (N= 2,721 without removal of estimated SVCT Repeat Testing) |
Number of First Time HIV Tests = ((B + D) – (C + E))
Information on repeat testing was not collected in Thailand in SVCT Venues. Value estimated to be 50% of SVCT venue tests for CBVCT community residents. Value estimated to be 15% of SVCT venue tests for SVCT community residents.
Figure 1.
Percentage of Population Aged 16–32 Years Receiving HIV Testing and Counseling
The patterns of HIV testing with regard to the number of total and repeat HIV tests by venue type among residents of each study arm are shown in Table 2. These values were used to calculate the estimated percentage of the total population aged 16–32 years who received at least one HIV test over the intervention period, shown in Figure 1. In Tanzania 37% (2,341 / 6,250) of residents in intervention communities received at least one HIV test, while in control communities the frequency was approximately four times lower at 9% (579 / 6,733). In Zimbabwe 51% (5,437 / 10,700) of intervention community residents tested for HIV as compared to 5% (602 / 12,150) of residents in control communities. In Thailand 69% (7,802 / 11,290) of intervention community residents received HIV counseling and testing compared to 23% (2,319 / 10,033) of control community residents. Note that for Thailand results we have adjusted values to reflect the estimated frequency of repeat testing that may have occurred at Thai SVCT venues, which lowers the frequency of testing from the unadjusted values.
In cross-site analysis there was a mean difference of 40.2% (95% CI: 15.8% – 64.7%) in the percentage of the population aged 16–32 years residing in CBVCT communities versus SVCT communities who were tested and counseled for HIV; t(2)=7.07, p = 0.019. This indicates that the difference in proportions tested across study arm is significant, even in a crude site-level analysis based on 3 observations (one pair per site).
The results shown in Table 2 also highlight that there were few clients who crossed over the randomized community boundaries to receive services. The exception is in Thailand, were a substantial number of clients in CBVCT communities opted to test in SVCT community venues (an estimated 20% of all HIV tests, excluding repeat testing).
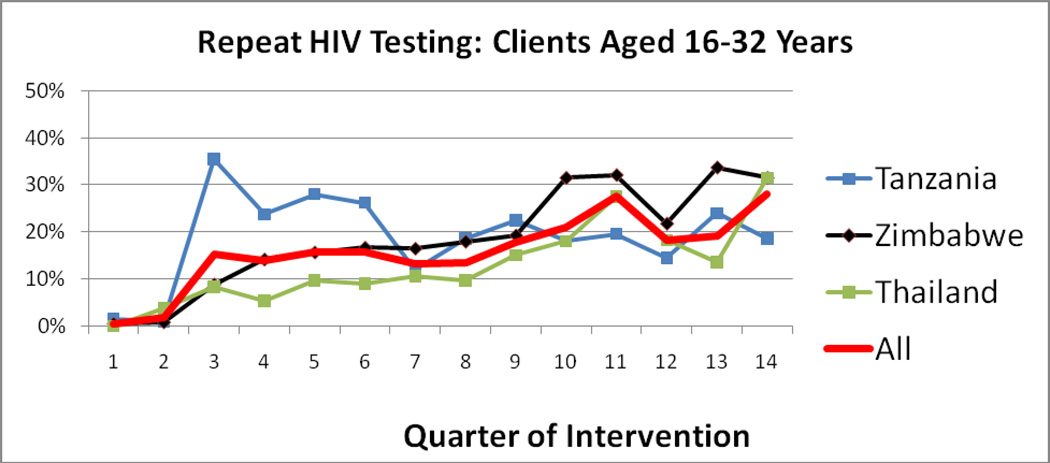
Percentage of CBVCT Residents Aged 16–32 Repeating HIV Test at Project Accept CBVCT Service Venues (Figure 2)
Figure 2.
Percentage of CBVCT Residents Repeating HIV Test at Project Accept CBVCT Service Venues for Persons Aged 16–32 Years
In Tanzania during the first year of intervention delivery the project experienced a high frequency of repeat testing among those residing in CBVCT communities, approaching 35% of all HIV tests provided by Project Accept. Over time this rate dropped, and fluctuated between 15% and 20%. In Thailand and Zimbabwe there was a consistent increase in repeat HIV testing over time, reaching 28% of all HIV testing in Project Accept CBVCT venues by the end of the intervention period for residents of those communities.
DISCUSSION
The results demonstrate that very large proportions of communities can be mobilized around HIV prevention and prompted to learn their HIV infection status, including in remote rural communities with limited infrastructure. Moreover, these results were consistent across different regions, epidemic settings, and cultures included in this multi-site community randomized trial. In Tanzania over four times as many people tested for HIV from intervention communities versus comparison communities. In Zimbabwe there were approximately nine times more persons testing in intervention communities, and in Thailand approximately 3.5 times more. We believe that the extremely high uptake of HIV testing in Thailand was supported to some degree by the many years of national support for HIV testing, which likely has made it more acceptable and normative. The lower, albeit still impressive, uptake in Tanzania is likely due to the more isolated nature of the communities where the study was based. In the study area in Tanzania there has been minimal media exposure (limited radio service, and no TV service) to HIV prevention campaigns, and little previous access to HIV testing services.
We believe that the intervention strategy succeeded at achieving an average of 55% HIV testing rates across the three sites among those 16–32 years old due largely to the multi-component, comprehensive, and integrated nature of the intervention. We also see evidence that the mobilization component stimulated demand for HIV testing, independent of improved access alone, given the number of persons residing in intervention communities who sought testing in SVCT venues as compared to control community members who sought HIV testing in CBVCT venues.
Important differences in the characteristics of clients who utilized CBVCT versus SVCT were found. In Tanzania, those testing in SVCT venues were more likely to be couples and pregnant women than in CBVCT venues. It is likely that the lower percentage of pregnant women in intervention communities in Tanzania seeking testing services is because SVCT services there are linked to public health clinics, which are also a major source of antenatal care for women. It is also notable that so few persons testing for HIV among those residing in intervention communities went to SVCT venues for HIV testing, indicating that ease of access for HIV testing services has a major impact on uptake.
In Zimbabwe, persons testing for HIV from intervention communities, versus comparison communities, were more likely to be adolescents, and have tested as an individual rather than couple. Standard clinic-based VCT in Zimbabwe has been criticized for not being youth friendly,28 which may explain the higher rates of youth attending mobile services. The higher rate of testing for couples at SVCT venues may partially be due to the coexistence of antenatal services, which may promote males to test for HIV when their pregnant partner also tests for HIV. As well, it may be that couples prefer to test away from their home community over fears of loss of confidentiality.
In Thailand, VCT has been available in all government hospitals with only a minimal fee since 1992. All pregnant women receive VCT during antenatal care and financial institutions require loan applicants to provide proof of negative HIV infection status. Hence, independent of the Project Accept intervention, a large percentage of the Thai population has prior experience with HIV testing. Studies have shown that more than 40% of persons aged 18–35 have a history of HIV testing in northern Thailand.27, 29 This may account for the much higher general frequency of testing observed in both study arms in Thailand. There were also higher rates of testing for females and couples from SVCT communities than CBVCT communities, and like Tanzania this may be due to the concurrent availability of antenatal testing available in Thai SVCT venues.
Against this backdrop of dramatically increased HIV testing associated with the intervention, we found couple testing to be infrequent. There is evidence that when clients test for HIV together as a couple they are more likely to reduce risk behavior.3–6 In all three countries couple testing was more frequent among clients from SVCT communities, especially in Zimbabwe and Thailand. Couples may prefer to test for HIV outside their home communities to enhance privacy, and may find the SVCT testing sites more private.
The experience of Project Accept also elucidates program evolution in the uptake of testing, and repeat testing, associated with community-based VCT. We believe that both community mobilization and social networking dynamics promoted uptake of HIV testing in intervention communities. As increasing numbers of people learn their HIV serostatus the likelihood of personally knowing someone who has tested grows, instilling trust in the safety and benefits of learning your serostatus. As well, by the end of the of the intervention period approximately 40% of persons testing presenting at CBVCT venues were repeating a previous HIV test conducted by Project Accept. This trend towards very high proportion of repeat testers over time is also correlated with reduced HIV infection case detection, as case detection is associated most commonly with first time testing. Thus, as a community-based VCT program matures the epidemiological benefits of the program also evolve from case detection towards behavioral reinforcement and prevention.
The utilization data for the study were carefully collected, and there were multiple layers of quality assurance to ensure that the results were accurate. However, the methodological challenges of measuring the complex patterns of HIV testing at the population level should not be underestimated. While the study results should be treated with some caution, we believe that they are reliable. The utilization information collected was based on self-report at intake to each service, and there was little reason for clients to provide inaccurate information. The questions asked were neither highly sensitive nor stigmatizing, and there was no discouragement to access services for those residing outside of geographic community boundaries defined for the study. Everyone coming to the intervention sites, regardless of where they lived, was allowed to access all services. We carefully mapped the communities prior to the study, and staff reported few problems determining whether clients resided in intervention, comparison, or other communities. Since we did not collect identifying information on clients we were unable to determine the individual pattern of repeat testing, other than by asking clients whether they had tested before, and whether Project Accept was the source of previous testing. One possible source of error is that community members may have tested for HIV in venues from which we did not have access to data. However, all of the study sites, both intervention and control, were rural, and access to alternative testing venues outside our data catchment was difficult. Additional testing outside our data catchment would only increase the numbers of people estimated to have tested for HIV, and there is little reason to believe that traveling to distant VCT testing venues would occur differentially across study arm communities. There are several additional study limitations that are noteworthy. Cost data for the project intervention is currently being collected, and is not yet available. The study also had some missing data for some variables. In most sites the number of cases with missing data is small, although in Thailand substantial cases in the intervention arm were missing data on time of day services were rendered, and pregnancy which likely does introduce a bias in these descriptive statistics. In Tanzania and Zimbabwe the study provided both the CBVCT and SVCT services, enhancing our ability to track uptake patterns. In Thailand the project relied on existing SVCT services, and we had excellent access to well maintained utilization data at these clinics. The reliance on SVCT clinics in Thailand not affiliated with our project is also a potential source of bias that should be recognized. Yet the VCT provided at Thai government clinics is recognized to be of high quality, and it conforms closely to the same international standards applied by study-supported HIV testing services. The study results are also partially based on self-report data on repeat testing, and it is possible that some may have denied previous testing. In Tanzania and Zimbabwe few community members crossed over the randomized community boundaries to receive services. In Thailand approximately 20% of CBVCT community members received HIV testing in SVCT venues. Very few SVCT community members traveled to testing venues located in CBVCT communities in any site. Whether these events constitute contamination of the study design is most relevant to our main study outcome of HIV incidence, which is forthcoming. It can be said that clearly in some sites, especially Thailand, some people made a concerted effort to receive their HIV test in a less convenient venue, implying that publically seeking an HIV test does not appeal to all clients.
We also recognize that the main study design included matching community pairs for randomization, and intraclass correlation may be present at the community, household, and couple levels which should ideally be adjusted for in analysis. Thus, the P-values presented may be overly optimistic, and inferring statistical significance should be taken with caution. The utilization data included in this analysis did not include a level of geographic and individual measures that would allow us to identify intraclass correlation. To preserve client anonymity we opted to not collect information at the time of service delivery which could identify clients. Yet we would point out that the main effect presented on the differential percentage of the population who tested for HIV across study communities was enormous. The level of intraclass correlation that would be required to obviate statistical significance in this effect is highly unlikely to be present.
Our ultimate conclusion is that bringing VCT directly to communities and linking it with mobilization efforts and post-test support services results in substantially greater uptake of both HIV testing and HIV case detection than standard clinic-based VCT. This has important implications for both prevention and treatment of HIV in developing countries, especially in rural communities such as those in which this study was conducted, which are often neglected in the provision of HIV programming due to logistical and health systems challenges. The project’s ability to mobilize such large proportions of the population to go through the difficult process of learning whether they are infected with HIV in such a short time speaks to the capacity of local communities to respond to HIV epidemics when comprehensive, user-friendly services are provided.
PANEL: Research in Context
Systematic Review
We searched PUBMED and the Cochrane Library using the following Boolean search terms with no date limitations: ((“HIV voluntary counseling and testing” OR “VCT” or “HIV Testing”) AND (“utilization” OR “uptake”) AND (“trial” OR “randomized” OR “campaign”)). We screened the results for randomized controlled trials of HIV testing interventions that reported on the uptake of HIV testing. Our search identified six citations on five relevant trials 30–35.
Interpretation
A total of 16,585 participants were involved in these trials, four of which were conducted in Sub-Saharan Africa, and one in Thailand. The study populations, endpoint measures, and interventions varied across trials making generation of a pooled effect size estimate infeasible. A trial among antenatal women found individual VCT resulted in significantly greater uptake over couples VCT (71% vs. 39% respectively; p<0.001). A workplace-based study found that onsite VCT resulted in higher uptake than off-site VCT (RR 2.8; 95% CI, 1.8–3.8). A study of community-based education and mobilization reported higher uptake of VCT compared to no intervention (RR: 2.9; 95% CI, 1.27–6.74). Two trials examined variants of home-based VCT, and both demonstrated significantly greater uptake of home-based VCT over clinic based-VCT (RR: 4.7; 95% CI, 3.62–6.21; OR: 2.76; 95% CI, 1.97–3.86). Our trial is the first to examine the effects of community-based VCT on uptake of HIV testing among a probability-based sample of community members. The intervention effect we found is consistent with the limited studies that have been conducted on home-based VCT in developing countries with generalized HIV epidemics.
Supplementary Material
Acknowledgments
This research was sponsored by the U.S. National Institute of Mental Health as a cooperative agreement, through contracts U01MH066687 (Johns Hopkins University – David Celentano, PI); U01MH066688 (Medical University of South Carolina – Michael Sweat, PI); U01MH066701 (University of California, Los Angeles – Thomas J. Coates, PI); and U01MH066702 (University of California, San Francisco – Stephen F. Morin, PI). In addition, this work was supported as HPTN Protocol 043 through contracts U01AI068613 (HPTN Network Laboratory – Susan Eshleman, PI); U01AI068617 (SCHARP – Deborah Donnell, PI); and U01AI068619 (HIV Prevention Trials Network – Sten Vermund, PI) of the Division of AIDS of the U.S. National Institute of Allergy and Infectious Diseases; and by the Office of AIDS Research of the U.S. National Institutes of Health. Views expressed are those of the authors, and not necessarily those of sponsoring agencies.
Appendix: NIMH Project Accept Study Group
Laurie Abler, MPH1
Christopher Bamanyisa, MA, AD2
Chris Beyrer, MD, MPH3
Adam W. Carrico, PhD4
David Celentano, ScD, MHS3
Suwat Chariyalertsak, MD, DrPH5
Alfred Chingono, MSc6
Lillianne Chovenye, MA2
Thomas J. Coates, PhD8
Kathryn Curran, MHS7
Deborah Donnell, PhD9
Susan Eshleman MD, PhD3
Agnès Fiamma, MIPH8
Katherine Fritz, PhD, MPH10
Janet Frohlich, Dcur11
Becky Genberg, MPH3
Glenda Gray, MBBCH, FCPaeds(SA)12
Amy Gregowski, MHS10
Harry Hausler, MD, MPH13
Zdenek Hlavka, PhD14
Daniel Hlubinka, PhD14
Nora Margaret Hogan, PsyD2
Philip Joseph11
Salim Abdool Karim, MBChB, PhD11
Sebastian Kevany, MPH4
Gertrude Khumalo-Sakutukwa, MSW, MMS4
G.P. Kilonzo, MD, FRCP, Mmed, MBChB, BA2
Michal Kulich, PhD14
Oliver Laeyendecker, MS, MBA15,3
Tim Lane, PhD, MPH4
Florence P. Lema, MSc, MPH2
Benjamin Link, MPH, MSW3
Tserayi Machinda, BSC Admin ACCA, MBA(wip)6
Suzanne Maman, PhD1
Jessie Mbwambo, MD2
Nuala McGrath, ScD, MSc, BSc13
James McIntyre, MBChB, MRCOG12
Joanne Mickalian, MA4
Precious Modiba, MA(SW)12
Simon Morfit, MPH, BA4
Stephen F. Morin, PhD4
Khalifa M. Mrumbi, MSc. PhD2
Marta I. Mulawa, MHS7
Oliver Murima, MSc6
Thulani Ngubani, BTh, Hons11
Audrey Pettifor, PhD, MPH1
Estelle Piwowar-Manning, BS MT(ASCP)SI3
Linda Richter, PhD11
Andrew M. Sadowski7
Memory Sendah, MSc6
Basant Singh, Bsc, Msc3
Michael Sweat, PhD7
Greg Szekeres8
Andrew Timbe, Med6
Heidi Van Rooyen, PhD11
Surasing Visrutaratna, PhD5
Godfrey Woelk, PhD, MCOMMH, BSc6
Carla E. Zelaya, PhD, MSc3
1 University of North Carolina at Chapel Hill
2 Muhimbili University of Health and Allied Sciences
3 Johns Hopkins University
4 University of California, San Francisco
5 Chiang Mai University, Research Institute for Health Sciences
6 University of Zimbabwe
7 Medical University of South Carolina
8 University of California, Los Angeles
9 Statistical Center for HIV/AIDS Research & Prevention, Fred Hutchinson Cancer Research Center
10 International Center for Research on Women
11 Human Sciences Research Council
12 University of the Witwatersrand/Chris Hani Baragwanath Hospital
13 London School of Hygiene and Tropical Medicine
14 Charles University, Department of Probability and Statistics
15 National Institute of Allergy and Infectious Diseases
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Denison JA, O'Reilly KR, Schmid GP, Kennedy CE, Sweat MD. HIV voluntary counseling and testing and behavioral risk reduction in developing countries: a meta-analysis, 1990–2005. AIDS Behav. 2008 May;12(3):363–373. doi: 10.1007/s10461-007-9349-x. [DOI] [PubMed] [Google Scholar]
- 2.The Voluntary HIV-1 Counseling and Testing Efficacy Study Group. Efficacy of voluntary HIV-1 counselling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: a randomised trial. Lancet. 2000 Jul 8;356(9224):103–112. [PubMed] [Google Scholar]
- 3.Allen S, Karita E, Chomba E, Roth DL, Telfair J, Zulu I, et al. Promotion of couples' voluntary counselling and testing for HIV through influential networks in two African capital cities. BMC Public Health. 2007;7:349. doi: 10.1186/1471-2458-7-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen S, Meinzen-Derr J, Kautzman M, Zulu I, Trask S, Fideli U, et al. Sexual behavior of HIV discordant couples after HIV counseling and testing. AIDS. 2003 Mar 28;17(5):733–740. doi: 10.1097/00002030-200303280-00012. [DOI] [PubMed] [Google Scholar]
- 5.King R, Allen S, Serufilira A, Karita E, Van de Perre P. Voluntary confidential HIV testing for couples in Kigali, Rwanda. AIDS. 1993 Oct;7(10):1393–1394. doi: 10.1097/00002030-199310000-00018. [DOI] [PubMed] [Google Scholar]
- 6.King R, Katuntu D, Lifshay J, Packel L, Batamwita R, Nakayiwa S, et al. Processes and outcomes of HIV serostatus disclosure to sexual partners among people living with HIV in Uganda. AIDS Behav. 2008 Mar;12(2):232–243. doi: 10.1007/s10461-007-9307-7. [DOI] [PubMed] [Google Scholar]
- 7.Mshana GH, Wamoyi J, Busza J, Zaba B, Changalucha J, Kaluvya S, et al. Barriers to accessing antiretroviral therapy in Kisesa, Tanzania: a qualitative study of early rural referrals to the national program. AIDS Patient Care STDS. 2006 Sep;20(9):649–657. doi: 10.1089/apc.2006.20.649. [DOI] [PubMed] [Google Scholar]
- 8.Nsigaye R, Wringe A, Roura M, Kalluvya S, Urassa M, Busza J, et al. From HIV diagnosis to treatment: evaluation of a referral system to promote and monitor access to antiretroviral therapy in rural Tanzania. J Int AIDS Soc. 2009 Nov 11;2(1):6. doi: 10.1186/1758-2652-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perbost I, Malafronte B, Pradier C, Santo LD, Dunais B, Counillon E, et al. In the era of highly active antiretroviral therapy, why are HIV-infected patients still admitted to hospital for an inaugural opportunistic infection? HIV Med. 2005 Jul;6(4):232–239. doi: 10.1111/j.1468-1293.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 10.Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, Cohen CR, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010 Jun 12;375(9731):2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009 Jan 3;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 12.UNIAIDS. AIDS epidemic update. Geneva: UNAIDS; 2009. Dec, 2009. [Google Scholar]
- 13.Bunnell R, Cherutich P. Universal HIV testing and counselling in Africa. Lancet. 2008 Jun 28;371(9631):2148–2150. doi: 10.1016/S0140-6736(08)60929-0. [DOI] [PubMed] [Google Scholar]
- 14.Ganguli I, Bassett IV, Dong KL, Walensky RP. Home testing for HIV infection in resource-limited settings. Curr HIV/AIDS Rep. 2009 Nov;6(4):217–223. doi: 10.1007/s11904-009-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helleringer S, Kohler HP, Frimpong JA, Mkandawire J. Increasing uptake of HIV testing and counseling among the poorest in sub-Saharan countries through home-based service provision. J Acquir Immune Defic Syndr. 2009 Jun 1;51(2):185–193. doi: 10.1097/QAI.0b013e31819c1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denison JA, McCauley AP, Dunnett-Dagg WA, Lungu N, Sweat MD. The HIV testing experiences of adolescents in Ndola, Zambia: do families and friends matter? AIDS Care. 2008 Jan;20(1):101–105. doi: 10.1080/09540120701427498. [DOI] [PubMed] [Google Scholar]
- 17.Denison JA, McCauley AP, Dunnett-Dagg WA, Lungu N, Sweat MD. HIV testing among adolescents in Ndola, Zambia: how individual, relational, and environmental factors relate to demand. AIDS Educ Prev. 2009 Aug;21(4):314–324. doi: 10.1521/aeap.2009.21.4.314. [DOI] [PubMed] [Google Scholar]
- 18.Holmes C, Preko P, Bolds R, Baidoo J, Jolly P. Acceptance of Voluntary Counselling, Testing and Treatment for HIV Among Pregnant Women in Kumasi, Ghana. Ghana Med J. 2008 Mar;42(1):8–15. [PMC free article] [PubMed] [Google Scholar]
- 19.Jurgens R, Cohen J, Girard F, Beyrer C. Increasing access to HIV testing and counselling while respecting human rights. HIV AIDS Policy Law Rev. 2007 Dec;12(2–3):63–66. [PubMed] [Google Scholar]
- 20.Swamy M. UN agencies issue new guidelines for HIV testing. HIV AIDS Policy Law Rev. 2007 Dec;12(2–3):39–40. [PubMed] [Google Scholar]
- 21.De Cock KM, Bunnell R, Mermin J. Unfinished Business -- Expanding HIV Testing in Developing Countries. N Engl J Med. 2006 February 2;354(5):440–442. doi: 10.1056/NEJMp058327. 2006. [DOI] [PubMed] [Google Scholar]
- 22.Matovu JK, Makumbi FE. Expanding access to voluntary HIV counselling and testing in sub-Saharan Africa: alternative approaches for improving uptake, 2001–2007. Trop Med Int Health. 2007 Nov;12(11):1315–1322. doi: 10.1111/j.1365-3156.2007.01923.x. [DOI] [PubMed] [Google Scholar]
- 23.Peltzer K, Matseke G, Mzolo TMM. Determinants of knowledge of HIV status in South Africa: results from a population-based HIV survey. MC Public Health. 2009;5(9):174. doi: 10.1186/1471-2458-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CLINICALTRIALS.GOV. Trial Registry: Community Mobilization, Mobile Testing, Same-Day Results, and Post-Test Support for HIV in Sub-Saharan Africa and Thailand. [cited 2010 December];2010 Available from: http://clinicaltrials.gov/ct2/show/NCT00203749.
- 25.The Lancet. Protocol 05PRT/33: NIMH Project Accept: a phase III randomized controlled trial of community mobilization, mobile testing, same-day results, and post-test support for HIV in sub-Saharan Africa and Thailand (HPTN 043; NCT00203749) 2010 doi: 10.1371/journal.pone.0149335. [cited; Available from: http://www.thelancet.com/protocol-reviews/05PRT-33. [DOI] [PMC free article] [PubMed]
- 26.Khumalo-Sakutukwa G, Morin SF, Fritz K, Charlebois ED, van Rooyen H, Chingono A, et al. Project Accept (HPTN 043): a community-based intervention to reduce HIV incidence in populations at risk for HIV in sub-Saharan Africa and Thailand. J Acquir Immune Defic Syndr. 2008 Dec 1;49(4):422–431. doi: 10.1097/QAI.0b013e31818a6cb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genberg BL, Kulich M, Kawichai S, Modiba P, Chingono A, Kilonzo GP, et al. HIV risk behaviors in sub-Saharan Africa and Northern Thailand: baseline behavioral data from Project Accept. J Acquir Immune Defic Syndr. 2008 Nov 1;49(3):309–319. doi: 10.1097/QAI.0b013e3181893ed0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boswell D, Baggaley R. Voluntary Counsleing and Testing (VCT) for Young People: A Summary Overview. Arlington, VA: Family Health International; 2002. Jun, [Google Scholar]
- 29.Kawichai S, Celentano DD, Vongchak T, Beyrer C, Suriyanon V, Razak MH, et al. HIV voluntary counseling and testing and HIV incidence in male injecting drug users in northern Thailand: evidence of an urgent need for HIV prevention. J Acquir Immune Defic Syndr. 2006 Feb 1;41(2):186–193. doi: 10.1097/01.qai.0000179431.42284.3e. [DOI] [PubMed] [Google Scholar]
- 30.Becker S, Mlay R, Schwandt HM, Lyamuya E. Comparing couples' and individual voluntary counseling and testing for HIV at antenatal clinics in Tanzania: a randomized trial. AIDS Behav. 2010 Jun;14(3):558–566. doi: 10.1007/s10461-009-9607-1. [DOI] [PubMed] [Google Scholar]
- 31.Corbett EL, Dauya E, Matambo R, Cheung YB, Makamure B, Bassett MT, et al. Uptake of workplace HIV counselling and testing: a cluster-randomised trial in Zimbabwe. PLoS Med. 2006 Jul;3(7):e238. doi: 10.1371/journal.pmed.0030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corbett EL, Makamure B, Cheung YB, Dauya E, Matambo R, Bandason T, et al. HIV incidence during a cluster-randomized trial of two strategies providing voluntary counselling and testing at the workplace, Zimbabwe. AIDS. 2007 Feb 19;21(4):483–489. doi: 10.1097/QAD.0b013e3280115402. [DOI] [PubMed] [Google Scholar]
- 33.Fylkesnes K, Siziya S. A randomized trial on acceptability of voluntary HIV counselling and testing. Trop Med Int Health. 2004 May;9(5):566–572. doi: 10.1111/j.1365-3156.2004.01231.x. [DOI] [PubMed] [Google Scholar]
- 34.Jiraphongsa C, Danmoensawat W, Greenland S, Frerichs R, Siraprapasiri T, Glik DC, et al. Acceptance of HIV testing and counseling among unmarried young adults in Northern Thailand. AIDS Educ Prev. 2002 Apr;14(2):89–101. doi: 10.1521/aeap.14.2.89.23897. [DOI] [PubMed] [Google Scholar]
- 35.Lugada E, Levin J, Abang B, Mermin J, Mugalanzi E, Namara G, et al. Comparison of home and clinic-based HIV testing among household members of persons taking antiretroviral therapy in Uganda: results from a randomized trial. J Acquir Immune Defic Syndr. 2010 Oct 1;55(2):245–252. doi: 10.1097/QAI.0b013e3181e9e069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.