Abstract
Background.
The prevalence of the metabolic syndrome (MetSyn) approaches 50% in postmenopausal women. This study examines the efficacy of lifestyle modification for the treatment of MetSyn and its associated risk for cardiovascular disease and diabetes in this population.
Methods.
This prospective controlled study examines the effects of a 6-month weight loss and low-intensity exercise program (WL+LEX) on body composition (dual-energy X-ray absorptiometry and abdominal computed tomography scans), fasting glucose and lipid levels, cytokines, and blood pressure in postmenopausal women with and without MetSyn.
Results.
WL+LEX reduced body weight (MetSyn: −5% vs non-MetSyn: −7%) and fat mass (−11% vs −15%) and increased VO2max (+2% vs +3%) in both MetSyn (N = 35) and non-MetSyn (N = 41) groups. Constituents of MetSyn decreased comparably in both groups. Fifteen (45%) MetSyn participants responded (R) by converting to non-MetSyn, 18 remained MetSyn (NR), and 2 had missing data. Reduction in fat mass (−15% vs −8%, p = .02) was greater in R than NR, but there were no between-group differences in changes in VO2max, cytokines, or other variables. The decrease in the number of MetSyn criteria was greater in R than in NR (−27 vs −13, p < .0001) due to decreases in blood pressure (p < .01), glucose (p = .02), and with a trend for triglyceride (p = .07). Reductions in fat mass best predicted resolution of MetSyn (p = .04).
Conclusions.
Women who lose more fat are more likely to lower blood pressure, glucose, and triglyceride levels to resolve MetSyn. Thus, a WL+LEX program effectively treats postmenopausal women with MetSyn.
Keywords: Exercise, Dieting, Metabolic syndrome, Inflammation
THE increased proportion of overweight and obese older individuals in Western societies over the past several decades is largely due to a sedentary lifestyle and dietary excess. These individuals have a high prevalence of the metabolic syndrome (MetSyn), a constellation of metabolic abnormalities including abdominal obesity, dyslipidemia, hypertension, and glucose intolerance (1). The prevalence of MetSyn increases with age, affecting ∼40% of the U.S. population older than 50 years with 56% of women compared with 46% of men aged 60 years or older having MetSyn (2,3). A recent analysis of the Third National Health and Nutrition Examination Survey shows that the MetSyn is associated with a significantly higher all-cause, cardiovascular, and even noncardiac mortality in postmenopausal women that is not apparent in men over an observation period as long as 12 years (4). The high prevalence of MetSyn in older women and its strong association with diabetes, cardiovascular disease (CVD) (5–9), and decreased mobility (10–12), risk factors for disability and mortality in older people, makes its treatment a public health priority.
Pharmacological interventions are effective in treating dyslipidemia, hypertension, and elevated glucose levels, but they fail to adequately address the risk factors of the 21st century sedentary obesogenic lifestyle. In contrast, lifestyle changes that directly address inactivity and overeating show encouraging results in the treatment of MetSyn. The Diabetes Prevention Program (13) and other studies (14,15) showed that diet-induced weight loss and exercise were more effective than placebo in resolution of MetSyn across the age span, but our study focuses on obese postmenopausal women where diabetes, CVD risk (3), and morbidity/mortality are high (16). The purpose of this study was to evaluate the effectiveness of a diet-induced weight loss and low-intensity exercise program (WL+LEX) to reduce individual constituents and prevalence of MetSyn in obese postmenopausal women, as well as reduce CVD risk in obese postmenopausal women without MetSyn. We hypothesized that this lifestyle program would reduce multiple indices of CVD risk and the prevalence of the MetSyn in obese postmenopausal women primarily through reductions in body fat and that the metabolic improvements would be greater in women with worse atherogenic metabolic profiles.
METHODS
Overweight and obese postmenopausal women were recruited from the Baltimore, MD, to Washington, DC, area who met the following inclusion criteria: (a) no menstruation for more than 1 year, follicle stimulating hormone more than 30 IU/L, and no hormone replacement therapy; (b) body mass index 27–40 kg/m2; (c) weight-stable (<2.0 kg weight change during the previous year); and (d) sedentary (<20-minute exercise three times per week) but able to walk 2 mph on a treadmill for 5 minutes. Participants were healthy, with a normal physical examination; 12-lead resting electrocardiogram; graded exercise test; and normal liver, renal, and hematological blood assays. Exclusion criteria included diabetes (fasting glucose > 7 mmol/L or 2 hours after 75 g oral glucose >11 mmol/L), poorly controlled blood pressure (>160/90) or high triglyceride ([TG] > 400 mg/dL), orthopedic limitations that would limit physical activity, or significant metabolic disorders such as untreated hypothyroidism. Participants using medications that affect glucose metabolism were excluded; however, participants were permitted to take antihypertensive (N = 6) and lipid-lowering medications (N = 2). No tobacco product use was permitted for the previous 5 years. This manuscript presents a new hypothesis and adds cytokine data to a previous report (17). Participants provided informed consent according to the University of Maryland Institutional Review Board for Human Research.
Study Design
To minimize the effect of dietary composition as a confounder of metabolic changes with WL+LEX, women underwent a 6- to 8-week American Heart Association Step 1 diet (18) stabilization period during which they met weekly with a registered dietician. Women then underwent baseline testing including measurement of body composition, aerobic fitness (VO2max), sitting blood pressure on three occasions, and overnight 12-hour fasted plasma lipids, glucose and 2-hour postprandial glucose, and insulin levels before beginning a 6-month WL+LEX intervention. There were weekly dietitian-led WL classes in the principles of 250–350 kcal/d caloric restriction that focused on eating behavior, stress management, modification of binge eating, and control of portion sizes. The participants participated in a low-intensity aerobic exercise program that involved walking 1 d/wk on a treadmill at 50%–60% heart rate reserve for 45 minutes under the supervision of an exercise physiologist and instruction to walk a minimum of 2 d/wk at the same intensity on their own. At the end of the 6-month program, women were weight-stabilized on an isocaloric diet (<0.5 kg change) for 2 weeks prior to retesting while continuing the exercise program. The same series of tests performed at baseline were repeated 36–48 hours after the last exercise session. Participants were classified as having MetSyn according to the Adult Treatment Panel III guidelines (1).
Procedures
Participants were weighed in kilograms and height measured in meters using a stadiometer; body mass index was computed as kilograms per square meter. Waist circumference was measured at the narrowest point superior to the hip. Body composition was determined using dual-energy x-ray absorptiometry (DPX-L; Lunar Radiation Corp., Madison, WI), and fat and lean tissue mass were determined with the LUNAR version 1.3z DPX-L extended analysis program. Visceral abdominal (VAT) and subcutaneous adipose tissue (SAT) areas were measured using a single-slice computed tomography scan taken between L4 and L5 (17). Maximum aerobic capacity (VO2max) was measured using a modified Balke treadmill test as previously described (19). A valid measurement required that at least two criteria were met: (a) maximum heart rate greater than 90% of age-predicted maximum computed as 220 − age; (b) respiratory exchange ratio of 1.10 or greater; and (c) plateau in oxygen consumption (<200 mL/min change) with increasing work rate. Blood for fasting glucose, insulin, and lipid profiles were obtained after a 12-hour overnight fast three times, and results averaged. Two-hour glucose and insulin levels were measured after ingestion of 75 g glucose. Other blood chemistries were obtained from a single fasting blood sample. Attendance was taken at all classes, and compliance to diet was judged monthly on a 1–5 scale (5 is best) based on quality of weekly food records, class participation, knowledge of nutrition principles for WL, and WL and averaged. During metabolic testing, compliance and weight were monitored twice weekly for weight stability (<0.5 kg weight change) and weekly by review of 7-day food records and 24-hour dietary recalls.
Laboratory Methods
Glucose concentrations were measured by a glucose oxidase method (YSI Instruments, Yellow Springs, OH) and insulin concentrations by radioimmunoassay (Linco, St Louis, MO). Tumor necrosis factor (TNF), TNF receptor superfamily 1A and 1B, and interleukin-6 (IL-6) concentrations were determined by enzyme-linked immunosorbent assays using Quantikine immunoassay kits from R&D Systems (Minneapolis, MN). The inter- and intra-assay Coefficient of Variation were less than 6% for IL-6 and the soluble receptors and less than 12% for TNF. High-sensitive C-reactive protein was measured by automated immunoanalyzer (Immulite; Diagnostics Products, Los Angeles, CA) with inter- and intra-assays CVs of 7.5% and 4.4%, respectively. Plasma TG and cholesterol were analyzed by enzymatic methods (Hitachi model 917 analyzer), and high-density lipoprotein cholesterol was measured in the supernatant after precipitation with dextran sulfate (20). Low-density lipoprotein was calculated using the Friedewald equation: LDL-C = total cholesterol − (TG/5 + HDL-C).
Statistical Analyses
Homeostasis model assessment—insulin resistance was computed as (glucose × insulin)/22.5 with glucose expressed in millimolar and insulin in microunits per milliliter. Comparisons between groups were performed using linear least-squares regression with groups identified by dummy variables and race included as a covariable. These equations were solved using a modified Applied Statistics Algorithm 274 (21). Change data are expressed as a percentage of the initial value. Outlying and influential data were identified according to Belsley and colleagues (22). The categorical analysis for baseline criteria count between groups compares the proportion of participants satisfying criteria with those who do not. The categorical analysis for response to WL+LEX compares the proportion of participants who no longer satisfy a criterion with those who continue to satisfy a criterion using Fisher's exact test. Stepwise logistic regression was performed using the R statistical program. Data are mean ± SD with significance at p ≤ .05.
RESULTS
Participants
The 115 participants included 83 (72%) Caucasians(C) and 32 (28%) African Americans (AA) who were 11 ± 8 years postmenopausal. The 39 participants (34%) who dropped out due to time constraints or personal reasons included 25 of the 83 C and 14 of the 32 AA (p = .19 between races). Participants were divided into two groups at baseline based on MetSyn status. Dropouts did not differ by MetSyn status (MetSyn = 21/56 vs non-MetSyn = 14/55, p = .22; data missing in four participants). The dropouts were heavier (91 + 14 vs 86 + 13 kg, p = .05), had higher VAT (176 + 59 vs 152 + 50 cm2, p = .03), and were more insulin resistant (homeostasis model assessment: 4.2 + 1.9 vs 3.1 + 1.5, p = .003) than the 76 participants who completed the intervention. Overall attendance to intervention was 76% with mean compliance score of 3.33; there was no difference by MetSyn status, with non-MetSyn participants attending 76% and the MetSyn 76% of classes with average compliance scores of 3.5 and 3.2 (p = Not Significant [NS]), respectively. Participants reported that they walked at home two to three times per week for 30–45 minutes at the prescribed heart rate.
Baseline Differences Between MetSyn and Non-MetSyn
When compared as continuous variables (Table 1), most of the metabolic indices and MetSyn constituents differed between the MetSyn and non-MetSyn groups. There were significant between-group differences in all MetSyn criteria when compared as categorical variables (p ≤ .001), except for blood pressure (p = .07).
Table 1.
Baseline Values and Response to WL+LEX in Women With and Without MetSyn
| Baseline Values |
Percent Change After WL+LEX |
|||||||
| Non-MetSyn (n = 41) | MetSyn (n = 35) | Difference (95% CL) | p Value | Non-MetSyn | MetSyn | Difference (95% CL) | p Value† | |
| Age, y | 60 ± 6 | 59 ± 6 | −1 (−4 to 1) | .38 | ||||
| Weight, kg | 83 ± 12 | 90 ± 13 | 7 (2–13) | .008 | −7 ± 4* | −5 ± 5* | 2 (0–4) | .06 |
| BMI, kg/m2 | 31 ± 4 | 34 ± 5 | 3 (1–5) | .003 | −7 ± 4* | −5 ± 5* | 2 (0–4) | .09 |
| % Body fat | 47 ± 5 | 48 ± 5 | 1 (−1 to 3) | .52 | −4.1 ± 3* | −3.1 ± 2* | 0.9 (−0.4 to 2.2) | .15 |
| Fat mass, kg | 38 ± 8 | 42 ± 9 | 4 (0–8) | .03 | −15 ± 10* | −11 ± 8* | 3 (−1 to 8) | .12 |
| Lean mass, kg | 40 ± 5 | 43 ± 6 | 3 (1–6) | .003 | 1 ± 4 | 0 ± 4 | −1 (−3 to 1) | .28 |
| Waist, cm | 93 ± 9 | 101 ± 9 | 8 (4–12) | <.001 | −5 ± 6* | −3 ± 4* | 1 (−1 to 4) | .30 |
| VAT, cm2 | 135 ± 41 | 172 ± 54 | 37 (16–58) | .001 | −17 ± 14* | −9 ± 19* | 9 (1–17) | .03 |
| SAT, cm2 | 457 ± 111 | 472 ± 120 | 15 (−39 to 69) | .58 | −12 ± 13* | −12 ± 11* | −1(−6 to 4) | .77 |
| VO2max, mL/kg/m | 20.2 ± 3 | 19.6 ± 3 | −0.7 (−2.0 to 0.7) | .33 | 11 ± 9* | 9 ± 13* | −2 (−8 to 4) | .52 |
| VO2max, L/min | 1.66 ± 0.3 | 1.76 ± 0.2 | 0.1 (−0.02 to 0.23) | .09 | 3 ± 7* | 2 ± 10 | 0 (−4 to 3) | .80 |
| SBP, mmHg | 121 ± 17 | 130 ± 12 | 8 (2–15) | .01 | 0 ± 12 | −1 ± 12 | −1 (−7 to 6) | .85 |
| DBP, mmHg | 78 ± 11 | 81 ± 6 | 3 (−1 to 7) | .16 | −3 ± 16* | 0 ± 11 | 5 (−1 to 11) | .09 |
| Total cholesterol, mg/dL | 207 ± 39 | 198 ± 35 | −9 (−26 to 8) | .29 | −4 ± 9* | 1 ± 9 | 5 (1–9) | .02 |
| TG, mg/dL | 110 ± 37 | 149 ± 50 | 38 (20–56) | <.001 | −10 ± 19* | −13 ± 16* | −3 (−11 to 6) | .52 |
| HDL-C, mg/dL | 54 ± 14 | 45 ± 10 | −9 (−14 to −3) | .002 | 6 ± 14* | 10 ± 10* | 4 (−2 to 9) | .21 |
| LDL-C, mg/dL | 131 ± 33 | 123 ± 31 | −8 (−23 to 7) | .27 | −6 ± 12* | 3 ± 12 | 9 (3–15) | .002 |
| Glu0, mg/dL | 94 ± 7 | 100 ± 17 | 4 (1–8) | .02 | −4 ± 9* | −3 ± 8 | 2 (−2 to 6) | .36 |
| Glu120, mg/dL | 124 ± 34 | 145 ± 30 | 20 (6–35) | .008 | −3 ± 25 | −11 ± 16* | −8 (−18 to 2) | .13 |
| Ins0, pmol/L | 63 ± 21 | 98 ± 37 | 34 (21–47) | <.001 | −9 ± 22* | −4 ± 20 | 5 (−5 to 15) | .30 |
| HOMA-IR | 2.5 ± 0.9 | 4.0 ± 1.6 | 1.5 (0.9–2.1) | <.001 | −13 ± 26 | −6 ± 24 | 7 (−5 to 18) | .28 |
| hsCRP, μg/mL | 0.5 ± 0.4 | 0.6 ± 0.3 | 0.1 (−0.1 to 0.3) | .51 | −14 ± 58 | −25 ± 40* | −11 (−44 to 22) | .49 |
| IL-6, pg/mL | 2.5 ± 1.3 | 2.4 ± 1.1 | −0.1 (−0.9 to 0.6) | .70 | −14 ± 36* | 28 ± 52* | 35 (14–56) | .002 |
| TNF-α, pg/mL | 0.9 ± 0.4 | 1.0 ± 0.4 | 0.1 (−0.1 to 0.3) | .33 | 8 ± 71 | −14 ± 25* | −12 (−33 to 8) | .23 |
| sTNFR1A, pg/mL | 1028 ± 235 | 1177 ± 307 | 148 (2–295) | .05 | −3 ± 15 | −4 ± 12 | −2 (−11 to 7) | .71 |
| sTNFR1B, pg/mL | 2143 ± 405 | 2336 ± 493 | 193 (−54 to 440) | .12 | −2 ± 10 | −4 ± 10 | −2 (−8 to 4) | .43 |
Notes: Values are mean ± SD. BMI = body mass index; CL = confidence limits; Glu0 = glucose at Time 0; Glu120 = glucose at time 120 minutes; HDL-C = high-density lipoprotein cholesterol; HOMA-IR = homeostasis model assessment—insulin resistance; hsCRP = high-sensitive C-reactive protein; IL-6 = interleukin-6; Ins0 = insulin concentration at Time 0; LDL-C = low-density lipoprotein cholesterol; SAT = subcutaneous abdominal fat, S/DBP = systolic/diastolic blood pressure; sTNFR = soluble tumor necrosis factor receptor; TG = triglyceride; TNF-α = tumor necrosis factor alpha; VAT = visceral adipose tissue.
*Indicates significant change (p < .05) in response to WL+LEX. †p Value of non-MetSyn versus MetSyn.
Response to WL+LEX in MetSyn and non-MetSyn groups.—
We compared the responses of non-MetSyn with MetSyn groups to test whether participants with the worse metabolic profile at baseline would show the greatest improvement. The response in both groups was robust, with most variables showing a highly significant change (Table 1). The non-MetSyn group showed significantly greater improvements in VAT, total cholesterol, low-density lipoprotein cholesterol, and IL-6 responses, but there were no differences in the responses of the MetSyn variables. We then determined whether the two groups differed in the number of MetSyn criteria that changed with intervention (ie, a categorical analysis). This showed that there were significant reductions in the waist, TG, and glucose constituents in the MetSyn group (Table 3) but no difference between MetSyn and non-MetSyn in the relative number of criteria that changed (non-MetSyn: 22 of 61 vs MetSyn: 40 of 118, p = Not Significant).
Table 3.
Responses of MetSyn Constituents by Category to WL-LEX in Responders (R) and Nonresponders (NR)
| MetSyn at Baseline |
Nonresponders |
Responders |
p Value† | ||||
| Baseline (N = 33) | Change Due to WL+LEX | Baseline (N = 18) | Change Due to WL+LEX | Baseline (N = 15) | Change Due to WL+LEX | ||
| MetSyn waist | 33 | −5* | 18 | −1 | 15 | −4* | NS |
| MetSyn TG | 20 | −12* | 12 | −5 | 8 | −7* | NS (.07) |
| MetSyn HDL-C | 28 | −6 | 17 | −2 | 11 | −4 | NS |
| MetSyn BP | 21 | −6 | 13 | −1 | 8 | −5 | .007 |
| MetSyn glucose | 16 | −11* | 9 | −4 | 7 | −7* | .02 |
| MetSyn total | 118 | −40* | 69 | −13 | 49 | −27* | <.0001 |
Notes: Values represent number of participants. Two participants with baseline data were missing postintervention data and MetSyn status could not be determined after WL+LEX. BP = blood pressure; HDL-C = high-density lipoprotein cholesterol; TG = triglyceride; NS = Not Significant.
*Indicates that the difference (pre- and post-WL+LEX) in the number of MetSyn participants with each MetSyn constituent is significant (p < .05). †p Value of the response in NR versus R.
Baseline differences between responder (R) and nonresponder (NR) groups.—
Of the 33 MetSyn participants, 15 responded to WL+LEX by converting to non-MetSyn status and were classified as responders (R) and the 18 who remained MetSyn were classified as nonresponders (NR). There were no significant differences between R and NR participants at baseline in any of the MetSyn components, either when expressed as continuous (Table 2) or categorical variables. The only variable that differed between the R and NR groups at baseline was VO2max expressed per kilogram body weight (p = .001), with NR being more deconditioned prior to intervention.
Table 2.
Physiological and Metabolic Responses to WL+LEX in Responders (R) and Nonresponders (NR)
| Baseline Values |
Percent Change After WL+LEX |
|||||||
| NR (n = 18) | R (n = 15) | Change | p Value | NR | R | Change | p Value† | |
| Age, y | 58 ± 6 | 59 ± 5 | 1 (−4 to 6) | .71 | ||||
| Weight, kg | 93 ± 15 | 86 ± 11 | −6 (−17 to 4) | .21 | −4 ± 4* | −7 ± 5* | −2 (−6 to 1) | .14 |
| BMI, kg/m2 | 35 ± 5 | 32 ± 4 | −3 (−6 to 1) | .15 | −4 ± 4* | −7 ± 5* | −2 (−6 to 1) | .14 |
| % Body fat | 49 ± 6 | 47 ± 4 | −2 (−6 to 1) | .19 | −2.4 ± 1.6* | −4.1 ± 2.9* | −1.6 (−3.2 to −0.1) | .05 |
| Fat mass, kg | 45 ± 10 | 40 ± 8 | −5 (−12 to 1) | .13 | −8 ± 4* | −15 ± 10* | −7 (−12 to −1) | .02 |
| Lean mass, kg | 44 ± 7 | 42 ± 4 | −1 (−7 to 5) | .65 | 0 ± 4 | −1 ± 4 | −1 (−4 to 2) | .37 |
| Waist, cm | 103 ± 10 | 98 ± 8 | −5 (−12 to 2) | .15 | −2 ± 4* | −4 ± 4* | −2 (−5 to 1) | .14 |
| VAT, cm2 | 166 ± 59 | 180 ± 50 | 14 (−32 to 60) | .54 | −6 ± 18 | −14 ± 21* | −9 (−23 to 6) | .24 |
| SAF, cm2 | 489 ± 114 | 467 ± 131 | −22 (−140 to 96) | .71 | −14 ± 10* | −12 ± 12* | 2 (−7 to 11) | .71 |
| VO2max mL/kg/m | 18 ± 3 | 21 ± 2 | 3 (1–5) | .001 | 9 ± 14* | 12 ± 12* | 4 (−7 to 14) | .50 |
| VO2max, L/m | 1.69 ± 0.3 | 1.81 ± 0.2 | 0.12 (−0.1 to 0.3) | .16 | 2 ± 10 | 4 ± 10 | 2 (−6 to 9) | .67 |
| SBP, mmHg | 133 ± 13 | 126 ± 12 | −7 (−16 to 2) | .15 | 1 ± 13 | −3 ± 11 | −4 (−13 to 4) | .29 |
| DBP, mmHg | 82 ± 6 | 79 ± 6 | −2 (−7 to 3) | .35 | 4 ± 13 | −3 ± 8 | −6 (−14 to 1) | .11 |
| Total cholesterol, mg/dL | 196 ± 34 | 208 ± 32 | 12 (−14 to 39) | .35 | 1 ± 8 | −1 ± 10 | −2 (−8 to 4) | .52 |
| TG, mg/dL | 154 ± 51 | 147 ± 48 | −8 (−26 to 11) | .42 | −12 ± 12* | −15 ± 18* | −2 (−12 to 7) | .63 |
| HDL-C, mg/dL | 43 ± 8 | 45 ± 7 | 2 (−3 to 6) | .45 | 7 ± 11* | 13 ± 9* | 6 (−2 to 13) | .14 |
| LDL-C, mg/dL | 121 ± 27 | 133 ± 28 | 12 (−8 to 32) | .25 | 3 ± 11 | 0 ± 12 | −3 (−10 to 4) | .46 |
| Glu0, mg/dL | 98 ± 10 | 97 ± 8 | −1 (−8 to 5) | .76 | −1 ± 9 | −5 ± 7* | −4 (−10 to 2) | .20 |
| Glu120, mg/dL | 147 ± 24 | 140 ± 36 | −7 (−31 to 16) | .55 | −11 ± 14* | −11 ± 18* | 0 (−17 to 17) | .98 |
| Ins0, pmol/L | 102 ± 35 | 90 ± 40 | −12 (−43 to 18) | .43 | −4 ± 19 | −8 ± 17 | −3 (−16 to 10) | .61 |
| HOMA-IR | 4.2 ± 1.6 | 3.6 ± 1.7 | −0.5 (−1.8 to 0.8) | .43 | −5 ± 24 | −13 ± 16* | −8 (−23 to 7) | .30 |
| hsCRP, μg/mL | 0.5 ± 0.3 | 0.6 ± 0.4 | 0.1 (−0.2 to 0.4) | .57 | −25 ± 38 | −25 ± 44 | 0 (−7 to 6) | .89 |
| IL-6, pg/mL | 2.2 ± 1.0 | 2.5 ± 1.2 | 0.4 (−0.4 to 1.1) | .32 | 42 ± 57* | 11 ± 43 | −31(−75 to 13) | .18 |
| TNF-α, pg/mL | 1.0 ± 0.5 | 1.1 ± 0.3 | 0.1 (−0.4 to 0.7) | .67 | −15 ± 27 | −13 ± 23 | 1 (−34 to 37) | .94 |
| sTNFR1A, pg/mL | 1196 ± 342 | 1159 ± 287 | −37 (−285 to 212) | .77 | −5 ± 11 | −4 ± 13 | 2 (−5 to 8) | .65 |
| sTNFR1B, pg/mL | 2420 ± 561 | 2252 ± 429 | −168 (−589 to 252) | .43 | −7 ± 5* | −1 ± 13 | 7 (−1 to 14) | .09 |
Notes: Values are mean ± SD. BMI = body mass index; Glu0 = glucose at Time 0; Glu120 = glucose at time 120 minutes; HDL-C = high-density lipoprotein cholesterol; HOMA-IR = homeostasis model assessment—insulin resistance; hsCRP = high-sensitive C-reactive protein; IL-6 = interleukin-6; Ins0 = insulin concentration at Time 0; LDL-C = low-density lipoprotein cholesterol; SAT = subcutaneous abdominal fat, S/DBP = systolic/diastolic blood pressure; sTNFR = soluble tumor necrosis factor receptor; TG = triglyceride; TNF-α = tumor necrosis factor alpha; VAT = visceral adipose tissue.
*Indicates significant change (p < .05) in response to WL+LEX. †p Value of nonresponders versus responders.
Response to WL+LEX in responder (R) and nonresponder (NR) groups.—
There was no difference between the NR and R groups in attendance (NR: 77% ± 13%, R: 78% ± 12%, p = .67) or compliance (NR: 3.1 ± 1.0, R: 3.4 ± 1.1, p = .47). At baseline, the NR consumed slightly less carbohydrate than the R (57% vs 61%, p < .04), but there were no differences in total calories or other dietary variables. There were no differences between NR and R in the changes in total calories; carbohydrate; protein; fat; saturated, monounsaturated, or polyunsaturated fat; fiber; or sodium with WL+LEX; but dietary cholesterol intake decreased less in the NR than in the R (26% vs 37%, p = .004).
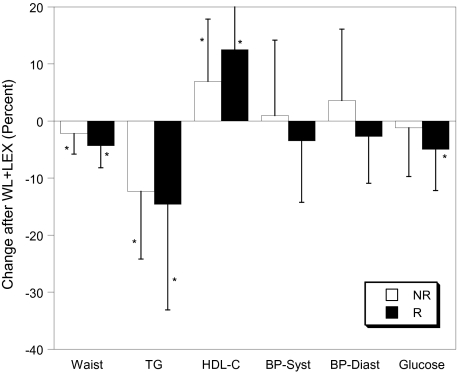
The WL+LEX intervention did not change VO2max (liters per minute) significantly in either group; however, all anthropometric and many of the metabolic constituents in both the NR and R groups decreased (Table 2; Figure 1). Reductions in fat mass (−8% vs −15%, p = .02) and percent fat (2.4% vs −4.0%, p = .05) were greater in R, and the reduction in visceral fat was more than twofold greater in R (−6% vs −14%, p = Not Significant). There were no between-group differences in changes in VO2max, cytokines, or other variables. Of the MetSyn variables, waist, TG, and high-density lipoprotein cholesterol showed significant improvements in both NR and R participants, fasting glucose decreased only in R, and blood pressure did not change significantly in either group (Table 2). As expected, the change in the total number of MetSyn criteria was significantly greater in the R group (−27 vs −13, p < .0001), and when the individual criteria were examined (Table 3), there were differences in the changes in blood pressure (p < .01) and glucose (p = .03) criteria, with a trend toward significance for TG (p = .07).
Figure 1.
Response of metabolic syndrome variables to weight loss and low-intensity exercise (WL+LEX) in responders (R) and nonresponders (NR). Data are mean ± SD. BP = blood pressure; HDL-C = high-density lipoprotein cholesterol; TG = triglyceride.
Stepwise logistic regression using candidate variables of body composition and fitness showed that the change in fat mass was the best predictor of resolution of MetSyn (p = .04).
DISCUSSION
The metabolic syndrome is a cluster of abnormalities that are associated with increased risk of developing CVD and type 2 diabetes (5–7). Results of this study show that a 6-month lifestyle intervention of moderate weight loss and low-intensity exercise effectively reduces both CVD risk factors and the prevalence of the MetSyn by 45%. A novel outcome of our study is that the reductions in TG, glucose, and blood pressure are the MetSyn criteria primarily associated with conversion from MetSyn to non-MetSyn status. This is similar to National Health and Nutrition Examination Survey study data which suggests that hypertriglyceridemia and hypertension are the cardiometabolic abnormalities responsible for the heightened prevalence of MetSyn among older women (3) and supports the possibility that the high prevalence of MetSyn is related to weight gain in midlife (23) leading to abdominal obesity, high TGs, and low high-density lipoprotein (24). Our results also show that WL+LEX reduces the constituents of the MetSyn in obese postmenopausal women both with and without MetSyn to an equivalent degree, which suggests that this intervention may be effective in preventing as well as reversing the MetSyn. Although most of the MetSyn variables decreased in response to the intervention, there were greater reductions in the non-MetSyn criteria of total cholesterol and low-density lipoprotein cholesterol, IL-6, and VAT in the non-MetSyn than in the MetSyn participants, suggesting an overall health benefit of this lifestyle intervention for obese postmenopausal women.
Interestingly, none of the changes in MetSyn variables after WL+LEX differed between R and NR when analyzed as continuous variables. Although MetSyn is predictive of the development of diabetes and CVD, both the American and the European Diabetes Associations and others express concern over the arbitrary and dichotomous nature of the cut points for its constituents (25–28), which is supported by the discordance between the categorical and continuous variable analysis in this study.
The participants who achieved larger reductions in percent body fat and fat mass with WL+LEX were more likely to reverse MetSyn status. The small change in VO2max, when calculated as absolute O2 consumption (liters per minute) to minimize the inflated increase in VO2max if expressed per kilogram body weight, supports the primary role of WL in resolution of MetSyn. However, the exercise may have prevented significant loss of lean mass seen with WL alone in older people, which may predispose to sarcopenia (29). Thus, both WL and meeting the physical activity guidelines for Americans seem to reduce the incidence and development of MetSyn components in older adults (30,31). The moderate dropout rate and resultant small sample size probably precluded determination whether the resolution of the MetSyn in these women is related primarily to the decrease in visceral body fat. Although there was no difference in the attendance to the WL+LEX classes between the responders and the nonresponders, the responders did lose more body fat and may have complied better with the intervention suggesting that behavioral factors may be important. Other possible contributing factors to these responses include genetic susceptibility, as the effects of the fat mass and obesity associated (FTO) gene variants on obesity can be blunted through physical activity (32).
The post hoc analysis is a weakness in the design, but our results are congruent with the findings that a 6-month moderate intensity exercise regimen consisting of flexibility, balance, aerobic and strength training, and 8% weight loss is associated with a 59% reduction in the prevalence of the MetSyn in older men and women (14). Interestingly, the 41% reduction in the prevalence of MetSyn in the Diabetes Prevention Program population of glucose intolerant men and women (13) was observed after 3 years of a WL and exercise intervention in contrast to the 6-month intervention in the current study and that of Villareal and colleagues (14). In another study examining the effects of a Mediterranean diet with instructions to increase daily physical activity by 30 minutes, there was a 60% reduction in the MetSyn in middle-aged men and women after 2 years (33). In a recent study (34), there were even greater reductions in MetSyn variables after a more intensive walking program in which energy intake is kept constant or when exercise is combined with caloric restriction to increase VO2max by ∼15% in a smaller group of 24 older men and women with MetSyn. Furthermore, in an observational study in older Japanese men and women, the risk of MetSyn was 4.3 times greater in the least active quartile compared with the most active (35). This suggests that the intensity, duration, and likelihood of compliance to the lifestyle intervention should be considered when designing treatment program for MetSyn.
Several investigators postulate that a chronic low-grade inflammation is a key component of MetSyn in older people (36), especially given the relationship between elevated cytokine concentrations, obesity, and insulin resistance (37). However, inflammation is not included in the definition of the MetSyn in the United States (1) or Europe (38,39). Similar to our previous work, there are no differences in the majority of the cytokine concentrations at baseline other than soluble tumor necrosis factor receptor (sTNFR1A) between the MetSyn and the non-MetSyn groups (40). There were significant reductions in both high-sensitive C-reactive protein and TNF-α levels in the MetSyn and IL-6 in the non-MetSyn participants, confirming our earlier reports (36,40). Contrary to the reductions found in response to a 12-month Mediterranean diet and physical activity intervention (33), IL-6 increased significantly in the MetSyn and NR groups. Although we anticipated a decrease in sTNFR1A, but not in sTNFR1B receptors based on our prior work (41,42), there were only small decreases in TNF receptors with intervention. It is possible that the low-intensity walking exercise chosen in the current study did not improve fitness to the same extent as in our previous interventions, given the inverse relationship of the changes in sTNFR1A with VO2max (41). Further research in a larger sample is necessary to assess the independent and combined effects of weight loss and aerobic exercise of different intensities and duration on cytokines, and their role in the CVD risk and complications associated with MetSyn in older adults.
Thus, a lifestyle intervention that employs a weight-reducing diet coupled with low-intensity exercise reduces the constituents of the MetSyn in overweight and obese middle-aged to older Caucasian and African American postmenopausal women. This intervention is effective in women with and without MetSyn, suggesting a beneficial role for WL+LEX in preventing the development of MetSyn as well as treating MetSyn in these high CVD risk individuals. Future studies are necessary to elucidate the mechanisms underlying these metabolic changes, the most effective exercise and weight loss programs and the predictors of the most desirable metabolic and health-related outcomes in older women with MetSyn.
FUNDING
Supported by the National Institutes of Health (K01 AG021457 to L.J.J., R01 AG20116 to A.P.G., AG019310 to A.S.R., P30 AG028747 to A.P.G., P60 DK078637, and DK072488) and the Department of Veterans Affairs (Baltimore VA Medical Center, Geriatric Research, Education and Clinical Center, VA Research Career Scientist Award to A.S.R., Advanced Career Development Award to J.B.B., and the Department of Veteran Affairs Medical Research Service, Baltimore, Maryland).
Acknowledgments
L.J.J. contributed to this article as an employee of the University of Maryland. L.J.J. is currently employed at the National Institute on Aging, National Institutes of Health. The views expressed are his own and do not necessarily represent the views of the National Institutes of Health or the U.S. Government.
References
- 1.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among U.S. adults. Diabetes Care. 2004;27(10):2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 4.Lin JW, Caffrey JL, Chang MH, Lin YS. Sex, menopause, metabolic syndrome, and all-cause and cause-specific mortality—cohort analysis from the Third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2010;95(9):4258–4267. doi: 10.1210/jc.2010-0332. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107(3):391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 6.Sattar N, Gaw A, Scherbakova O, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108(4):414–419. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 7.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 8.Monami M, Lamanna C, Balzi D, et al. Metabolic syndrome and cardiovascular mortality in older type 2 diabetic patients: a longitudinal study. J Gerontol A Biol Sci Med Sci. 2008;63(6):646–649. doi: 10.1093/gerona/63.6.646. [DOI] [PubMed] [Google Scholar]
- 9.Maggi S, Noale M, Gallina P, et al. Metabolic syndrome, diabetes, and cardiovascular disease in an elderly Caucasian cohort: the Italian Longitudinal Study on Aging. J Gerontol A Biol Sci Med Sci. 2006;61(5):505–510. doi: 10.1093/gerona/61.5.505. [DOI] [PubMed] [Google Scholar]
- 10.Stenholm S, Koster A, Alley DE, et al. Joint association of obesity and metabolic syndrome with incident mobility limitation in older men and women—results from the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2010;65(1):84–92. doi: 10.1093/gerona/glp150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penninx BW, Nicklas BJ, Newman AB, et al. Metabolic syndrome and physical decline in older persons: results from the Health, Aging And Body Composition Study. J Gerontol A Biol Sci Med Sci. 2009;64(1):96–102. doi: 10.1093/gerona/gln005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blaum CS, West NA, Haan MN. Is the metabolic syndrome, with or without diabetes, associated with progressive disability in older Mexican Americans? J Gerontol A Biol Sci Med Sci. 2007;62(7):766–773. doi: 10.1093/gerona/62.7.766. [DOI] [PubMed] [Google Scholar]
- 13.Orchard TJ, Temprosa M, Goldberg R, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142(8):611–619. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villareal DT, Miller BV, III, Banks M, Fontana L, Sinacore DR, Klein S. Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. Am J Clin Nutr. 2006;84(6):1317–1323. doi: 10.1093/ajcn/84.6.1317. [DOI] [PubMed] [Google Scholar]
- 15.Katzmarzyk PT, Leon AS, Wilmore JH, et al. Targeting the metabolic syndrome with exercise: evidence from the HERITAGE Family Study. Med Sci Sports Exerc. 2003;35(10):1703–1709. doi: 10.1249/01.MSS.0000089337.73244.9B. [DOI] [PubMed] [Google Scholar]
- 16.Harris T, Cook EF, Garrison R, Higgins M, Kannel W, Goldman L. Body mass index and mortality among nonsmoking older persons. The Framingham Heart Study. JAMA. 1988;259(10):1520–1524. [PubMed] [Google Scholar]
- 17.Nicklas BJ, Dennis KE, Berman DM, Sorkin J, Ryan AS, Goldberg AP. Lifestyle intervention of hypocaloric dieting and walking reduces abdominal obesity and improves coronary heart disease risk factors in obese, postmenopausal, African-American and Caucasian women. J Gerontol A Biol Sci Med Sci. 2003;58(2):181–189. doi: 10.1093/gerona/58.2.m181. [DOI] [PubMed] [Google Scholar]
- 18.American Heart Association. Dietary guidelines for healthy American adults. A statement for physicians and health professionals by the Nutrition Committee, American Heart Association. Circulation. 1988;77(3):721A–724A. [PubMed] [Google Scholar]
- 19.Nicklas BJ, Rogus EM, Colman EG, Goldberg AP. Visceral adiposity, increased adipocyte lipolysis, and metabolic dysfunction in obese postmenopausal women. Am J Physiol. 1996;270(1 Pt 1):E72–E78. doi: 10.1152/ajpendo.1996.270.1.E72. [DOI] [PubMed] [Google Scholar]
- 20.Halverstadt A, Phares DA, Wilund KR, Goldberg AP, Hagberg JM. Endurance exercise training raises high-density lipoprotein cholesterol and lowers small low-density lipoprotein and very low-density lipoprotein independent of body fat phenotypes in older men and women. Metabolism. 2007;56(4):444–450. doi: 10.1016/j.metabol.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Miller AJ. Algorithm AS 274: least squares routines to supplement those of Gentleman. J Royal Stat Soc (App Stat) 1992;41(2):458–478. [Google Scholar]
- 22.Belsley DA, Kuh E, Welsch RE. Regression Diagnostics. New York: Wiley; 1980. [Google Scholar]
- 23.Alley DE, Chang VW. Metabolic syndrome and weight gain in adulthood. J Gerontol A Biol Sci Med Sci. 2010;65(1):111–117. doi: 10.1093/gerona/glp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scuteri A, Morrell CH, Najjar SS, et al. Longitudinal paths to the metabolic syndrome: can the incidence of the metabolic syndrome be predicted? The Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2009;64(5):590–598. doi: 10.1093/gerona/glp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau DC. Metabolic syndrome: perception or reality? Curr Atheroscler Rep. 2009;11(4):264–271. doi: 10.1007/s11883-009-0041-7. [DOI] [PubMed] [Google Scholar]
- 26.Mente A, Yusuf S, Islam S, et al. Metabolic syndrome and risk of acute myocardial infarction a case-control study of 26,903 subjects from 52 countries. J Am Coll Cardiol. 2010;55(21):2390–2398. doi: 10.1016/j.jacc.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 27.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28(9):2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 28.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal. Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2005;48(9):1684–1699. doi: 10.1007/s00125-005-1876-2. [DOI] [PubMed] [Google Scholar]
- 29.Weiss EP, Racette SB, Villareal DT, et al. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol. 2007;102(2):634–640. doi: 10.1152/japplphysiol.00853.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Physical Activity Guidelines for Americans. Washington, DC: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- 31.Peterson MJ, Morey MC, Giuliani C, et al. Walking in old age and development of metabolic syndrome: the Health, Aging, and Body Composition Study. Metab Syndr Relat Disord. 2010;8(4):317–322. doi: 10.1089/met.2009.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rampersaud E, Mitchell BD, Pollin TI, et al. Physical activity and the association of common FTO gene variants with body mass index and obesity. Arch Intern Med. 2008;168(16):1791–1797. doi: 10.1001/archinte.168.16.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292(12):1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 34.Yassine HN, Marchetti CM, Krishnan RK, Vrobel TR, Gonzalez F, Kirwan JP. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults—a randomized clinical trial. J Gerontol A Biol Sci Med Sci. 2009;64(1):90–95. doi: 10.1093/gerona/gln032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park S, Park H, Togo F, et al. Year-long physical activity and metabolic syndrome in older Japanese adults: cross-sectional data from the Nakanojo Study. J Gerontol A Biol Sci Med Sci. 2008;63(10):1119–1123. doi: 10.1093/gerona/63.10.1119. [DOI] [PubMed] [Google Scholar]
- 36.You T, Nicklas BJ, Ding J, et al. The metabolic syndrome is associated with circulating adipokines in older adults across a wide range of adiposity. J Gerontol A Biol Sci Med Sci. 2008;63(4):414–419. doi: 10.1093/gerona/63.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruotsalainen E, Salmenniemi U, Vauhkonen I, et al. Changes in inflammatory cytokines are related to impaired glucose tolerance in offspring of type 2 diabetic subjects. Diabetes Care. 2006;29(12):2714–2720. doi: 10.2337/dc06-0147. [DOI] [PubMed] [Google Scholar]
- 38.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 39.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 40.You T, Ryan AS, Nicklas BJ. The metabolic syndrome in obese postmenopausal women: relationship to body composition, visceral fat, and inflammation. J Clin Endocrinol Metab. 2004;89(11):5517–5522. doi: 10.1210/jc.2004-0480. [DOI] [PubMed] [Google Scholar]
- 41.You T, Berman DM, Ryan AS, Nicklas BJ. Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J Clin Endocrinol Metab. 2004;89(4):1739–1746. doi: 10.1210/jc.2003-031310. [DOI] [PubMed] [Google Scholar]
- 42.Ryan AS, Nicklas BJ. Reductions in plasma cytokine levels with weight loss improve insulin sensitivity in overweight and obese postmenopausal women. Diabetes Care. 2004;27(7):1699–1705. doi: 10.2337/diacare.27.7.1699. [DOI] [PubMed] [Google Scholar]