Abstract
Stromal interaction molecules (STIM) were identified as the endoplasmic-reticulum (ER) Ca2+ sensor controlling store-operated Ca2+ entry (SOCE) and Ca2+-release-activated Ca2+ (CRAC) channels in non-excitable cells. STIM proteins target Orai1-3, tetrameric Ca2+-permeable channels in the plasma membrane. Structure-function analysis revealed the molecular determinants and the key steps in the activation process of Orai by STIM. Recently, STIM1 was found to be expressed at high levels in skeletal muscle controlling muscle function and properties. Novel STIM targets besides Orai channels are emerging.
Here, we will focus on the role of STIM1 in skeletal-muscle structure, development and function. The molecular mechanism underpinning skeletal-muscle physiology points toward an essential role for STIM1-controlled SOCE to drive Ca2+/calcineurin/nuclear factor of activated T cells (NFAT)-dependent morphogenetic remodeling programs and to support adequate sarcoplasmic-reticulum (SR) Ca2+-store filling. Also in our hands, STIM1 is transiently up-regulated during the initial phase of in vitro myogenesis of C2C12 cells. The molecular targets of STIM1 in these cells likely involve Orai channels and canonical transient receptor potential (TRPC) channels TRPC1 and TRPC3. The fast kinetics of SOCE activation in skeletal muscle seem to depend on the triad-junction formation, favoring a pre-localization and/or pre-formation of STIM1-protein complexes with the plasma-membrane Ca2+-influx channels. Moreover, Orai1-mediated Ca2+ influx seems to be essential for controlling the resting Ca2+ concentration and for proper SR Ca2+ filling. Hence, Ca2+ influx through STIM1-dependent activation of SOCE from the T-tubule system may recycle extracellular Ca2+ losses during muscle stimulation, thereby maintaining proper filling of the SR Ca2+ stores and muscle function. Importantly, mouse models for dystrophic pathologies, like Duchenne muscular dystrophy, point towards an enhanced Ca2+ influx through Orai1 and/or TRPC channels, leading to Ca2+-dependent apoptosis and muscle degeneration. In addition, human myopathies have been associated with dysfunctional SOCE. Immunodeficient patients harboring loss-of-function Orai1 mutations develop myopathies, while patients suffering from Duchenne muscular dystrophy display alterations in their Ca2+-handling proteins, including STIM proteins. In any case, the molecular determinants responsible for SOCE in human skeletal muscle and for dysregulated SOCE in patients of muscular dystrophy require further examination.
Review
STIM is the ER Ca2+ sensor that controls Orai-mediated store-operated Ca2+ influx
For about 20 years after the initial concept of store-operated Ca2+ entry (SOCE) was proposed by Putney [1,2], the molecular candidates underpinning SOCE remained elusive. In 2005 and 2006, key players for SOCE in non-excitable cells were identified via RNAi screens in Drosophila [3] and HeLa cells [4], which elucidated STIM proteins as the endoplasmic-reticulum (ER) Ca2+ sensor and Orai proteins [5-7] as the Ca2+-permeable store-operated Ca2+ channel or Ca2+-release activated Ca2+ (CRAC) channel [8,9]. In mammals, two STIM genes, STIM1 and STIM2, and three ORAI genes, ORAI1, ORAI2 and ORAI3, have been identified [5-7]. Different reports confirmed that STIM1 and Orai1 are the molecular candidates for currents with the electrophysiological properties of the CRAC channel [10-12]. These properties include a high selectivity for Ca2+ over monovalent ions, like Na+ and K+, and a single-channel Ca2+ conductance of about 30 fS, which is about 100 times smaller than the conductance of L-type Ca2+ channels [13,14].
STIM1 is predominantly located in the ER, the main intracellular Ca2+ store [4,15-17]. The ER-resident STIM1 controls Orai function by activating these channels upon ER Ca2+-store depletion [3,12,17-19]. Recently, the different steps involved in the STIM1-dependent activation of Ca2+ influx upon store depletion have been identified [15,16,20,21]. These steps include the sensing of Ca2+ depletion from the ER, dissociation of Ca2+ from the EF-hand motif of STIM1, the rapid oligomerization of STIM1, the translocation of STIM1 into punctae consisting of close ER/plasma-membrane junctions and the activation of plasma-membrane Ca2+-influx channels (see [13,14] for recent reviews).
STIM1 (approximately 75 kDa) contains an intraluminal region of approximately 22 kDa, a single transmembrane domain and a cytosolic region of approximately 51 kDa. The functional intraluminal ER Ca2+ sensor of STIM1 is the first of two EF-hand domains (EF1; aa 63-96), which precedes a second EF-hand domain (EF2; aa 97-128) and a sterile α-motif domain (SAM; aa 131-200) [13,22]. The cytosolic domain consists of two or three coiled-coil domains within an ezrin-radixin moesin (ERM) domain, a serine/proline-rich (S/P) domain and a polybasic lysine-rich (KKK) domain. Structural analysis of the recombinantly expressed EF-SAM domain (aa 58-201) revealed that Ca2+ is bound to the first EF-hand domain. EF-SAM exists in a monomeric state when Ca2+ is bound because of close interaction of the paired EF hands and SAM [22,23]. When Ca2+ dissociates, protein unfolding triggers major structural rearrangements of EF-SAM and the accumulation of dimer and aggregated forms of EF-SAM are observed [22,23]. In vitro experiments revealed a Kd of about 500-600 μM for Ca2+ binding to EF-SAM [23], which is in the range of Ca2+ concentration ([Ca2+]) in the ER [21].
Ca2+ dissociation from EF1 of STIM1 causes its oligomerization [22,23] and underpins the sequential changes upon ER Ca2+-store depletion [20]. Mutations in EF1 disrupting the Ca2+-binding properties of STIM1 or mutations in EF2 and SAM domain destabilizing the interaction between the EF-hand domains and SAM, result in a constitutively active STIM1 and activation of Orai proteins, resembling their state during depleted ER Ca2+ stores [4,17]. Oligomerization of STIM1 is closely related to activation of CRAC, since artificial oligomerization of the STIM1 cytosolic domains was sufficient to trigger punctae formation and Ca2+ influx [21]. These results indicate that the oligomerization of STIM1 is the switch that controls SOCE upon ER Ca2+-store depletion via STIM1/Orai1 clustering at ER/plasma-membrane junctions [21].
Upon STIM1 oligomerization, STIM1 redistributes to sites of close apposition of ER and plasma membrane [15,16,24]. The kinetics of STIM1 redistribution is in the order of tens of seconds and involves local diffusion in the ER membranes, while interaction with specific lipids or proteins may facilitate the accumulation of ER/plasma-membrane contact sites [4,16,20]. Two factors seem to contribute to STIM1 relocalization: i) protein-lipid interactions, mediated by the interaction of the polybasic C-terminus of STIM1 with plasmalemmal phospholipids like phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate, and ii) protein-protein interactions, mediated by the direct interaction of STIM1 with the C-terminus of Orai [20,25-28]. The latter interactions are proposed to contribute to the recruitment of Orai1 to ER/plasma-membrane junctions. Indeed, C-terminal truncation of Orai1 fails to co-localize with STIM1 punctae and hence fails to mediate CRAC currents upon store depletion [29,30]. A final step in the activation process is the opening of the tetrameric, Ca2+-selective Orai1 channels. In vitro Ca2+-flux assays revealed that Orai1 channels are directly gated by STIM1 [31]. The N-terminal cytosolic domain of Orai1 seems critical for Orai1-channel opening [30], possibly involving a direct binding of STIM1 to aa 65-91 of Orai1 [26,29,31]. Recently, the minimal region of STIM1 involved in CRAC activation was identified as the CRAC-activating domain (CAD, aa 342-448), also known as the STIM1-Orai1-activating region (SOAR, aa 344-442) [26,32-34].
Very recently, Orai1 channels were shown to be directly activated by the SPCA2, a Ca2+ pump belonging to the secretory-pathway Ca2+ ATPases [35]. SPCA2 expression potentiated Ca2+ influx through Orai1 channels, independently of STIM proteins or SPCA2 Ca2+-ATPase activity. The mechanism involves a two-step activation mechanism and interaction of two parts of SPCA2: binding of the N-terminal region of SPCA2 to Orai1 enables SPCA2's C-terminus to access and activate Orai1 [35]. These findings are clinically relevant, since SPCA2 is up-regulated in breast tumors and SPCA2 knockdown decreases tumorigenicity.
STIM2 is very similar to STIM1 in basic structure and functional properties [4,36]. STIM2 senses luminal ER Ca2+ via two EF-hands. Ca2+ dissociation from STIM2 leads to a conformational change, oligomerization and redistribution to ER/plasma-membrane contact sites [4]. The redistribution of STIM2 seems to occur at higher ER [Ca2+], that is, at smaller decreases in ER, than the redistribution of STIM1 [Ca2+] [37]. In this perspective, STIM2 has been identified in an siRNA screen as a critical feedback regulator of basal cytosolic and ER [Ca2+] [37]. Knockdown of STIM2 markedly lowers the basal cytosolic and ER [Ca2+], while knockdown of STIM1 has less effect. However, these features may be dependent on the cell type and/or levels of STIM2, since basal [Ca2+] as well as the thapsigargin-releasable Ca2+ did not differ between wild-type mouse embryonic fibroblast (MEF) cells and MEF cells deficient for STIM2 [38]. Similar findings were observed using an overexpression approach, in which STIM2 overexpression increased basal cytosolic [Ca2+], markedly more than STIM1 overexpression. Consistent with these findings, small reductions in ER [Ca2+] caused STIM2, but not STIM1, translocation and redistribution to punctae, with subsequent activation of Ca2+ influx [37]. While both STIM1 and STIM2 have been implicated in triggering Ca2+ influx following receptor-mediated ER Ca2+-store depletion [37,39], STIM1-/- cells displayed more severe defects in SOCE than STIM2-/- cells [38,39]. From agonist-induced Ca2+ release in STIM1-/- and STIM2-/- cells, it was found that STIM2, but not STIM1, is dispensable for agonist-induced Ca2+ signaling [38]. Nevertheless, the sustained nuclear localization of NFAT and production of cytokines are severely hampered in STIM2-/- T cells [39].
The fundamental biological role of STIM/Orai signaling is indicated by the fact that recessive mutations in STIM or Orai affecting their molecular function lead to severe hereditary immunodeficiency in humans [13]. Different mutations in STIM1 as well as Orai1 leading to loss-of-function or loss-of-expression of these proteins have been identified in immunodeficient patients [5,40-42]. Importantly, these defects can be overcome by STIM1 or Orai1 overexpression, but not by STIM2 or Orai2/3, indicating a predominant role for STIM1/Orai1 in T-cell Ca2+-signaling function. Loss of STIM1/Orai1 signaling in patients results in severe T-cell immunodeficiency and concomitant viral, bacterial and fungal infections [41-44]. Strikingly, a congenital, nonprogressive myopathy is consistently observed in infant patients, indicating that STIM1 not only plays a crucial role in T-cell activation and proliferation, but also in skeletal-muscle function and/or development (see part 3) [42].
Other targets of STIM proteins
A detailed discussion on STIM targets can be found in [13].
Microtubule-plus-end-tracking protein EB1
STIM1 is recruited by end-binding protein-1 (EB1) to sites of physical contact between growing microtubule tips and ER [45,46]. However, the contribution of microtubule-associated STIM1 to Ca2+ signaling is not clear. Preventing STIM1 localization at the microtubule does not directly affect SOCE [45] and microtubules are not essential for initial CRAC channel gating in T cells and mast cells [47,48]. Indirect effects of the association of STIM1 with the microtubule may be caused by remodeling of the ER or ER/plasma-membrane contact sites or the availability of STIM1.
Canonical transient receptor potential (TRPC) channels
Other candidates for SOCE include TRPC channels [25,49]. Biochemical and functional experiments revealed that STIM1 directly interacts with TRPC channels through electrostatic interaction, which involves the K864/K865 in the polybasic lysine-rich region of STIM1 and two negative charges that are conserved in all TRPC channels, that is, D639/D640 in TRPC1 and D697/D698 in TRPC3 [50]. STIM1 was shown to directly bind and regulate TRPC1, TRPC4 and TRPC5, while indirect actions of STIM1 on TRPC3 and TRPC6 have been proposed [49]. STIM1-dependent gating of TRPC channels seem to differ from their gating of Orai channels, pointing towards an independent gating mechanism of TRPCs versus Orai channels by STIM1 [50]. Nevertheless, the regulation of TRPC channels by STIM1 has been questioned in a very diligent study performed by the Putney lab [51], in which endogenous and ectopic TRPC1-, TRPC3-, TRPC5-, TRPC6- and TRPC7-mediated Ca2+ entry was unaffected by increased or decreased STIM1 levels. A detailed discussion on the role of STIM1 in regulating Orai versus TRPC channels can be found in a recent review [52].
Arachidonate-regulated Ca2+-selective (ARC) channel
Another target of STIM1 is the ARC channel, a receptor-operated Ca2+-entry channel whose activation is completely independent of store depletion or of translocation of ER-resident STIM1 [53,54]. It is proposed that a fraction of STIM1 constitutively residing at the plasma membrane is responsible for the regulation of the activity of the ARC channels, since antibodies targeting plasmalemmal STIM1 or mutating its N-linked glycosylation sites essential for its cell-surface expression inhibited ARC-channel activity. Recent work identified the molecular architecture of ARC channels, revealing a pentameric organization consisting of three Orai1 and two Orai3 subunits [55]. This deviates from the tetrameric structure of Orai channels mediating CRAC currents.
Adenylate cyclase (AC)
A recent study revealed a novel role for STIM1 where it participated in the store-operated recruitment of AC [56]. Indeed, depleting ER Ca2+ stores led to the recruitment of AC to the intracellular Ca2+ stores, resulting in increased cAMP levels and enhanced signaling by protein kinase A. This process was shown to be independent of increases in cytosolic [Ca2+], but required the translocation of STIM1. This study therefore points out that STIM1 may be an important integrator molecule that mediates cross-talk between Ca2+-dependent signaling and cAMP-dependent signaling. Recently, STIM1-mediated store-operated cAMP signaling has been implicated in the downstream effects of Ca2+ signaling induced by eicosapentaenoic acid, an omega-3 polyunsaturated fatty acid present in fish oil [57].
The L-type Ca2+ channel Cav1.2
In addition to the well-described Orai targets of STIM1, two very recent studies identified the voltage-operated Ca2+ channel Cav1.2 as a novel target of STIM1 [58,59]. STIM1 targeted the α1c pore-forming subunit via a direct interaction, thereby imposing an inhibitory control over Cav1.2 upon ER Ca2+-store depletion, independently of Orai1-channel activity or changes in cytosolic [Ca2+]. The mechanism involves the direct binding of CAD or SOAR to a region encompassing aa 1809-1908, located in the C-terminus of Cav1.2.
Importantly, while CAD or SOAR activates Orai1-mediated currents, they inhibit Cav1.2-mediated currents. The interaction of STIM1 with the C-terminus of Cav1.2 is critical for its inhibition. This process is independent of functional Orai1-channel activity, but Orai1 may help STIM1-mediated Cav1.2 inhibition by trapping STIM1 in punctae that contain Cav1.2 channels, thereby recruiting STIM1 in the vicinity of Cav1.2 channels. In addition, STIM1 expression seems to regulate the plasma-membrane level of Cav1.2 [59]. Overexpression of STIM1 caused a prominent decrease in the amount of Cav1.2 at the plasma membrane, leading to STIM1/Cav1.2 co-localization in intracellular vesicles and the internalization of the channels.
The molecular targeting of STIM1 in Cav1.2 and Orai1-related Ca2+-influx pathways may be the molecular switch that accounts for the reciprocal regulation of voltage-gated Ca2+ influx (inhibition) and store-operated Ca2+ influx (activation) [58,59]. Interestingly, only excitable cells are able to increase cytosolic [Ca2+] in response to membrane depolarization, although immune cells (T cells, B cells, dendritic cells and mast cells) also express voltage-gated Ca2+ channels [60,61]. In contrast, only non-excitable cells display prominent CRAC-channel activity upon store depletion, while SOCE is only a minor component of Ca2+ influx in excitable cells [62,63]. However, excitable cells, like smooth-muscle cells [64,65], neurons [66] and skeletal-muscle cells [63], do express STIM1. The high expression level of STIM1 in skeletal muscle is supported by data obtained from BioGPS (http://biogps.gnf.org/), an online gene annotation portal (Figure 1) [67,68]. Since Cav1.2 and Orai activate different downstream Ca2+-signaling cascades that control growth [65], differentiation [63] and cell death [66], it is likely that STIM1 is a novel key player with different functions in these cell types.
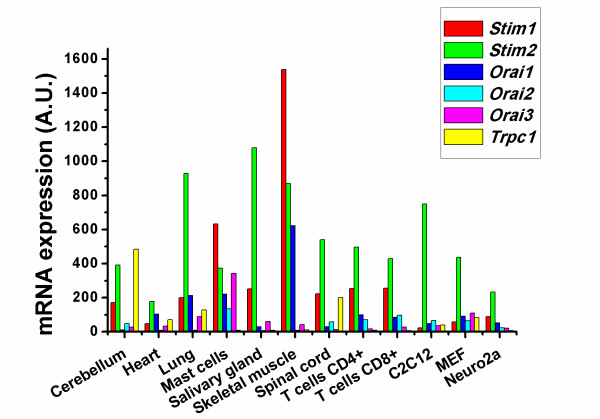
Figure 1.
Tissue distribution of Stim1, Stim2, Orai1-3 and Trpc1 in mouse. Data were extracted from the BioGPS database (http://biogps.gnf.org/) and represent the mRNA expression patterns in selected mouse tissues [67,68]. Stim1 is strongly expressed in skeletal muscle and mast cells, while Stim2 exhibits a relatively higher expression level in all tissues tested with pronounced expression in salivary-gland tissue. Orai1 expression is stronger in skeletal muscle, while there is low expression of all Orai in other tissues presented here. Trpc1 expression is relatively low in both heart and skeletal-muscle tissue, but higher in tissue of the central nervous system, such as in the cerebellum. These patterns do not necessarily reflect the expression at the protein level or the situation in humans, but provide a good view of the patterns observed in mouse models.
STIM1 in skeletal muscle
SOCE mechanism in skeletal muscle
SOCE in skeletal muscle was originally described in a study from Kurebayashi and Ogawa [69]. They discovered in thin muscle-fiber bundles of the extensor digitorum longus (EDL) muscle of adult mice, that the depletion of the SR by repetitive high-K+ stimulation in the presence of sarcoplasmic/endoplasmic-reticulum Ca2+ ATPase (SERCA) inhibitors triggered SOCE with the same characteristics as the CRAC current. This pathway is distinct from the excitation-coupled Ca2+ entry, which is store-independent [62]. Both pathways consist of distinct molecular components and activation mechanisms [62]. Ca2+ entry is important for store repletion [69], limiting fatigue under conditions of extensive exercise [70], activation of NFAT [63,71] and muscle differentiation [72]. Hence, it is becoming increasingly clear that dysregulation of Ca2+ entry may lead to severe muscle pathologies [73-75]. In general, we will limit our discussion to SOCE and we will refer to other reviews for excitation-coupled Ca2+ entry [76,77].
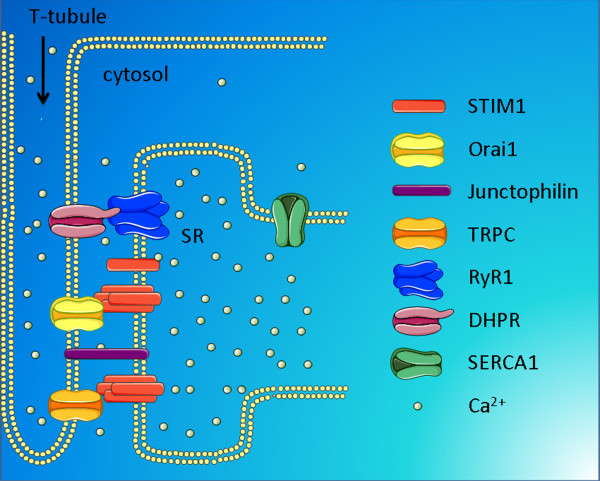
Four models for SOCE in skeletal muscle have been proposed, including the conformation coupling between i) ryanodine receptors (RyRs) and TRPCs, ii) inositol 1,4,5-trisphosphate receptors (IP3Rs) and TRPCs, iii) STIM1 and Orai1 and iv) STIM1 and TRPC channels [76]. Figure 2 shows the molecular determinants involved in SOCE in skeletal muscle with indication of the outside-inside coupling between the dihydropyridine receptor (DHPR), the skeletal-muscle type-1 RyR (RyR1) and the inside-outside SOCE-coupling mechanisms via STIM1/Orai1 and STIM1/TRPC.
Figure 2.
Regulators of SOCE in skeletal muscle. At the triad junction, the voltage-sensitive DHPR (Cav1.1) and RyR physically interact. Depolarization of the plasma membrane causes activation of DHPR and subsequent opening of intracellular Ca2+-release channels, like the most abundant RyR isoform in skeletal muscle, RyR1. In addition, STIM1 monomers and/or oligomers accumulate at the terminal cisternae of the SR, thereby being in close proximity or in complex with Orai1. This pre-localization of STIM1 with Orai1 likely accounts for the fast-activation kinetics of SOCE. Furthermore, it may also account for a basal Ca2+ influx through Orai1 channels in resting conditions. As a consequence, STIM1/Orai1 complexes are an integral part of the Ca2+-homeostasis mechanisms responsible for maintaining proper SERCA1-mediated filling of the SR Ca2+ stores and sustaining resting [Ca2+] in the cytosol. The highly specialized and structural organization of the triad in close proximity of the SR to plasma-membrane SOCE, which involves junctophilin, seems to be essential for proper STIM1/Orai1-mediated Ca2+ homeostasis. A similar mechanism involving TRPC channels and STIM1 oligomers have also been implicated in Ca2+-influx mechanisms in the skeletal muscle, although STIM1-dependent regulation of TRPCs is a matter of debate. Abbreviations: DHPR, dihydropyridine receptor; RyR, ryanodine receptor; SOCE, store-operated Ca2+ entry; STIM, stromal interaction molecule; SR, sacroplasmic reticulum; TRPC, canonical transient receptor potential.
Different studies proposed a role for conformational coupling of RyRs to TRPCs, thereby activating SOCE through TRPC channels [71,73,78]. However, RyRs are likely not essential for SOCE in skeletal muscle, since myotubes of mice lacking RyR1/RyR3 still display prominent SOCE [62,70]. Consistent with this, Lee and et al. did not find any role for TRPC3 in Ca2+ entry in skeletal muscle although its expression level increased during differentiation [79]. The authors proposed that the functional interaction between RyR1 and TRPC3 enhances RyR1 Ca2+-release-channel activity and is thus required for adequate SR Ca2+ release.
Another candidate proposed was the coupling between IP3Rs and TRP-family members [80,81], like TRPC3 [82]. However, the expression level of IP3Rs in myotubes is relatively low and their localization is rather around the nuclear envelope than at the SR terminal cisternae (TC) [83,84].
The identification of STIM1 as the ER Ca2+-sensor protein and its conformational coupling to Orai1 channels controlling SOCE in T lymphocytes spurred the idea that STIM1/Orai1 may be the molecular players underlying SOCE also in skeletal muscle. Different lines of evidence support the idea that STIM1 is critical for SOCE in skeletal muscle: i) STIM1 and Orai1 are highly expressed in skeletal muscle (Figure 1) [63,85], ii) STIM1 is pre-localized at the SR junctions with the T-tubule system which contains pre-localized Orai1 [63,85], iii) mice lacking STIM1 or Orai1 display myopathy [63], iv) severe combined immunodeficiency (SCID) patients characterized by loss-of-function mutations in STIM1/Orai1 signaling display skeletal-muscle myopathy [5], and v) knockdown of STIM1 or expression of the dominant negative Orai1 E106Q caused a marked decline in SOCE in skeletal-muscle myotubes [62].
Besides Orai channels, a role for a conformational coupling between STIM1 and TRPC channels in skeletal muscle can however not be excluded. TRPC1 and TRPC3 channels have been shown to be expressed in skeletal muscle and have been implicated in SOCE in lymphocytes [25]. Moreover, STIM1/Orai1/TRPC1-ternary complexes have been shown to assemble during store depletion, thereby contributing to SOCE [49,50,86]. The C-terminal domain of STIM1 has been shown to directly bind and activate TRPC1 upon ER Ca2+-store depletion. Furthermore, in a recent study using whole-cell patch-clamp recordings approximately 60% of primary myotubes displayed an inwardly rectifying current with characteristics typical for CRAC current, while approximately 40% of myotubes displayed linear current-voltage relationships, which may be related to store-operated activation of TRPC channels [63]. These observations are in line with evidence obtained from myoblasts, which displayed a decrease in SOCE upon repression of TRPC1 expression, thereby affecting myoblast migration and differentiation [87]. However, while mice lacking TRPC1 display a muscle phenotype with muscle fibers that have a decreased cross-sectional area, a reduced force generation, a decline in the level of myofibrillar proteins and a decreased resistance towards muscle fatigue, the role of TRPC1 in adult fibers seemed independent of the Ca2+-store content [88]. The work of Gailly and others indicates that the role of TRPC1 in SOCE is dependent on factors that differ among myoblasts and adult fibers [87,88]. A pivotal role for the a-isoform of the inhibitor of myogenic family (I-mfa) has been proposed, since I-mfa binds and inhibits TRPC1 and myogenic factors interfere with these complexes to alleviate TRPC1 inhibition by I-mfa [89,90]. Hence, in myoblasts, TRPC1 may drive the onset of differentiation through a store-controlled mechanism, while in adult fibers TRPC1 may sustain endurance by maintaining force production upon repeated stimulation and proper muscle development through a store-independent mechanism.
STIM1/Orai1 pre-localization in skeletal muscle and consequences for SOCE activation
While the molecular determinants responsible for SOCE are very similar among T lymphocytes and skeletal-muscle cells, there are also some striking differences, which may be related to the pre-localization of STIM1/Orai1 and the putative contribution of voltage-gated Ca2+ channels in the targeting of STIM1 to the TC/T-tubule junctions. Indeed, a clear distinction between SOCE in T lymphocytes and in skeletal muscle is the kinetics of CRAC-channel activation. In T lymphocytes, the kinetics of SOCE activation by store depletion is relatively slow with a delay of tens of seconds between store depletion and SOCE. In contrast, in skeletal muscle the local activation of SOCE by store depletion is almost instant (less than 1 second delay) and graded in nature, as shown in recent studies by Launikonis [91,92]. Hence, it was proposed that pre-localization of STIM1 and Orai1 at TC/T-tubule junctions under basal conditions when SR stores are fully loaded with Ca2+ underpins the fast kinetics (Figure 2). The pre-localization of STIM1 in the SR at triad junctions was observed in differentiated myotubes of the C2C12 cell line as well as in native skeletal muscle from the hind limbs of adult mice [63,85]. The exact molecular architecture of the inactive STIM1/Orai1 complexes in resting skeletal-muscle cells remains to be elucidated. Two models that allow for rapid SOCE activation upon SR Ca2+-store depletion were proposed by Dirksen [76]. In model 1, STIM1 monomers are localized in the vicinity of inactive Orai1 channels at the triad junctions. A decrease in the SR [Ca2+] will cause rapid dissociation of Ca2+ from STIM1, resulting in conformational changes in STIM1, its oligomerization and activation of the pre-localized Orai1 channels. In model 2, STIM1 exists in pre-formed complexes with the C-terminal region of inactive Orai1 channels, which remain silent until a decrease in SR [Ca2+] triggers conformational changes in STIM1 and direct activation of Orai1-mediated Ca2+ influx, for example through an interaction of STIM1 with the N-terminus of Orai1. The latter would allow for an ultra-fast, efficient and tightly controlled activation of Orai1.
In any case, the spatial organization of triad-junction structure, which allows close contacts between the TC of the SR and the transverse tubular invaginations of the plasma membrane, seems to be the key for efficient SOCE in skeletal muscle [93]. Indeed, disrupting the triad structure in skeletal-muscle fibers by acute suppression of junctophilin 1/2, a protein responsible for forming the close contacts between the intracellular stores and the plasma membrane (Figure 2), leads to reduced SOCE, a decrease in the SR Ca2+ content and altered caffeine-triggered RyR-mediated Ca2+ response. Hence, structural properties of the triad junctions seem to be responsible for the efficient coupling of retrograde signaling from the SR to T-tubules, thereby controlling SOCE, and overall Ca2+ homeostasis and muscle physiology. These features may be important in explaining the effect of pathophysiological mutations in junctophilins or altered junctophilin-expression profiles that are associated with cardiac failure or muscle aging [94,95]. Very recently, reduced SOCE in junctophilin-1 knock out myotubes was associated with a decline in STIM1/Orai1-expression levels and a reduced resting cytosolic [Ca2+] and SR Ca2+ content [96]. Using a Ca2+-entry blocker, BTP2, Eltit and co-workers showed that Ca2+ influx was essential for maintaining proper resting [Ca2+], since treating wild-type myotubes with BTP2 caused a decrease in SOCE and in resting [Ca2+], resembling the situation in junctophilin-1 knock out myotubes [96]. Since different Ca2+-entry mechanisms may account for this pharmacological effect, the authors elegantly used the dominant negative Orai1 form, Orai1 E190Q. Strikingly, expression of Orai1 E190Q was sufficient to inhibit SOCE in wild-type myotubes and to decrease the resting [Ca2+], while it had no effect in junctophilin-1 knock out myotubes. As a consequence, wild-type myotubes expressing Orai1 E190Q displayed a reduced SR Ca2+ content. Hence, this study is one of the first to show that Orai1-mediated SOCE is critical to control resting cytosolic [Ca2+] and SR Ca2+ content. This further supports the concept that SOCE is an essential feature for proper muscle function, not only during conditions of intensive stimulation, but also during resting conditions.
It is also conceivable that other plasma-membrane channels may contribute to the pre-localization of STIM1 to the TC/t-tubule junctions. The identification of the α1c subunit of the voltage-gated Cav1.2 channel as a novel target of STIM1 [58,59] may point towards a more general role for voltage-gated Ca2+ channels to target STIM1 in spatially or functionally restricted domains. Although the DHPR L-type Ca2+-channel (α1s) differs in properties with α1c, it is possible that STIM1 interacts with both.
STIM1 in physiological signaling
Recently, it became clear that SOCE plays a prominent role in muscle development and muscle function and that STIM1 hereby has a central role [63,93,96,97] (Figure 3). For example, mice deficient in STIM1 signaling displayed defects in muscle differentiation and contractile activity [63]. Homozygous STIM1-deficient neonatal mice died from a perinatal myopathy, whereas STIM1-haploinsufficient mice displayed increased susceptibility to fatigue. These data indicate that STIM1 controls both chronic Ca2+-controlled signaling processes, like muscle differentiation and remodeling through the activation of a genetic program, as well as acute Ca2+-signaling processes such as muscle contraction, by supporting the adequate function of the contractile system under conditions of prolonged motor-nerve activity.
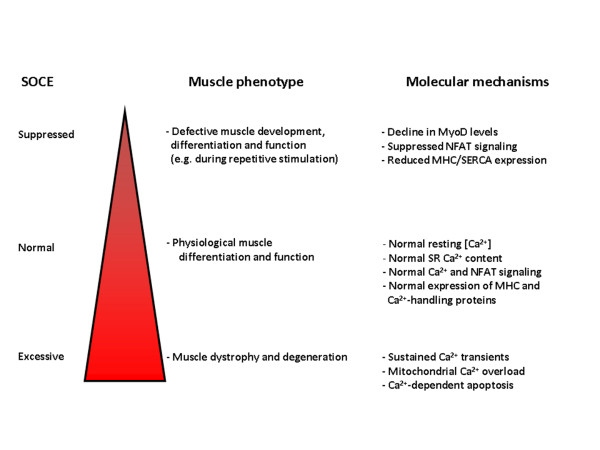
Figure 3.
Schematic overview of the physiological role of SOCE in skeletal muscle and pathophysiological consequences of Ca2+-influx dysregulation based on mouse-model studies. STIM1-controlled Ca2+ influx, either through Orai1 or TRPC channels, ought to be tightly regulated for proper muscle development and function. STIM1-gated Orai1-mediated Ca2+ influx seems to be a requisite for proper Ca2+ homeostasis in the skeletal muscle, maintaining resting cytosolic [Ca2+] sufficiently high and adequately filling the SR Ca2+ stores. This constitutes an essential mechanism that compensates for Ca2+ losses to the extracellular space. Importantly, these phenomena may not only act during periods of intense stimulation, but may also be required during basal conditions and regular muscle function. On the one hand, suppressing Ca2+ influx, for example in STIM1-deficient muscle fibers, leads to improper muscle development and function. This is likely due to hampered activation of NFAT-dependent signaling and defective expression of Ca2+-handling proteins, such as SERCAs and RyRs, as well as other proteins involved in muscle contraction. On the other hand, events such as mutations in dystrophin or overexpression of TRPC channels, lead to muscle dystrophy and degeneration, likely due to mitochondrial Ca2+ overload and Ca2+-dependent activation of the apoptotic program. Abbreviations: NFAT, nuclear factor of activated T cells; RyR, ryanodine receptor; SERCA, sarcoplasmic/endoplasmic-reticulum Ca2+-ATPase; SR, sacroplasmic reticulum; STIM, stromal interaction molecule; TRPC, canonical transient receptor potential.
Underlying these phenomena is STIM1-dependent activation of NFAT in skeletal muscle, which drives myogenesis and muscle differentiation [63]. It has been known for a long time that a dramatic remodeling of Ca2+-transport mechanisms underlies and accompanies skeletal-muscle differentiation. Differentiation of BC3H1 cells to a muscular phenotype was characterized by a decrease in IP3-induced Ca2+ release and an increase in Ca2+-pump activity as well as in caffeine-induced Ca2+ release [98]. Also during C2C12 differentiation from myoblasts to multinucleated myotubes, IP3R-expression levels declined, while the expression levels of RyR1 Ca2+-release channels and SERCA2a/SERCA1 Ca2+ pumps dramatically augmented [99]. A recent study of Stiber et al. [63] now adds STIM1 as another molecular factor, whose expression level increases and whose localization changes from perinuclear to cell peripheral upon differentiation of C2C12 myoblasts into myotubes. These molecular findings correlate with the increased rate of Ca2+ influx in these cells upon thapsigargin-induced SR Ca2+-store depletion.
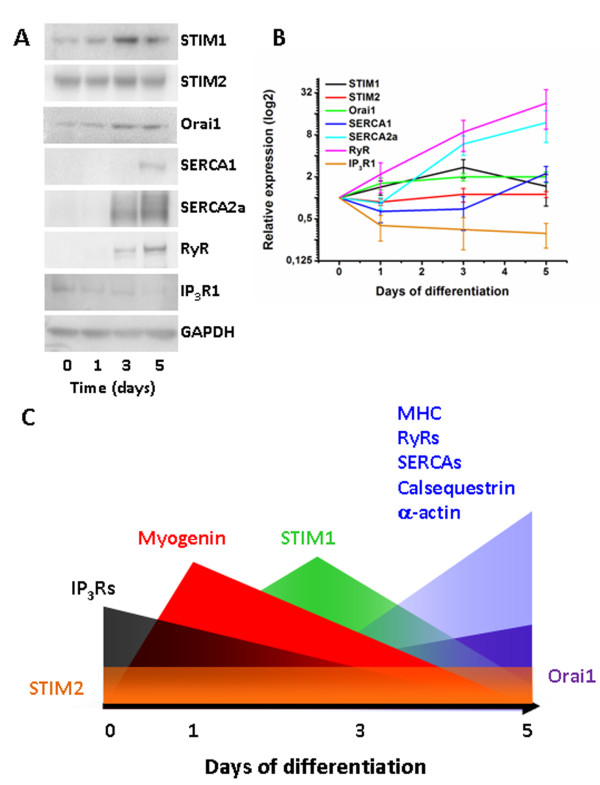
Independently, we have examined STIM1- and STIM2-protein levels together with the expression of other Ca2+-handling proteins, like Orai1, SERCA1, SERCA2a, RyRs and IP3Rs in differentiating C2C12 cells (Figures 4A and 4B). We could confirm the up-regulation of STIM1 and Orai1 in C2C12 cells undergoing differentiation. However we observed a transient up-regulation of STIM1, while Orai1 is permanently augmented. Strikingly, the maximal STIM1 levels correlate with the up-regulation of Orai1, but precede the up-regulation of SERCA1, SERCA2a and RyR. In contrast, STIM2 levels did not significantly change during C2C12 differentiation, suggesting a selective role for STIM1 in skeletal-muscle differentiation. STIM1 up-regulation seems to be a very proximal event in skeletal-muscle differentiation. Nevertheless, this transient STIM1 up-regulation seems to correlate with the observations of Stiber et al. in muscle formation during development in vivo [63].
Figure 4.
Expression of Ca2+-handling proteins including STIM1, STIM2 and Orai1 during myogenic differentiation of C2C12 cells. A: Representative panel of Western blots for each protein of interest in lysates from C2C12 cells in an undifferentiated state and different stages of differentiation. B: Graph represents the average expression of three independent biological samples (mean ± S.E.M.) of C2C12 cells undergoing differentiation. All values were normalized to GAPDH, which was used as a loading control. C: A model for key proteins involved in the proper remodeling of myoblasts into myotubes, based on previous reports [99] and own data. The expression pattern of myogenin, taken from the study of MacLennan and co-workers [99], was up-regulated starting from day 1 of myoblast differentiation, which would mark the first wave of differentiation [99]. Orai1, SERCA2a and RyR1 started to emerge at day 3, while cells exhibit a transient cardiac phenotype. Interestingly, we also observed an even shorter transient of STIM1 up-regulation, which seemed to decline at the same time as SERCA1 levels increased. Remarkably, while STIM1 was transiently up-regulated, STIM2 did not significantly alter and Orai1 continued to increase during differentiation. Materials and methods: Cells were collected 0, 1, 3 or 5 days after replacing myoblast culture medium with myotube differentiation medium [99,113]. Cell lysates were analyzed for protein expression by Western blot and expression was each time normalized to GAPDH expression. Signals were detected with ECF and analyzed with ImageJ. Antibodies were from ProSci Inc., Poway, CA, USA (Orai1), Sigma, St Louis, MO, USA (STIM2 and GAPDH), Abnova, Taipei City, Taiwan (STIM1) and Thermo Scientific, Rockford, IL, USA (SERCA1 and RyR). The SERCA2a and IP3R1 antibodies are described elsewhere [114,115]. Abbreviations: ECF, enhanced chemifluorescence; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IP3R, inositol 1,4,5-trisphosphate receptor; RyR, ryanodine receptor; SERCA, sarcoplasmic/endoplasmic-reticulum Ca2+-ATPase; STIM, stromal interaction molecule.
It is possible that the transient STIM1 up-regulation, which seems to occur as an intermediate step between myogenin and SERCA/RyR up-regulation, is needed for increasing Ca2+ influx and is required for driving the remodeling of the Ca2+-handling proteins through Ca2+-dependent NFAT signaling (Figure 4C). Indeed, Stiber et al. have shown that STIM1 silencing with short hairpin RNA decreased basal NFAT trans-activation in differentiated myotubes, while constitutively active STIM1 expression increased basal NFAT trans-activation [63]. Furthermore, NFAT is known to control muscle formation through morphogenetic events that depend on Ca2+-dependent signaling through the activation of calcineurin/NFAT [100,101].
The critical role of STIM1 in skeletal-muscle development was further demonstrated by Stiber et al. using a gene trap approach resulting in the expression of STIM1/LacZ-fusion proteins (STIM1gt), which contained the N-terminal EF-SAM domain and the transmembrane domain of STIM1 [63]. Most homozygous STIM1gt/gt mice died at a neonatal stage. However, surviving mice displayed decreased body weight, impaired muscle formation and increased fatigue. Studies revealed that the SOC currents in response to thapsigargin were completely abolished in primary myotubes deficient of STIM1.
STIM1 deficiency caused a severe impairment of the skeletal-muscle structure and function. STIM1 loss also resulted in increased central nucleation, a reduced muscle cross-sectional area, swollen mitochondria and a decline in the muscle-specific proteins in the SR, like SERCA1 and myosin heavy chain. In addition, the levels of MyoD, one of the master regulatory genes that controls muscle differentiation, were lower in the muscle of STIM1-deficient mice. At the functional level, loss of STIM1 led to a decrease in the maximal tetanic force and in the fatigue resistance. The latter could be attributed to an impaired SR Ca2+-store filling in the myotubes. After on-going depolarization pulses, the content of the SR Ca2+ stores was severely reduced in STIM1gt/gt myotubes in contrast to their wild-type counterparts. This indicates that STIM1-controlled SOCE is required to refill the internal SR Ca2+ stores during repeated stimulations. The latter has been confirmed by another study showing the rapid efflux of Ca2+ in the T-tubule system upon muscle-cell depolarizations [91]. A critical function of STIM1 in SR Ca2+-store filling in human skeletal muscle needs to be confirmed. However, the fact that SCID patients carrying loss-of-protein expression mutations in the STIM1 gene, manifest atrophy of the type-II skeletal-muscle fibers resulting in severe chronic pulmonary disease [41], supports the concept that STIM1-mediated SOCE plays a critical role in sustaining proper SR Ca2+-store filling and skeletal-muscle function in humans.
STIM1 in pathophysiological signaling
While STIM1 and SOCE seem to be essential for skeletal-muscle development, excessive store-operated Ca2+ influx may underpin pathological conditions, like Duchenne muscular dystrophy [74]. A crucial feature of this disease is defective expression of dystrophin. Myotubes lacking functional dystrophin display altered Ca2+ dynamics, characterized by exaggerated SOCE [74,102]. The latter may be responsible for the observed sustained cytosolic Ca2+ transients and increased Ca2+ uptake by the mitochondria. Importantly, re-introduction of minidystrophin reduces Ca2+ entry to its normal level, which leads to shorter Ca2+ transients and decreased mitochondrial Ca2+ uptake [74]. Although these studies did not clarify the involvement or role of STIM1, they point towards a critical regulation of proper SOCE for the physiological function of skeletal cells. Indeed, SOCE apparently needs to be tightly regulated, since suppressed as well as exaggerated SOCE underpin skeletal-muscle dysfunction [63,74]. An elegant study recently published by the Molkentin lab indicated that increased Ca2+ entry by itself is sufficient to induce muscular dystrophy in vivo, since transgenic mice overexpressing TRPC3 channels are characterized by features similar to the dystrophic disease models [75].
Different molecular mechanisms of increased Ca2+ entry underpinning this disease model have been proposed, including store-operated and stretch-operated ion channels [103]. On the one hand, TRPC channels seem important candidates [75]. Indeed, mdx dystrophic skeletal-muscle fibers displayed increased TRPC-mediated Ca2+ influx [73,75]. Interestingly, TRPC1 has been shown to associate with the dystrophin-protein complex [104,105]. Moreover, dystrophic skeletal-muscle disease models associated with mutations in the dystrophin or mutations in the delta-Sarcoglycan (Scgd) genes were rescued by transgene-mediated inhibition of TRPC channels, thereby reducing Ca2+ influx and preventing the development of muscular-dystrophy features [75]. On the other hand, Launikonis and co-workers demonstrated that while SOCE functions normally in mdx muscle fibers, the thresholds for activation and deactivation of SOCE have been shifted to higher SR [Ca2+] [106]. This may contribute to higher Ca2+ influx during long periods of stimulation. The molecular basis for these shifts in threshold may be associated with the dramatic increase in STIM1/Orai-protein levels found in the mdx muscle fibers [106]. Interestingly, these data may correlate with our observations suggesting a transient up-regulation of STIM1 during muscle differentiation, while a permanent up-regulation of STIM1 may contribute to deleterious muscle events.
Independently of the mechanism, preventing Ca2+ influx may be beneficial and holds potential for future therapeutic strategies to tackle muscular dystrophy either by targeting STIM1/Orai1 or TRPC channels, since both channel complexes have been shown to be implicated and/or up-regulated in Duchenne muscular dystrophy models [75,106]. All studies seem to agree on the fact that excessive SOCE is an early event in this pathology and that inhibiting SOCE seems to be beneficial. This paradigm is supported by different studies: i) TRPC suppression rescues muscular dystrophic features in mouse models [75]; ii) blockers of stretch-activated channels prevent muscle degeneration in mdx mice [107]; iii) inhibitors of phospholipase A2, which is overexpressed in skeletal muscle of a mouse model for Duchenne muscular dystrophy, attenuate the exaggerated SOCE and the subsequent muscle damage [102].
Furthermore, while reducing Ca2+ influx may be critical for targeting this pathology, inhibiting Ca2+ release from the ER has also been shown to be beneficial [108]. For instance, overexpression of anti-apoptotic Bcl-2 prevented IP3R-mediated Ca2+ release [109,110] and subsequent mitochondrial Ca2+ overload, thereby protecting dystrophic muscle cells against Ca2+-dependent apoptosis [108]. Hence, a concerted strategy to alleviate muscular dystrophy likely requires the dampening of both Ca2+ influx as well as ER Ca2+ release.
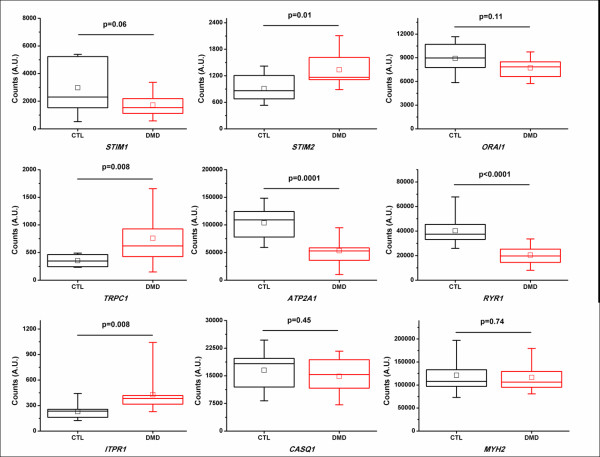
Finally, it is important to note that most of the evidence that points towards excessive Ca2+ influx as an early event in the development of muscular dystrophy has been obtained from mouse models. We need to keep in mind that the need and physiological role of SOCE might be different for mouse and human skeletal muscle. For instance, a micro-array analysis of human skeletal-muscle biopsies from control patients and Duchenne muscular dystrophy patients (obtained from the Gene Expression Omnibus; http://www.ncbi.nlm.nih.gov/geo/) indicated that the STIM1-mRNA levels were not increased, but rather tended to decrease in the muscle of the dystrophic patients, while Orai1-mRNA levels were not significantly changed (Figure 5). This seems in contrast with the up-regulation of STIM1 and Orai1 and the increased SOCE reported in mouse models for Duchenne muscular dystrophy. These contrasting findings may indicate that mechanisms underpinning SOCE are differently affected in the human muscular pathologies versus the mouse models for these pathologies. In addition, the relative importance of STIM1 versus STIM2 for SOCE in human muscles and their contribution to myopathies may differ among human and mice. In this respect, Duchenne muscular dystrophy patients did show an up-regulation of STIM2-mRNA levels, which is activated at more modest decreases in [Ca2+]ER than STIM1. Therefore, STIM2 up-regulation may be another critical factor that needs to be taken into account in the development of muscular dystrophy in human patients. In any case, it is clear that the role of both STIM proteins for the development of myopathies in human patients must be further explored.
Figure 5.
Gene expression involved in Ca2+ and/or contractility. Plots represent gene expression in quadriceps skeletal-muscle samples of controls (CTL, n = 10-12) and patients with Duchenne muscular dystrophy (DMD, n = 10 - 12) in arbitrary units (A.U.). Data were obtained from GEO reference series GSE1007 (STIM1, STIM2, ORAI1, ITPR1 and CASQ1) and GSE1004 (TRPC1, ATP2A1, RYR1 and MYH2) [116-118] comparing the mRNA-expression levels in normal and dystrophic patients (http://www.ncbi.nlm.nih.gov/geo/). Graphs represent box plots, indicating the mean (square symbol), the median (line), the 25th and 75th percentiles (bottom and top of the box), and the 5th and 95th percentiles (whisker range). Strikingly, STIM1-, SERCA1-, RyR1-mRNA levels tended to decline, while STIM2-, TRPC1- and IP3R1-mRNA levels tended to increase. Orai1-, calsequestrin-1- and myosin heavy chain-2-mRNA levels did not significantly alter. This seems opposite to what has been observed in mouse models for Duchenne muscular dystrophy, which displayed excessive Ca2+ influx and up-regulation of STIM1/Orai1 [106]. In human patients suffering from Duchenne muscular dystrophy, TRPC1 elevations may account for the increase in Ca2+ influx, leading to Ca2+-dependent apoptosis and muscle degeneration. This indicates that caution should be taken from extrapolating results from mouse models for pathophysiological conditions to human pathophysiological conditions. Abbreviations indicate the gene names for stromal interaction molecule 1 (STIM1), stromal interaction molecule 2 (STIM2), Orai1 (ORAI1), canonical transient receptor potential 1 (TRPC1), sarcoplasmic/endoplasmic-reticulum Ca2+-ATPase 1 (ATP2A1), ryanodine receptor 1 (RYR1), inositol 1,4,5-trisphosphate receptor 1 (ITPR1), calsequestrin 1 (CASQ1), and myosin heavy chain IIa (MYH2).
Moreover, other mechanisms may account for the excessive Ca2+ influx that leads to muscle degeneration in human patients. Therefore, it is important to note that TRPC1-mRNA levels are also significantly up-regulated in the patients suffering from muscular dystrophy. Importantly, excessive TRPC1 activity has been implicated in spontaneous Ca2+ influx and the activation of Ca2+-dependent proteolysis, leading to the degradation of cytoskeletal proteins and the development of myopathies in Homer 1-deficient mice [111]. Decreased levels of Homer 1 in mdx mouse models may contribute to the reported TRPC1 hyperactivity in response to store depletion [73]. However, the contribution of STIM1 in this process remains unknown.
Finally, these micro-array analyses also revealed that SERCA1- and RyR1-mRNA levels declined, IP3R1-mRNA levels increased and Orai1-, calsequestrin-1- and myosin heavy chain-2-mRNA levels did not significantly alter. Changes in IP3R-expression level have previously been shown to occur in patient samples of Duchenne muscular dystrophy [112]. This indicates a remodeling of the Ca2+-handling mechanisms that tends to shift to the undifferentiated state, while proteins involved in the contractile mechanism are not profoundly affected. In any case, it would be interesting to elucidate whether the changes in Ca2+-transport mechanisms correlate with the severity of the disease.
Conclusions
While the role of SOCE is well established in T cells and other non-excitable cells, it is becoming clear that SOCE may play a very important role in skeletal-muscle physiology, ranging from roles in development, differentiation, contractile function and resistance against fatigue. STIM1 plays a central role in these processes by controlling SOCE channels, like Orai1 and/or TRPCs in a rapid and highly regulated fashion. While physiological SOCE is required for proper muscle development and function, excessive SOCE seems to be detrimental for skeletal muscle. Indeed, promoting Ca2+ influx by overexpressing TRPCs is sufficient to degenerate healthy muscle, while genetic mouse models for muscular dystrophy have been characterized by excessive SOCE. From these studies, it is clear that STIM1 function and thus SOCE ought to be tightly regulated for proper muscle physiology.
List of Abbreviations
CRAC: Ca2+-release-activated Ca2+; DMD: Duchenne muscular dystrophy; ER: endoplasmic reticulum; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; IP3R: inositol 1,4,5-trisphosphate receptor; NFAT: nuclear factor of activated T cells; RyR: ryanodine receptor; SCID: severe combined immunodeficiency; SERCA: sarcoplasmic/endoplasmic-reticulum Ca2+-ATPase; SOCE: store-operated Ca2+ entry; SPCA: secretory-pathway Ca2+ ATPase; SR: sarcoplasmic reticulum; STIM: stromal interaction molecule; TC: terminal cisterna(e); TRPC: canonical transient receptor potential.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
GB conceived the experiments, analyzed the data and wrote the manuscript. SK performed the experiments, analyzed the data and wrote the manuscript. JPD, HDS, LM and JBP discussed the data and revised the manuscript.
Contributor Information
Santeri Kiviluoto, Email: santeri.kiviluoto@med.kuleuven.be.
Jean-Paul Decuypere, Email: jeanpaul.decuypere@med.kuleuven.be.
Humbert De Smedt, Email: humbert.desmedt@med.kuleuven.be.
Ludwig Missiaen, Email: ludwig.missiaen@med.kuleuven.be.
Jan B Parys, Email: jan.parys@med.kuleuven.be.
Geert Bultynck, Email: geert.bultynck@med.kuleuven.be.
Acknowledgements
Work in the authors' laboratory was supported by the Research Foundation-Flanders (F.W.O.) grant G.0604.07N to HDS and G.0788.11N to GB, by the Research Council of the K.U. Leuven via the Concerted Actions program GOA 09/012 and via OT START grant SRT/10/044, and by the Interuniversity Attraction Poles Program (Belgian Science Policy; P6/28 to HDS, JBP and LM). JPD is a Ph.D. fellow of the Agency for Innovation by Science and Technology (IWT). The authors wish to thank Dr. Peter Vangheluwe for providing materials.
References
- Putney JW Jr. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Takemura H, Hughes AR, Thastrup O, Putney JW Jr. Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989;264:12266–12271. [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Veliçelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci USA. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- Smyth JT, Hwang SY, Tomita T, DeHaven WI, Mercer JC, Putney JW. Activation and regulation of store-operated calcium entry. J Cell Mol Med. 2010;14:2337–2349. doi: 10.1111/j.1582-4934.2010.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW Jr. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan PG, Rao A. Dissecting ICRAC, a store-operated calcium current. Trends Biochem Sci. 2007;32:235–245. doi: 10.1016/j.tibs.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Lu J, Li Z, Yu X, Chen L, Xu T. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem Biophys Res Commun. 2006;350:969–976. doi: 10.1016/j.bbrc.2006.09.134. [DOI] [PubMed] [Google Scholar]
- Baba Y, Hayashi K, Fujii Y, Mizushima A, Watarai H, Wakamori M, Numaga T, Mori Y, Iino M, Hikida M, Kurosaki T. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci USA. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci USA. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–122. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J Biol Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- Wu MM, Luik RM, Lewis RS. Some assembly required: constructing the elementary units of store-operated Ca2+ entry. Cell Calcium. 2007;42:163–172. doi: 10.1016/j.ceca.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, ICRAC and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CM, Chvanov M, Haynes LP, Petersen OH, Tepikin AV, Burgoyne RD. Role of phosphoinositides in STIM1 dynamics and store-operated calcium entry. Biochem J. 2009;425:159–168. doi: 10.1042/BJ20090884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan E, Momburg F, Engel U, Temmerman K, Nickel W, Seedorf M. A conserved, lipid-mediated sorting mechanism of yeast Ist2 and mammalian STIM proteins to the peripheral ER. Traffic. 2009;10:1802–1818. doi: 10.1111/j.1600-0854.2009.00995.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Lu J, Xu P, Xie X, Chen L, Xu T. Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J Biol Chem. 2007;282:29448–29456. doi: 10.1074/jbc.M703573200. [DOI] [PubMed] [Google Scholar]
- Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, Groschner K, Romanin C. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem. 2008;283:8014–8022. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Meraner P, Kwon HT, Machnes D, Oh-hora M, Zimmer J, Huang Y, Stura A, Rao A, Hogan PG. STIM1 gates the store-operated calcium channel ORAI1 in vitro. Nat Struct Mol Biol. 2010;17:112–116. doi: 10.1038/nsmb.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik M, Fahrner M, Derler I, Schindl R, Bergsmann J, Frischauf I, Groschner K, Romanin C. A cytosolic homomerization and a modulatory domain within STIM1 C terminus determine coupling to ORAI1 channels. J Biol Chem. 2009;284:8421–8426. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Lange I, Feske S. A minimal regulatory domain in the C terminus of STIM1 binds to and activates ORAI1 CRAC channels. Biochem Biophys Res Commun. 2009;385:49–54. doi: 10.1016/j.bbrc.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M, Grice DM, Faddy HM, Nguyen N, Leitch S, Wang Y, Muend S, Kenny PA, Sukumar S, Roberts-Thomson SJ, Monteith GR, Rao R. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell. 2010;143:84–98. doi: 10.1016/j.cell.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RT, Manji SS, Parker NJ, Hancock MS, Van Stekelenburg L, Eid JP, Senior PV, Kazenwadel JS, Shandala T, Saint R, Smith PJ, Dziadek MA. Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem J. 2001;357:673–685. doi: 10.1042/0264-6021:3570673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decuypere JP, Monaco G, Kiviluoto S, Oh-hora M, Luyten T, De Smedt H, Parys JB, Missiaen L, Bultynck G. STIM1, but not STIM2, is required for proper agonist-induced Ca2+ signaling. Cell Calcium. 2010;48:161–167. doi: 10.1016/j.ceca.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Prakriya M, Rao A, Lewis RS. A severe defect in CRAC Ca2+ channel activation and altered K+ channel gating in T cells from immunodeficient patients. J Exp Med. 2005;202:651–662. doi: 10.1084/jem.20050687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarl CA, Picard C, Khalil S, Kawasaki T, Rother J, Papolos A, Kutok J, Hivroz C, Ledeist F, Plogmann K, Ehl S, Notheis G, Albert MH, Belohradsky BH, Kirschner J, Rao A, Fischer A, Feske S. ORAI1 deficiency and lack of store-operated Ca2+ entry cause immunodeficiency, myopathy, and ectodermal dysplasia. J Allergy Clin Immunol. 2009;124:1311–1318 e1317. doi: 10.1016/j.jaci.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C, McCarl CA, Papolos A, Khalil S, Luthy K, Hivroz C, LeDeist F, Rieux-Laucat F, Rechavi G, Rao A, Fischer A, Feske S. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N Engl J Med. 2009;360:1971–1980. doi: 10.1056/NEJMoa0900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Draeger R, Peter HH, Eichmann K, Rao A. The duration of nuclear residence of NFAT determines the pattern of cytokine expression in human SCID T cells. J Immunol. 2000;165:297–305. doi: 10.4049/jimmunol.165.1.297. [DOI] [PubMed] [Google Scholar]
- Feske S. ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev. 2009;231:189–209. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriev I, Gouveia SM, van der Vaart B, Demmers J, Smyth JT, Honnappa S, Splinter D, Steinmetz MO, Putney JW Jr, Hoogenraad CC, Akhmanova A. STIM1 is a MT-plus-end-tracking protein involved in remodeling of the ER. Curr Biol. 2008;18:177–182. doi: 10.1016/j.cub.2007.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honnappa S, Gouveia SM, Weisbrich A, Damberger FF, Bhavesh NS, Jawhari H, Grigoriev I, van Rijssel FJ, Buey RM, Lawera A, Jelesarov I, Winkler FK, Wüthrich K, Akhmanova A, Steinmetz MO. An EB1-binding motif acts as a microtubule tip localization signal. Cell. 2009;138:366–376. doi: 10.1016/j.cell.2009.04.065. [DOI] [PubMed] [Google Scholar]
- Bakowski D, Glitsch MD, Parekh AB. An examination of the secretion-like coupling model for the activation of the Ca2+ release-activated Ca2+ current ICRAC in RBL-1 cells. J Physiol. 2001;532:55–71. doi: 10.1111/j.1469-7793.2001.0055g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Schwarz EC, Schwindling C, Lipp P, Kaestner L, Hoth M. Sustained activity of calcium release-activated calcium channels requires translocation of mitochondria to the plasma membrane. J Biol Chem. 2006;281:40302–40309. doi: 10.1074/jbc.M607896200. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell. 2008;32:439–448. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHaven WI, Jones BF, Petranka JG, Smyth JT, Tomita T, Bird GS, Putney JW Jr. TRPC channels function independently of STIM1 and Orai1. J Physiol. 2009;587:2275–2298. doi: 10.1113/jphysiol.2009.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Yuan JP, Hong JH, So I, Worley PF, Muallem S. An endoplasmic reticulum/plasma membrane junction: STIM1/Orai1/TRPCs. FEBS Lett. 2010;584:2022–2027. doi: 10.1016/j.febslet.2009.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth TJ, Thompson JL, Mignen O. STIM1 and the noncapacitative ARC channels. Cell Calcium. 2007;42:183–191. doi: 10.1016/j.ceca.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store depletion or translocation to the plasma membrane. J Physiol. 2007;579:703–715. doi: 10.1113/jphysiol.2006.122432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignen O, Thompson JL, Shuttleworth TJ. Both Orai1 and Orai3 are essential components of the arachidonate-regulated Ca2+-selective (ARC) channels. J Physiol. 2008;586:185–195. doi: 10.1113/jphysiol.2007.146258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkimmiatis K, Srikanthan M, Maiellaro I, Moyer MP, Curci S, Hofer AM. Store-operated cyclic AMP signalling mediated by STIM1. Nat Cell Biol. 2009;11:433–442. doi: 10.1038/ncb1850. [DOI] [PubMed] [Google Scholar]
- Roy J, Lefkimmiatis K, Moyer MP, Curci S, Hofer AM. The {omega}-3 fatty acid eicosapentaenoic acid elicits cAMP generation in colonic epithelial cells via a "store-operated" mechanism. Am J Physiol Gastrointest Liver Physiol. 2010;299:G715–722. doi: 10.1152/ajpgi.00028.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, Tang XD, Gill DL. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science. 2010;330:105–109. doi: 10.1126/science.1191086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Shcheglovitov A, Dolmetsch R. The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science. 2010;330:101–105. doi: 10.1126/science.1191027. [DOI] [PubMed] [Google Scholar]
- Kotturi MF, Carlow DA, Lee JC, Ziltener HJ, Jefferies WA. Identification and functional characterization of voltage-dependent calcium channels in T lymphocytes. J Biol Chem. 2003;278:46949–46960. doi: 10.1074/jbc.M309268200. [DOI] [PubMed] [Google Scholar]
- Kotturi MF, Jefferies WA. Molecular characterization of L-type calcium channel splice variants expressed in human T lymphocytes. Mol Immunol. 2005;42:1461–1474. doi: 10.1016/j.molimm.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Lyfenko AD, Dirksen RT. Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. J Physiol. 2008;586:4815–4824. doi: 10.1113/jphysiol.2008.160481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiber J, Hawkins A, Zhang ZS, Wang S, Burch J, Graham V, Ward CC, Seth M, Finch E, Malouf N, Williams RS, Eu JP, Rosenberg P. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol. 2008;10:688–697. doi: 10.1038/ncb1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Deng X, Hewavitharana T, Soboloff J, Gill DL. Stim, ORAI and TRPC channels in the control of calcium entry signals in smooth muscle. Clin Exp Pharmacol Physiol. 2008;35:1127–1133. doi: 10.1111/j.1440-1681.2008.05018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J. 2009;23:2425–2437. doi: 10.1096/fj.09-131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna-Erro A, Braun A, Kraft R, Kleinschnitz C, Schuhmann MK, Stegner D, Wultsch T, Eilers J, Meuth SG, Stoll G, Nieswandt B. STIM2 regulates capacitive Ca2+ entry in neurons and plays a key role in hypoxic neuronal cell death. Sci Signal. 2009;2:ra67. doi: 10.1126/scisignal.2000522. [DOI] [PubMed] [Google Scholar]
- Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge C, Haase J, Janes J, Huss J, Su A. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009;10:R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattin J, Schroder K, Su A, Walker J, Zhang J, Wiltshire T, Saijo K, Glass C, Hume D, Kellie S, Sweet M. Expression analysis of G Protein-Coupled Receptors in mouse macrophages. Immunome Res. 2008;4:5. doi: 10.1186/1745-7580-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi N, Ogawa Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J Physiol. 2001;533:185–199. doi: 10.1111/j.1469-7793.2001.0185b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Yang D, Nagaraj RY, Nosek TA, Nishi M, Takeshima H, Cheng H, Ma J. Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat Cell Biol. 2002;4:379–383. doi: 10.1038/ncb788. [DOI] [PubMed] [Google Scholar]
- Rosenberg P, Hawkins A, Stiber J, Shelton JM, Hutcheson K, Bassel-Duby R, Shin DM, Yan Z, Williams RS. TRPC3 channels confer cellular memory of recent neuromuscular activity. Proc Natl Acad Sci USA. 2004;101:9387–9392. doi: 10.1073/pnas.0308179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbellay B, Arnaudeau S, Konig S, Jousset H, Bader C, Demaurex N, Bernheim L. STIM1- and Orai1-dependent store-operated calcium entry regulates human myoblast differentiation. J Biol Chem. 2009;284:5370–5380. doi: 10.1074/jbc.M806726200. [DOI] [PubMed] [Google Scholar]
- Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J Cell Biol. 2002;158:1089–1096. doi: 10.1083/jcb.200203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandebrouck A, Ducret T, Basset O, Sebille S, Raymond G, Ruegg U, Gailly P, Cognard C, Constantin B. Regulation of store-operated calcium entries and mitochondrial uptake by minidystrophin expression in cultured myotubes. FASEB J. 2006;20:136–138. doi: 10.1096/fj.04-3633fje. [DOI] [PubMed] [Google Scholar]
- Millay DP, Goonasekera SA, Sargent MA, Maillet M, Aronow BJ, Molkentin JD. Calcium influx is sufficient to induce muscular dystrophy through a TRPC-dependent mechanism. Proc Natl Acad Sci USA. 2009;106:19023–19028. doi: 10.1073/pnas.0906591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen RT. Checking your SOCCs and feet: the molecular mechanisms of Ca2+ entry in skeletal muscle. J Physiol. 2009;587:3139–3147. doi: 10.1113/jphysiol.2009.172148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Murphy RM, Edwards JN. Toward the roles of store-operated Ca2+ entry in skeletal muscle. Pflugers Arch. 2010;460:813–823. doi: 10.1007/s00424-010-0856-7. [DOI] [PubMed] [Google Scholar]
- Kiselyov KI, Shin DM, Wang Y, Pessah IN, Allen PD, Muallem S. Gating of store-operated channels by conformational coupling to ryanodine receptors. Mol Cell. 2000;6:421–431. doi: 10.1016/S1097-2765(00)00041-1. [DOI] [PubMed] [Google Scholar]
- Lee EH, Cherednichenko G, Pessah IN, Allen PD. Functional coupling between TRPC3 and RyR1 regulates the expressions of key triadic proteins. J Biol Chem. 2006;281:10042–10048. doi: 10.1074/jbc.M600981200. [DOI] [PubMed] [Google Scholar]
- Launikonis BS, Barnes M, Stephenson DG. Identification of the coupling between skeletal muscle store-operated Ca2+ entry and the inositol trisphosphate receptor. Proc Natl Acad Sci USA. 2003;100:2941–2944. doi: 10.1073/pnas.0536227100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada M, Espinosa A, Gibson CJ, Uhlen P, Jaimovich E. Capacitative calcium entry in testosterone-induced intracellular calcium oscillations in myotubes. J Endocrinol. 2005;184:371–379. doi: 10.1677/joe.1.05921. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- Powell JA, Carrasco MA, Adams DS, Drouet B, Rios J, Muller M, Estrada M, Jaimovich E. IP3 receptor function and localization in myotubes: an unexplored Ca2+ signaling pathway in skeletal muscle. J Cell Sci. 2001;114:3673–3683. doi: 10.1242/jcs.114.20.3673. [DOI] [PubMed] [Google Scholar]
- Cardenas C, Liberona JL, Molgo J, Colasante C, Mignery GA, Jaimovich E. Nuclear inositol 1,4,5-trisphosphate receptors regulate local Ca2+ transients and modulate cAMP response element binding protein phosphorylation. J Cell Sci. 2005;118:3131–3140. doi: 10.1242/jcs.02446. [DOI] [PubMed] [Google Scholar]
- Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JT, Dehaven WI, Bird GS, Putney JW Jr. Ca2+-store-dependent and -independent reversal of Stim1 localization and function. J Cell Sci. 2008;121:762–772. doi: 10.1242/jcs.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis M, Zanou N, Van Schoor M, Gailly P. TRPC1 regulates skeletal myoblast migration and differentiation. J Cell Sci. 2008;121:3951–3959. doi: 10.1242/jcs.037218. [DOI] [PubMed] [Google Scholar]
- Zanou Ng, Shapovalov G, Louis M, Tajeddine N, Gallo C, Van Schoor M, Anguish I, Cao ML, Schakman O, Dietrich A, Lebacq J, Ruegg U, Roulet E, Birnbaumer L, Gailly P. Role of TRPC1 channel in skeletal muscle function. Am J Physiol - Cell Physiol 2010. pp. C149–162. [DOI] [PMC free article] [PubMed]
- Chen CMA, Kraut N, Groudine M, Weintraub H. I-mf, a novel myogenic repressor, interacts with members of the MyoD family. Cell. 1996;86:731–741. doi: 10.1016/S0092-8674(00)80148-8. [DOI] [PubMed] [Google Scholar]
- Ma R, Rundle D, Jacks J, Koch M, Downs T, Tsiokas L. Inhibitor of myogenic family, a novel suppressor of store-operated currents through an interaction with TRPC1. J Biol Chem. 2003;278:52763–52772. doi: 10.1074/jbc.M309610200. [DOI] [PubMed] [Google Scholar]
- Launikonis BS, Rios E. Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle. J Physiol. 2007;583:81–97. doi: 10.1113/jphysiol.2007.135046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JN, Murphy RM, Cully TR, von Wegner F, Friedrich O, Launikonis BS. Ultra-rapid activation and deactivation of store-operated Ca2+ entry in skeletal muscle. Cell Calcium. 2010;47:458–467. doi: 10.1016/j.ceca.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Brotto M, Weisleder N, Chu Y, Lin P, Zhao X, Thornton A, Komazaki S, Takeshima H, Ma J, Pan Z. Uncoupling store-operated Ca2+ entry and altered Ca2+ release from sarcoplasmic reticulum through silencing of junctophilin genes. Biophys J. 2006;90:4418–4427. doi: 10.1529/biophysj.105.076570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landstrom AP, Weisleder N, Batalden KB, Martijn Bos J, Tester DJ, Ommen SR, Wehrens XHT, Claycomb WC, Ko JK, Hwang M, Pan Z, Ma J, Ackerman MJ. Mutations in JPH2-encoded junctophilin-2 associated with hypertrophic cardiomyopathy in humans. J Mol Cell Cardiol. 2007;42:1026–1035. doi: 10.1016/j.yjmcc.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo JS, Hwang JH, Ko JK, Weisleder N, Kim DH, Ma J, Lee EH. S165F mutation of junctophilin 2 affects Ca2+ signalling in skeletal muscle. Biochem J. 2010;427:125–134. doi: 10.1042/BJ20091225. [DOI] [PubMed] [Google Scholar]
- Li H, Ding X, Lopez JR, Takeshima H, Ma J, Allen PD, Eltit JM. Impaired Orai1-mediated resting Ca2+ entry reduces the cytosolic [Ca2+] and sarcoplasmic reticulum Ca2+ loading in quiescent junctophilin 1 knock-out myotubes. J Biol Chem. 2010;285:39171–39179. doi: 10.1074/jbc.M110.149690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Muallem S. Skeletal muscle dressed in SOCs. Nat Cell Biol. 2008;10:639–641. doi: 10.1038/ncb0608-639. [DOI] [PubMed] [Google Scholar]
- De Smedt H, Parys JB, Himpens B, Missiaen L, Borghgraef R. Changes in the mechanism of Ca2+ mobilization during the differentiation of BC3H1 muscle cells. Biochem J. 1991;273:219–223. doi: 10.1042/bj2730219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai M, Otsu K, MacLennan DH, Periasamy M. Regulation of sarcoplasmic reticulum gene expression during cardiac and skeletal muscle development. Am J Physiol. 1992;262:C614–620. doi: 10.1152/ajpcell.1992.262.3.C614. [DOI] [PubMed] [Google Scholar]
- Friday BB, Horsley V, Pavlath GK. Calcineurin activity is required for the initiation of skeletal muscle differentiation. J Cell Biol. 2000;149:657–666. doi: 10.1083/jcb.149.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegley KM, Gephart J, Warren GL, Pavlath GK. Altered primary myogenesis in NFATC3-/- mice leads to decreased muscle size in the adult. Dev Biol. 2001;232:115–126. doi: 10.1006/dbio.2001.0179. [DOI] [PubMed] [Google Scholar]
- Boittin FX, Petermann O, Hirn C, Mittaud P, Dorchies OM, Roulet E, Ruegg UT. Ca2+-independent phospholipase A2 enhances store-operated Ca2+ entry in dystrophic skeletal muscle fibers. J Cell Sci. 2006;119:3733–3742. doi: 10.1242/jcs.03184. [DOI] [PubMed] [Google Scholar]
- Allen DG, Gervasio OL, Yeung EW, Whitehead NP. Calcium and the damage pathways in muscular dystrophy. Can J Physiol Pharmacol. 2010;88:83–91. doi: 10.1139/Y09-058. [DOI] [PubMed] [Google Scholar]
- Vandebrouck A, Sabourin J, Rivet J, Balghi H, Sebille S, Kitzis A, Raymond G, Cognard C, Bourmeyster N, Constantin B. Regulation of capacitative calcium entries by alpha1-syntrophin: association of TRPC1 with dystrophin complex and the PDZ domain of alpha1-syntrophin. FASEB J. 2007;21:608–617. doi: 10.1096/fj.06-6683com. [DOI] [PubMed] [Google Scholar]
- Sabourin J, Lamiche C, Vandebrouck A, Magaud C, Rivet J, Cognard C, Bourmeyster N, Constantin B. Regulation of TRPC1 and TRPC4 cation channels requires an alpha1-syntrophin-dependent complex in skeletal mouse myotubes. J Biol Chem. 2009;284:36248–36261. doi: 10.1074/jbc.M109.012872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JN, Friedrich O, Cully TR, von Wegner F, Murphy RM, Launikonis BS. Upregulation of store-operated Ca2+ entry in dystrophic mdx mouse muscle. Am J Physiol Cell Physiol. 2010;299:C42–50. doi: 10.1152/ajpcell.00524.2009. [DOI] [PubMed] [Google Scholar]
- Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol. 2005;562:367–380. doi: 10.1113/jphysiol.2004.075275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basset O, Boittin FX, Cognard C, Constantin B, Ruegg UT. Bcl-2 overexpression prevents calcium overload and subsequent apoptosis in dystrophic myotubes. Biochem J. 2006;395:267–276. doi: 10.1042/BJ20051265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong YP, Aromolaran AS, Bultynck G, Zhong F, Li X, McColl K, Matsuyama S, Herlitze S, Roderick HL, Bootman MD, Mignery GA, Parys JB, De Smedt H, Distelhorst CW. Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2's inhibition of apoptotic calcium signals. Mol Cell. 2008;31:255–265. doi: 10.1016/j.molcel.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong YP, Bultynck G, Aromolaran AS, Zhong F, Parys JB, De Smedt H, Mignery GA, Roderick HL, Bootman MD, Distelhorst CW. The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc Natl Acad Sci USA. 2009;106:14397–14402. doi: 10.1073/pnas.0907555106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiber JA, Zhang ZS, Burch J, Eu JP, Zhang S, Truskey GA, Seth M, Yamaguchi N, Meissner G, Shah R, Worley PF, Williams RS, Rosenberg PB. Mice lacking Homer 1 exhibit a skeletal myopathy characterized by abnormal transient receptor potential channel activity. Mol Cell Biol. 2008;28:2637–2647. doi: 10.1128/MCB.01601-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas C, Juretic N, Bevilacqua JA, Garcia IE, Figueroa R, Hartley R, Taratuto AL, Gejman R, Riveros N, Molgō J, Jaimovich E. Abnormal distribution of inositol 1,4,5-trisphosphate receptors in human muscle can be related to altered calcium signals and gene expression in Duchenne dystrophy-derived cells. FASEB J. 2010;24:3210–3221. doi: 10.1096/fj.09-152017. [DOI] [PubMed] [Google Scholar]
- Bultynck G, De Smet P, Rossi D, Callewaert G, Missiaen L, Sorrentino V, De Smedt H, Parys JB. Characterization and mapping of the 12 kDa FK506-binding protein (FKBP12)-binding site on different isoforms of the ryanodine receptor and of the inositol 1,4,5-trisphosphate receptor. Biochem J. 2001;354:413–422. doi: 10.1042/0264-6021:3540413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangheluwe P, Schuermans M, Zādor E, Waelkens E, Raeymaekers L, Wuytack F. Sarcolipin and phospholamban mRNA and protein expression in cardiac and skeletal muscle of different species. Biochem J. 2005;389:151–159. doi: 10.1042/BJ20050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parys JB, de Smedt H, Missiaen L, Bootman MD, Sienaert I, Casteels R. Rat basophilic leukemia cells as model system for inositol 1,4,5-trisphosphate receptor IV, a receptor of the type II family: functional comparison and immunological detection. Cell Calcium. 1995;17:239–249. doi: 10.1016/0143-4160(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett J, Sanoudou D, Kho A, Han M, Bennett R, Kohane I, Beggs A, Kunkel L. Gene expression profiling of Duchenne muscular dystrophy skeletal muscle. Neurogenetics. 2003;4:163–171. doi: 10.1007/s10048-003-0148-x. [DOI] [PubMed] [Google Scholar]
- Haslett JN, Sanoudou D, Kho AT, Bennett RR, Greenberg SA, Kohane IS, Beggs AH, Kunkel LM. Gene expression comparison of biopsies from Duchenne muscular dystrophy (DMD) and normal skeletal muscle. Proc Natl Acad Sci USA. 2002;99:15000–15005. doi: 10.1073/pnas.192571199. [DOI] [PMC free article] [PubMed] [Google Scholar]