Abstract
Introduction
Insomnia is common, persistent, and increases the risk for relapse in alcohol-dependent (AD) patients. Abstinence has long been considered the best strategy for allowing sleep to normalize, although how many and which patients respond to abstinence is unknown. The aims of this study were to investigate the prevalence and correlates of both baseline and persistent insomnia in AD patients. The course of sleep problems in response to abstinence, moderate drinking, or relapse following treatment was also examined.
Methods
A naturalistic longitudinal outcomes study interviewed 267 patients (69% male; mean age 44 yr) with DSM-IV alcohol dependence at baseline and 6 months later (84% follow-up rate). The Sleep Problems Questionnaire, Time-Line Follow-Back Interview, and Brief Symptom Inventory measured insomnia, drinking, and psychiatric symptoms, respectively. Simple correlations, logistic regression, and repeated-measures analysis of variance were used to analyze the data.
Results
At baseline, 47% of patients were classified with insomnia, which was independently predicted by female gender and psychiatric severity. Both abstinence and moderate drinking outcomes significantly predicted a reduction of insomnia symptoms after controlling for gender and psychiatric severity. Among patients with baseline insomnia, however, insomnia persisted in over 60% of cases, which was also predicted by psychiatric severity. Moreover, insomnia persisted in one-quarter of patients despite abstinence.
Conclusions
Treatment aimed at preventing relapse to heavy drinking provides good first-line therapy for insomnia in AD patients, but some may require insomnia-specific evaluation and treatment in addition to substance-focused treatment and psychiatric care.
Introduction
Many patients entering treatment for alcohol and other drug use disorders report problems sleeping that persist beyond the initial phase of substance withdrawal.1, 2 Insomnia is also common in the general population,3 and is frequently comorbid with a variety of psychiatric and medical disorders.2, 4 As a symptom, insomnia refers to complaints of trouble falling asleep and staying asleep, or feeling dissatisfied with sleep. As a DSM-IV disorder, insomnia symptoms must be accompanied by either significant distress or impairments in daytime functioning in order to fulfill diagnostic criteria.5 DSM-IV also specifies that insomnia may either be a primary disorder or induced by substances, medication, or a medical disorder. When treating alcohol-dependent (AD) patients, addiction-specialized clinicians commonly assume that their patients’ insomnia is substance-induced, although psychiatric disorders such as depression and activating antidepressants such as bupropion are also well-appreciated causes of insomnia in this population.
Management of insomnia in AD patients, of course, depends on an assessment of etiology and perpetuating factors.3 Prevailing conventional wisdom in the addiction field holds that abstinence from alcohol and other drugs of abuse is necessary and often sufficient treatment for insomnia. Few studies, however, provide data to inform clinicians regarding the course of insomnia after starting treatment for alcohol dependence, and whether insomnia responds only to abstinence or if it also may respond to a reduction in drinking to moderate levels.
The purpose of this study was to examine the 6-month course of insomnia in AD patients. Questions to be answered included: (1) How prevalent is insomnia when starting treatment and what factors distinguish between patients with and without insomnia? (2) For what proportion of patients does insomnia at baseline persist when assessed 6 months later, and what factors distinguish patients with and without persistent insomnia? (3) How does the course of drinking influence the course of insomnia over a 6-month period? In particular, what effect does abstinence, moderate drinking, and heavy drinking have on insomnia outcomes? (4) How often does insomnia persist despite achieving good drinking outcomes?
Methods
A total of 364 volunteers who met DSM-IV criteria for alcohol dependence were recruited to participate in a study of spiritual change during recovery. Participation required written informed consent and the study was approved by the University of Michigan Institutional Review Board. This study is a secondary analysis that excluded 93 individuals recruited from the community who were not seeking treatment for their disorder, leaving 271 individuals who were seeking help for their drinking. Four of them were excluded because they had missing data regarding their baseline sleep. Of the remaining 267 patients, 154 (57.7%), 80 (30.0%), and 33 (12.4%) were recruited from a university hospital clinic, Veterans Administration clinic, and moderation drinking program, respectively. Baseline insomnia and follow-up rates did not differ by recruitment site. Overall, follow-up assessments at 6 months were conducted with 228 (85.4%) patients, of which three patients had incomplete 6-month sleep data. Therefore, baseline data pertain to 267 patients and 6-month data pertain to 225 (84.3%) patients. Table 1 shows baseline demographic and clinical characteristics for the sample.
Table 1.
Correlates of Baseline Insomnia
| Baseline Study Variables /Patient Characteristics | Baseline Insomnia
|
Total (N=267) | |
|---|---|---|---|
| Yes (n=125) | No (n=142) | ||
| Demographics | |||
| Age (yr) | 44.8 (12.2) | 44.1 (12.9) | 44.5 (12.5) |
| Gender (Male/Female, %)** | 62.4 / 37.6 | 74.6 / 25.4 | 68.9 / 31.1 |
| Race (White/Other, %) | 85.6 / 14.4 | 90.1 / 9.9 | 88.0 / 12.0 |
| Education (yr) | 14.3 (2.3) | 14.4 (2.5) | 14.3 (2.4) |
| Marital Status (n=264)a | |||
| Never married (%) | 23.6 | 26.2 | 25.0 |
| Married/Living Together (%) | 38.2 | 43.3 | 40.9 |
| Separated/Divorced (%) | 38.2 | 30.5 | 34.1 |
| Employed (%)* | 49.6 | 62.0 | 56.2 |
| Clinical | |||
| Last Drink (days) | 29.9 (27.5) | 33.8 (26.5) | 32.0 (26.9) |
| PDA in past 90 days | 59.0 (30.0) | 61.2 (29.2) | 60.2 (29.5) |
| PHDD in past 90 days | 32.0 (28.5) | 30.3 (27.0) | 31.1 (27.7) |
| DDD in past 90 days | 10.3 (9.2) | 9.2 (6.5) | 9.7 (7.9) |
| SIP score*** | 23.4 (10.8) | 19.4 (11.3) | 21.3 (11.2) |
| Age of onset (yr) | 29.4 (13.0) | 29.1 (11.4) | 29.2 (12.1) |
| DSM-IV symptom count** | 5.8 (1.3) | 5.4 (1.4) | 5.6 (1.4) |
| Psychiatric Symptoms (#)**** | 28.3 (13.0) | 19.6 (12.2) | 23.7 (13.3) |
| Total SPQ Score**** | 14.0 (4.2) | 5.0 (3.1) | 9.2 (5.8) |
Three patients were widowed and not included in analysis.
PDA = percent days abstinent; PHDD = percent heavy drinking days; DDD = drinks per drinking day; SIP = Short Inventory of Problems; SPQ = Sleep Problems Questionnaire
p<.10
p<.05
p<.005
p<.0005
Measures
The diagnosis of alcohol dependence was confirmed at baseline using the Structured Clinical Interview for DSM-IV Diagnoses (SCID).6 Symptoms of insomnia were assessed at both time points with the 4-item, self-administered Sleep Problems Questionnaire (SPQ), characterized by scores ranging from 0 to 20 with higher scores indicating greater sleep disturbance.7 The items ask about trouble falling asleep, trouble staying asleep, waking too early, and awakening in the morning feeling tired and worn out. Patients rate each item on a scale of 0 to 5, based on the number of days a symptom was experienced, with 4 representing 15–21 days and 5 representing 22–31 days in the past month, respectively. Because no cut-off score for the SPQ is available, patients were classified as having insomnia if they rated any one item as a 4 or 5. This method of classification was designed to reflect a common convention that clinically significant insomnia occurs at least 3 days weekly for at least 1 month.8–10
The Brief Symptom Inventory (BSI) is a 53-item self-administered scale that measures psychiatric distress.11 Each item represents one symptom and is rated on 0–4 Likert scale. The BSI has six subscales and three global scales that are highly correlated with one another. Of particular interest for this study were symptoms of depression and anxiety, which are associated in the literature with insomnia.12, 13 Because the BSI anxiety and depression subscales were highly correlated in this sample at baseline (r=. 73, P<.0005), one of the global BSI scales was selected to represent them, thereby reducing the number of comparisons and preventing the entry of highly correlated measures into regression analyses. The Positive Symptom Total scale is a numerical count of items endorsed (range 0–53), and correlated strongly with both the depression (r=.77, p<.0005) and anxiety (r=.82, p<.0005) subscales in this sample. It also has the advantage of simple scoring and interpretation. Thus, it was used to measure psychiatric severity.
The Timeline Follow-Back Interview was used to measure drinking in the 90 days prior to baseline as well as 90 days prior to the 6-month follow-up assessment.14, 15 Derived variables at baseline included percent days abstinent (PDA), percent heavy drinking days (PHDD), drinks per drinking day (DDD), and time since last drink (TSLD). Derived variables at the 6-month follow-up assessment included three mutually exclusive categories: no drinking (abstinence), moderate drinking, and any heavy drinking (relapse). Moderate drinking was defined as no more than 3 standard drinks in any one day for women, and no more than 4 standard drinks in any one day for men. Patients who exceeded these limits were defined as having relapsed to heavy drinking (4 or more drinks in a day for women and 5 or more drinks in a day for men). The category of moderate drinking was included to examine the response of insomnia to achieving that status, because one of the programs was designed to facilitate that treatment goal.
The Short Inventory of Problems (SIP) is a 15-item self-administered measure of alcohol-related problems in the previous month.16 It was included to characterize alcohol problem severity and to examine if insomnia was potentially related to concerns about adverse consequences. In addition, a count of DSM-IV symptoms was derived from the SCID and included as a measure of dependence severity to determine if it correlated with insomnia.
Analyses
Descriptive statistics for continuous variables are expressed as means and standard deviations (SDs), and categorical variables are expressed as frequencies and/or percentages. The baseline sample was next divided into patients with and without baseline insomnia, and compared on demographic and clinical characteristics using chi square tests for categorical variables and independent samples t-tests for continuous variables, respectively. Variables that differentiated groups at a level of p<.10, with the exception of total SPQ scores, were then entered simultaneously into a logistic regression analysis to determine the best predictors of baseline insomnia. For follow-up analyses, the proportion of patients with persistent insomnia was calculated, defined as meeting study criteria for insomnia at both baseline and follow-up. To determine the correlates of persistent insomnia, patients with persistent insomnia were compared to patients with insomnia at baseline only, using the same procedures described above for comparing patients with and without baseline insomnia. In addition, baseline SPQ scores were compared between those with and without persistent insomnia. To examine the effect of drinking status (no, moderate, or heavy drinking) in the 90 days prior to follow-up on the course of insomnia from baseline to follow-up, a repeated-measures analysis of variance (ANOVA) was run using total SPQ scores at baseline and follow-up as the repeated measure and drinking group as the between subjects factor. Significant correlates of baseline and follow-up insomnia were entered in the ANOVA as covariates.
Results
At baseline, 125 (46.8%) of 267 patients met study criteria for insomnia. Baseline correlates of insomnia were female gender, unemployment, alcohol problem severity (SIP score), DSM-IV dependence symptom count, and psychiatric symptom count (Table 1). Variables not associated with baseline insomnia were age, race, marital status, education, and all alcohol consumption variables. When positive correlates (p<.10) were entered simultaneously into a logistic regression analysis, gender and psychiatric severity remained as significant predictors of baseline insomnia.
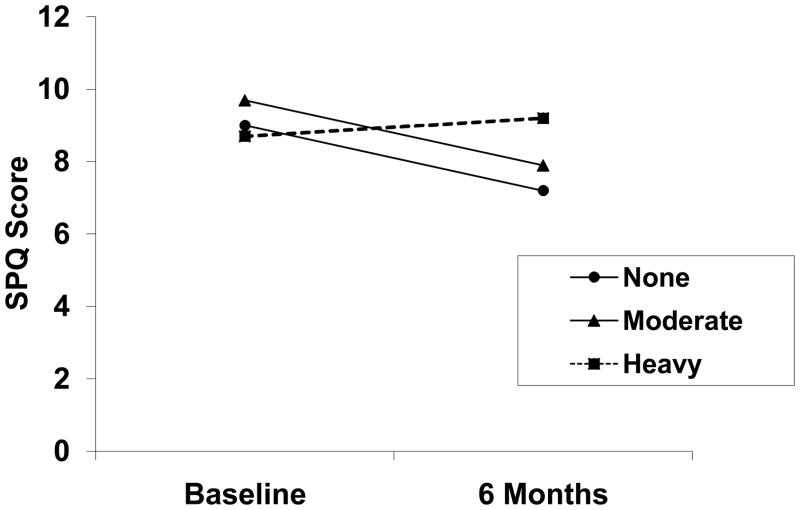
At the 6-month follow-up (n=225), 107 (47.6%) patients were abstinent in the previous 90 days, 29 (12.9%) were drinking moderately, and 89 (39.6%) had relapsed to heavy drinking. Results of the repeated-measures ANOVA are shown in Figure 1. A significant time by group interaction was found. Post-hoc analyses revealed that total SPQ scores decreased (improved) significantly in abstinent and moderate-drinking patients (paired samples t=3.97, df=135, p<.0005), whereas no significant change over time occurred in patients who relapsed to heavy drinking.
Figure 1.
A depiction of change in total scores on the Sleep Problems Questionnaire (SPQ) from baseline to 6 months as a function of drinking status at 6-months. Adjusting for gender and psychiatric severity, a significant interaction between time and drinking group is shown by parallel decreases in scores (improvement in sleep) among patients achieving abstinence (n=107) or drinking moderately (n=29), whereas patients who relapsed to heavy drinking (n=89) show no improvement in sleep (F=5.71, df=2,220, p=.004). Groups did not differ significantly in baseline sleep scores.
Among the 225 followed patients, 103 (45.8%) had insomnia at baseline, and 64 of those (62.1%) had persistent insomnia. Patients with (n=64) and without (n=39) persistent insomnia were compared to look for baseline predictors of persistent insomnia. Only baseline SPQ scores and DSM-IV symptom count differed significantly (p<.05) between groups. Those with persistent insomnia had significantly higher SPQ scores (mean 14.6 [4.1]) than those without (mean 12.1 [4.2], p=.003), and significantly higher DSM-IV symptom counts (mean 6.0 [1.3]) than those without (mean 5.3 [1.3], p=.009). In addition, the mean (SD) Positive Symptom Total Scores for those with and without persistent insomnia were 31.0 (12.8) vs. 26.0 (11.3), respectively (t=1.99, df=101, p=.05); and the mean (SD) SIP scores were 24.2 (10.9) and 20.3 (10.6), respectively (t=1.84, df=101, p=.07). When these four variables were entered into the logistic regression analysis, only baseline SPQ scores predicted persistent insomnia.
Of the 64 patients with persistent insomnia, 26 (40.6%) achieved abstinence, 8 (12.5%) were drinking moderately, and 30 (46.9%) had relapsed to heavy drinking. Thus, 34 (53.1%) of 64 patients with persistent insomnia had achieved good drinking outcomes, meaning they did not relapse to heavy drinking. Of the 103 patients with baseline insomnia, 26 (25.2%) and 8 (7.8%) had persistent insomnia despite achieving abstinence and reducing drinking to moderate levels, respectively. In other words, 34 (33%) of the 103 patients with baseline insomnia had persistent insomnia despite achieving good drinking outcomes.
Discussion
The main findings of this study were: (1) about one-half of patients near the time of treatment entry had symptoms of insomnia that occurred at least 15 days or more in the preceding month; (2) these insomnia symptoms were independently associated with female gender and psychiatric symptom severity, but not alcohol-related variables; (3) insomnia symptoms improved significantly over 6 months time with either abstinence or reduction in drinking to moderate levels, whereas symptoms did not improve among patients who relapsed to heavy drinking; (4) insomnia symptoms also failed to improve in one-third of patients with baseline insomnia (i.e., one-half of patients with persistent insomnia) despite abstinence or moderate drinking levels, indicating that good drinking outcomes do not necessarily and always insure improvement in sleep; and (5) persistent insomnia was independently associated only with baseline insomnia.
The prevalence of insomnia in this sample is consistent with other studies, which have reported rates ranging from 36% to 91%,17, 18 with an average rate across studies of 58%. As with this study, these other studies were based on clinical convenience samples. In a general population study, by contrast, insomnia was reported by 28.4% of alcohol-dependent individuals, compared to an overall rate of 18.5%.19 In the general population, insomnia is also more common in women than men,13, 20 which most likely explains the gender difference found at baseline in this study.
A bivariate association between severity of dependence and insomnia has been reported previously,21 although the association in this study was not significant in the multivariate analysis. Cohn et al18 also did not find an association between severity of dependence and sleep disturbance. Other studies have found an association between alcohol consumption variables and insomnia,22, 23 although this study did not. The absence of an association between alcohol consumption and insomnia at baseline seems inconsistent with the finding that insomnia improved with both abstinence and reduction in drinking to moderate levels. Several overlapping possibilities may explain these discrepancies. First, insomnia can precede the development of alcohol-related problems,24, 25 which might explain why its severity at baseline was independent of drinking parameters. Unless drinking was contributing to insomnia at least in part, however, improvement with reduced drinking as occurred in this study would seem unlikely. Nevertheless, the persistence of insomnia found in some cases despite reduction in drinking would be expected with premorbid insomnia. Second, insomnia may have been caused in large part by comorbid factors such as psychiatric severity, and improvement in sleep with reduced drinking under these conditions would vary in accordance with the degree of persisting psychiatric symptoms. A third explanation is that alcohol consumption contributes substantially to insomnia, but not beyond a threshold level of consumption, which treatment-seeking patients have already surpassed. When drinking is reduced, therefore, the alcohol-related “portion” of insomnia would significantly improve, but other factors would better explain the variation in baseline insomnia as well as its persistence in some cases.
The seemingly high level of persistent insomnia in over 60% of patients is confounded by relapse to heavy drinking in about half of them. Nevertheless, the other half of patients had persistent insomnia despite achieving either abstinence (25%) or moderate drinking levels (8%). This is important information for clinicians, because such patients may require additional diagnostic assessment and specialized treatment approaches for either insomnia or other comorbid conditions. Obstructive sleep apnea is a good example. Such patients may complain of awakening and feeling unrefreshed and, thus, report dissatisfaction with their sleep. Sleep apnea may also contribute to treatment-resistant depression,26, 27 to which persistent sleep complaints may be misattributed. Only by ordering polysomnography can sleep apnea be diagnosed and treated.
The parallel improvement in insomnia with either abstinence or moderate drinking levels has key clinical implications. Importantly, the study was not designed to address the issue of whether moderate drinking is a reasonable treatment goal. Nevertheless, by including moderate drinking as a treatment outcome, the study extends the previous literature and suggests the following: insomnia that persists despite moderate levels of drinking should be approached in the same manner as insomnia that persists despite abstinence. In other words, assessing insomnia in more detail and planning for its treatment should not be delayed until the patient achieves complete abstinence. Although recommending abstinence is certainly preferable for most AD patients, the findings of this study suggest that further improvement in insomnia is unlikely to result once moderate drinking is stable.
This study has several limitations. Like other studies of its kind, it is based on a convenience sample, and may not generalize to the full spectrum of alcohol use disorders. Nevertheless, by recruiting from three different sites, which varied by socioeconomic status and treatment goals (abstinence vs. moderate drinking), the results may have broader applicability than other studies in the field. Second, drinking outcomes were based on self-report without collateral or biochemical corroboration, although differences in self-report between those with and without insomnia would not be expected. Drinking outcomes were also limited to the 90 days prior to the 6-month follow-up interval; however, those 90 days might be expected to correlate most strongly with insomnia in the 1 month prior to follow-up. Third, insomnia as defined in this study does not fully capture insomnia as defined by DSM-IV, which requires either significant distress about insomnia or diminished daytime function resulting from insomnia, in addition to symptoms about sleep quality. Fourth, information about psychiatric severity, while important, did not extend to assessing co-occurring psychiatric disorders such as major depression and nicotine dependence that are strongly associated with both insomnia and alcohol dependence. Similarly, information about medical conditions or medications that might contribute to insomnia was not obtained. Fifth, details of treatment received including its duration, frequency of visits, and use of medications for alcohol dependence, insomnia, and psychiatric disorders were not analyzed.
Limitations such as these stem in part from analyzing data from a study designed to study spiritual factors in recovery, not sleep. Still, the study also has several strengths, including a reasonable sample size, multisite recruitment, and inclusion of moderate drinking as a drinking outcome. Future studies should include more frequent assessment of insomnia symptoms during the first 6 months to better describe how quickly symptoms remit, as well as more detailed information on comorbid disorders and their treatment.
In summary, nearly one-half of AD patients reported frequent symptoms of insomnia in the month prior to seeking treatment, especially women and those with concurrent psychiatric symptoms. Most patients who achieved abstinence or reduced their drinking to moderate levels had significantly improved sleep when measured 6 months later, while sleep did not improve among those who relapsed to heavy drinking. Among those with baseline insomnia, however, sleep problems persisted in about one-third of them despite good drinking outcomes. Such patients are candidates for further diagnostic assessment and consideration for insomnia-specific treatment.
Acknowledgments
Supported by R01AA014442-05, 2K24AA00304-10, T32 AA007477
Footnotes
The authors report no conflicts of interest
References
- 1.Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Res Health. 2001;25:110–125. [PMC free article] [PubMed] [Google Scholar]
- 2.Krystal AD, Thakur M, Roth T. Sleep disturbance in psychiatric disorders: effects on function and quality of life in mood disorders, alcoholism, and schizophrenia. Ann Clin Psychiatry. 2008;20:39–46. doi: 10.1080/10401230701844661. [DOI] [PubMed] [Google Scholar]
- 3.Roth T, Roehrs T, Pies R. Insomnia: pathophysiology and implications for treatment. Sleep Med Rev. 2007;11:71–79. doi: 10.1016/j.smrv.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Wallander MA, Johansson S, Ruigomez A, Garcia Rodriguez LA, Jones R. Morbidity associated with sleep disorders in primary care: a longitudinal cohort study. Prim Care Companion J Clin Psychiatry. 2007;9:338–345. doi: 10.4088/pcc.v09n0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- 6.First MB, Spitzer RL, Gibbon M, Williams JBW, editors. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinician Version: User's Guide. Washington, D.C.: American Psychiatric Press; 1997. [Google Scholar]
- 7.Jenkins CD, Stanton BA, Niemcryk SJ, Rose RM. A scale for the estimation of sleep problems in clinical research. J Clin Epidemiol. 1988;41:313–321. doi: 10.1016/0895-4356(88)90138-2. [DOI] [PubMed] [Google Scholar]
- 8.Roth T, Hajak G, Ustun TB. Consensus for the pharmacological management of insomnia in the new millennium. Int J Clin Pract. 2001;55:42–52. [PubMed] [Google Scholar]
- 9.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281:991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 10.Jansson M, Linton SJ. Cognitive-behavioral group therapy as an early intervention for insomnia: a randomized controlled trial. J Occup Rehabil. 2005;15:177–190. doi: 10.1007/s10926-005-1217-9. [DOI] [PubMed] [Google Scholar]
- 11.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 12.Morphy H, Dunn KM, Lewis M, Boardman HF, Croft PR. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007;30:274–280. [PubMed] [Google Scholar]
- 13.Sivertsen B, Krokstad S, Overland S, Mykletun A. The epidemiology of insomnia: associations with physical and mental health. The HUNT-2 study. J Psychosom Res. 2009;67:109–116. doi: 10.1016/j.jpsychores.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Maisto SA, Sobell MB, Cooper AM, Sobell LC. Test-retest reliability of retrospective self-reports in three populations of alcohol abusers. Journal of Behavioral Assessment. 1979;1:315–326. [Google Scholar]
- 15.Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. Br J Addiction. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 16.Miller WR, Tonigan JS, Longabaugh RL. Publication No. 95-3911. 4. Rockville, MD: National Institutes of Health; 1995. The Drinker Inventory of Consequences (DrInc): an instrument for assessing adverse consequences of alcohol abuse. [Google Scholar]
- 17.Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7:523–539. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- 18.Cohn TJ, Foster JH, Peters TJ. Sequential studies of sleep disturbance and quality of life in abstaining alcoholics. Addiction Biology. 2003;8:455–462. doi: 10.1080/13556210310001646439. [DOI] [PubMed] [Google Scholar]
- 19.Crum RM, Ford DE, Storr CL, Chan YF. Association of sleep disturbance with chronicity and remission of alcohol dependence: data from a population-based prospective study. Alcohol Clin Exp Res. 2004;28:1533–1540. doi: 10.1097/01.alc.0000141915.56236.40. [DOI] [PubMed] [Google Scholar]
- 20.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbance and psychiatric disorders. JAMA. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 21.Brower KJ, Aldrich M, Robinson EAR, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. American Journal of Psychiatry. 2001;158:399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baekeland F, Lundwall L, Shanahan TJ, Kissin B. Clinical correlates of reported sleep disturbance in alcoholics. Q J Stud Alcohol. 1974;35:1230–1241. [PubMed] [Google Scholar]
- 23.Shinba T, Murashima Y, Yamamoto K-I. Alcohol consumption and insomnia in a sample of Japanese alcoholics. Addiction. 1994;89:587–591. doi: 10.1111/j.1360-0443.1994.tb03335.x. [DOI] [PubMed] [Google Scholar]
- 24.Crum RM, Storr CL, Chan YF, Ford DE. Sleep disturbance and risk for alcohol- related problems. Am J Psychiatry. 2004;161:1197–1203. doi: 10.1176/appi.ajp.161.7.1197. [DOI] [PubMed] [Google Scholar]
- 25.Gillin JC. Are sleep disturbances risk factors for anxiety, depressive and addictive disorders? Acta Psychiatrica Scandinavica, Supplementum. 1998;393:39–43. doi: 10.1111/j.1600-0447.1998.tb05965.x. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz DJ, Karatinos G. For individuals with obstructive sleep apnea, institution of CPAP therapy is associated with an amelioration of symptoms of depression which is sustained long term. J Clin Sleep Med. 2007;3:631–635. [PMC free article] [PubMed] [Google Scholar]
- 27.Habukawa M, Uchimura N, Kakuma T, et al. Effect of CPAP treatment on residual depressive symptoms in patients with major depression and coexisting sleep apnea: Contribution of daytime sleepiness to residual depressive symptoms. Sleep Med. 2010;11:552–557. doi: 10.1016/j.sleep.2010.02.007. [DOI] [PubMed] [Google Scholar]