Abstract
Stressful experiences during adolescence can alter the trajectory of neural development and contribute to psychiatric disorders in adulthood. We previously demonstrated that adolescent male rats exposed to repeated social defeat stress show changes in mesocorticolimbic dopamine content both at baseline and in response to amphetamine when tested in adulthood. In the present study we examined whether markers of adult dopamine function are also compromised by adolescent experience of social defeat. Given that the dopamine transporter as well as dopamine D1 receptors act as regulators of psychostimulant action, are stress sensitive and undergo changes during adolescence, quantitative autoradiography was used to measure [3H]-GBR12935 binding to the dopamine transporter and [3H]-SCH23390 binding to dopamine D1 receptors, respectively. Our results indicate that social defeat during adolescence led to higher dopamine transporter binding in the infralimbic region of the medial prefrontal cortex and higher dopamine D1 receptor binding in the caudate putamen, while other brain regions analyzed were comparable to controls. Thus it appears that social defeat during adolescence causes specific changes to the adult DA system, which may contribute to behavioral alterations and increased drug seeking.
Keywords: adolescent stress; dopamine, social defeat; dopamine transporter; dopamine D1 receptor
1. Introduction
Adolescence marks a period of critical change in which the mesocorticolimbic dopamine (DA) system undergoes substantial reorganization, enabling emotional and cognitive development that aids in the transition to adulthood [61, 70]. While such changes are inherently adaptive to survival, they also make the adolescent brain particularly vulnerable to insults from the experience of stressors [2, 3, 61]. Stress is a potent activator of the mesocorticolimbic DA system [1, 6], and evidence suggests that stressful experiences during adolescence can lead to long-term changes in this system that may contribute to increased incidence of psychiatric disorders in adulthood [7, 14, 23, 39, 47, 49, 62, 72, 74].
One particularly common yet severe stressor that adolescents encounter is bullying [45]. Along with its immediate consequences to psychological well-being, bullying is associated with a greater incidence of psychiatric disorders that may emerge either in adolescence or in later life [8, 25, 27, 36, 45, 65, 71]. In order to gain insight into the potential neural mechanisms by which bullying might contribute to later psychopathology, we have developed a rodent model of adolescent social defeat that mimics the victimization and imbalance of power defining human adolescent bullying [11, 72]. In line with the aforementioned developmental vulnerability of the mesocorticolimbic DA system, rats undergoing repeated social defeat in adolescence exhibit reduced dopamine content in the medial prefrontal cortex (mPFC) as adults [72]. Furthermore, amphetamine-induced increases in DA responses are attenuated in the mPFC of adult rats that had experienced adolescent social defeat [14]. Conversely, previously defeated rats showed an enhanced increase in amphetamine-induced DA responses in the nucleus accumbens (NAc) core compared to controls [14]. This particular pattern of low mPFC DA activity and high NAc DA activity has been associated with enhanced locomotion responses to both novelty and amphetamine, as well as increased psychostimulant self-administration [13, 51, 57, 67]. Indeed, rats exposed to social defeat in adolescence do show greater locomotion activity in novel environments as adults [14, 72], along with enhanced conditioned place preference for amphetamine [15]. Together, these findings suggest that the experience of social defeat in adolescence may have long term consequences on mesocorticolimbic DA regulatory processes that contribute to altered novelty and psychostimulant responses.
One point of DA regulation is the DA transporter (DAT), which acts as both a mechanism to clear synaptic DA and as a pharmacological target for amphetamine [55, 77]. Differences in DAT function and expression have also been found in rats with high versus low locomotion responses to novelty [28, 76]. Given findings of altered psychostimulant and novelty responses in previously defeated rats [14, 72], and that DAT levels are also affected by social defeat stress [22, 30, 40], we hypothesized that adolescent social defeat may lead to long-term changes in DAT expression. Besides DAT, the DA D1 receptor plays a role in facilitating amphetamine-induced locomotion behavior [26, 29, 68, 69, 75] and also participates in modulating the balance between mPFC and NAc DA levels [21, 48, 68, 69]. As rats defeated in adolescence also show decreased amphetamine-induced locomotion in adulthood compared to non-defeated controls [14], it was additionally hypothesized that DA D1 receptors may be altered by adolescent defeat experience. In order to investigate potential changes to these dopaminergic markers, we sought to analyze DAT and DA D1 receptors in mPFC, NAc, and striatum, as DA activity is affected by social defeat in these regions [30, 40, 66]. In addition, these structures are principally involved in mediating novelty and amphetamine-evoked responses on both a pharmacological and behavioral level [19, 51, 67, 77]. Thus, the present study utilized quantitative autoradiography to measure the binding of [3H]-GBR12935 to DAT sites and of [3H]-SCH23390 to DA D1 receptors in the mPFC, NAc, and striatum of adult rats that had undergone repeated social defeat in adolescence.
2. Materials and Methods
2.1. Subjects
Male juvenile post-weanling Sprague-Dawley rats (Postnatal day [P]21, n=20) were obtained from the University of South Dakota Laboratory Animal Services. All rats were pair-housed such that cage-mates were in the same treatment group (social defeat or control) and kept at 22 °C on a reverse 12-hr light-dark cycle (lights off 10.00). Food and water were available ad libitum. Behavioral experiments were conducted between 11.00 and 15.00 under red lighting. All procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and received approval from the Institutional Animal Care and Use Committee of the University of South Dakota. Every effort was made to minimize the number of animals used and their suffering.
2.2. Social defeat
The adolescent social defeat procedure used in this study is a modification of the resident-intruder paradigm [34, 42, 43] and has been described in detail previously [14, 72]. Briefly, male rats (n=10), starting at P35 (mid-adolescence, [2, 61]), were introduced to the home cage of a larger aggressive resident adult male Sprague Dawley rat once daily for 5 consecutive days. After the adolescent intruder exhibited 3 consecutive submissive postures, it was considered defeated, and promptly confined behind a wire-mesh barrier within the resident’s cage for 35 minutes. The adolescent rat was then subsequently returned to its home cage. Age-matched controls (n=10) did not experience social defeat, but were instead placed into a novel empty cage for the duration of the defeat trial in order to control for handling and novel environment stress. After the final defeat trial, all animals were allowed to mature undisturbed in their home cages until early adulthood (P56).
2.3. Brain Section Preparation
At P56, all subjects underwent rapid decapitation and brains were collected and frozen at −80 °C until use. Brain sections (16μm) were cut at −18°C in a cryostat microtome and mounted on gelatin-coated microscope slides (two brain sections per slide). Before storing at −80°C, all slides were kept overnight at 4°C under vacuum. For autoradiographic analysis, quadruplicate brain sections from each animal were used encompassing the mPFC (infralimbic, prelimbic and cingulate cortices) and corresponding to Plate 8 as listed in the brain atlas of Paxinos and Watson [50]. Similarly, quadruplicate sections from each subject that contained the nucleus accumbens (core and shell) and striatal caudate putamen (CPu) equivalent to Plate 12 of the Paxinos and Watson atlas [50] were also analyzed.
2.4. DAT binding
DAT binding was assessed with the radioligand [3H]-GBR12935 according to the method described previously by Jiao et al. [31] with minor modifications. Slides containing sections were preincubated for 15 minutes at 4°C in a 7.5 pH buffer solution containing 50 mM NaH2PO4, 70 mMNaCl, 0.025% bovine serum albumin (BSA), 0.001% ascorbate, and 1 μMcis-flupentixol. This was followed by a 23 hour incubation in the same buffer solution with the addition of 2nM [3H]-GBR12935. Non-specific binding was determined with the addition of 50μM mazindol. In order to the end the incubation, slides were placed in ice-cold buffer solution without [3H]-GBR12935 for 2 hours. Slides were then dried at 4°C, transferred into cassettes and exposed to BioMax MS film with [3H] standards. The exposure times were 14 days and 35 days for plates 12 and 8, respectively.
2.5. DA D1 receptor binding
DA D1 receptors were labeled with [3H]-SCH23390 based on the method of Savasta et al. [56] and similar to that described previously [46]. Specifically, sections were preincubated for 15 minutes in a 7.4 pH buffer solution containing 50mM Tris-HCl, 120mM NaCl, 5mM KCl, and 1mM MgCl2 at room temperature. The sections were then incubated for 60 minutes at room temperature in a similar buffer solution with the addition of 3.5nM [3H]-SCH23390 and 30nM ketanserin tartrate (to block 5-HT2 receptors). Non-specific binding was determined with the addition of 1μM (+)− butaclamol. The incubation was ended by dipping the slides in ice-cold buffer, followed by two consecutive 10 minute washes in ice-cold buffer and a final dip in cold de-ionized water. The sections were then dried at room temperature and transferred into cassettes and exposed to Kodak BioMax MS film with [3H] standards. The exposure times were 14 and 28 days for Plates 12 and 8, respectively.
2.6. Quantification and Statistics
Autoradiographic films were analyzed using the computer software program, ImageJ [54]. Nonspecific binding was subtracted from the total binding to provide the specific binding to either DAT or DA D1 receptors in the regions of interest.
Statistical analysis was performed using SigmaStat 3.5 for Windows. Data were expressed as mean ± S.E.M specific binding (fmol/mg brain protein), where protein levels are based on a standard curve of optical densities generated from a series of tritiated standards of known concentrations [31, 46]. Levels of DAT and DA D1 receptor binding in each region of interest were compared between previously defeated and control rats using separate one way ANOVA. The level of significance was set a priori at p<0.05.
3. Results
3.1. DAT binding to [3H]-GBR12935
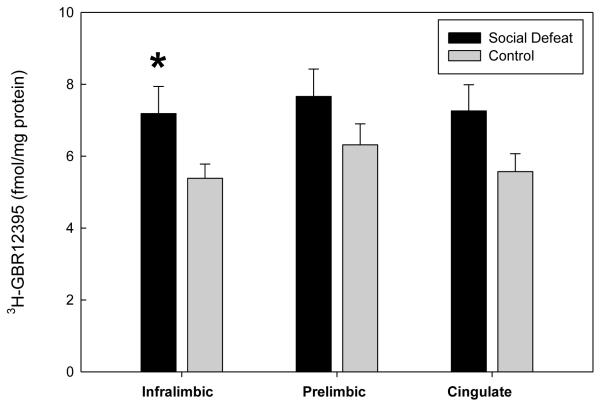
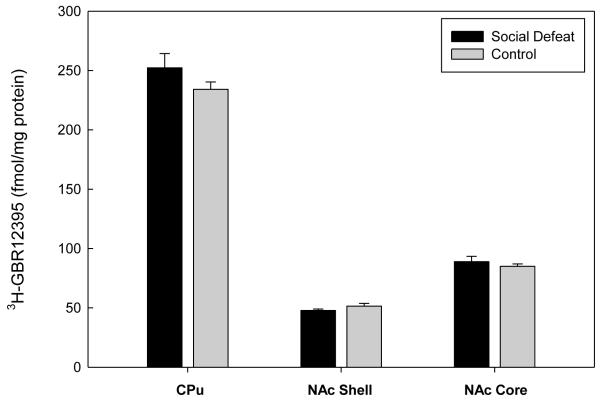
In the mPFC, significant increases in DAT binding density were found in the infralimbic cortex of adult rats that had undergone social defeat during adolescence (F(1,17)=4.685, p=0.045; Figure 1). However, previously defeated rats and controls showed equivalent levels of DAT binding in the prelimbic cortex (F(1,17)=2.527, p=0.130) and the cingulate cortex (F(1,17)=4.373, p=0.052) (Figure 1). No significant differences in DAT binding were found in the subcortical regions of the CPu (F(1,17)=1.670, p=0.213), NAc Shell (F(1,17)=2.020, p=0.173), or NAc Core (F(1,17)=0.590, p=0.453) (Figure 2).
Figure 1.
Specific binding of [3H]-GBR12935 to DAT sites in regions of the mPFC of adult rats that underwent adolescent social defeat versus controls. Data are expressed as the mean±S.E.M. of measurements from 10 rats from each group with determinations made in quadruplicate sections from each brain. * Significant difference between treatment groups (p<0.05).
Figure 2.
Specific binding of [3H]-GBR12935 to DAT sites in subcortical regions of adult rats that underwent adolescent social defeat versus controls. Data are expressed as the mean±S.E.M. of measurements from 10 rats from each group with determinations made in quadruplicate sections from each brain.
3.2. DA D1 binding to [3H]-SCH23390
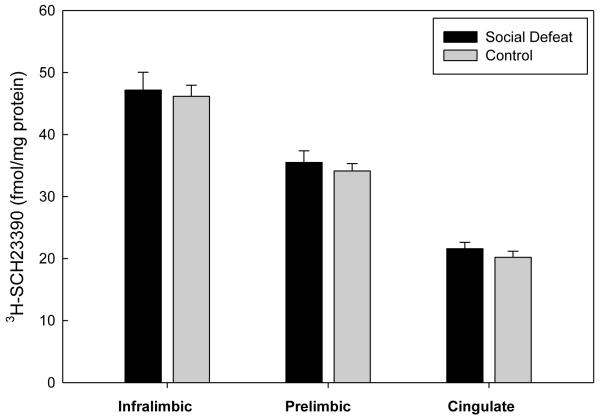
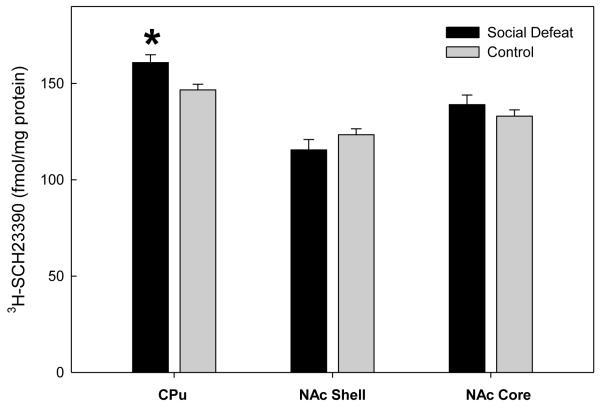
In contrast to DAT binding density, no significant differences in DA D1 receptor binding between previously defeated rats and controls were found in the subregions of the mPFC (infralimbic (F(1,18)=0.0891, p=0.769); prelimbic (F(1,18)=0.379, p=0.546); cingulate F(1,18)=0.947, p=.343) (Figure 3). Likewise, DA D1 receptor binding did not differ between defeated and control rats in either the NAc core (F(1,17)=0.939, p=0.346) or the NAc shell (F(1,17)=1.527, p=0.233). However, previously defeated rats demonstrated increased DA D1 receptor binding density in the CPu (F(1,17)=7.634, p=0.013) as adults (Figure 4).
Figure 3.
Specific binding of [3H]-SCH23390 to DA D1 receptor sites in regions of the mPFC of adult rats that underwent adolescent social defeat versus controls. Data are expressed as the mean±S.E.M. of measurements from 10 rats from each group with determinations made in quadruplicate sections from each brain.
Figure 4.
Specific binding of [3H]-SCH23390 to DA D1 receptor sites in subcortical regions of adult rats that underwent adolescent social defeat versus controls. Data are expressed as the mean±S.E.M. of measurements from 10 rats from each group with determinations made in quadruplicate sections from each brain. * Significant difference between treatment groups (p<0.05).
4. Discussion
4.1. Adolescent Social Defeat induces Changes in Adult DAT Binding
Rats that had experienced repeated social defeat in adolescence showed increased DAT binding in the infralimbic mPFC as adults. Given that a blunted mPFC DA response to acute amphetamine was previously observed in adult rats exposed to adolescent defeat [14], findings of the current study suggest that DAT availability is not a limiting factor for amphetamine action in the mPFC in these animals. Rather, this dampened DA response exhibited by previously defeated rats is most likely a function of reduced basal mPFC DA content caused by adolescent defeat [72].
Both physical and social stressors cause excessive mPFC DA release [1, 18, 66] in adult rats. Similarly, preliminary data using our model indicate that adolescent rats undergoing repeated defeat exhibit acute increases in mPFC DA release upon subsequent exposure to social threat [73], and it is also known that stress experienced during adolescence induces greater mPFC neuronal activity than in adulthood [41]. Combined, this suggests that increased DAT density in the mPFC may reflect a compensatory mechanism to enhance clearance of excessive mPFC DA release caused by the stressful adolescent defeat experience, as has been observed in adult rats exposed to physical stressors [60]. While this would theoretically serve to help maintain efficient DA regulation in the face of repeated social defeat, its persistence beyond the stressful period into young adulthood could potentially contribute to the mPFC DA hypofunction and related behaviors of defeated rats we have described previously [14, 72]. In addition, heightened mPFC DA clearance resulting from increased DAT activity could directly enhance end-product inhibition of tyrosine hydroxylase to reduce DA synthesis [9], explaining why rats defeated in adolescence show decreased mPFC DA content as adults [72]. Evidence suggests the norepinephrine transporter (NET) plays an important role in mPFC DA clearance, possibly as a result of the relatively sparse distribution of DAT in the mPFC compared to other regions [44, 52]. Therefore, future studies should investigate whether mPFC NET expression and function is also affected by adolescent defeat and if this contributes to alterations in mPFC DA and related behaviors.
Rats defeated in adolescence show heightened locomotion responses in novel environments as adults [14, 72]. Zhu et al. [76] found that mPFC DAT function and cell surface expression in the mPFC were lower in rats with a high locomotion response to an inescapable novel environment, while no differences in total mPFC DAT binding were observed. With regards to DAT binding, the discrepancy of our findings with those of Zhu et al. [76] may be related to the method and model used in the current work, as our study used quantitative autoradiography to measure DAT binding in discrete mPFC subregions rather than homogenate binding within the entire mPFC. Furthermore, social defeat stress during adolescence may produce differential effects on mPFC DAT as compared to DAT profiles associated with naturally-occurring predispositions for high or low novelty responses without prior adolescent stress.
To our knowledge, this is the first study to evaluate the long-term effects of a social stressor in adolescence on DAT binding density, and the first to demonstrate that this effect on DAT is specific to the mPFC. Previous studies have shown that exposure to social defeat in adulthood results in changes to DAT expression, but unlike the current findings, these appear to be restricted to subcortical components of the limbic DA system, with no reported alterations to cortical DAT. Further, the direction of DAT change appears to depend on the defeat paradigm used. For instance, chronic social stress in adulthood causes an upregulation of accumbal and striatal DAT binding in subordinate male rats [40], while VTA DAT mRNA levels are increased in adult male mice that repeatedly experience social defeat [22]. In contrast, adult male rats exposed to a single social defeat show decreased striatal DAT binding, with this effect only apparent after being housed in isolation for >24 hr immediately following defeat [30]. The prefrontal cortex DA system undergoes dynamic alterations during adolescence [10, 20, 41, 64], components of which are delayed compared to subcortical DA structures [4, 12, 17]. Of relevance to the current findings, Leussis et al. [38] found that social isolation stress during adolescence produced decreases in synaptic density in both the infralimbic and cingulate cortex. Given that adolescent social defeat produced changes in DAT within similar regions, the present work supports literature in both animals [37, 38] and humans [5] that the developing prefrontal cortex is particularly vulnerable during adolescence to social stress, and as such may be responsible for the differing patterns of DAT expression seen in the current study compared with those using adult social stress paradigms.
4.2. DA D1 receptor binding
Adult rats that had undergone repeated social defeat during adolescence exhibited increased DA D1 receptor binding in the CPu, with no changes in regions of the NAc or mPFC. The DA D1 receptor is particularly important for facilitating locomotion responses to psychostimulants [75]. Although the current study found that previously defeated rats demonstrated increased CPu DA D1 receptor binding, these rats exhibit attenuated locomotion responses to amphetamine [14]. This is likely due to the fact that while the CPu is involved in amphetamine-induced stereotypy, DA activity in the NAc plays a more fundamental role in amphetamine-induced locomotion activity [32, 33, 58].
In viewing the increased DA D1 receptor binding in defeated rats from a developmental perspective, it is interesting to note that DA D1 receptors in the CPu undergo extensive pruning between adolescence and young adulthood [4, 24, 63]. These studies have shown that DA D1 receptor binding density reaches a peak in the CPu at P40 with an approximate 35-40% reduction by P60 [4, 63]. It is thus tempting to speculate that repeated social defeat stress ending at P39 prevented the normal DA D1 receptor pruning in the CPu that would be evident by the time the receptors were assayed at P56. The failure of defeated rats to demonstrate this pruning phenomenon may be due to changes in the mPFC DA system. It has been found that 6-OHDA lesions of the mPFC can produce upregulation of DA D1 receptors in the striatum [53], suggesting that the previously observed deficits in mPFC DA content following social defeat [72] may influence subcortical receptor content.
Andersen et al. [4] have suggested that DA receptor pruning in the CPu may be related to decreases in hyperactivity symptoms seen in attention deficit hyperactivity disorder (ADHD) after periadolescence. Such reasoning is in line with observations that the spontaneously hypertensive rat (SHR), which is used as model for ADHD, has higher DA D1 receptor binding in the CPu compared to other strains [16, 35] (but see [59]). Given the hyperactive-like nature of previously defeated rats [14, 72], assessing DA receptor binding at multiple time points would provide more insight into how the experience of stressors during adolescence affect normal development of the DA system and might contribute to disorders such as ADHD.
4.3. Conclusions
When exposed to repeated social defeat in adolescence, male rats demonstrate regional alterations in DAT and DA D1 receptor binding density as adults. Specifically, previously defeated rats were found to have significant increases in DAT binding in the infralimbic region of the mPFC, while DA D1 receptor binding was significantly increased in the CPu. The persistence of these changes into adulthood is likely reminiscent of the developmental vulnerability of the adolescent brain to stress [2, 3, 61]. Given the role of DAT and DA D1 receptors in regulating DA, the alterations found in the present study may contribute to the long-term changes in behavior and psychostimulant responses seen previously in rats exposed to adolescent defeat [14, 72]. In order to further our understanding of the long-term consequences of severe adolescent stressors, future studies utilizing pharmacological challenges to target DAT and DA D1 receptors will better characterize the mechanisms by which changes in binding density found in the present study relate to alterations in brain function and behavior.
Novick et al – Research Highlights.
Stressful experiences in adolescence can disrupt neural development.
We show adult prefrontal cortex dopamine transporter increases after adolescent social defeat.
Previously defeated rats also showed increased striatal dopamine-1 receptors.
These changes may underlie altered drug responses seen after adolescent defeat.
Acknowledgements
This work was supported by NIDA RO1 DA019921 (Forster), NIAAA R15 AA015921 (Tejani-Butt) and NIH P20 RR015567, which is designated a Center of Biomedical Research Excellence (COBRE).
Abbreviations
- DA
Dopamine
- DAT
Dopamine Transporter
- mPFC
medial prefrontal cortex
- NAc
nucleus accumbens
- CPu
caudate putamen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- [2].Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- [3].Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- [4].Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- [5].Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20:292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Anisman H, Zacharko RM. Behavioral and neurochemical consequences associated with stressors. Ann N Y Acad Sci. 1986;467:205–225. doi: 10.1111/j.1749-6632.1986.tb14630.x. [DOI] [PubMed] [Google Scholar]
- [7].Arnsten AF, Shansky RM. Adolescence: vulnerable period for stress-induced prefrontal cortical function? Introduction to part IV. Ann N Y Acad Sci. 2004;1021:143–147. doi: 10.1196/annals.1308.017. [DOI] [PubMed] [Google Scholar]
- [8].Arseneault L, Bowes L, Shakoor S. Bullying victimization in youths and mental health problems: ‘much ado about nothing’? Psychol Med. 2010;40:717–729. doi: 10.1017/S0033291709991383. [DOI] [PubMed] [Google Scholar]
- [9].Bannon MJ, Roth RH. Pharmacology of mesocortical dopamine neurons. Pharmacol Rev. 1983;35:53–68. [PubMed] [Google Scholar]
- [10].Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cereb Cortex. 2000;10:1014–1027. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- [11].Bjorkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- [12].Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bubser M, Schmidt WJ. 6-Hydroxydopamine lesion of the rat prefrontal cortex increases locomotor activity, impairs acquisition of delayed alternation tasks, but does not affect uninterrupted tasks in the radial maze. Behav Brain Res. 1990;37:157–168. doi: 10.1016/0166-4328(90)90091-r. [DOI] [PubMed] [Google Scholar]
- [14].Burke AR, Renner KJ, Forster GL, Watt MJ. Adolescent social defeat alters neural, endocrine and behavioral responses to amphetamine in adult male rats. Brain Res. 2010;1352:147–156. doi: 10.1016/j.brainres.2010.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Burke AR, Watt MJ, Forster GL. Adolescent social defeat increases conditioned place preferences for amphetamine in adulthood; Soc for Neurosci Meeting Abstracts; 2008; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Carey MP, Diewald LM, Esposito FJ, Pellicano MP, Gironi Carnevale UA, Sergeant JA, Papa M, Sadile AG. Differential distribution, affinity and plasticity of dopamine D-1 and D-2 receptors in the target sites of the mesolimbic system in an animal model of ADHD. Behav Brain Res. 1998;94:173–185. doi: 10.1016/s0166-4328(97)00178-2. [DOI] [PubMed] [Google Scholar]
- [17].Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- [18].Cenci MA, Kalen P, Mandel RJ, Bjorklund A. Regional differences in the regulation of dopamine and noradrenaline release in medial frontal cortex, nucleus accumbens and caudate-putamen: a microdialysis study in the rat. Brain Res. 1992;581:217–228. doi: 10.1016/0006-8993(92)90711-h. [DOI] [PubMed] [Google Scholar]
- [19].Creese I, Iversen SD. The role of forebrain dopamine systems in amphetamine induced stereotyped behavior in the rat. Psychopharmacologia. 1974;39:345–357. doi: 10.1007/BF00422974. [DOI] [PubMed] [Google Scholar]
- [20].Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Doherty MD, Gratton A. Medial prefrontal cortical D1 receptor modulation of the meso-accumbens dopamine response to stress: an electrochemical study in freely-behaving rats. Brain Res. 1996;715:86–97. doi: 10.1016/0006-8993(95)01557-4. [DOI] [PubMed] [Google Scholar]
- [22].Filipenko ML, Alekseyenko OV, Beilina AG, Kamynina TP, Kudryavtseva NN. Increase of tyrosine hydroxylase and dopamine transporter mRNA levels in ventral tegmental area of male mice under influence of repeated aggression experience. Brain Res Mol Brain Res. 2001;96:77–81. doi: 10.1016/s0169-328x(01)00270-4. [DOI] [PubMed] [Google Scholar]
- [23].Finlay JM. Mesoprefrontal dopamine neurons and schizophrenia: role of developmental abnormalities. Schizophr Bull. 2001;27:431–442. doi: 10.1093/oxfordjournals.schbul.a006885. [DOI] [PubMed] [Google Scholar]
- [24].Gelbard HA, Teicher MH, Faedda G, Baldessarini RJ. Postnatal development of dopamine D1 and D2 receptor sites in rat striatum. Brain Res Dev Brain Res. 1989;49:123–130. doi: 10.1016/0165-3806(89)90065-5. [DOI] [PubMed] [Google Scholar]
- [25].Gladstone GL, Parker GB, Malhi GS. Do bullied children become anxious and depressed adults?: A cross-sectional investigation of the correlates of bullying and anxious depression. J Nerv Ment Dis. 2006;194:201–208. doi: 10.1097/01.nmd.0000202491.99719.c3. [DOI] [PubMed] [Google Scholar]
- [26].Hall DA, Powers JP, Gulley JM. Blockade of D1 dopamine receptors in the medial prefrontal cortex attenuates amphetamine- and methamphetamine-induced locomotor activity in the rat. Brain Res. 2009;1300:51–57. doi: 10.1016/j.brainres.2009.08.084. [DOI] [PubMed] [Google Scholar]
- [27].Hoffmann JP, Cerbone FG, Su SS. A growth curve analysis of stress and adolescent drug use. Subst Use Misuse. 2000;35:687–716. doi: 10.3109/10826080009148417. [DOI] [PubMed] [Google Scholar]
- [28].Hooks MS, Juncos JL, Justice JB, Jr., Meiergerd SM, Povlock SL, Schenk JO, Kalivas PW. Individual locomotor response to novelty predicts selective alterations in D1 and D2 receptors and mRNAs. J Neurosci. 1994;14:6144–6152. doi: 10.1523/JNEUROSCI.14-10-06144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Isacson R, Kull B, Wahlestedt C, Salmi P. A 68930 and dihydrexidine inhibit locomotor activity and d-amphetamine-induced hyperactivity in rats: a role of inhibitory dopamine D(1/5) receptors in the prefrontal cortex? Neuroscience. 2004;124:33–42. doi: 10.1016/j.neuroscience.2003.11.016. [DOI] [PubMed] [Google Scholar]
- [30].Isovich E, Engelmann M, Landgraf R, Fuchs E. Social isolation after a single defeat reduces striatal dopamine transporter binding in rats. Eur J Neurosci. 2001;13:1254–1256. doi: 10.1046/j.0953-816x.2001.01492.x. [DOI] [PubMed] [Google Scholar]
- [31].Jiao X, Pare WP, Tejani-Butt S. Strain differences in the distribution of dopamine transporter sites in rat brain. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:913–919. doi: 10.1016/S0278-5846(03)00150-7. [DOI] [PubMed] [Google Scholar]
- [32].Jones GH, Robbins TW. Differential effects of mesocortical, mesolimbic, and mesostriatal dopamine depletion on spontaneous, conditioned, and drug-induced locomotor activity. Pharmacol Biochem Behav. 1992;43:887–895. doi: 10.1016/0091-3057(92)90422-c. [DOI] [PubMed] [Google Scholar]
- [33].Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- [34].Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta Physiol Scand Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- [35].Kujirai K, Przedborski S, Kostic V, Jackson-Lewis V, Fahn S, Cadet JL. Autoradiography of dopamine receptors and dopamine uptake sites in the spontaneously hypertensive rat. Brain Res Bull. 1990;25:703–709. doi: 10.1016/0361-9230(90)90046-3. [DOI] [PubMed] [Google Scholar]
- [36].Kuntsche EN, Gmel G. Emotional wellbeing and violence among social and solitary risky single occasion drinkers in adolescence. Addiction. 2004;99:331–339. doi: 10.1111/j.1360-0443.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- [37].Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- [38].Leussis MP, Lawson K, Stone K, Andersen SL. The enduring effects of an adolescent social stressor on synaptic density, part II: Poststress reversal of synaptic loss in the cortex by adinazolam and MK-801. Synapse. 2008;62:185–192. doi: 10.1002/syn.20483. [DOI] [PubMed] [Google Scholar]
- [39].Lewis DA. Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16:385–398. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- [40].Lucas LR, Celen Z, Tamashiro KL, Blanchard RJ, Blanchard DC, Markham C, Sakai RR, McEwen BS. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience. 2004;124:449–457. doi: 10.1016/j.neuroscience.2003.12.009. [DOI] [PubMed] [Google Scholar]
- [41].Lyss PJ, Andersen SL, LeBlanc CJ, Teicher MH. Degree of neuronal activation following FG-7142 changes across regions during development. Brain Res Dev Brain Res. 1999;116:201–203. doi: 10.1016/s0165-3806(99)00069-3. [DOI] [PubMed] [Google Scholar]
- [42].Martinez M, Calvo-Torrent A, Herbert J. Mapping brain response to social stress in rodents with c-fos expression: a review. Stress (Amsterdam, Netherlands) 2002;5:3–13. doi: 10.1080/102538902900012369. [DOI] [PubMed] [Google Scholar]
- [43].Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- [44].Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nansel TR, Overpeck M, Pilla RS, Ruan WJ, Simons-Morton B, Scheidt P. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA. 2001;285:2094–2100. doi: 10.1001/jama.285.16.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Novick A, Yaroslavsky I, Tejani-Butt S. Strain differences in the expression of dopamine D1 receptors in Wistar-Kyoto (WKY) and Wistar rats. Life Sci. 2008;83:74–78. doi: 10.1016/j.lfs.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].O’Donnell P. Adolescent maturation of cortical dopamine. Neurotox Res. 2010;18:306–312. doi: 10.1007/s12640-010-9157-3. [DOI] [PubMed] [Google Scholar]
- [48].Olsen CM, Duvauchelle CL. Intra-prefrontal cortex injections of SCH 23390 influence nucleus accumbens dopamine levels 24 h post-infusion. Brain Res. 2001;922:80–86. doi: 10.1016/s0006-8993(01)03152-3. [DOI] [PubMed] [Google Scholar]
- [49].Pani L, Porcella A, Gessa GL. The role of stress in the pathophysiology of the dopaminergic system. Mol Psychiatry. 2000;5:14–21. doi: 10.1038/sj.mp.4000589. [DOI] [PubMed] [Google Scholar]
- [50].Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. Academic Press; San Diego: 1998. [Google Scholar]
- [51].Piazza PV, Rouge-Pont F, Deminiere JM, Kharoubi M, Le Moal M, Simon H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res. 1991;567:169–174. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- [52].Pozzi L, Invernizzi R, Cervo L, Vallebuona F, Samanin R. Evidence that extracellular concentrations of dopamine are regulated by noradrenergic neurons in the frontal cortex of rats. J Neurochem. 1994;63:195–200. doi: 10.1046/j.1471-4159.1994.63010195.x. [DOI] [PubMed] [Google Scholar]
- [53].Pycock CJ, Kerwin RW, Carter CJ. Effect of lesion of cortical dopamine terminals on subcortical dopamine receptors in rats. Nature. 1980;286:74–76. doi: 10.1038/286074a0. [DOI] [PubMed] [Google Scholar]
- [54].Rasband WS. ImageJ. U. S. National Institutes of Health; Bethesda, Maryland: 1997-2009. [Google Scholar]
- [55].Riddle EL, Fleckenstein AE, Hanson GR. Role of monoamine transporters in mediating psychostimulant effects. AAPS J. 2005;7:E847–851. doi: 10.1208/aapsj070481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Savasta M, Dubois A, Scatton B. Autoradiographic localization of D1 dopamine receptors in the rat brain with [3H]SCH 23390. Brain Res. 1986;375:291–301. doi: 10.1016/0006-8993(86)90749-3. [DOI] [PubMed] [Google Scholar]
- [57].Schenk S, Horger BA, Peltier R, Shelton K. Supersensitivity to the reinforcing effects of cocaine following 6-hydroxydopamine lesions to the medial prefrontal cortex in rats. Brain Res. 1991;543:227–235. doi: 10.1016/0006-8993(91)90032-q. [DOI] [PubMed] [Google Scholar]
- [58].Sellings LH, Clarke PB. 6-Hydroxydopamine lesions of nucleus accumbens core abolish amphetamine-induced conditioned activity. Synapse. 2006;59:374–377. doi: 10.1002/syn.20247. [DOI] [PubMed] [Google Scholar]
- [59].Siemiatkowski M, Rokicki D, Czlonkowska AI, Sienkiewicz-Jarosz H, Bidzinski A, Plaznik A. Locomotor activity and a conditioned fear response: correlation with cortical and subcortical binding of the dopamine D1 receptor antagonist. Neuroreport. 2000;11:3953–3956. [PubMed] [Google Scholar]
- [60].Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the medial prefrontal cortex. Neuroscience. 1993;53:695–703. doi: 10.1016/0306-4522(93)90617-o. [DOI] [PubMed] [Google Scholar]
- [61].Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- [62].Sullivan RM, Brake WG. What the rodent prefrontal cortex can teach us about attention-deficit/hyperactivity disorder: the critical role of early developmental events on prefrontal function. Behav Brain Res. 2003;146:43–55. doi: 10.1016/j.bbr.2003.09.015. [DOI] [PubMed] [Google Scholar]
- [63].Teicher MH, Andersen SL, Hostetter JC., Jr. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- [64].Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP. Developmental neurobiology of childhood stress and trauma. Psychiatr Clin North Am. 2002;25:397–426. vii–viii. doi: 10.1016/s0193-953x(01)00003-x. [DOI] [PubMed] [Google Scholar]
- [65].Tharp-Taylor S, Haviland A, D’Amico EJ. Victimization from mental and physical bullying and substance use in early adolescence. Addict Behav. 2009;34:561–567. doi: 10.1016/j.addbeh.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- [67].Ventura R, Alcaro A, Cabib S, Conversi D, Mandolesi L, Puglisi-Allegra S. Dopamine in the medial prefrontal cortex controls genotype-dependent effects of amphetamine on mesoaccumbens dopamine release and locomotion. Neuropsychopharmacology. 2004;29:72–80. doi: 10.1038/sj.npp.1300300. [DOI] [PubMed] [Google Scholar]
- [68].Vezina P, Blanc G, Glowinski J, Tassin JP. Opposed Behavioural Outputs of Increased Dopamine Transmission in Prefrontocortical and Subcortical Areas: A Role for the Cortical D-1 Dopamine Receptor. Eur J Neurosci. 1991;3:1001–1007. doi: 10.1111/j.1460-9568.1991.tb00036.x. [DOI] [PubMed] [Google Scholar]
- [69].Vezina P, Blanc G, Glowinski J, Tassin JP. Blockade of D-1 dopamine receptors in the medial prefrontal cortex produces delayed effects on pre- and postsynaptic indices of dopamine function in the nucleus accumbens. Synapse. 1994;16:104–112. doi: 10.1002/syn.890160204. [DOI] [PubMed] [Google Scholar]
- [70].Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain Cogn. 2010;72:146–159. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wals M, Verhulst F. Child and adolescent antecedents of adult mood disorders. Curr Opin Psychiatry. 2005;18:15–19. [PubMed] [Google Scholar]
- [72].Watt MJ, Burke AR, Renner KJ, Forster GL. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behav Neurosci. 2009;123:564–576. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Watt MJ, Scholl JL, Haaland EJ, Forster GL. Activation of cortical D2 dopamine autoreceptors during adolescence increases novelty seeking; Soc for Neurosci Meeting Abstracts; 2009. [Google Scholar]
- [74].Wright LD, Hebert KE, Perrot-Sinal TS. Periadolescent stress exposure exerts long-term effects on adult stress responding and expression of prefrontal dopamine receptors in male and female rats. Psychoneuroendocrinology. 2008;33:130–142. doi: 10.1016/j.psyneuen.2007.10.009. [DOI] [PubMed] [Google Scholar]
- [75].Zhang J, Walsh RR, Xu M. Probing the role of the dopamine D1 receptor in psychostimulant addiction. Ann N Y Acad Sci. 2000;914:13–21. doi: 10.1111/j.1749-6632.2000.tb05179.x. [DOI] [PubMed] [Google Scholar]
- [76].Zhu J, Bardo MT, Bruntz RC, Stairs DJ, Dwoskin LP. Individual differences in response to novelty predict prefrontal cortex dopamine transporter function and cell surface expression. Eur J Neurosci. 2007;26:717–728. doi: 10.1111/j.1460-9568.2007.05690.x. [DOI] [PubMed] [Google Scholar]
- [77].Zhu J, Reith ME. Role of the dopamine transporter in the action of psychostimulants, nicotine, and other drugs of abuse. CNS Neurol Disord Drug Targets. 2008;7:393–409. doi: 10.2174/187152708786927877. [DOI] [PMC free article] [PubMed] [Google Scholar]