Abstract
The tumor microenvironment in pancreatic ductal adenocarcinoma (PDAC) is dynamic, with an extensive interaction between the stroma and tumor cells. The aim of this study was to delineate the cross talk between PDAC and cancer-associated fibroblasts (CAFs), with a focus on the mechanism creating the chronic inflammatory tumor milieu. We assessed the effects of the cross talk between PDAC and CAF cell lines on the creation and sustenance of the inflammatory tumor microenvironment in pancreatic cancer. The coculture of PDAC and CAF cell lines enhanced the levels of inflammatory factors including IL-1α, IL-6, CXCL8, VEGF-A, CCL20, and COX-2. CAFs were superior to tumor cells regarding the production of most inflammatory factors, and tumor cell-associated IL-1α was established as the initiator of the enhanced production of inflammatory factors through the binding of IL-1α to IL-1 receptor 1 (IL-1R1) expressed predominantly by CAFs. Furthermore, we found a correlation between IL-1α and CXCL8 expression levels in PDAC tissues and correlation between IL-1α expression and the clinical outcome of the patients. This confirmed an important role for the IL-1 signaling cascade in the creation and sustenance of a tumor favorable microenvironment. Neutralization of the IL-1α signaling efficiently diminished the cross talk-induced production of inflammatory factors. These data suggest that the cross talk between PDAC cells and the main stroma cell type, i.e. CAFs, is one essential factor in the formation of the inflammatory tumor environment, and we propose that neutralization of the IL-1α signaling might be a potential therapy for this cancer.
Introduction
Survival rates for the most prevalent cancers, such as breast and colon cancers, have improved during the last two decades, whereas only minor advances have been reported regarding pancreatic ductal adenocarcinoma (PDAC) [1]. Adenocarcinomas, in particular PDAC, have a vast stromal reaction, that is, desmoplasia, which infiltrates and enwraps the cancer cells [2,3] and may account for 70%of the total tumor mass [4]. New evidence points to an interlinked relationship between PDAC and its stroma, which promotes tumor growth and metastasis by supporting vascularization, recruitment of inflammatory cells, and activation of fibroblasts [5]. The transformation of fibroblasts in PDAC into cancer-associated fibroblasts (CAFs) is linked with several genetic and morphologic changes and seems to be driven by the tumor cells [6]. The mechanisms underlying the assembly and maintenance of the tumor stroma are complex and not fully understood. In PDAC, the tumor cells are believed to produce cytokines, such as interleukins (IL) 1 and 6, CXCL8, and tumor necrosis factor α, and growth factors, such as PDGF and transforming growth factor β with the potential to activate CAFs [7]. When activated, CAFs produce high levels of growth factors, and inflammatory molecules that maintain their activated phenotype and influence the surrounding cells [8]. The inflammatory environment created by the CAFs supports tumor growth and progression and the recruitment of leukocytes such as macrophages, dendritic cells, T cells, and neutrophils [9].
In PDAC, several inflammatory factors, such as COX-2 and CXCL8, have been investigated to determine their role in tumor development, angiogenesis, and correlation to disease severity [10,11]. Production of many of these factors by tumor cells, stroma, and immune cells is initiated by proinflammatory factors, such as IL-1 and tumor necrosis factor α. IL-1 is proposed to be involved in the earliest stages of carcinogenesis by stimulating phagocytes and fibroblasts to produce mutagenic reactive oxygen intermediates and to stimulate proliferation of the premalignant cells [12]. The exact pathways involved in this inflammatory cascade in the tumor are not known. The levels of IL-1α expression have been shown to associate with a virulent tumor phenotype and liver metastases for gastric cancers, for example [13].
Recently, the research focus has shifted from studying mainly the tumor cells to investigating the tumor microenvironment as an entity consisting of several components that are tightly interlinked. Few studies exist where the interactions between cancer cells and stroma components, such as CAFs, have been investigated and include breast, prostate, and pancreatic carcinomas [14–17]. We hypothesized that the cross talk between PDAC cells and stroma, that is, CAFs, may be the initiator and sustainer of cancer-associated inflammation. The aims of this study were to characterize the inflammatory factors involved in and upregulated by the cross talk between tumor cells and CAFs and to delineate the pathway responsible for creating the inflammatory environment.
Our findings show that the inflammatory environment observed in PDAC is dependent on the interaction between cancer cells and CAFs. The inflammatory profiles detected in vivo in tumor tissues were comparable to the findings from the in vitro cross talk model with a high expression of inflammatory factors in the fibrotic stroma, namely, CAFs. Our data implicate a vital role for the IL-1α signaling pathway in the sustainment of the inflammatory microenvironment in the tumor and for the patient's survival time. This observation suggests that treatment of the chronic inflammation in individuals with PDAC by neutralizing IL-1 may be beneficial because it is expected to drastically decrease the amount of angiogenic and metastatic associated factors and may possibly even restore the immune system's ability to fight the cancer.
Materials and Methods
PDAC Patients
Tumor tissues from a total of 30 patients with PDAC and 10 normal pancreatic tissues from individuals who died of hypothermia or from patients with benign pancreatic diseases were used in this study. PDAC tissues were collected from patients undergoing pancreatic Whipple resections at Linköping University Hospital. PDAC was histologically confirmed by two pathologists, independently investigating the samples. The PDACs were staged according to the 1997 International Union Against Cancer classification (TNM = tumor, node, metastasis) and they ranged from T1 to T4 (T1 [n = 3], T2 [n = 14], T3 [n = 12], and T4 [n = 1]), N0 (n = 6), N1 (n = 24), and M0 (n = 30) stage. Consent documents and study protocols for both patient and control samples were approved by the regional ethics committee in Linköping, Sweden (Dnr. M38-06).
Propagation of PDAC Cell Lines and CAFs
Primary PDAC or CAF cell lines were propagated from PDAC tumor tissue biopsies obtained from patients PC013, PC039, PC055, PC065, PC073, and PC077. Tumor samples were cut into small pieces and incubated in Hank's balanced salt solution buffer (Invitrogen, Stockholm, Sweden) supplemented with 0.3 M CaCl2 and 1 mg/ml Collagenase II (Invitrogen) under gentle agitation at 37°C for 1 hour. The samples were centrifuged, and the cell pellet was resuspended in RPMI 1640 (Fisher Scientific, Pittsburgh, PA); supplemented with 20% fetal calf serum (Invitrogen), 2 mM HEPES (Invitrogen), 30 µg/ml gentamicin (Invitrogen), and 1% Fungizone (Invitrogen); and cultured in tissue flasks. The cells were detached using trypsin-EDTA (Invitrogen) when they reached confluence and labeled with CD326 microbeads, and tumor cells were positively selected according to the manufacturer's description (Miltenyi Biotec, Bergisch Gladbach, Germany). The primary PDAC cell lines were cultured for five passages, harvested, and cryopreserved. CD326-negative cells, that is, CAFs, were cultured for two passages, harvested, and cryopreserved until later use. The PDAC cell lines used were our primary cell lines PC013, PC065, and PC077, and the commercial PDAC cell line BXPC-3 was purchased from ATCC (LGC Standards, Stockholm, Sweden). Three PDAC-derived CAF cell lines, CAF039, CAF055, and CAF073, were used in the experiments performed in the present study.
In Vitro Coculture of PDAC Cells and Fibroblasts
PDAC and CAF cell lines were cultured together or alone for 5 days in RPMI 1640 supplemented with 10% fetal calf serum, 2 mM HEPES, and 30 µg/ml gentamicin (R10). The amount of CAFs in the cocultures was kept constant, whereas the amount of tumor cells differed between the PDAC cell lines to best mimic the natural environment of the tumor, that is, CAFs constituted most of the culturing area (60%–70%) after 5 days of culturing. After coculture, the PDAC cells were separated from the CAFs by staining with Allophycocyanin (APC)-conjugated anti-CD326 antibodies (Miltenyi Biotec) followed by sorting on a FACSAria Cell Sorter (BD Biosciences, San Jose, CA). PDAC cells and CAFs were also indirectly cocultured in R10 medium for 5 days using Transwell culturing inserts (BD Biosciences) to examine the importance of cell contact. Singly cultured CAFs were incubated for 5 days in R10 medium supplemented with 10 pg/ml human recombinant IL-1α (200-LA-002; R&D Systems, Abingdon, United Kingdom) to investigate the involvement of IL-1α in the up-regulation of inflammatory factors in CAFs. The cells were then lysed in RLT Buffer (Qiagen, Sollentuna, Sweden) containing 1% β-mercaptoethanol (Sigma Aldrich, Inc, St Louis, MO) and stored at -70°C until preparation of RNA.
Neutralization of IL-1
For complete inhibition of IL-1 activity, PDAC cells and CAFs were cultured as single and cocultures for 5 days in R10 containing 10 µg/ml recombinant IL-1RA (Kineret 100 mg; Biovitrum AB, Stockholm, Sweden). To distinguish between the two IL-1 agonists, the IL-1 activity was blocked by incubating cocultured PC013 and CAF039 cells for 5 days with 25 µg/ml IL-1α- (MAB200; R&D Systems) or IL-1β- (AB-201-NA; R&D Systems) neutralizing antibodies. The cell cultures were then treated as described previously.
Gene Array Analysis of Single and Cocultured PDAC Cell Line and CAF
The PC013 and CAF039 cell lines were cocultured or cultured separately for 5 days in R10 culture medium. After culture, the cells were harvested, sorted into pure population of PDACs and CAFs, and RNA was extracted using the RNeasy Mini Kit according to the manufacturer's description (Qiagen). The quality of the total RNA was assessed using Agilent RNA 6000 Nano kit (Agilent Technologies, Böblingen, Germany), and the RNA was used to make first-strand complementary DNA (cDNA) followed by second-strand cDNA synthesis according to the manufacturer's protocol (Affymetrix, Woodburn Green, United Kingdom). The double-stranded cDNA was purified using the Sample Cleanup Module and cDNA Cleanup Spin Columns (Affymetrix) and biotin-labeled antisense complementary RNA was synthesized using GeneChip IVT labeling kit (Affymetrix). The biotin labeled complementary RNA was purified, fragmented, hybridized to the gene chips (GeneChip HG U133 Plus 2.0; Affymetrix), stained with streptavidin-phycoerythrin in an Affymetrix Fluidics Station 450 using the Affymetrix GeneChip protocol, and scanned using the Affymetrix GeneChip Scanner 3000. The acquisition and initial quantification of array images were done using the GCOS software (Affymetrix). Subsequent data analysis involved the normalization of array values using PMA software followed by nonsupervised hierarchical clustering using d-Chip software (www.dchip.org; gene array accession no. E-MEXP-2826).
Quantification with Real-time Polymerase Chain Reaction
Total RNA was prepared from the samples using RNA Easy Mini kit (Qiagen), and cDNA was synthesized with SuperScript III Reverse Transcriptase First-Strand cDNA Synthesis kit according to the manufacturer's protocol (Invitrogen). Quantitative polymerase chain reaction (PCR) was performed with Fast SYBR Green Master Mix (Version 09/2007; Applied Biosystems, Foster City, CA) on 7900 Fast Real-time PCR System with 7900 System SDS 2.3 Software (Applied Biosystems) according to the manufacturer's protocol. The final concentration of the primers was 100 nM. In the negative controls, cDNA was replaced by distilled water. The final amount of cDNA in each reaction was comparable to 1 ng of RNA. The PCR program was set for 40 cycles at 95°C for 20 seconds, 95°C for 1 second, and 60°C for 20 seconds. At the end of the reaction, a dissociation curve analysis was performed. Specific primers for CXCL8, CCL20, IL-1α, IL-1β, IL-6, IL-11, IL-24, IL-32, IL-1R1, IL-1RA, vascular endothelial growth factor (VEGF-A; CyberGene AB, Stockholm, Sweden), and COX-2 (Invitrogen) were used. β-Actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; CyberGene AB) were used as housekeeping genes. The primers were designed using Primer Express (Applied Biosystems) if not otherwise indicated. Real-time PCRs for the detection of CXCL chemokines were performed using TaqMan Gene Expression Assays (Applied BioSystems) according to the manufacturer's protocol. All reactions were performed in triplicates including none-template controls and two endogenous control probes. FAM-conjugated gene-specific assays were Hs00236937_m1 (CXCL1), Hs00236966_m1 (CXCL2) Hs00171061_m1 (CXCL3), Hs00171085_m1 (CXCL5), Hs00237017_m1 (CXCL6), and the endogenous controls Hs01003267_m1 (HPRT1) and Hs99999905_m1 (GAPDH). The results were analyzed using the ΔΔCt method [18] and presented as either normalized data or as relative gene expression.
Enzyme-Linked Immunosorbent Assay and Cytokine Array
Supernatants from single or cocultured PDAC and CAF cell lines were harvested and cryopreserved until assessment for inflammatory factors. The concentrations of IL-1α, IL-1β (Nordic Biosite, Taby, Sweden), IL-1RA (R&D Systems), IL-6, and CXCL8 (eBioscience, San Diego, CA) were evaluated by enzyme-linked immunosorbent assay according to the manufacturers' protocols. IL-1β was analyzed in supernatants after 5 days of culturing and in plasma from 25 PDAC patients by Bio-Plex Human cytokine 27-plex panel (Biorad Laboratories, Inc, Hercules, CA) as explained elsewhere [19].
Immunohistochemical Analysis of PDAC Tissue Samples
Formalin-fixed paraffin-embedded samples of tumor tissue from PDAC patients (n = 7) and normal pancreas tissues obtained from individuals (n = 7) who had died of hypothermia were used. The samples were pretreated with H2O2, followed by 1% albumin solution, and immunostained using mouse anti-human monoclonal antibodies (mAbs), Ki-67 (DakoCytomation, Glostrup, Denmark), and CXCL8 (BD Biosciences), and rabbit anti-human IL-1α (ab7632), IL-1RA (ab2573), and IL-1R1 (ab59995), all from Abcam (Cambridge, United Kingdom) and incubated overnight at room temperature. Samples were then incubated with alkaline phosphatase-conjugated and/or biotin-conjugated anti-mouse or anti-rabbit secondary Abs (Jackson ImmunoResearch, Suffolk, United Kingdom). Samples stained with biotin conjugated secondary Abs were further incubated with avidin-biotin complex (DakoCytomation) according to the manufacturer's protocol. Peroxidase was detected by development in Tris buffer containing diaminobenzidine tetrahydrochloride (Saveen-Werner AB, Limhamn, Sweden) and 10 µl of 30% H2O2. Alkaline phosphatase was detected by Vulcan fast red chromogen 2 solution (Biocare Medical, Concord, CA) according to the manufacture's protocol. All tissue sections were counterstained with methyl green solution (0.1 M sodium acetate buffer, pH 4.2) containing 1% methyl green (Sigma Aldrich). Images representative of patients were processed using Quantimet 500 MC image processing analysis systems linked to a Leica DMLB microscope (Leica Cambridge, United Kingdom) supported by Leica QWin software version 3 (Leica).
Statistical Analysis
Statistical analysis was performed with GraphPad Prism 5 (GraphPad Software, La Jolla, CA). P < .05 was considered statistically significant, and error bars throughout indicate SEM. Nonparametric data were analyzed using the Wilcoxon matched-pairs test followed by Mann-Whitney test, and paired t test was used for normalized data. Correlation was determined using Spearman nonparametric correlation. The Kaplan-Meier estimator was used to compare different patient groups, and P values were calculated using the log-rank (Mantel-Cox) test.
Results
Cross Talk between PDAC and CAF Creates an Inflammatory Microenvironment
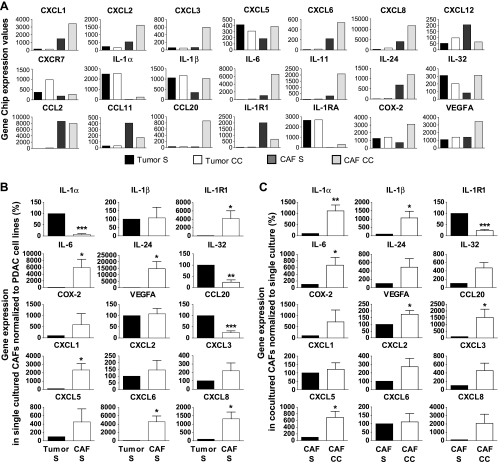
The PDAC-CAF coculture system used for primary PDAC and CAF cell lines created a pattern similar to the one seen in PDAC tumor tissues with the formation of “tumor nests” surrounded by stroma, that is, fibroblasts (Figure W1A). Characterization of the primary CAF cell lines propagated from PDAC tissues, demonstrating that these cells expressed markers well established for myofibroblasts/CAFs, alpha smooth muscle actin (α-SMA), and vimentin (Figure W1B) [20,21]. Tumor cells were positive and CAFs negative for the epithelial cell marker, EpCAM (CD326) (Figure W1, C and D), and this marker was used to obtain pure populations of tumor cells and CAFs from the cocultures by cell sorting (FACS) (Figure W1E). We first sought to assess the gene profile induced by the cross talk between PDAC cells and CAFs. Gene arrays (n = 2) were performed on primary PDAC cell line PC013 and CAF cell line CAF039 after 5 days of culture, either single or cultured together (cocultured cells were sorted into pure PDAC cells and CAFs; Figure W1E). The gene array profiles proved up-regulation of several inflammatory genes in CAF039 after cross talk with tumor cells, for example, IL-1α, IL-1β, CXCL1, CXCL8, IL-6, and CCL20 (Figures 1A and W1F). Furthermore, COX-2, an enzyme that produces prostaglandins and is upregulated at sites of inflammation, was elevated both in CAF039 and in PC013 due to their cross talk (Figures 1A and W1F). The inflammatory signatures generated by the cross talk initiated by the PC013 and CAF039 cell line were confirmed using four different PDAC cell lines, namely, three primary (PC013, PC065, and PC077) propagated from our tumor tissues and one commercial (BXPC-3), as well as three primary CAF cell lines (CAF039, CAF055, and CAF073). Levels of inflammatory factors expressed by singly cultured CAFs were compared with PDAC cell lines (Figure 1B). CAFs were the superior producers of most inflammatory factors, e.g., CXCL1 (P = .025), CXCL6 (P = .013), CXCL8 (P = .015), IL-24 (P = .028), IL-1R1 (P = .026), and IL-6 (P = .042), whereas PDAC cells expressed high levels of proinflammatory factors IL-1α (P < .001), IL-32 (P = .0049), and CCL20 (P < .001) (Figure 1B). After the PDAC-CAF cross talk, CAFs had significantly increased expression levels of inflammatory factors, namely, IL-1α (P = .004), IL-1β (P = .035), IL-6 (P = .031), CCL20 (P = .043), VEGF-A (P = .023), and CXCL5 (P = .001), whereas the expression of IL-1R1 (P < .001) was significantly decreased (Figure 1C). Noteworthy, our findings indicate that the PDAC cells act as the instigator of the inflammatory factors, known to affect different aspects of tumor progression, survival, and metastasis, expressed by the CAFs (Figure 1C). Furthermore, Gene Set Enrichment Analysis (GSEA) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway maps derived from our gene array data indicated an important role for the IL-1 family as the initiator/sustainer of the inflammation.
Figure 1.
Inflammatory profile induced by cross talk between PDAC and CAF cell lines. Gene expression levels from one representative gene array experiment of two assessed in single (S) and cocultured (CC) PC013 and CAF39 for several inflammatory factors (A). PDAC cell lines PC013, BXPC-3, PC065, and PC077 and CAF cell lines CAF039, CAF055, and CAF073 were cultured alone (S) (B) or cocultured (CC) (C) for 5 days and assessed for expression levels of inflammatory factors (IL-1α, IL-1β, IL-1R1, IL-6, IL-24, IL-32, CCL20, VEGF-A, COX-2, CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, and CXCL8) by quantitative RT-PCR. (B) The gene levels in CAFs were compared against the levels in PDAC cell lines as percent difference. (C) All data were pooled together, and the levels in (S) CAFs were compared against the levels in (CC) CAFs as percent difference (n = 10). Data were analyzed using paired t test: *P < .05, **P < .005, and ***P < .001.
Expression of IL-1 and IL-RA by PDAC and Their Receptor IL-1R1 by CAF Cell Lines
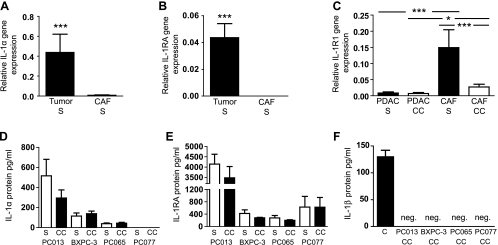
IL-1 is a known regulator and determinator of immune and inflammatory responses, and in light of our findings from the cross talk gene array and quantitative PCR, we investigated in detail the IL-1 family expression profile in PDAC and CAF cell lines. Analysis of all PDAC and CAF cell lines revealed tumor cells as the primary source of IL-1α (P < .001; Figure 2A). The tumor cell lines showed similar expression profiles for IL-1α at both gene and protein levels, with the highest production in PC013 followed by BXPC-3, PC065, and PC077 (Figures 2D and W2B). High expression levels of IL-1RA were observed in PDAC cell lines, whereas the CAF cell lines had no or very low levels of IL-1RA (P < .001; Figure 2B). The IL-1 receptor (IL-1R1) was expressed by all PDAC cell lines tested, although the expression was significantly higher in the CAFs (P < .001; Figure 2C). CAFs cocultured in presence of PDAC cell lines drastically reduced their expression of IL-1R1 (P < .001; Figure 2C). Furthermore, the antagonist IL-1RA levels were low to moderate in BXPC-3 and PC065, whereas PC077 and PC013 secreted high amounts (Figure 2E). Of note, none of the PDAC or CAF cell lines expressed or secreted IL-1β proteins, either in single cultures or in cocultures (Figure 2F), even if all of them expressed this cytokine at the gene level (Figure W2A). Taken together, IL-1α might initiate a signaling cascade through IL-1R1 resulting in the production of inflammatory factors necessary for the development and growth of the PDAC tumor.
Figure 2.
Expression of IL-1 agonists, IL-1RA, and IL-1R1 by PDAC and CAF cell lines. Relative IL-1α (A) and IL-1RA (B) gene expression analyzed by quantitative RT-PCR in PDAC and CAF cell lines cultured alone (S). (C) Relative IL-1R1 gene expression in PDAC and CAF cell lines from cells cultured alone (S) or cocultured (CC) for 5 days and analyzed by quantitative RT-PCR. IL-1α (D) and IL-1RA (E) protein secretion profile in PDAC cell lines cultured alone or cocultured with CAF cell lines. IL-1β protein (F) in supernatants from cocultured PDAC and CAF cell lines. The amount 140 pg/ml of recombinant IL-1β was used as positive control. (A–F) The PC013, BXPC-3, PC065, and PC077 PDAC and CAF039, CAF055, and CAF073 CAF cell lines were used in these experiments. Data were analyzed using the Wilcoxon matched-pairs test followed by Mann-Whitney test. *P < .05, **P < .005, and ***P < .001.
PDAC-Derived IL-1α Is Responsible for the Inflammatory Profiles Induced in CAF Cell Lines after Coculture
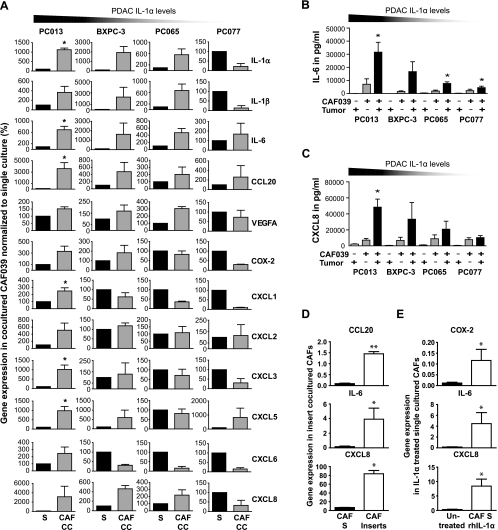
Seeing the diverse expression of IL-1α in PDAC cell lines, with the highest expression in PC013 and lowest in PC077 (Figures 2D and W2B), we examined the profile of inflammatory factors induced in the CAF039 cell line after cross talk with different PDAC cell lines (PC013, PC065, PC077, and BXPC-3) (Figure 3A). In addition, the interactions between the high (PC013) and medium (BXPC-3) IL-1α-expressing cell lines and two additional primary CAF cell lines (CAF073 and CAF055; Figure W3, A and B) were also assessed to validate that the inflammatory profile we detected was not unique for one cell line, that is, CAF039. The cross talk enhanced the expression of inflammatory factors in CAFs, including IL-1α, IL-6, CCL20, VEGF-A, CXCL5, and CXCL8. The highest increase in inflammatory factors was observed in CAF cell lines cocultured together with tumor cells expressing medium to high levels of IL-1α (Figures 3A and W3, A and B). For instance, all IL-1α-positive PDAC cell lines caused up-regulation of the chemoattractant CXCL8 and the cytokine IL-6 (Figure 3A) in CAFs, and the increase correlated to levels of IL-1α produced by the PDAC cells (Figure 2D). Moreover, the inflammatory profile in CAFs revealed increased expression of ELR-negative CXC chemokines after coculture with the high IL-1α-expressing cell line PC013, whereas intermediate IL-1α-expressing cell line BXPC-3 enhanced the levels of CXCL2, CXCL3, CXCL5, and CXCL8 but not to the same degree (Figures 3A and W3B). We confirmed CXCL8 and IL-6 at protein levels and found the highest levels produced in cocultures containing PC013 and the lowest in cocultures containing PC077 (Figure 3, B and C). To investigate if the up-regulation of inflammatory factors was contact dependent, we cocultured PC013 and CAF039 cells using cell culturing inserts and found up-regulation of several inflammatory factors in CAFs including CCL20 (P = .004), IL-6 (P = .036), and CXCL8 (P = .010) (Figure 3D). The involvement of IL-1α was also confirmed by adding recombinant human IL-1α to singly cultured CAFs, which resulted in significantly increased levels of inflammatory factors such as COX-2 (P = .022), IL-6 (P = .036), and CXCL8 (P = .036) (Figure 3E). Our data suggest that the IL-1α expression level in PDAC was the determining factor for the level of inflammatory factors produced by the CAFs underscoring the importance for the IL-1 signaling cascade in this process.
Figure 3.
PDAC-derived IL-1α is responsible for the inflammatory profiles induced in CAFs after coculture. (A) PDAC cell lines PC013, BXPC-3, PC065, and PC077 were cultured alone (S) or cocultured (CC) with the CAF cell line CAF039 (n = 10) for 5 days and assessed for gene expression levels of inflammatory factors IL-1α, IL-1β, IL-6, CCL20, VEGF-A, COX-2, CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, and CXCL8 by quantitative RT-PCR. Protein secretion of IL-6 (B), and CXCL8 (C) from the CAF cell line CAF039 cultured alone or cocultured for 5 days with the PDAC cell lines PC013, BXPC-3, PC065, and PC077 and (n = 13). (D) The PDAC cell line PC013 was cocultured with the CAF cell line CAF039 using cell culture transwell inserts for 5 days and analyzed by quantitative RT-PCR. The CAF gene expressions of CCL20, IL-6, and CXCL8 were analyzed and compared with singly cultured CAFs. (E) CAF039 cells were cultured alone for 5 days in medium supplemented with 10 pg/ml of recombinant human IL-1α and evaluated for the gene expression of COX-2, IL-6, and CXCL8 by quantitative RT-PCR. Data were analyzed using the Wilcoxon matched-pairs test followed by Mann-Whitney test, and paired t test was used for normalized data. *P < .05 and **P < .005.
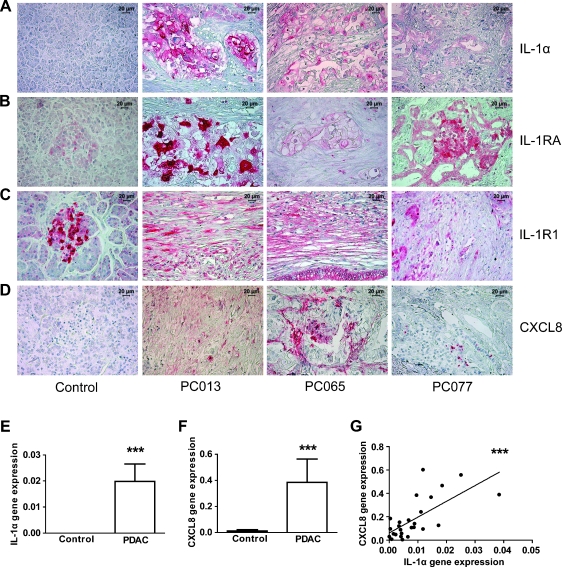
IL-1α Expression in Tumor Tissue Correlates with Location and Level of Inflammatory Factor CXCL8 In Vivo
Because the PDAC microenvironment is known to have a high degree of inflammation, we examined the profile and location of inflammatory factors in PDAC tissue samples ex vivo to establish if our in vitro findings correlated to the actual in vivo situation. An immunohistochemical analysis of the tumor tissue, obtained from patients PC013, PC065, and PC077, confirmed the expression profile found in the primary PDAC cell lines with IL-1α (Figures 4A and 2D), and IL-1RA (Figures 4B and 2E) localized in the tumor cells, and IL-1R1 (Figures 4C and 2C), and CXCL8 (Figure 4D) principally expressed by the fibrotic stroma. Neither tumor cells nor CAFs expressed IL-1β, but Langerhans islets in a few of the tumor tissues obtain from PDAC patients stained positive for IL-1β (n = 30) (Figure W4A). Moreover, all PDAC patients tested (n = 25) negative for IL-1β in plasma (not shown). The controls, that is, normal pancreatic tissues, were negative for IL-1α (Figure 4A), IL-1β (Figure W4B), and CXCL8 (Figure 4D), whereas the cells in the Langerhans islets were positive for both IL-1RA and IL-1R1 (Figure 4, B and C) in accordance with previous findings [22]. We examined the gene expression levels of IL-1α and CXCL8 in PDAC tissues and compared them to the levels found in control pancreas tissues. Gene expression levels of IL-1α (P < .001) and CXCL8 (P < .001) were significantly increased in PDAC tissues compared with those of controls (Figure 4, E and F). The gene expression of CXCL8 showed a positive correlation to the gene expression levels of IL-1α in PDAC tissue (Figure 4G: P < .001, r2 = 0.46). Taken together, the ex vivo expression of inflammatory factors in the PDAC microenvironment corresponded to the findings in our in vitro PDAC-CAF coculture model with its high expression of inflammatory factors, especially by the fibrotic stroma, namely, CAFs. Moreover, these data support IL-1α as an important initiator of inflammatory factors such as CXCL8.
Figure 4.
PDAC tissues express high levels of inflammatory factors. PDAC and normal pancreas tissue samples were fixed and paraffin embedded. Tissue sections from PDAC patients PC013, PC065, and PC077 and normal tissues were immunostained with anti-IL-1α, IL-1RA, IL-1RI, and CXCL8 mAbs followed by secondary mAbs. Images visualize the staining (red) for IL-1α (A), IL-1RA (B), IL-1RI (C) and CXCL8 (D) Size bar 20 µm. RNA was extracted from PDAC (N = 28) and normal pancreatic tissue samples (N = 10) and assessed for relative gene expression levels of the inflammatory factors, IL-1α (E) and, CXCL8 (F) by quantitative RT-PCR. IL-1α levels were correlated to the gene expression of CXCL8 (G). Data were analyzed using the Mann-Whitney test, and correlation was determined using Spearman nonparametric correlation. *P < .05.
Exogenous IL-1RA- and IL-1α-Neutralizing Antibodies Diminished the Inflammatory Environment in PDAC and CAF Cocultures
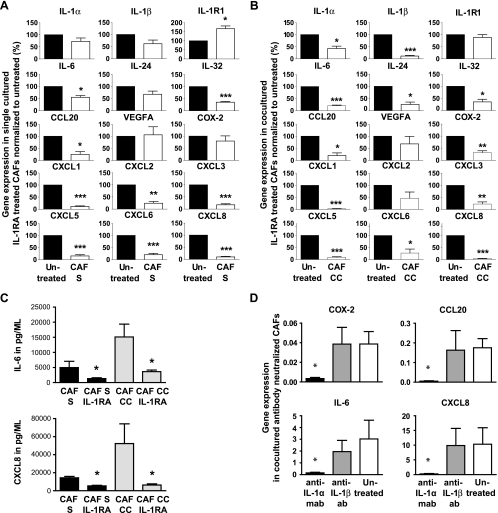
To prove that the enhanced inflammatory milieu induced by the PDAC and CAF cross talk was created by the IL-1α/IL-1R1 signaling pathway, as indicated by our findings, we blocked this pathway with recombinant human IL-1RA. The inflammatory gene profiles from the different PDAC cell lines (PC013, BXPC-3, PC065, and PC077) cocultured with CAF039 cells, established that IL-6 (single [S], P = .009; cocultured [CC], P < .001), IL-32 (S, P < .001; CC, P = .011), CXCL1 (S and CC, P < .001), CXCL3 (S, P < .001; CC, P = .025), CXCL5 (S and CC, P < .001), CXCL6 (S, P < .001; CC, P = .020), CXCL8 (S and CC, P < .001), and CCL20 (S and CC, P = .007) were significantly decreased in both single and cocultured CAF039 after IL-1α neutralization. These findings for single and cocultured CAFs point to both the autocrine and paracrine effects of IL-1α on CAFs. COX-2 (P = .003), IL-1α (P = .011), IL-1β (P < .001), and IL-24 (P = .005) were all decreased significantly in the cocultured CAFs (Figure 5, A and B) indicating a paracrine up-regulation of these factors. Of note, the IL-1R1 was significantly increased in CAFs after neutralization with IL-1RA (Figure 5A), indicating that the low degree of IL-1α production by the CAFs downregulated this receptor in an autocrine manner. In addition, we established that the secretion of IL-6 (S, P = .028; CC, P = .018) and CXCL8 (S, P = .029; CC, P = .013) was significantly decreased in the CAFs cultured alone or cocultured with PDAC cell lines after neutralization (Figure 5C). IL-1RA does not distinguish between IL-1α and IL-1β; hence, to further investigate the active role of IL-1α in the up-regulation of inflammatory factors in CAFs, we performed additional experiments using neutralizing antibodies against IL-1α and IL-1β. Only IL-1α-neutralizing antibodies decreased the levels of inflammatory factors compared with those of controls, COX-2 (P = .036), CCL20 (P = .024), IL-6 (P = .036), and CXCL8 (P = .024), confirming IL-1α and not IL-1β as the key initiator of inflammatory factors in CAFs (Figure 5D). These findings showed that tumor cell-derived IL-1α provokes and maintains the highly inflammatory environment observed in our in vitro cross talk assays and most likely is the initiator of the chronic inflammation observed in vivo.
Figure 5.
Neutralization of IL-1α signaling with IL-1RA diminishes the inflammation created by the PDAC and CAF cross talk. The CAF cell line CAF039 was cultured separately (S; left) (A) or together (CC; right) (B) with PDAC cell lines (PC013, BXPC-3, PC065, and PC077) for 5 days with or without IL-1RA and assessed for inflammatory factors (IL-1α, IL-1β, IL-6, CCL20, VEGF-A, COX-2, CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, and CXCL8) (n = 4) by quantitative RT-PCR. CXCL8 and IL-6 protein secretion (C) measured in supernatants from single CAF and coculture (CAF039 and PDAC cell lines PC013, PC065, BXPC-3, and PC077) with or without exogenous added IL-1RA. (D) The PDAC cell line PC013 was cocultured with the CAF cell line CAF039 for 5 days in medium supplemented with 25 µg/ml IL-1α- or IL-1β-neutralizing antibodies, and the gene expressions of COX-2, IL-6, CCL20 and CXCL8 were analyzed by quantitative RT-PCR and compared with those of controls. Data were analyzed using the Wilcoxon matched-pairs test followed by Mann-Whitney test, and paired t test was used for normalized data. *P < .05, **P < .005, and ***P < .001.
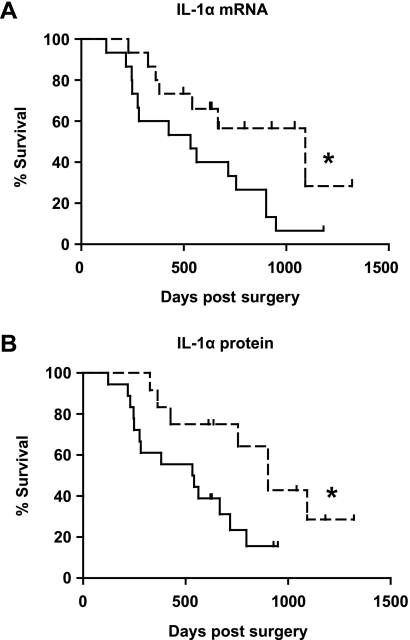
IL-1α Expression Levels in PDAC Tissue Correlated to Poor Clinical Outcome
When analyzing the relative IL-1α gene expression in PDAC tissues from 30 patients, we found that the patient group expressing the highest gene levels of IL-1α (>median) (n = 15) had a shorter survival than the patient group expressing the lowest levels (<median) (n = 15) (Figure 6A; P = .04). To confirm these findings, IL-1α protein expression by tumor cells in the PDAC tissue samples was assessed by immunohistochemistry. The negative/low- (n = 12) expressing PDAC tissue samples showed a better clinical outcome than the moderate/high- (n = 18) expressing patients (Figure 6B; P = .03).
Figure 6.
IL-1α levels in tumor tissues correlate to patient survival time. The patients were divided into two groups, comparing the survival between the PDAC patient group with the lowest (scattered line) (n = 15) and highest (n = 15) PDAC tissue gene expression levels of IL-1α (± median) analyzed by quantitative RT-PCR (A). IL-1α protein expression in PDAC tissue samples (n = 30 patients and each patient in three different experiments) were assessed by immunohistochemistry staining using anti-IL-1α Abs. The tissue samples were semiquantified using a microscope and the patients divided into two groups: one group expressing no or low levels of IL-1α (scattered line) (n = 12) and the other group expressing moderate to high levels of IL-1α (n = 18) (B). All PDAC tissue samples were examined in a blinded manner. Log-rank (Mantel-Cox) test was used for calculation of P values. *P < .05, **P < .005, and ***P < .001.
Discussion
Several studies suggest that inflammation functions as a promoter of tumorigenesis, and identification of the factors contributing to a tumor supporting microenvironment is of vital importance for prevention and treatment of cancer. Given that the fibrosis is a large component in the PDAC tumor mass [4], it is logical to assume that PDAC and CAF cross talk plays an important role in the production and maintenance of the inflammatory tumor microenvironment. Our study shows that the chronic inflammation observed in PDAC was mainly produced by the CAFs and maintained by the IL-1α provided by the tumor cells. It was clear that the PDAC cells controlled their environment through an IL-1α/IL-1R1 cross talk with stromal cells, that is, in a paracrine manner, thereby promoting the production of high levels of inflammatory factors from CAFs. Of note, we also found that the low degree of inflammation seen in single CAFs was IL-1α dependent in an autocrine manner. It should be noted that treatment with an IL-1α blocker inhibiting the IL-1 signaling cascade diminished the inflammation and the production of factors known to be important for supporting tumor growth and spread.
The role of CAFs as a major player in tumor progression is supported by the fact that many tumors fail to develop unless the stroma is modified [23], and these changes are induced in a paracrine manner by adjacent tumor cells [14,24]. Several factors have been shown to be involved in tumor and stroma interactions including CXCL8, transforming growth factor β, and metalloproteases [25–27], all observed in our PDAC-CAF cross talk system.
Tumor cells have been shown to express and secrete IL-1, either constitutively or in response to cytokines [28]. One explanation is the polymorphisms of the IL-1 gene or IL-1β gene promoter, which have been shown to be associated with breast and pancreatic adenocarcinoma respectively [29,30]. Indeed, we found IL-1α protein expressed by PDAC cells both in vitro and in tumor tissue but detected no expression of the IL-1β protein. These findings were further confirmed by treating tumor cell and CAF cocultures with neutralizing antibodies against IL-1α and IL-1β, showing effects only when blocking IL-1α signaling. This is in contrast to breast cancer cells, which expressed both IL-1α and IL-1β proteins [31]. These observations indicate that IL-1α, and not IL-1β, is the activator of the tumor stroma inflammation in PDAC, and to the best of our knowledge, this has not previously been shown for PDAC. Mouse tumor models showed that autocrine production of IL-1β by tumor cells increased their invasive pattern and potential to metastasize [32,33]. Moreover, induction of IL-1α expression in PDAC cell lines has been shown to favor their metastatic and invasive behavior in an orthotopic mouse model consisting of only PDAC tumor cells [34] and in an in vitro models [35,36]. A correlation between liver metastasis and expression levels of IL-1α in the tumor has been found for human gastric and colon cancers [13,37,38], which reveal how favorable this factor is for the tumor. Most studies focus on how tumor cells use IL-1 as an autocrine growth factor [34,39]. Our observations expand these results and point to an even more important paracrine effect, where the tumor cells control their environment through an IL-1α/IL-1R1 cross talk with stromal cells, thereby promoting the production of inflammatory factors from CAFs.
A prostate cancer study revealed an IL-1-dependent up-regulation of CXCL1, CXCL2, CXCL3, and CXCL8 in stroma cells [16]. These findings confirm our results with induction of high levels of CXC chemokines in CAFs by high/intermediate IL-1α-expressing PDAC cell lines and a correlation between IL-1α and CXCL8 mRNA levels in PDAC tumor tissues. Furthermore, neutralization of IL-1 signaling decreased the expression of CXC chemokines in both PDAC cells and CAFs, which is further evidence for an important role of IL-1α in chemokine expression. In breast cancer, the highest expression of CXC chemokines was found in the metastases, whereas breast cancers without metastatic abilities had low chemokine levels [40]. Our finding of enhanced expression of angiogenesis associated ELR- chemokines in CAFs cocultured with high levels of tumor-associated IL-1α, supports a mechanism where the tumor cells use IL-1α in the generation of a microenvironment favoring tumor cell migration and angiogenesis. A factor important in angiogenesis, as tumors outgrow their blood supply and become hypoxic, is VEGF [41], and the levels of VEGF-A increased after PDAC and CAF cross talk, indicating the supportive role of cellular interactions have on the tumor growth.
COX-2, CXCL8, CCL20, and IL-6 were increased in CAFs cocultured with PDAC cell lines expressing IL-1α. Both CXCL8 and CCL20 are potent angiogenic factors, and CCL20 has also the ability to promote the survival and proliferation of cancer cells by increasing their cell adhesion [42]. This shows clearly that the tumor, that is, PDAC, directs the fibroblasts to secrete tumor-promoting factors. IL-6 is another tumor-promoting factor that was increased in CAFs after coculture with PDAC cell lines. IL-6 increases the motility of tumor cells by diminishing their ability for adherence [43], and many of the genes targeted by IL-6 are involved in creating an invasive tumor cell phenotype [44].
COX-2 is normally expressed in association with inflammation, but it is also an inducible early gene involved in differentiation, apoptosis, metastasis, angiogenesis, and tumor development [45]. In several types of cancer, including PDAC and breast cancer, the expression of COX-2 enzyme has been associated with poor prognosis [46,47]. The levels of this enzyme were enhanced only in cocultures with PDAC cell lines expressing medium to high levels of IL-1α. Neutralization of the IL-1α pathway significantly decreased COX-2 expression in the cocultured CAFs. Selective COX-2 inhibitors are considered for targeted treatment of PDAC in patients [48]. Our findings point to the fact that COX-2 inhibition through blocking of the IL-1α signaling could have similar effects on the tumor.
Our findings show that treating the tumor and stroma with IL-1RA- or IL-1α-neutralizing antibodies diminished the inflammation and production of factors important for supporting tumor growth and spread. A previous mouse study showed that the use of IL-1RA treatment decreased metastasis and tumor proliferation in vivo, as well as decreasing the gene expression and production of VEGF and CXCL8 [49]. The neutralization of IL-1α using IL-1RA- or IL-1α-neutralizing antibodies revealed that several inflammatory factors produced by CAFs are highly dependent of the IL-1 pathway for their expression. Moreover, we confirmed and even expanded the IL-1 tumor-associated gene fingerprint in CAFs suggested by Erez et al. [50] to include IL-24, IL-32, and CCL20 and the ELR- CXC chemokines CXCL3, CXCL5, CXCL6, and CXCL8.
In summary, we found that the cross talk between PDAC and its stroma cells, namely, CAFs, contributes to the generation of the inflammatory environment through the IL-1α signaling cascade. The microenvironment created by IL-1α was beneficial for tumor survival because it contains factors associated with, for example, angiogenesis, and tumor cell migration and IL-1α levels correlated to the severity of the disease. Neutralization of IL-1α decreased the inflammation, and thus, targeting this pathway could be a new strategy of treating PDAC seeing that its expression has a negative effect on the patients' survival. Of note, new candidates for blocking IL-1, namely, cytokine traps specific for IL-1α, could facilitate this considerably.
Supplementary Material
Footnotes
This article refers to supplementary materials, which are designated by Figures W1 to W4 and are available online at www.neoplasia.com.
References
- 1.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 2.Korc M. Pancreatic cancer-associated stroma production. Am J Surg. 2007;194:S84–S86. doi: 10.1016/j.amjsurg.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 4.Froeling FE, Marshall JF, Kocher HM. Pancreatic cancer organotypic cultures. J Biotechnol. 2010;148:16–23. doi: 10.1016/j.jbiotec.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One. 2009;4:e7965. doi: 10.1371/journal.pone.0007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachem MG, Schunemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–921. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 8.Hartel M, Di Mola FF, Gardini A, Zimmermann A, Di Sebastiano P, Guweidhi A, Innocenti P, Giese T, Giese N, Buchler MW, et al. Desmoplastic reaction influences pancreatic cancer growth behavior. World J Surg. 2004;28:818–825. doi: 10.1007/s00268-004-7147-4. [DOI] [PubMed] [Google Scholar]
- 9.Esposito I, Menicagli M, Funel N, Bergmann F, Boggi U, Mosca F, Bevilacqua G, Campani D. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J Clin Pathol. 2004;57:630–636. doi: 10.1136/jcp.2003.014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsubayashi H, Infante JR, Winter J, Klein AP, Schulick R, Hruban R, Visvanathan K, Goggins M. Tumor COX-2 expression and prognosis of patients with resectable pancreatic cancer. Cancer Biol Ther. 2007;6:1569–1575. doi: 10.4161/cbt.6.10.4711. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo Y, Ochi N, Sawai H, Yasuda A, Takahashi H, Funahashi H, Takeyama H, Tong Z, Guha S. CXCL8/IL-8 andCXCL12/SDF-1a co-operatively promote invasiveness and angiogenesis in pancreatic cancer. Int J Cancer. 2009;124:853–861. doi: 10.1002/ijc.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apte RN, Voronov E. Is interleukin-1 a good or bad “guy” in tumor immunobiology and immunotherapy? Immunol Rev. 2008;222:222–241. doi: 10.1111/j.1600-065X.2008.00615.x. [DOI] [PubMed] [Google Scholar]
- 13.Tomimatsu S, Ichikura T, Mochizuki H. Significant correlation between expression of interleukin-1a and liver metastasis in gastric carcinoma. Cancer. 2001;91:1272–1276. doi: 10.1002/1097-0142(20010401)91:7<1272::aid-cncr1128>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Sato N, Maehara N, Goggins M. Gene expression profiling of tumorstromal interactions between pancreatic cancer cells and stromal fibroblasts. Cancer Res. 2004;64:6950–6956. doi: 10.1158/0008-5472.CAN-04-0677. [DOI] [PubMed] [Google Scholar]
- 15.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 16.Kogan-Sakin I, Cohen M, Paland N, Madar S, Solomon H, Molchadsky A, Brosh R, Buganim Y, Goldfinger N, Klocker H, et al. Prostate stromal cells produce CXCL-1, CXCL-2, CXCL-3 and IL-8 in response to epithelia-secreted IL-1. Carcinogenesis. 2009;30:698–705. doi: 10.1093/carcin/bgp043. [DOI] [PubMed] [Google Scholar]
- 17.Pilarsky C, Ammerpohl O, Sipos B, Dahl E, Hartmann A, Wellmann A, Braunschweig T, Lohr M, Jesnowski R, Friess H, et al. Activation of Wnt signalling in stroma from pancreatic cancer identified by gene expression profiling. J Cell Mol Med. 2008;12:2823–2835. doi: 10.1111/j.1582-4934.2008.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Tjomsland V, Sandstrom P, Spangeus A, Messmer D, Emilsson J, Falkmer U, Falkmer S, Magnusson KE, Borch K, Larsson M. Pancreatic adenocarcinoma exerts systemic effects on the peripheral blood myeloid and plasmacytoid dendritic cells: an indicator of disease severity? BMC Cancer. 2010;10:87. doi: 10.1186/1471-2407-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonda TA, Varro A, Wang TC, Tycko B. Molecular biology of cancer-associated fibroblasts: can these cells be targeted in anti-cancer therapy? Semin Cell Dev Biol. 2009;21:2–10. doi: 10.1016/j.semcdb.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eyden B. The myofibroblast: phenotypic characterization as a prerequisite to understanding its functions in translational medicine. J Cell Mol Med. 2008;12:22–37. doi: 10.1111/j.1582-4934.2007.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, Dayer JM, Reinecke M, Halban PA, Donath MY. Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1β in human islets. Proc Natl Acad Sci USA. 2004;101:8138–8143. doi: 10.1073/pnas.0305683101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franco OE, Shaw AK, Strand DW, Hayward SW. Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol. 2010;21:33–39. doi: 10.1016/j.semcdb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somasundaram R, Herlyn D. Chemokines and the microenvironment in neuroectodermal tumor-host interaction. Semin Cancer Biol. 2009;19:92–96. doi: 10.1016/j.semcancer.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato N, Fukushima N, Maehara N, Matsubayashi H, Koopmann J, Su GH, Hruban RH, Goggins M. SPARC/osteonectin is a frequent target for aberrant methylation in pancreatic adenocarcinoma and a mediator of tumorstromal interactions. Oncogene. 2003;22:5021–5030. doi: 10.1038/sj.onc.1206807. [DOI] [PubMed] [Google Scholar]
- 26.Saad S, Gottlieb DJ, Bradstock KF, Overall CM, Bendall LJ. Cancer cell-associated fibronectin induces release of matrix metalloproteinase-2 from normal fibroblasts. Cancer Res. 2002;62:283–289. [PubMed] [Google Scholar]
- 27.Dong Z, Nemeth JA, Cher ML, Palmer KC, Bright RC, Fridman R. Differential regulation of matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1 (TIMP-1) and TIMP-2 expression in co-cultures of prostate cancer and stromal cells. Int J Cancer. 2001;93:507–515. doi: 10.1002/ijc.1358. [DOI] [PubMed] [Google Scholar]
- 28.Rhim JH, Kim SA, Lee JE, Kim DJ, Chung HK, Shin KJ, Chung J. Cancer cell-derived IL-1a induces IL-8 release in endothelial cells. J Cancer Res Clin Oncol. 2008;134:45–50. doi: 10.1007/s00432-007-0243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamacher R, Diersch S, Scheibel M, Eckel F, Mayr M, Rad R, Bajbouj M, Schmid RM, Saur D, Schneider G. Interleukin 1β gene promoter SNPs are associated with risk of pancreatic cancer. Cytokine. 2009;46:182–186. doi: 10.1016/j.cyto.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Snoussi K, Strosberg AD, Bouaouina N, Ben Ahmed S, Chouchane L. Genetic variation in pro-inflammatory cytokines (interleukin-1β, interleukin-1α and interleukin-6) associated with the aggressive forms, survival, and relapse prediction of breast carcinoma. Eur Cytokine Netw. 2005;16:253–260. [PubMed] [Google Scholar]
- 31.Pantschenko AG, Pushkar I, Anderson KH, Wang Y, Miller LJ, Kurtzman SH, Barrows G, Kreutzer DL. The interleukin-1 family of cytokines and receptors in human breast cancer: implications for tumor progression. Int J Oncol. 2003;23:269–284. [PubMed] [Google Scholar]
- 32.Chirivi RG, Garofalo A, Padura IM, Mantovani A, Giavazzi R. Interleukin 1 receptor antagonist inhibits the augmentation of metastasis induced by interleukin 1 or lipopolysaccharide in a human melanoma/nude mouse system. Cancer Res. 1993;53:5051–5054. [PubMed] [Google Scholar]
- 33.Vidal-Vanaclocha F, Amezaga C, Asumendi A, Kaplanski G, Dinarello CA. Interleukin-1 receptor blockade reduces the number and size of murine B16 melanoma hepatic metastases. Cancer Res. 1994;54:2667–2672. [PubMed] [Google Scholar]
- 34.Melisi D, Niu J, Chang Z, Xia Q, Peng B, Ishiyama S, Evans DB, Chiao PJ. Secreted interleukin-1α induces a metastatic phenotype in pancreatic cancer by sustaining a constitutive activation of nuclear factor-κB. Mol Cancer Res. 2009;7:624–633. doi: 10.1158/1541-7786.MCR-08-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuo Y, Sawai H, Ochi N, Yasuda A, Takahashi H, Funahashi H, Takeyama H, Guha S. Interleukin-1a secreted by pancreatic cancer cells promotes angiogenesis and its therapeutic implications. J Surg Res. 2009;153:274–281. doi: 10.1016/j.jss.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 36.Sawai H, Okada Y, Funahashi H, Matsuo Y, Takahashi H, Takeyama H, Manabe T. Interleukin-1α enhances the aggressive behavior of pancreatic cancer cells by regulating the α6β1-integrin and urokinase plasminogen activator receptor expression. BMC Cell Biol. 2006;7:8. doi: 10.1186/1471-2121-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuo Y, Sawai H, Ma J, Xu D, Ochi N, Yasuda A, Takahashi H, Funahashi H, Takeyama H. IL-1α secreted by colon cancer cells enhances angiogenesis: the relationship between IL-1α release and tumor cells' potential for liver metastasis. J Surg Oncol. 2009;99:361–367. doi: 10.1002/jso.21245. [DOI] [PubMed] [Google Scholar]
- 38.Ma J, Sawai H, Matsuo Y, Ochi N, Yasuda A, Takahashi H, Wakasugi T, Funahashi H, Sato M, Okada Y, et al. Interleukin-1α enhances angiogenesis and is associated with liver metastatic potential in human gastric cancer cell lines. J Surg Res. 2008;148:197–204. doi: 10.1016/j.jss.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 39.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bieche I, Chavey C, Andrieu C, Busson M, Vacher S, Le Corre L, Guinebretiere JM, Burlinchon S, Lidereau R, Lazennec G. CXC chemokines located in the 4q21 region are up-regulated in breast cancer. Endocr Relat Cancer. 2007;14:1039–1052. doi: 10.1677/erc.1.01301. [DOI] [PubMed] [Google Scholar]
- 41.Brown LF, Guidi AJ, Schnitt SJ, Van De Water L, Iruela-Arispe ML, Yeo TK, Tognazzi K, Dvorak HF. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res. 1999;5:1041–1056. [PubMed] [Google Scholar]
- 42.Beider K, Abraham M, Begin M, Wald H, Weiss ID, Wald O, Pikarsky E, Abramovitch R, Zeira E, Galun E, et al. Interaction between CXCR4 and CCL20 pathways regulates tumor growth. PLoS One. 2009;4:e5125. doi: 10.1371/journal.pone.0005125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamm I, Cardinale I, Murphy JS. Decreased adherence of interleukin 6-treated breast carcinoma cells can lead to separation from neighbors after mitosis. Proc Natl Acad Sci USA. 1991;88:4414–4418. doi: 10.1073/pnas.88.10.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin MT, Lin BR, Chang CC, Chu CY, Su HJ, Chen ST, Jeng YM, Kuo ML. IL-6 induces AGS gastric cancer cell invasion via activation of the c-Src/RhoA/ROCK signaling pathway. Int J Cancer. 2007;120:2600–2608. doi: 10.1002/ijc.22599. [DOI] [PubMed] [Google Scholar]
- 45.Singh B, Berry JA, Shoher A, Ayers GD, Wei C, Lucci A. COX-2 involvement in breast cancer metastasis to bone. Oncogene. 2007;26:3789–3796. doi: 10.1038/sj.onc.1210154. [DOI] [PubMed] [Google Scholar]
- 46.Nozaki S, Sledge GW, Jr, Nakshatri H. Cancer cell-derived interleukin 1α contributes to autocrine and paracrine induction of pro-metastatic genes in breast cancer. Biochem Biophys Res Commun. 2000;275:60–62. doi: 10.1006/bbrc.2000.3241. [DOI] [PubMed] [Google Scholar]
- 47.Juuti A, Louhimo J, Nordling S, Ristimaki A, Haglund C. Cyclooxygenase-2 expression correlates with poor prognosis in pancreatic cancer. J Clin Pathol. 2006;59:382–386. doi: 10.1136/jcp.2005.026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu XF, Xie CG, Wang XP, Liu J, Yu YC, Hu HL, Guo CY. Selective inhibition of cyclooxygenase-2 suppresses the growth of pancreatic cancer cells in vitro and in vivo. Tohoku J Exp Med. 2008;215:149–157. doi: 10.1620/tjem.215.149. [DOI] [PubMed] [Google Scholar]
- 49.Elaraj DM, Weinreich DM, Varghese S, Puhlmann M, Hewitt SM, Carroll NM, Feldman ED, Turner EM, Alexander HR. The role of interleukin 1 in growth and metastasis of human cancer xenografts. Clin Cancer Res. 2006;12:1088–1096. doi: 10.1158/1078-0432.CCR-05-1603. [DOI] [PubMed] [Google Scholar]
- 50.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.