Abstract
In prostate cancers, epidemiological data suggest a link between prostate inflammation and subsequent cancer development, but proof for this concept in a tumor model is lacking. A constitutively active version of IκB kinase 2 (IKK2), which is activated by many inflammatory stimuli, was expressed specifically in the prostate epithelium. Constitutive activation of the IKK2/nuclear factor κB axis was insufficient for prostate transformation. However, in combination with heterozygous loss of phosphatase and tensin homolog, IKK2 activation led to an increase in tumor size, formation of cribriform structures, and increase in fiber in the fibroblastic stroma. This phenotype was coupled with persistent inflammation evoked by chemokine expression in the epithelium and stroma. The hyperplastic and dysplastic epithelia correlated with changes evoked by decreased androgen receptor activation. Conversely, inflammation correlated with stromal changes highlighted by loss of smooth muscle cells around prostate ducts. Despite the loss of the smooth muscle barrier, tumors were rarely invasive in a C57BL/6 background. Data mining revealed that smooth muscle markers are also downregulated in human prostate cancers, and loss of these markers in primary tumors is associated with subsequent metastasis. In conclusion, our data show that loss of smooth muscle and invasiveness of the tumor are not coupled in our model, with inflammation leading to increased tumor size and a dedifferentiated stroma.
Introduction
Inflammation as a causal agent has been linked to approximately 20% of human cancers [1]. The type and nature of inflammatory infiltrates are different for different cancer types, and thus, a number of different mechanisms for the tumor-promoting effect of inflammation have been proposed [2]. In prostate cancer, a growing amount of evidence suggests a link between chronic or persistent inflammation and tumor development [3]. First, epidemiological data indicate that people diagnosed with chronic prostatitis have an increased risk of developing prostate cancer at a later age. Moreover, people receiving long-term treatment with nonsteroidal anti-inflammatory drugs have a reduced risk of developing prostate cancer [4–6]. Second, histologic data imply that certain lesions (sometimes referred to as prostate inflammatory atrophy) are frequently found near prostate intraepithelial neoplasias (PINs), precursor lesions of prostate cancer [7]. Third, treatment of rodent prostates with bacteria induces prostatitis and hyperplastic and dysplastic epithelial alterations [8,9]. Although all these data suggest a link between prostatitis and tumor formation, the cause of prostate inflammation in humans is largely unknown. However, many possible causes for an inflammatory phenotype, such as bacterial or viral infections or production of inflammatory cytokines and chemokines, share common signaling pathways that are activated in the affected tissue. All of the aforementioned stimuli activate the IκB kinase 2 (IKK2), a key mediator between extracellular signaling and the transcription factor NF-κB [10]. Although IKK2 has some other targets, its main function is phosphorylation of IκB (inhibitors of κB) molecules, rendering them subject for degradation and thus activating NF-κB. Moreover, IKK2 has been described as an oncogenic kinase; activated IKK2 correlates with poor clinical outcome in breast cancer patients, and the enzyme targeting IKK2 for ubiquitination and degradation is lost or mutated in different human cancers [11,12]. For the prostate, it is interesting to note that the aforementioned relationship between longterm drug treatment and reduced prostate cancer risk is more significant for aspirin, which is also an IKK2 inhibitor.
In this work, we describe a novel genetic mouse model for inflammatory signaling in the prostate epithelium based on the expression of a constitutively active version of the IκB kinase 2 (IKK2ca). Using the Cre/LoxP system and the well-described Probasin-Cre mice [13], we expressed IKK2ca in epithelial cells of the mouse prostate with or without a monoallelic deletion of the tumor suppressor phosphatase and tensin homolog (PTEN; monoallelic loxP-f lanked PTEN) in the same cells. PTEN is a tumor suppressor frequently mutated or lost in prostate cancer. In mouse models, PTEN deficiency in the epithelium shows a dose-dependent transformation of prostate tissue, with low-grade PIN late in mouse life in monoallelic deletions and lesions with ability to form invasive and eventually metastasizing carcinomas in full deletions [14–16]. We wanted to answer the questions whether signaling through IKK2/NF-κB is sufficient to transform prostate tissue and whether IKK2 activation in mild neoplastic lesions can advance tumor formation. We will refer to prostates carrying normal or modified epithelia as wild-type, prostate epithelium (PE-)IKK2ca, PE- PTEN+/-, or PE-PTEN+/-IKK2ca.
Materials and Methods
Mice
Probasin-Cre (PB-Cre4) mice were obtained from the National Cancer Institute — Frederick Mouse Repository. Mice with a floxed PTEN allele [17] were a gift from Prof Tak Mak. R26StopFlIKK2ca mice were generated by one of the authors (M.S.-S.) and were described previously [18]. Mice were bred on a C57BL/6 background. Their housing and euthanasia were in accordance with institutional guidelines.
RNA Extraction and Quantitative Polymerase Chain Reaction Analysis
Harvested tissue was stored in RNA later, then homogenized using a Polytron homogenizer, and purified on RNA binding columns according to the manufacturer's instructions (RNeasy [Qiagen, Hilden, Germany] or Total RNA Kit [Peqlab, Erlangen, Germany]). Complementary DNA (cDNA) synthesis was performed using the RevertAid H Minus First Strand cDNA Synthesis Kit (Fermentas, St. Leon-Rot, Germany) and random hexamer primers. Quantitative polymerase chain reaction (PCR) was done on a StepOne Plus Real-time PCR Cycler (Applied Biosystems, Vienna, Austria). HPRT was used as normalization gene. Primer sequences are listed in Table W1.
Microarray Experiment
Total RNA was extracted from lateral prostates of three animals (12 months) with PTEN+/-IKK2ca/ca prostates and three age-matched animals with PTEN+/- prostates using RNeasy (Qiagen). RNA quality control, labeling, hybridization, and initial data analysis were done by an Affymetrix service provider (KFB Regensburg, Regensburg, Germany). Complete array data are stored in GEO (accession no. GSE26410). For presentation in tables, log2 signals from the Robust Multichip Analysis were transformed into linear signals, and the mean expression fold was calculated by dividing the average signal from the PE-PTEN+/- IKK2ca/ca by the average signal from the PE-PTEN+/- group.
Explant Culture
Dissected prostate tissue was cut into pieces of 0.2 to 1 mm, placed on collagenated tissue culture plates, and supplied with minimal amount of epithelial growth medium containing 1% serum as described by Barclay and Cramer [19]. After 24 hours, more media were added. After further 24 to 48 hours, cells began to grow out from the prostate tissue. Clean epithelial or stromal cultures were obtained by removing the unwanted cell type with a cell scraper or pipette tip. Cells were passaged using Accutase (PAA, Pasching, Austria). For generation of cell lines, cells were passaged until they survived crisis. Cell lines were treated in some experiments with tumor necrosis factor (TNF; 20 ng/ml; Immunotools, Friesoythe, Germany) or BMS-345541 (10 µM; Sigma, Schnelldorf, Germany).
Tissue Sectioning and Immunostaining
For paraffin sections, harvested tissue was fixed overnight in 4% paraformaldehyde. Tissues were then dehydrated, embedded in paraffin in small tissue blocks, cut (2-µm sections) using a Leica microtome (Leica Microsystems, Vienna, Austria), and collected on SuperFrost Plus slides (Thermo Scientific, Braunschweig, Germany). Antibody staining procedure has been described [20]. Apoptosis was assessed using the TUNEL method and the In Situ Cell Death Detection Kit (Roche, Vienna, Austria).
For frozen sections, harvested tissue was directly frozen in isopentane/liquid nitrogen and stored at -80°C until cutting. Staining was done as described [20]. For biotinylated antibodies, sections were blocked with Avidin/Biotin Blocking Kit (Vector Laboratories, Burlingame, CA). Antibodies used are described in Table W2.
Histologic Assessment
Hematoxylin and eosin (H&E) staining from paraffin sections was judged in a blind fashion by an expert pathologist (N.K.).
Microscopy and Image Analysis
Images were acquired on an Olympus AX470 microscope (Olympus, Vienna, Austria) equipped with F-View II (grayscale) and ColorViewII (color) digital cameras using the manufacturer's software (CellP; Olympus) and appropriate filter sets for fluorescence.
For the quantification of stromal elements, individual ducts (>5) were randomly chosen on H&E staining of lateral prostates, and the number of fibers between the gland and the neighboring gland was counted. The average number of fibers was calculated for each prostate tissue. The numbers given in the figures represent the arithmetic average and SD over the individual values.
For the quantification of smooth muscle thickness versus CD11b count, parameters were quantified using ImageJ (National Institutes of Health, Bethesda, MD). The diameter of smooth muscle was quantified using the anti-actin staining and the line selection of ImageJ. CD11b-positive cells were counted within the duct and in the stroma closer to the investigated duct than the neighboring one and were then normalized to the area of the respective duct.
For quantification of Ki67- and TUNEL-positive cells, polymorphonuclear inflammatory cells were excluded from the analysis.
For quantification of androgen receptor (AR) nuclear/cytoplasmic ratio, AR fluorescent staining was analyzed using elliptical regions inside and just outside the nucleus and determining the mean gray value of the regions in ImageJ. Nuclear regions were identified by overlaying Hoechst staining.
Statistical Analysis
One-way analysis of variance was used to determine statistical significance, where applicable. P < .05 was considered statistically significant. Error bars in figures represent SEM.
Results
IKK2 Activity Is Insufficient to Transform Prostate Epithelium
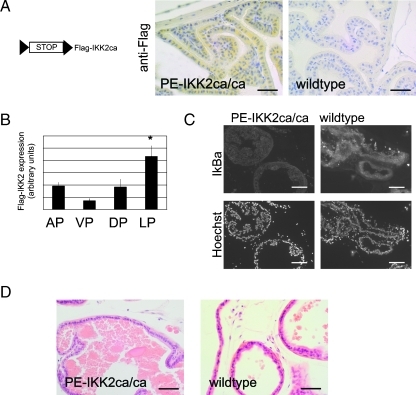
Given the central role of IKK2 as a signal mediator from inflammatory agents to transcription factors and its description as an oncogenic kinase, we wanted to determine whether chronic IKK2 activity leads to transformation of the prostate epithelium. On excision of the Stop cassette in R26StopFlIKK2ca mice, transgene expression was confirmed by means of the Flag-tag of the IKK2 transgene (Figure 1A). As expected, the expression was restricted to the epithelium. Dissection of the different prostate lobes and expression analysis using quantitative reverse transcription-polymerase chain reaction (RT-PCR) showed that transgene expression was highest in the lateral prostate, followed by the anterior and dorsal prostates, and was lowest in the ventral prostate (Figure 1B). Down-regulation of IκBα, which is degraded after phosphorylation by IKKs, indicated IKK2 activity (Figure 1C). However, no histologic phenotype of the prostate tissue was noticed after 4 (n = 3), 8 (n = 4), or 12 (n = 10) months of age either in animals expressing IKK2ca from a single allele or in animals expressing the transgene from both alleles (Figure 1D). We conclude that constitutively active IKK2 is insufficient to transform prostate tissue.
Figure 1.
IKK2 signaling is insufficient to transform prostate epithelium. (A) schematic drawing of the R26StopFlIKK2ca mouse line (loxP sites: triangles) and anti-Flag staining. (B) Quantitative RT-PCR for the Flag-IKK2 transgene in different prostate lobes of IKK2ca/ca expressing mice (n = 4). (C) Immunostaining of frozen sections of wild-type or PE-IKK2ca/ca prostates with IκBα antibody. (D) H&E stain from lateral prostate of IKK2ca/ca expressing mouse (12 months). Scale bars, 50 µm (A, D); 100 µm (C). Error bars, SEM. *P < .05. AP indicates anterior prostate; DP, dorsal prostate; LP, lateral prostate; VP, ventral prostate.
IKK2 Activity on PTEN+/- Background Increases Tumor Size and Epithelial and Stromal Proliferation
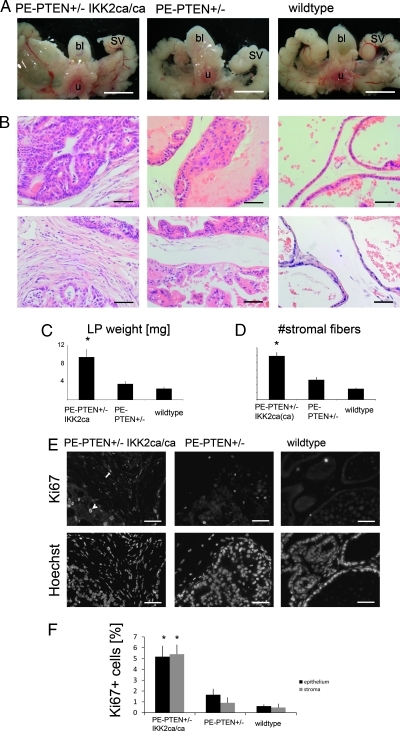
Transgenic prostates with monoallelic deletion of the PTEN tumor suppressor (PTEN+/-) in the epithelium develop low-grade PIN late in mouse life (around 10–12 months). We wanted to know whether IKK2 signaling could enhance this tumor development and alter either tumor size or invasiveness. Expression of constitutively active IKK2 in PTEN+/- prostate epithelium led to a clearly visible increase in prostate size (Figure 2A, after 12 months). The difference was significant after 8 months and not seen at 4 months (not shown). PTEN+/- epithelium expressing IKK2ca on both alleles (IKK2ca/ca) had all prostate lobes enlarged at 8 months and older, whereas PE-PTEN+/-IKK2ca (expressing IKK2ca from one allele) only showed enlarged lateral prostates, in line with transgene expression being highest in the lateral lobe (Figure 2C, cf., Figure 1B). Histologic staining of these prostates showed normal, smooth epithelium in prostates from wild-type littermates, whereas most PE-PTEN+/- prostates had some hyperplasia and focal nuclear abnormalities judged as murine PIN. PTEN+/- IKK2ca (or ca/ca) epithelium was severely hyperplastic, often leading to cribriform structures, but had an equal incidence of nuclear atypia as PTEN+/- epithelium (Figure 2B, upper panels). Moreover, PE-PTEN+/-IKK2ca(ca) prostates showed a dramatic stromal response, indicated by dense and numerous collagen fibers in comparison to the loose stroma with few fibers in normal or PE-PTEN+/- prostates (Figure 2B, lower panels, quantified in Figure 2D). This increase in tumor size was due to an increase in proliferation of both epithelial and stromal cells, as indicated by more Ki67-positive cells in epithelium and stroma, respectively (Figure 2, E and F). Apoptosis assessed by TUNEL stain was low and not significantly changed in one of the genotypes (Figure W1).
Figure 2.
IKK2 activity on PTEN+/- background increases tumor size and epithelial and stromal proliferation. (A) Steromicroscopical images of the structures surrounding the urethra (u), bladder (bl), and seminal vesicles (SV) from 12-month-old mice. The prostate lobes are seen between the noted structures. (B) H&E stain of lateral prostates at 12 months, focusing on epithelium (upper panels) and stroma (lower panels). The genotypes are according to labeling in A. (C) Quantification of dry prostate weight of lateral prostates at 12 months. (D) Stromal fiber number in between epithelial ducts (see Materials and Methods) of 12-month-old lateral prostates. (E) Paraffin sections of lateral prostates (12 months) stained for proliferation marker Ki67. Positive cells in the epithelium (arrowhead) and stroma (arrow) are seen. Hoechst staining of the same sections (lower panels) are given to indicate tissue structure. (F) Quantification of Ki67-positive cells in the epithelium and stroma for the indicated genotypes. Scale bars, 5 mm (A); 50 µm (B, E). Error bars, SEM. *P < .01.
Persistent Inflammation in PE-PTEN+/- IKK2ca Prostates
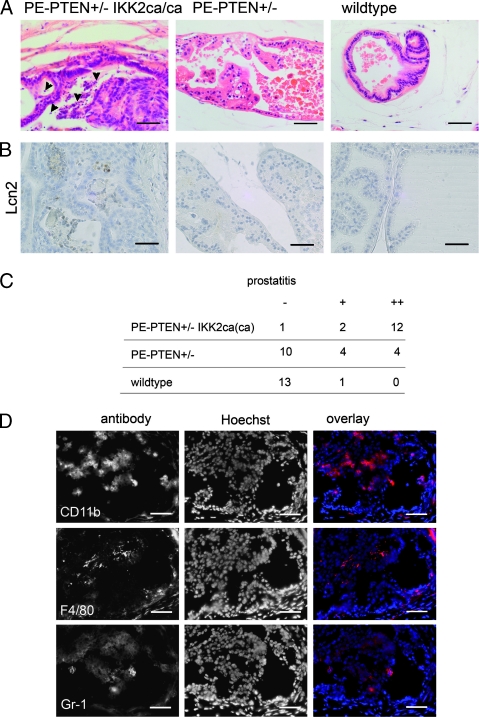
The proliferative epithelium and stromal response were coupled with signs of severe prostatitis in PE-PTEN+/- IKK2ca(ca) prostates. Prostate tissue showed infiltration of granulocytes characterized by their polymorphic nuclei and positive staining with the neutrophil marker Lipocalin2 (Figure 3, A and B). The infiltrates were seen in the stroma but also in the epithelium where they could be easily spotted in the lumina of cribriform structures (Figure 3A). Wild-type tissue and younger PE-PTEN+/- (4 months, n = 3; 8 months, n = 5) were devoid of infiltrates, although with advanced age (>12 months), some PE-PTEN+/- prostates also developed signs of prostatitis (analysis in Figure 3C). Immunofluorescence analysis revealed positive staining of infiltrates with the macrophage/monocyte antigen CD11b, the macrophage marker F4/80, and the neutrophil marker Gr-1 (Figure 3D). Leukocytes positive for CD3e were also seen but were generally rare (not shown). Control tissues from PE-PTEN+/-IKK2ca/ca mice did not show any signs of inflammation, and noninflamed prostates did not show positive immunoreactivity with immune cell marker antibodies (Figure W2). We conclude that the prostate-specific expression of constitutively active IKK2 on a PE-PTEN+/- background leads to severe persistent inflammation characterized by infiltration of neutrophil granulocytes and macrophages.
Figure 3.
Inflammation in PE-PTEN+/-IKK2ca transgenic prostates. (A) H&E staining of lateral prostates from 12-month-old mice. Arrow-heads indicate polymorphonuclear granulocytes. (B) Staining for the neutrophil marker Lipocalin2 (12 months). (C) Analysis of prostatitis seen in transgenic or wild-type prostates at 12 months. - indicates no prostatitis; +, focal prostatitis; ++, prostatitis in the major part of the prostate gland. (D) Antibody staining of consecutive cryosections from dorsal prostates of PE-PTEN+/-IKK2ca/ca mice using marker genes CD11b, F4/80, and Gr-1. Scale bars, 50 µm.
Loss of Smooth Muscle but No Invasion in PE-PTEN+/- IKK2ca(ca) Prostates
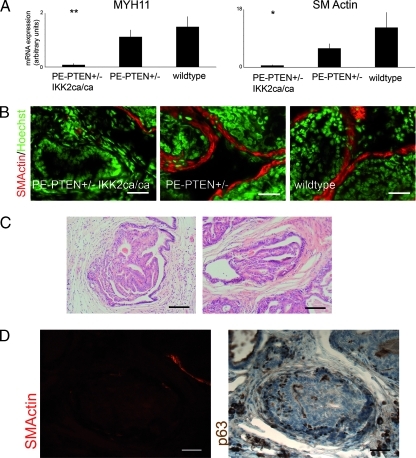
Because histologic staining of prostate tissue showed a dramatic change in the stroma, we investigated the expression of various candidate genes known or thought to be expressed in the prostate stroma. We found a dramatic down-regulation of smooth muscle cell markers, smooth muscle myosin heavy chain (MYH11), and smooth muscle actin (SMA) in PE-PTEN+/- IKK2ca prostates compared with wildtype or PE-PTEN+/- (Figure 4A). This was confirmed by staining lateral prostates with SMA antibody, demonstrating a complete loss of smooth muscle cells around many prostatic ducts (Figure 4B). In many tumor models, loss of smooth muscle around the stratified epithelium is indicative of an invasive phenotype. Thus, we reinvestigated our samples for invasive carcinoma formation. Histologically, the larger tumors in PE-PTEN+/- IKK2ca(ca) prostates were still well confined, with no signs of “dripping-off” of smaller epithelial structures or even invasion into the neighboring tissue (Figure 4C). Another indication of invasiveness is loss of basal cells in the epithelium. Costaining of smooth muscle marker and the basal cell marker p63 confirmed that, even in glands with complete loss of smooth muscle, basal cell expression is maintained (Figure 4D).
Figure 4.
Loss of smooth muscle cells, but no invasion in PE-PTEN+/- IKK2ca(ca) prostates. (A) Quantitative RT-PCR for smooth muscle cell markers from lateral prostates (12 months), n = 6 per group. (B) Colored immunofluorescence staining for smooth muscle actin (SM Actin) and Hoechst counterstain in dorsal prostates (12 months). (C) H&E staining of paraffin sections from PE-PTEN+/- IKK2ca/ca prostates. (D) Costaining for SMA and basal cell marker p63 in paraffin-embedded sections from PE-PTEN+/- IKK2ca/ca prostates (12 months). Scale bars, 50 µm (B, D); 100 µm (C). Error bars, SEM. *P < .05, **P < .01.
Rarely observed carcinomas formed unilaterally in PE-PTEN+/- IKK2ca(ca) prostates, suggestive of a further mutation or transforming event. We examined such a carcinoma and did not find overexpression of factors known to be associated with invasiveness such as Spink genes, Ets transcription factors, or myc, indicating that a different set of genes was responsible for invasiveness (Figure W3).
Taken together, these data show that PE- PTEN+/- IKK2ca prostates exhibit a profound change in the stroma highlighted by a loss of smooth muscle around the stratified epithelium but without regular formation of invasive structures.
Expression Profiling Details the Inflammatory Tumor Phenotype
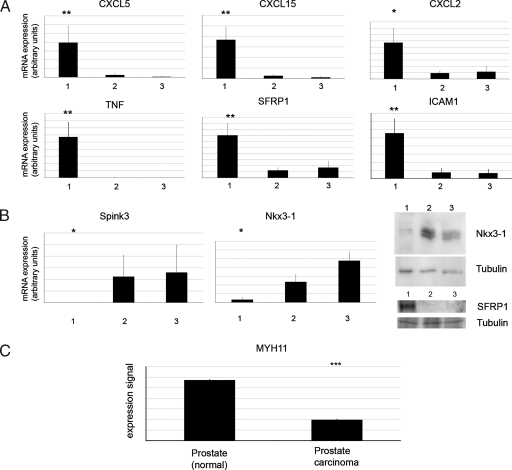
To gain insight into the molecular mechanism of inflammation and tumor development in the transgenic prostates, we performed microarray analysis on PE-PTEN+/-IKK2ca/ca or PE-PTEN+/- lateral prostates. Overexpressed (PE-PTEN+/- IKK2ca/ca vs PE-PTEN+/-) or downregulated genes were determined and grouped according to function (Table 1; the 100 most overexpressed and downregulated genes are listed in Tables W3 and W4). We then confirmed misexpression of many of these genes by quantitative RT-PCR or immunoblot analysis (Figure 5). Consistent with the absence of a histologic phenotype, PE-IKK2ca prostates did not overexpress these candidate genes (Figure W4). One group of genes overexpressed in PE-PTEN+/-IKK2ca/ca prostates constituted chemokines such as CXCL5, CXCL15, CCL3, CXCL10, and CXCL2. These chemokines are known to attract immune cells, particularly neutrophil granulocytes and monocytes/macrophages, the cells found in the inflamed tissue (Figure 3). Consistently, another group of genes comprised genes found predominantly or exclusively in granulocytes or macrophages. Inflammatory cytokines, particularly TNF but also IL-1b, were also overexpressed in the tissues exhibiting prostatitis. These cytokines are known target genes of the transcription factor NF-κB. Other classic NF-κB targets identified included IκB proteins and the adhesion molecule ICAM1. Of note, we also identified the secreted frizzle-related proteins 1 and 4 (SFRP1 and 4) as overexpressed in PE-PTEN+/-IKK2ca/ca. SFRP1 has been described as a candidate gene expressed by the stroma to modulate the epithelium in prostate cancer progression [21]. This shows that the activated stroma in our intraepithelial tumors bears resemblance to human prostate cancer stroma.
Table 1.
Selected Genes from the 100 Most Overexpressed or Underexpressed Genes from Microarray Analysis.
| Gene (Abbreviation) | Full Name | Fold Expression |
| Upregulated genes | ||
| Immune cell associated | ||
| Cd14 | Cd14 antigen | 6.97 |
| Csf3r | Colony-stimulating factor 3 receptor (granulocyte) | 4.83 |
| Mpeg1 | Macrophage expressed gene 1 | 3.64 |
| Chemokines and receptors | ||
| Cxcl5 | Chemokine (C-X-C motif) ligand 5 | 11.68 |
| Cxcl15 | Chemokine (C-X-C motif) ligand 15 | 7.22 |
| Cxcl10 | Chemokine (C-X-C motif) ligand 10 | 5.35 |
| Cxcl2 | Chemokine (C-X-C motif) ligand 2 | 4.10 |
| Ccl3 | Chemokine (C-C motif) ligand 3 | 5.11 |
| Cxcr4 | Chemokine (C-X-C motif) receptor 4 | 3.60 |
| Cytokines and receptors | ||
| Tnf | Tumor necrosis factor | 4.28 |
| Il1f9 | Interleukin 1 family, member 9 | 3.85 |
| Il1b | Interleukin 1β | 3.62 |
| Il8rb | Interleukin 8 receptor, β | 5.15 |
| NF-κB target genes | ||
| C3 | Complement component 3 | |
| Nfkbia | IκBα | 3.52 |
| Nfkbie | IκBε | 4.38 |
| Icam1 | Intercellular adhesion molecule 1 | 6.94 |
| Other | ||
| Sfrp4 | Secreted frizzle-related protein 4 | 7.76 |
| Sfrp1 | Secreted frizzle-related protein 1 | 3.54 |
| Downregulated genes | ||
| Smooth muscle cell markers | ||
| Myh11 | Myosin, heavy polypeptide 11, smooth muscle | 0.12 |
| Cnn1 | Calponin 1 | 0.18 |
| Spink genes | ||
| Spink3 | Serine peptidase inhibitor, Kazal type 3 | 0.005 |
| Spink5 | Serine peptidase inhibitor, Kazal type 5 | 0.03 |
| Spink11 | Serine peptidase inhibitor, Kazal type 11 | 0.16 |
| Spink8 | Serine peptidase inhibitor, Kazal type 8 | 0.20 |
| Prostate-specific genes | ||
| Pbsn | Probasin | 0.06 |
| Tgm4 | Transglutaminase 4 (prostate) | 0.07 |
| Nkx3-1 | NK-3 transcription factor, locus 1 (Drosophila) | 0.13 |
RNA was extracted from 12-month-old PE-PTEN+/-IKK2ca/ca or PE-PTEN+/- lateral prostates.
Figure 5.
Confirmation of gene expression changes in transgenic prostates. (A, B) Quantitative RT-PCR experiments on samples from PE- PTEN+/-IKK2ca/ca prostates (group 1), PE-PTEN+/- prostates (group 2), and wild-type littermate controls (group 3) (n = 6 per group, extracted from 12-month-old tissues). (B) Immunoblot for Nkx3-1 and SFRP-1 (right panel). (C) Data mining of normal prostate versus human prostate cancer. The data set of Vanaja et al. was queried by use of Oncomine, and the expression data were transformed to the linear expression signal plotted in the bar graph. Error bars, SEM. *P < .05; **P < .01; ***P < .001.
In line with the analysis of the changes in stroma (Figure 4), some of the most downregulated genes were identified to be smooth muscle cell markers myosin heavy chain 11 (MYH11) and calponin 1. Moreover, we found genes associated with prostate tumor progression to be changed: First, the tumor suppressor Nkx3-1 was downregulated. Nkx3-1 is known to be frequently lost in human prostate cancer, and genetic ablation of the gene in the mouse resulted in hyperplasia and PIN formation [22]. This correlates with our finding of increased tumor proliferation in PE-PTEN+/-IKK2ca and is consistent with findings in a model of bacterial prostatitis [23]. Second, a group of serine protease inhibitors (Spink genes) was downregulated. Spink3, which topped the list of downregulated genes, is the murine homolog of human Spink1, identified as an upregulated gene in a subset of prostate cancers negative for translocation of Ets transcription factors. In these cancers, Spink1 overexpression is associated with invasiveness [24]. This correlates with our finding that the larger tumors in PE- PTEN+/-IKK2ca(ca) prostates failed to become invasive carcinomas. Furthermore, the consistent down-regulation of many Spink family members suggests a common regulatory mechanism for Spink genes.
The loss of smooth muscle was also analyzed in human prostate tumors. We used Oncomine (Compendia Bioscience), a database comprising a number of microarray data sets from human tumors, to determine regulation of the markers found in our transgenic mouse lines. We found a number of data sets with down-regulation of smooth muscle cell markers in carcinomas versus normal prostate indicating loss of smooth muscle as an event in carcinoma formation (one data set [25] is shown in Figure 5C). This confirms that tumors in the inflamed mouse prostate tissue show advanced tumor formation and share similarities with human cancers.
Epithelial and Stromal Cells Produce Inflammatory Chemokines
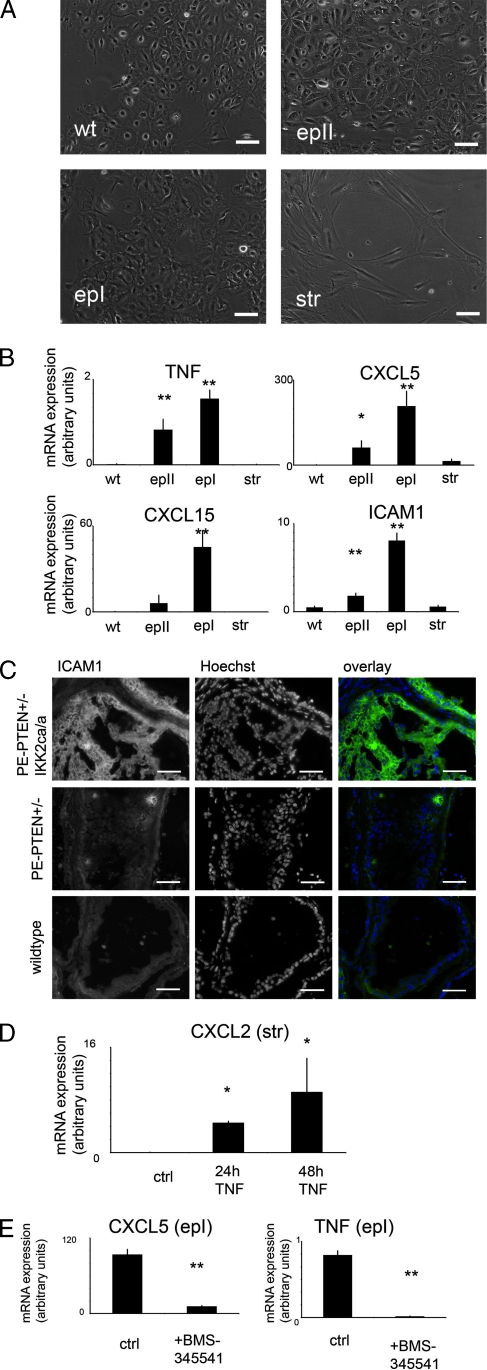
The inflammatory phenotype in PE-PTEN+/-IKK2ca/ca prostates is somehow evoked by the transgenically modified epithelium. We wanted to confirm that inflammatory chemokines or cytokines overexpressed in the whole prostate tissue samples are indeed produced by the transgenic epithelium. To this end, we established cell lines from transgenic mice and wild-type littermates by explant cultures (Figure 6A). Epithelial lines from PE-PTEN+/-IKKca/ca and wild-type prostates expressed E-cadherin and luminal cell marker cytokeratin 18, whereas stromal lines expressed collagen I (Figure W5). Interestingly, a wild-type epithelial (wt) and one transgenic epithelial line (epII) expressed basal cytokeratin 14, whereas another transgenic epithelial line (epI) did not. We conclude that the lines wt and epII show a more intermediate and that the line epI shows a more luminal phenotype based on cytokeratin expression. The Flag-IKK2 transgene was confirmed to be expressed in transgenic epithelium only. SFRP1, the reported stroma-to-epithelial modulator, was expressed in the stroma (Figure W5B). With respect to chemokine expression, we could show that most of the chemokines are indeed overexpressed in the transgenic epithelium, and their expression was higher in the luminal line compared with the intermediate line (Figure 6). CXCL5 and CXCL15 (Figure 6B) are strongly overexpressed in the epithelial lines, with moderate overexpression of CXCL10 and CXCL2 (not shown). Interestingly, the adhesion molecule ICAM1 is also strongly overexpressed in the epithelium (Figure 6B), and ICAM1 has been described as a receptor for neutrophil granulocytes [26]. This correlates with the finding that these inflammatory cells are located within the epithelium of PE- PTEN+/-IKK2ca/ca prostates (Figure 3). Overexpression of ICAM1 in the epithelium was confirmed by tissue section staining, indicating that it was no cell culture artifact (Figure 6C). Moreover, TNF was expressed in the transgenic epithelium. We tested the hypothesis that the stroma activated by cytokines from the epithelium could also express immune cell-attracting chemokines. Indeed, TNF treatment of stromal cultures induced messenger RNA (mRNA) of CXCL2 (Figure 6D) and, to a lesser extent, CXCL10 (Figure W5) but not CXCL5 or CXCL15. Furthermore, treatment of the epI cell line with the IKK/NF-κB inhibitor BMS-345541 decreased cytokine and chemokine expression, confirming that the transgenic activation of the IKK2/NF-κB axis is responsible for the overexpression of these molecules (Figure 6E).
Figure 6.
Inflammatory cytokines and chemokines are expressed in cultured epithelial and stromal cells. (A) Bright-field images from cell cultures of prostate epithelial cells from nontransgenic prostates (wt) or PE- PTEN+/- IKK2ca/ca prostates (epI, epII) and from cultures of prostate stromal cells from PE-PTEN+/- IKK2ca/ca prostates (str). (B) Quantitative RT-PCR data (n = 3 per group). (C) Immunostaining for ICAM1 in frozen sections of dorsal prostates (12 months) of the indicated genotypes. (D) Chemokine CXCL2 mRNA was determined in cultured stromal cells with or without TNF treatment for 24 or 48 hours (ctrl: untreated control). (E) mRNA was determined in epI epithelial line treated with mock control or BMS-345541 for 72 hours. Scale bars, 50 µm. Error bars, SEM. *P < .05; **P < .01.
Taken together, these data indicate that the transgenic PTEN+/- IKK2ca/ca epithelium can attract inflammatory cells by direct and/or indirect production of chemokines.
Loss of Smooth Muscle Correlates with Prostate Inflammation
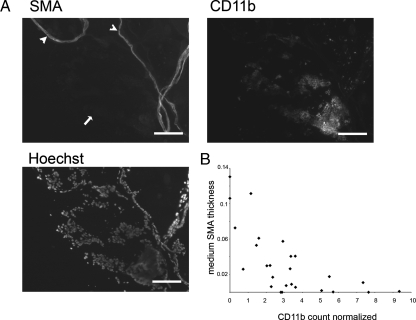
We wanted to establish a correlation between the inflammatory phenotype and loss of smooth muscle. To this end, we stained PE- PTEN+/- IKK2ca/ca dorsal prostates where smooth muscle was only partially lost, with both smooth muscle (SMA) and inflammatory cell marker (CD11b). In prostatic ducts with intense inflammatory cell infiltration, the smooth muscle was lost completely (Figure 7A, arrow), whereas in ducts with little or no signs of inflammation, the smooth muscle was maintained or partially intact (Figure 7A, arrowheads). Quantification over a number of prostatic ducts established a correlation between smooth muscle thickness and inflammatory cell infiltration (Figure 7B).
Figure 7.
Correlation of inflammatory cell infiltration and smooth muscle thickness. (A) Cryosections from 11-month-old PTEN+/-IKK2ca/ca dorsal prostate were stained as indicated. Arrow indicates duct with loss of smooth muscle and infiltration of inflammatory cells. Arrow-heads indicate ducts still surrounded by smooth muscle and less infiltration. (B) Quantification of dorsal prostate ducts (8–12 months). Scale bars, 100 µm.
Reduced Epithelial Androgen Receptor Activation in Transgenic Prostates
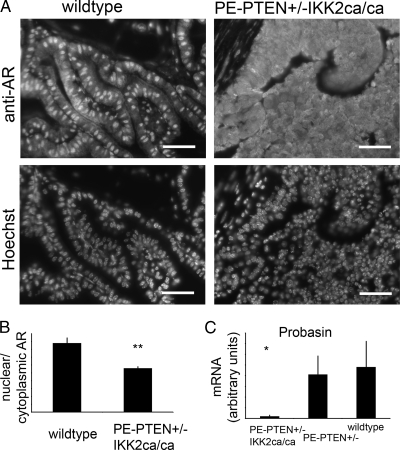
Down-regulation of Nkx3-1, an androgen-responsive gene, in PE- PTEN+/-IKK2ca prostates suggested reduced AR activation in the epithelium. Staining of wild-type and transgenic prostates showed less nuclear (activated) AR in the hyperplastic tissues (Figure 8, A and B). A decrease in AR activity was also indicated by down-regulation of other androgen-responsive genes such as Probasin (Figure 8C). These data suggest that decreased AR activity could be responsible for the hyperplastic epithelium by downregulating Nkx3-1.
Figure 8.
Reduced epithelial AR activation in PE-PTEN+/-IKK2ca/ca. (A) Paraffin sections of wild-type and transgenic tissue (anterior prostate, 12 months) stained for AR with Hoechst counterstain. (B) Quantification for wild-type and transgenic tissues (n = 4 each). (C) Quantitative PCR of androgen-responsive gene Probasin (n = 6 per group). Scale bars, 50 µm. **P < .01; *P < .05.
Discussion
In this study, we sought to elucidate the effects of inflammation and inflammatory signaling on early events of prostate tumorigenesis. The main reason for this is that epidemiological data suggest a role for inflammation in tumor initiation or early progression. Thus, we analyzed IKK2 signaling on a wild-type and PE-PTEN+/-, but not PE- PTEN-/- background, because the latter has been described to result in full carcinoma formation over time. The effect of NF-κB signaling on late-stage prostate tumorigenesis has been addressed by other groups and determined a role for NF-κB in promoting androgen-independent growth [27].
Inflammatory signaling through constitutive activation of the IKK2 axis in normal prostate epithelium is insufficient to transform prostate tissue. This is interesting because IKK2 has been shown to be an oncogenic kinase and activation on a PE-PTEN+/- background shows a clear additional phenotype. One possible reason for this lack of phenotype may be that IKK2/NF-κB transcription factors can activate promoters in proliferating epithelium that they cannot activate in quiescent epithelium such as the prostate epithelium. Consistent with this, some NF-κB dependent promoters were not activated in PE- IKK2ca prostates compared with wild-type prostates. Moreover, we have found a clear phenotype- and NF-κB-dependent gene expression when expressing the same transgene from the same strain in keratinocytes of the skin, a proliferating epithelium (Birbach et al., unpublished observations). The Probasin-Cre strain used in our study excises loxP-flanked sequences only in the adult, quiescent epithelium. Excision efficiency increases with age, in line with the age-dependent phenotype in PE-PTEN+/-IKK2ca(ca) [28].
Our experiments on PE-PTEN+/-IKK2ca(ca) indicate that some of the more upregulated genes in prostate epithelium in response to NF-κB are inflammatory cytokines and chemokines. This is in agreement with chemokine production from other epithelia in response to NF-κB [29]. The chemokines from the epithelium and cytokine-activated stroma (a finding consistent with data from human stromal cells [30]) are likely attractors for the infiltrating inflammatory cells. The inflammation evoked phenotype in both the epithelium and the stroma: the severe hyperplasia in dysplastic epithelial lesions was due to the increased proliferation, not apoptosis, consistent with expression data indicating no major change in regulators of apoptosis, but downregulation of Nkx3-1, a guardian of epithelial proliferation. Nkx3-1 loss in the epithelium is necessary for tumor initiation in tumor models and human cancer and could have a similar function in our model [31]. Reduced AR activation is a likely contributor to the decrease in Nkx3-1. In this context, the relationship between NF-κB and AR activity is complex, with different outcomes in in vitro experiments by different groups [32,33]. In our model, we hypothesize that the modified stromal-to-epithelial signaling could play a role in modifying AR activity.
In the stroma, increases in fibroblasts and collagen fibers were reminiscent of activated prostate cancer stroma, and expression data showed some upregulated genes known for their expression in cancer-associated fibroblasts. Loss of smooth muscle and concomitant increase of stromal fibroblasts suggest that the smooth muscle is lost by a dedifferentiation to fibroblastic cells. Various factors have been described to be involved in smooth muscle dedifferentiation, including inflammatory mediators [34,35]. Prostate smooth muscle cells can be partially dedifferentiated by LPS treatment, and partial vascular smooth muscle dedifferentiation by IL-1b can be enhanced by PGE-2. Along the same lines, we have observed a small decrease in smooth muscle markers by treating prostate stroma with TNF (not shown). Although neither of these experiments (during a time span of hours to days) result in complete loss of smooth muscle identity, it is conceivable that persistent inflammation for months and possible additive effects of different inflammatory mediators can lead to complete dedifferentiation.
Although the smooth muscle around the prostatic ducts was lost, most tumors were not invasive by histologic or expression parameters. We laid emphasis on maintaining our mouse colony on a strict C57BL/6 background, known as a strain with low tumor susceptibility [36]. It is well possible that more carcinomas, especially microinvasive structures, develop on a different inbred or mixed background; however, by using this approach, we were able to clearly distinguish the effects mediated by the inflammatory phenotype from the secondary events dependent on another genetic or epigenetic alteration in the tumor. Along these lines, tumor models with partial loss of Nkx3-1 on a PE-PTEN+/- background have been shown to be more frequently invasive than the tumors in our study but were bred on a mixed background [37].
Loss of smooth muscle has been used as a hallmark for carcinoma formation in mouse prostate tumor models [16,38]. Models of epithelial-stromal interactions have proposed smooth muscle integrity to be necessary to control epithelial proliferation and dysplasia [39,40]. Moreover, human prostate carcinoma is associated with downregulation of smooth muscle markers. Our data from the inflammatory model show that loss of smooth muscle is not sufficient for the tumor to become invasive but that the two events can be uncoupled.
One model of prostate carcinogenesis based on genetic analysis and mouse models of prostate tumors suggests that a distinct step associated with the overexpression of either Ets transcription factors, Spink1 gene, or other, yet unidentified genes must be taken to induce invasion through the basement membrane and carcinoma formation [24,41]. This is in agreement with our expression data from intraepithelial tumors of inflamed prostates with either unchanged (Ets factors) or even downregulated (Spink genes) invasion-associated genes.
In summary, as a genetic model, our model has advantages over bacterially induced models of inflammatory tumorigenesis. It is a reproducible model based on well-defined Cre-mediated deletion and dissects effects mediated by inflammation and genetic lesions. Inflamed prostates not only show enlarged tumor burden due to increased proliferation but also exhibit a clear change in stromal mass and composition. On a well-defined background, the model demonstrates what inflammation does by its own and what is due to genetic background or further genetic or epigenetic alterations in the tumor. Invasion, although rarely seen in our model, will be present in inflammatory tumors but is not a direct consequence of inflammation in our model. Loss of smooth muscle, an indicator of tumor progression, is seen in inflammatory lesions and correlates with inflammation. Interestingly, genetic data have revealed that loss of smooth muscle markers in primary tumors is part of a tumor signature predicting subsequent metastasis and poor clinical outcome [42]. Thus, the inflammatory tumors in our model, although not invasive without a subsequent transforming step, may be more dangerous in the long run when acquiring additional mutations to become carcinomas.
Supplementary Material
Acknowledgments
The authors thank Tak Mak for the loxP-PTEN mice, Gernot Schabbauer for sharing mice from his cohort, and Renate Kain for help with tissue processing for histology.
Abbreviations
- AR
androgen receptor
- IKK2
IκB kinase 2
- PTEN
phosphatase and tensin homolog
- SMA
smooth muscle actin
Footnotes
This work was supported by a grant from the Austrian Science Fund FWF (P21919-B13 to A.B.).
This article refers to supplementary materials, which are designated by Tables W1 to W4 and Figures W1 to W5 and are available online at www.neoplasia.com.
References
- 1.Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J InternMed. 2000;248:171–183. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 2.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacLennan GT, Eisenberg R, Fleshman RL, Taylor JM, Fu P, Resnick MI, Gupta S. The influence of chronic inflammation in prostatic carcinogenesis: a 5-year followup study. J Urol. 2006;176:1012–1016. doi: 10.1016/j.juro.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 5.Mahmud S, Franco E, Aprikian A. Prostate cancer and use of non-steroidal anti-inflammatory drugs: systematic review and meta-analysis. Br J Cancer. 2004;90:93–99. doi: 10.1038/sj.bjc.6601416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennis LK, Lynch CF, Torner JC. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002;60:78–83. doi: 10.1016/s0090-4295(02)01637-0. [DOI] [PubMed] [Google Scholar]
- 7.Putzi MJ, De Marzo AM. Morphologic transitions between proliferative inflammatory atrophy and high-grade prostatic intraepithelial neoplasia. Urology. 2000;56:828–832. doi: 10.1016/s0090-4295(00)00776-7. [DOI] [PubMed] [Google Scholar]
- 8.Quintar AA, Doll A, Leimgruber C, Palmeri CM, Roth FD, Maccioni M, Maldonado CA. Acute inflammation promotes early cellular stimulation of the epithelial and stromal compartments of the rat prostate. Prostate. 2010;70:1153–1165. doi: 10.1002/pros.21150. [DOI] [PubMed] [Google Scholar]
- 9.Elkahwaji JE, Hauke RJ, Brawner CM. Chronic bacterial inflammation induces prostatic intraepithelial neoplasia in mouse prostate. Br J Cancer. 2009;101:1740–1748. doi: 10.1038/sj.bjc.6605370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmid JA, Birbach A. IκB kinase beta (IKKβ/IKK2/IKBKB)—a key molecule in signaling to the transcription factor NF-κB. Cytokine Growth Factor Rev. 2008;19:157–165. doi: 10.1016/j.cytogfr.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B, Huang WC, et al. IKKβ suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 12.Lee DF, Kuo HP, Liu M, Chou CK, Xia W, Du Y, Shen J, Chen CT, Huo L, Hsu MC, et al. KEAP1 E3 ligase-mediated downregulation of NF-κB signaling by targeting IKKβ. Mol Cell. 2009;36:131–140. doi: 10.1016/j.molcel.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu X, Wu J, Huang J, Powell WC, Zhang J, Matusik RJ, Sangiorgi FO, Maxson RE, Sucov HM, Roy-Burman P. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101:61–69. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- 14.Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A, Khoo AS, Roy-Burman P, Greenberg NM, Van Dyke T, et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003;1:E59. doi: 10.1371/journal.pbio.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma X, Ziel-van der Made AC, Autar B, van der Korput HA, Vermeij M, van Duijn P, Cleutjens KB, de Krijger R, Krimpenfort P, Berns A, et al. Targeted biallelic inactivation of Pten in the mouse prostate leads to prostate cancer accompanied by increased epithelial cell proliferation but not by reduced apoptosis. Cancer Res. 2005;65:5730–5739. doi: 10.1158/0008-5472.CAN-04-4519. [DOI] [PubMed] [Google Scholar]
- 16.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, Yoshida R, Wakeham A, Higuchi T, Fukumoto M, et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki Y, Derudder E, Hobeika E, Pelanda R, Reth M, Rajewsky K, Schmidt-Supprian M. Canonical NF-κB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24:729–739. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Barclay WW, Cramer SD. Culture of mouse prostatic epithelial cells from genetically engineered mice. Prostate. 2005;63:291–298. doi: 10.1002/pros.20193. [DOI] [PubMed] [Google Scholar]
- 20.Birbach A, Casanova E, Schmid JA. A Probasin-MerCreMer BAC allows inducible recombination in the mouse prostate. Genesis. 2009;47:757–764. doi: 10.1002/dvg.20558. [DOI] [PubMed] [Google Scholar]
- 21.Joesting MS, Perrin S, Elenbaas B, Fawell SE, Rubin JS, Franco OE, Hayward SW, Cunha GR, Marker PC. Identification of SFRP1 as a candidate mediator of stromal-to-epithelial signaling in prostate cancer. Cancer Res. 2005;65:10423–10430. doi: 10.1158/0008-5472.CAN-05-0824. [DOI] [PubMed] [Google Scholar]
- 22.Kim MJ, Bhatia-Gaur R, Banach-Petrosky WA, Desai N, Wang Y, Hayward SW, Cunha GR, Cardiff RD, Shen MM, Abate-Shen C. Nkx3.1 mutant mice recapitulate early stages of prostate carcinogenesis. Cancer Res. 2002;62:2999–3004. [PubMed] [Google Scholar]
- 23.Khalili M, Mutton LN, Gurel B, Hicks JL, De Marzo AM, Bieberich CJ. Loss of Nkx3.1 expression in bacterial prostatitis: a potential link between inflammation and neoplasia. Am J Pathol. 2010;176:2259–2268. doi: 10.2353/ajpath.2010.080747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomlins SA, Rhodes DR, Yu J, Varambally S, Mehra R, Perner S, Demichelis F, Helgeson BE, Laxman B, Morris DS, et al. The role of SPINK1 in ETS rearrangement-negative prostate cancers. Cancer Cell. 2008;13:519–528. doi: 10.1016/j.ccr.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanaja DK, Cheville JC, Iturria SJ, Young CY. Transcriptional silencing of zinc finger protein 185 identified by expression profiling is associated with prostate cancer progression. Cancer Res. 2003;63:3877–3882. [PubMed] [Google Scholar]
- 26.Diamond MS, Staunton DE, de Fougerolles AR, Stacker SA, Garcia-Aguilar J, Hibbs ML, Springer TA. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18) J Cell Biol. 1990;111:3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin RJ, Lho Y, Connelly L, Wang Y, Yu X, Saint Jean L, Case TC, Ellwood-Yen K, Sawyers CL, Bhowmick NA, et al. The nuclear factor-κB pathway controls the progression of prostate cancer to androgen-independent growth. Cancer Res. 2008;68:6762–6769. doi: 10.1158/0008-5472.CAN-08-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy-Burman P, Wu H, Powell WC, Hagenkord J, Cohen MB. Genetically defined mouse models that mimic natural aspects of human prostate cancer development. Endocr Relat Cancer. 2004;11:225–254. doi: 10.1677/erc.0.0110225. [DOI] [PubMed] [Google Scholar]
- 29.Richmond A. Nf-κB, chemokine gene transcription and tumour growth. Nat Rev Immunol. 2002;2:664–674. doi: 10.1038/nri887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kogan-Sakin I, Cohen M, Paland N, Madar S, Solomon H, Molchadsky A, Brosh R, Buganim Y, Goldfinger N, Klocker H, et al. Prostate stromal cells produce CXCL-1, CXCL-2, CXCL-3 and IL-8 in response to epithelia-secreted IL-1. Carcinogenesis. 2009;30:698–705. doi: 10.1093/carcin/bgp043. [DOI] [PubMed] [Google Scholar]
- 31.Lei Q, Jiao J, Xin L, Chang CJ, Wang S, Gao J, Gleave ME, Witte ON, Liu X, Wu H. NKX3.1 stabilizes p53, inhibits AKT activation, and blocks prostate cancer initiation caused by PTEN loss. Cancer Cell. 2006;9:367–378. doi: 10.1016/j.ccr.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 32.Supakar PC, Jung MH, Song CS, Chatterjee B, Roy AK. Nuclear factor κB functions as a negative regulator for the rat androgen receptor gene and NF-κB activity increases during the age-dependent desensitization of the liver. J Biol Chem. 1995;270:837–842. doi: 10.1074/jbc.270.2.837. [DOI] [PubMed] [Google Scholar]
- 33.Delfino FJ, Boustead JN, Fix C, Walker WH. NF-κB and TNF-α stimulate androgen receptor expression in Sertoli cells. Mol Cell Endocrinol. 2003;201:1–12. doi: 10.1016/s0303-7207(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 34.Clement N, Glorian M, Raymondjean M, Andreani M, Limon I. PGE2 amplifies the effects of IL-1β on vascular smooth muscle cell de-differentiation: a consequence of the versatility of PGE2 receptors 3 due to the emerging expression of adenylyl cyclase 8. J Cell Physiol. 2006;208:495–505. doi: 10.1002/jcp.20673. [DOI] [PubMed] [Google Scholar]
- 35.Leimgruber C, Quintar AA, Sosa LD, Garcia LN, Figueredo M, Maldonado CA. Dedifferentiation of prostate smooth muscle cells in response to bacterial LPS. Prostate. 2010;71:1097–1107. doi: 10.1002/pros.21322. [DOI] [PubMed] [Google Scholar]
- 36.Deschner EE, Long FC, Hakissian M, Herrmann SL. Differential susceptibility of AKR, C57BL/6J, and CF1 mice to 1,2-dimethylhydrazine-induced colonic tumor formation predicted by proliferative characteristics of colonic epithelial cells. J Natl Cancer Inst. 1983;70:279–282. [PubMed] [Google Scholar]
- 37.Abate-Shen C, Banach-Petrosky WA, Sun X, Economides KD, Desai N, Gregg JP, Borowsky AD, Cardiff RD, Shen MM. Nkx3.1; Pten mutant mice develop invasive prostate adenocarcinoma and lymph node metastases. Cancer Res. 2003;63:3886–3890. [PubMed] [Google Scholar]
- 38.Wang Y, Hayward SW, Donjacour AA, Young P, Jacks T, Sage J, Dahiya R, Cardiff RD, Day ML, Cunha GR. Sex hormone-induced carcinogenesis in Rb-deficient prostate tissue. Cancer Res. 2000;60:6008–6017. [PubMed] [Google Scholar]
- 39.Cunha GR, Hayward SW, Dahiya R, Foster BA. Smooth muscle-epithelial interactions in normal and neoplastic prostatic development. Acta Anat (Basel) 1996;155:63–72. doi: 10.1159/000147791. [DOI] [PubMed] [Google Scholar]
- 40.Wong YC, Tam NN. Dedifferentiation of stromal smooth muscle as a factor in prostate carcinogenesis. Differentiation. 2002;70:633–645. doi: 10.1046/j.1432-0436.2002.700916.x. [DOI] [PubMed] [Google Scholar]
- 41.Tomlins SA, Laxman B, Varambally S, Cao X, Yu J, Helgeson BE, Cao Q, Prensner JR, Rubin MA, Shah RB, et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10:177–188. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.