Abstract
Ku70 was first characterized as a nuclear factor that binds DNA double-strand breaks in nonhomolog end-joining DNA repair. However, recent studies have shown that Ku70 is also found in the cytoplasm and binds Bax, preventing Bax-induced cell death. We have shown that, in neuroblastoma cells, the binding between Ku70 and Bax depends on the acetylation status of Ku70, such that, when Ku70 is acetylated, Bax is released from Ku70, triggering cell death. Thus, to survive, in neuroblastoma cells, cytoplasmic Ku70 acetylation status is carefully regulated such that Ku70 is maintained in a deacetylated state, keeping Bax complexed with Ku70. We have shown that overexpression of CREB-binding protein (CBP), a known acetyltransferase that acetylates Ku70, releases Bax from Ku70, triggering apoptosis. Although we have shown that blocking deacetylase activity using non-type-specific inhibitors also triggers Ku70 acetylation and Bax-dependent cell death, the targets of these deacetylase inhibitors in neuroblastoma cells remain unknown. Here, we demonstrate that, in neuroblastoma cells, histone deacetylase 6 (HDAC6) binds Ku70 and Bax in the cytoplasm and that knocking down HDAC6 or using an HDAC6-specific inhibitor triggers Bax-dependent cell death. Our results show that HDAC6 regulates the interaction between Ku70 and Bax in neuroblastoma cells and may be a therapeutic target in this pediatric solid tumor.
Introduction
Neuroblastoma (NB) is a cancer diagnosed in infants and children. It develops during embryogenesis and after birth from sympathoadrenal stem cells in the adrenal gland or paraspinal locations [1]. Compared with most other childhood cancers, NB is difficult to cure; half of the cases are classified as high risk of relapse, and for these patients, the best available treatment results in a survival rate of less than 40% [2]. Current treatment regimens are dose-intense, involve cytotoxic drugs, and pose significant risks of serious short-term and long-term morbidity [3].
To identify new pharmacological targets in NB, we have recently described a novel pharmacologic approach to unleash cytosolic Bax and trigger apoptosis by inhibiting histone deacetylases (HDACs) in NB cells [4,5]. HDACs regulate the function of histones and many nonhistone proteins by modulating their acetylation status [6]. The HDAC family of proteins is divided into two categories: zinc-dependent enzymes (HDAC1-11) and NAD+-dependent enzymes (SIRT1-7) [7]. The zinc-dependent HDACs are subdivided into two classes: class 1 and class 2. HDAC inhibitors (HDACIs) are a new class of anticancer compounds [8]. Trichostatin A (TSA) and vorinostat (SAHA), class 1 and class 2 HDAC inhibitors, have promising antitumor effects against NB in preclinical models [9].
Our model is that Bax activation is central to the mechanism by which HDACI work against NB. The expression of the proapoptotic cytosolic protein Bax is high in NB cells and is linked to unfavorable outcomes. It has been hypothesized that, as a survival mechanism of NB tumor cells, Bax-dependent apoptosis is suppressed, particularly in advanced stage disease where increased expression is linked to unfavorable outcomes [10]. Elevated levels of the antiapoptotic proteins Bcl-2 and Bcl-xL, which work by inhibiting Bax, are correlated with poor prognosis, MYCN amplification, and chemotherapy resistance [11,12]. Caspase 8, which normally activates Bax in response to extracellular death signals, is epigenetically silenced in poor prognosis disease, effectively reducing Bax activation [13,14]. These two common motifs of high-risk NB tumors, namely, high levels of Bax protein and failure of Bax activation, led us to hypothesize that Bax activation is restrained in NB and that exploiting mechanisms that release the restraints on Bax could have antitumor effects.
Our results have shown that HDAC inhibition causes Bax-induced cell death by increasing acetylation of cytosolic Ku70, a multifunctional nuclear and cytosolic protein best known for its role in the nucleus as a factor in DNA repair [15]. Cytosolic deacetylated Ku70 sequesters activated Bax and suppresses apoptosis [16]. When Ku70 is acetylated, it loses its ability to bind Bax. In tumorigenic neuroblastic cell models of NB, we showed that Ku70 acetylation is increased by HDACI treatment, disrupting Ku70 binding to Bax, thereby causing activated Bax to translocate from the cytosol to the mitochondria and triggering cell death [5]. NB cells are poised to undergo spontaneous cell death when Ku70-Bax binding is disrupted. Indeed, our studies have shown that Ku70 acetylation is necessary for HDACIs to kill tumorigenic neuroblastic-type (N-type) NB cells [4,5]. Non-NB-cell types tested do not require Ku70-Bax binding for survival (data not shown); therefore, treatments designed to disrupt Ku70-Bax have the potential to be selective on the basis of both Ku70 deacetylation and Ku70-Bax binding. Interestingly, nontumorigenic stromal-type (S-type) NB cells that fail to acetylate Ku70 in response to HDACIs are likewise resistant to these agents.
Although we and others have demonstrated that the CREB-binding protein (CBP) acetylates Ku70, the deacetylase(s) that deacetylates Ku70 in NB cells is unknown. Here, we provide experimental evidence demonstrating that tubulin deacetylase, HDAC6, associates with Ku70 in NB cells and that knocking down HDAC6 or using an HDAC6-specific inhibitor, tubacin, induces Ku70 acetylation, Bax release, and NB-cell death. These results are critical to establish which protein targets should be evaluated in studies designed to determine protein expression of relevant HDACs in NB tumors. These results will also guide development of type-specific HDAC inhibitors for use in NB.
Materials and Methods
Cell Culture and Cell Transfection
Human NB cell lines SH-EP1, SH-SY5Y, IMR32, and SK-N-AS were cultured in modified Eagle medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin and maintained at 37°C in a humidified 5% CO2 incubator. IMR32 medium was further supplemented with 1 mM pyruvate and 0.075% NaHCO3. SH-SY5Y cells were transiently transfected for immunoprecipitation with either wild-type pCMV-2B-Ku70-FLAG or mutant pCMV-2B Ku70 K539R/K542R using Nucleofector Kit V (Amexa, Lonza, Basel, Switzerland) as per the manufacturer's protocol [4]. SH-EP1 cells expressing stable full-length pCDNA3-HDAC6-FLAG protein or the empty vector were generated by cotransfecting the cells with vector and puromycin expression vector or full-length HDAC6 and puromycin expression vector. A clonal population expressing high levels of HDAC6 was obtained using puromycin selection (1 µg/ml).
Cell Viability Assay
N-type SH-SY5Y and S-type SH-EP1 human NB-cell lines were treated with varying concentrations of MS275, tubacin, and SAHA, and viability was determined after 24 and 48 hours by MTT assay as previously described [17]. All experiments were carried out three times with triplicates in each experiment, and means and SDs were calculated. Trypan blue exclusion assay was used to determine the viability of cells shown in Figures 3, 4, and 5. Two-tailed t test was used to determine the statistical significance between treated and control samples, *P ≤ .05 (n = 3).
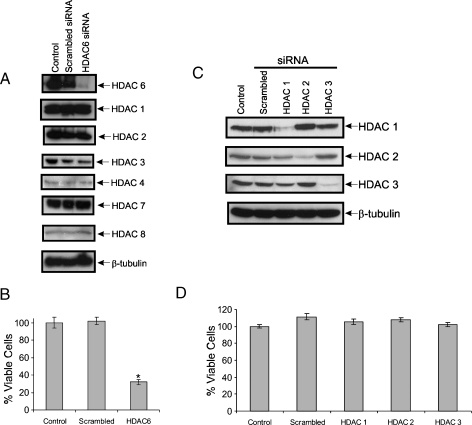
Figure 3.
Knocking down HDAC6, not class I HDACs, kills N-type NB cells. (A) SH-SY5Y cells were transfected with scrambled siRNA or HDAC6-specific siRNA. Cell lysates were prepared 48 hours after transfection and then blotted for HDAC6 and HDAC1, 2, 3, 4, 7, or 8. β-Tubulin was used as a loading control. (B) Cell viability after knockdown of HDAC6 was measured by trypan blue exclusion assay. Results are expressed as mean ± SD percent of control, n = 3. The P value of the significant difference between HDAC6-specific siRNA sample and the scrambled siRNA sample was .0006. (C) SH-SY5Y cells were transfected with scrambled siRNA, siRNA against HDAC1, 2, or 3. A mock transfection was used as a control. Cell lysates were prepared 48 hours after transfection and then blotted for HDAC1, 2, or 3. β-Tubulin was used as a loading control. (D) Cell viability was measured by trypan blue exclusion assay after HDAC1, 2, or 3 knockdown. Results are expressed as mean ± SD percent of control, n = 3.
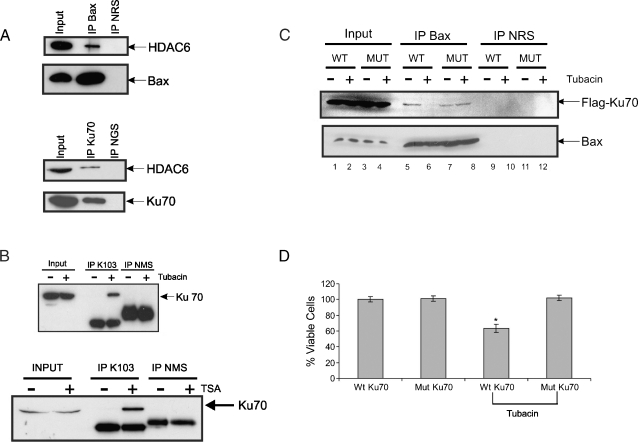
Figure 4.
Treatment of SH-SY5Y cells with tubacin results in Ku70 acetylation, which is blocked by Ku70 K539/542R mutant. (A) Wholecell SH-SY5Y extracts were immunoprecipitated with either Bax or Ku70 antibody. The immunocomplexes were separated by SDS-PAGE blotted for Ku70, Bax, or HDAC6. Normal rabbit serum and goat serum were used as immunoprecipitation controls. (B) SH-SY5Y cells were treated with 1 µM TSA, 10 µM tubacin, or vehicle for 24 hours as shown. Lysates were immunoprecipitated using acetyl lysine K103 antibody. The immune complexes were separated by SDS-PAGE and blotted for Ku70. (C) SH-SY5Y cells were transfected with Flag-Ku70 WT or Flag-Ku70 (K539/542R) mutant. Twenty-four hours later, the cells were treated with either 10 µM tubacin or vehicle. Lysates were prepared 24 hours after treatment and then immunoprecipitated with Bax antibodies. The immunocomplexes were then blotted for either Flag (for Flag-Ku70s) or Bax antibodies. Normal rabbit serum was used a control. (D) Cell viability of experiments shown in C was measured by trypan blue exclusion assay. Results are expressed as mean ± SD (n = 3) of percent of control (Ku70 WT). The P value of the significant difference between tubacin-treated Flag-Ku70 Wt transfected and Flag-Ku70 transfected alone was .012.
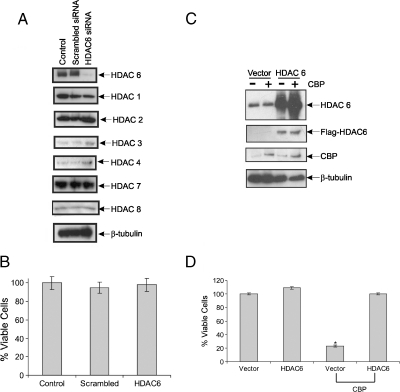
Figure 5.
Expressing HDAC6 rescues CBP-induced cell death in SH-EP1 cells. (A) SH-EP1 cells were transfected with scrambled siRNA or HDAC6 siRNA. Mock transfection is used as a control. Cell lysates were prepared 48 hours after transfection and then immunoblotted for HDACs as shown. β-Tubulin was used as a loading control. (B) Cell viability was measured using trypan blue exclusion assay. Results are expressed as percent of control, mean ± SD, n = 3. (C) SH-EP1 stable cell lines expressing HDAC6-Flag or control vector were transfected with CBP or the control vector. Twenty-four hours after transfection, the cells were lysed and then blotted for HDAC6, Flag-tagged (for Flag-HDAC6), or CBP. β-Tubulin was used as a loading control. (D) Cell viability was measured using trypan blue exclusion assay in experiment shown in C. Results are expressed as mean ± SD (n = 3) of percent of control.
Small Interfering RNA-Mediated Silencing
SH-SY5Y cells and SH-EP1 cells were plated at a density of ∼2 x 106 cells per 10-cm plate 24 hours before transfection. The following day, the cells were transfected either with smart pool HDAC6 small interfering RNA (siRNA) or HDAC1 siRNA or HDAC2 siRNA or HDAC3 siRNA or the scrambled nontargeting siRNA (Dharmacon, Inc, Chicago, IL) using Nucleofector Kit V (Amexa) as per the manufacturer's instruction. Mock transfection as well as the nontargeting siRNA transfection served as controls. The knockdowns of HDACs were measured 72 hours after transfection by immunoblot analysis using HDAC antibodies. The viability of cells after knockdown was measured by counting cells using trypan blue exclusion analysis.
Western Blot Analysis
For the immunoblot analysis, whole-cell extracts were prepared as described [4] from SH-SY5Y, SH-EP1, IMR32, or SK-N-AS human NB-cell lines. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to poly(vinylidene fluoride) membranes, and then blotted for different HDACs. The following antibodies were used for Western blot analyses: CBP (A-22), Ku70 (A-9), HDAC1 (H-51), HDAC2 (H-54), HDAC3 (H-99), HDAC7 (H-273), and HDAC8 (E-5) from Santa Cruz Biotechnology (Santa Cruz, CA); Flag (F-1804) and β-tubulin (T-4026) from Sigma (St Louis, MO); Bax NT (06-499) from Upstate (Millipore, Billerica, MA); HDAC6 (A-4006) from Epigentek (Farmingdale, NY); and HDAC4 (2072) and acetylated lysine (Ac-K-103) from Cell Signaling (Danvers, MA). The presence of protein was visualized by using Enhanced Chemiluminescence Plus (GE Healthcare, Piscataway, NJ).
Immunoprecipitation
Coimmunoprecipitation of Bax and Ku70 from SH-SY5Y and SH-EP1 cells was performed in CHAPS buffer according to the protocol described by Sawada et al. [18]. Immunoprecipitation of endogenous Bax before and after tubacin treatment for Ku70 wild-type- and mutant-transfected SH-SY5Y cells as well as immunoprecipitation with acetylated lysine antibody with and without TSA or tubacin treatment in SH-SY5Y cells was also performed using the above-mentioned protocol.
Immunohistochemistry on NB Tumors
HDAC6 expression in primary neuroblastic tumors was assessed by immunohistochemical staining of previously described tissue microarrays [4]. Briefly, these arrays are composed of triplicate samples of 1.0-mm cores taken from 48 paraffin-embedded, formalin-fixed neuroblastic tumors (32 NBs, 10 intermixed and nodular ganglio-neuroblastomas, and 6 ganglioneuromas) and 5 normal adrenal glands. Immunohistochemistry was performed using a commercially available polyclonal antibody (no. A4006; Epigentek) according to the manufacturer's recommended protocol at a 1:200 dilution using manual methods. Signals were evaluated for tumoral distribution (neuroblastic cells vs Schwann stromal cells), cellular distribution (nuclear vs cytoplasmic), and intensity (semiquantitatively graded as 0, 1+, 2+, or 3+). For each evaluated specimen, the mean neuroblastic component staining, the mean stromal component staining, and the mean component staining difference were calculated for all three cores.
Results
Knocking Down or Inhibiting HDAC6 Induces Cell Death in NB Cells
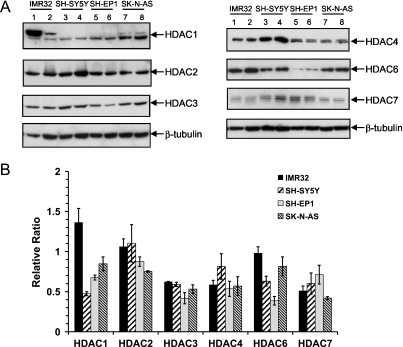
Our recent studies have shown that a non-type-specific HDACI, TSA, inhibits HDAC activity and induces cell death in N-type NB cells but not in S-type cells. Because TSA inhibits both class 1 and class 2 HDACs, to identify specific HDACs that deacetylate Ku70, we first determined whether there are any notable differences between class 1 and class 2 HDACs in NB cells. We determined the level of three class 1 HDACs (1, 2, and 3) and three class 2 HDACs (4, 6, 7) in two N-type NB cells (IMR-32 and SH-SY5Y) and two S-type NB cells (SH-EP1 and SK-N-AS). Figure 1A shows the immunoblots for all HDACs determined, and Figure 1B shows the densitometric scans of the results shown in Figure 1A. These results were normalized with β-tubulin as a loading control. These results indicate that there are no large differences among all except that there is a higher expression of HDAC1 in IMR32, one of two N-type NB cells, and that HDAC6 level in SH-EP1 cells is the lowest of all cell lines tested.
Figure 1.
Class I and II HDACs are expressed in N-type and S-type NB-cell lines. (A) Cell lysates from two N-type (IMR32 and SH-SY5Y) and two S-type (SH-EP1 and SK-N-AS) NB lines were blotted for class I HDACs (HDACs 1, 2, and 3) and class II HDACs (HDACs 4, 6, and 7). As a loading control, the same blot was stripped and reprobed for β-tubulin. (B) Densitometry scans for immunoblots shown in A. Results are expressed as a relative ratio to β-tubulin, and each bar presents the mean of three experiments.
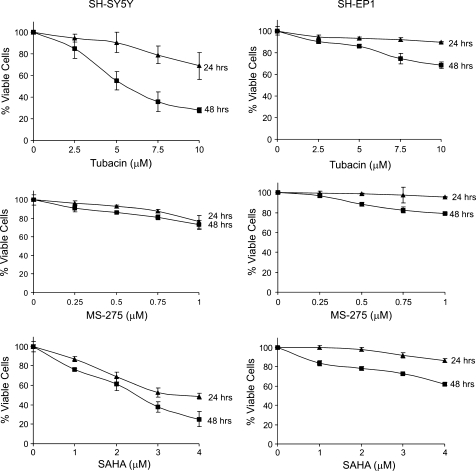
Next, we sought to use type-specific inhibitors to determine the HDACs involved in TSA-induced killing. Although there are many experimental type-specific HDACI, few are commercially available. The most common HDACIs available are MS275, which specifically inhibits HDACs 1, 2, and 3; and tubacin, an HDAC6 inhibitor. We used SAHA in these experiments as a control because it is in the same class of TSA and is one of the only two HDACIs approved by the US Food and Drug Administration (FDA). SAHA is also currently being evaluated in clinical trials for other tumors. As expected, SAHA significantly reduces N-type cell viability in a dose-dependent manner, whereas the SH-EP1 cells are less responsive to SAHA (Figure 2, bottom panels). Interestingly, both SH-SY5Y cells and SH-EP1 cells are not very responsive to MS275 treatment compared with tubacin treatment, which, like that of SAHA, reduces significantly SH-SY5Y cell viability but has a modest effect on SH-EP1 cells. These results strongly indicate that HDAC6 may be involved in HDACI-induced cell viability in NB cells.
Figure 2.
N-type (SH-SY5Y) and not S-type (SH-EP1) NB cells are sensitive to tubacin, an HDAC6-specific inhibitor. Cells are treated with various concentrations of a pan-HDAC inhibitor (SAHA), class I-specific HDAC inhibitor (MS275), or HDAC6-specific inhibitor (tubacin). The viability of the cells was assessed by MTT assay at 24 and 48 hours. Results are expressed as the percentage of viable cells compared with vehicle control (mean ± SD, n = 3). All 48-hour data points were significant from controls. The P values for all 48-hour data points were as follows: for SH-SY5Y cells, tubacin = .003, MS275 = .003, SAHA = .001; for SH-EP1 cells, tubacin = .005, MS275 = .002, SAHA = .0007.
To confirm that HDAC6 is playing a role in tubacin-induced cell death, we knocked down HDAC6 in SH-SY5Y cells using HDAC6-specific siRNA. Figure 3A shows that we have successfully knocked down HDAC6 in SH-SY5Y cells without affecting the level of other HDACs. More importantly, we have shown that knocking down HDAC6 in SH-SY5Y cells reduces the viability of these cells (Figure 3B). As controls, we also knocked down HDAC1, 2, or 3 individually (Figure 3C); blocking the activity of these enzymes with MS275 had not altered the cell's viability previously (Figure 2, middle panels). Indeed, we found that reducing the levels of these HDACs does not affect the cell's viability either (Figure 3D). Together, these results strongly suggest that HDAC6 in SH-SY5Y cells regulates a cell death mechanism.
HDAC6 Binds Ku70 and Bax and Inhibiting HDAC6 Increases Ku70 Acetylation
We reasoned that if HDAC6 regulates Ku70 acetylation, HDAC6 should complex with Ku70 and Bax. Indeed, in immunoprecipitation assays using either Bax-specific antibody or Ku70-specific antibody, we have shown that HDAC6 interacts with both Bax and Ku70 (Figure 4A). Furthermore, in immunoprecipitation assays using a pan-anti-acetyl-lysine antibody (K103) and then probing with Ku70-specific antibody, either tubacin or TSA treatment induced Ku70 acetylation (Figure 4B). Tubacin treatment also releases Bax from Ku70 (Figure 4C; compare lane 6 to lane 5 from the left) that is consistent with our previous finding using TSA [4,5]. The release of Bax from Ku70 after tubacin treatment can be rescued by overexpressing the Ku70 acetylation mutant (K539R/K542R). This mutant has been shown to rescue the TSA-induced cell death in NB cells [5]. This mutant also reverses the reduced cell viability induced by tubacin treatment (Figure 4D). Collectively, these results show that, in SH-SY5Y cells, HDAC6 inhibits Ku70 acetylation, and inhibition of HDAC6 increases Ku70 acetylation, triggering the release of Bax, resulting in Bax-dependent cell death.
HDAC6 Expression Blocks CBP-Induced Cell Death in SH-EP1 Cells
We have shown previously that S-type SH-EP1 cells are less responsive to HDACI-induced killing [4]. The unresponsiveness to HDACI inhibition is not due to the low level of Ku70 or Bax because we have shown that Ku70 and Bax levels are comparable to those in SH-SY5Y cells and that Ku70 and Bax form a complex in both cell types. One mechanism of the unresponsiveness to HDAC inhibition in SH-EP1 cells might be the low levels of CBP because we have shown that overexpressing CBP in these cells induces cell death and that this effect is blocked by the Ku70 acetylation mutant (K539R/K542R). Another possibility, suggested by our current findings, is that these cells have low HDAC6 levels (Figure 1). We showed that further depletion of HDAC6 level in these cells by HDAC6-specific siRNA did not affect the level of other HDACs (Figure 5A); also, it did not alter the cell's viability (Figure 5B). To further test the role of HDAC6 in the regulation of Ku70-Bax complex in these cells, we prepared SH-EP1 cells stably expressing HDAC6 (Figure 5C). We found that HDAC6 overexpression in these cells blocks CBP-induced cell death (Figure 5D). These results indicate that HDAC6 participates with CBP in regulating the Ku70-Bax complex in SH-EP1 cells.
HDAC6 in NB Patient Samples
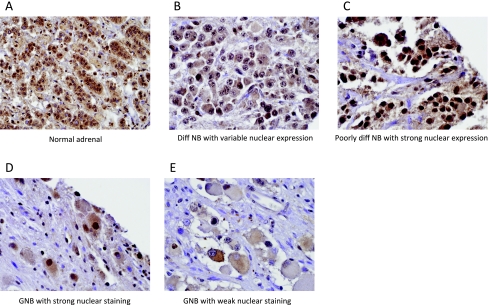
We have previously shown that the mediators of this proposed HDACI-sensitive cell death pathway—Bax, Ku70, and CBP—are expressed in NB cell lines and primary tumors [4,5]. We therefore sought to determine whether HDAC6 is also expressed in primary neuroblastic tumors. Normal adrenal medullae and all examined tumors stained positively for HDAC6 by immunohistochemistry (Figure 6). Nuclear expression of HDAC6 was modestly higher in the neuroblastic component of malignant tumors (NB, 1.5; ganglio-neuroblastoma, 1.6) than in benign ganglioneuromas (1.0) and normal adrenal glands (ND). However, there was a wide variability in staining patterns within each group (Table 1). Cytoplasmic staining was more uniform and seen in all specimen types.
Figure 6.
Immunohistochemical staining of primary neuroblastic tumors for HDAC6 protein. Normal adrenal medullae and all examined tumors stained positively. Although there was wide variation in staining patterns, nuclear HDAC6 expression was modestly higher in the neuroblastic component of malignant tumors; cytoplasmic staining was more uniform. (A) Normal adrenal gland. (B) Differentiating NB with variable nuclear expression. (C) Poorly differentiated NB with strong nuclear expression. (D) Ganglioneuroblastoma (GNB) with strong nuclear staining, and (E) GNB with weak nuclear staining.
Table 1.
Distribution of HDAC6 Protein Expression in NB Tumor Tissue Arrays.
| HDAC6 Protein Expression by IHC | ||||||
| Neuroblastic Component | Stromal Component | |||||
| 0–1 | 1–2 | 2–3 | 0–1 | 1–2 | 2–3 | |
| NB | 6 (24%) | 9 (36%) | 10 (40%) | 1 (25%) | 3 (75%) | 0 (0%) |
| Ganglioneuroblastoma | 1 (8.3%) | 4 (33.3%) | 7 (58.3%) | 3 (50%) | 3 (50%) | 0 (0%) |
| Ganglioneuroma | 0 (0%) | 2 (40%) | 3 (60%) | 0 (0%) | 2 (40%) | 3 (60%) |
| Normal adrenal medulla | 0 (0%) | 1 (20%) | 4 (80%) | N/A | N/A | N/A |
Immunohistochemistry (IHC) was performed using a commercially available polyclonal antibody and tissue microarrays containing triplicate cores from each primary tumor (32 NBs, 10 intermixed and nodular ganglioneuroblastomas, and 6 ganglioneuromas) and 5 normal adrenal glands. Each tumor core was scored semiquantitatively as 0, 1+, 2+, or 3+; results reported in the table reflect the mean for each tumor in each component across its three cores. The results are expressed as the average staining for each tumor scoring as 0, 1, 2, or 3 and are reported as the mean from three cores grouped by category of component (N- or S-) staining intensity (0–1, 1–2, or 2–3) and category of tumor type (NB, ganglioneuroblastoma, ganglioneuroma, or normal adrenal gland). The percentages under the categories are the percentage of tumors in a given category scoring with the indicated intensity. Not all tumors had cores evaluable for both components; hence, numbers in each table fall short of the overall tumor number.
Although nuclear HDAC6 protein expression was higher in biologically aggressive tumors, it is not yet known whether expression significantly correlates with clinical outcome. We expect that increased expression of specific HDACs that deacetylate Ku70 would be required for NB tumor survival and, thus, with worse patient outcome. We cannot yet predict whether expression will be correlated with any single specific prognostic marker; however, because the proposed mechanism by which HDACs contribute to tumorigenesis is via increased genomic instability, associations between HDAC expression and chromosomal changes or MYCN amplification are expected. An association between HDAC6 expression and decreased patient survival would spur investigations into clinical use of HDACIs for high-risk neuroblastic tumors. The absence of a correlation between HDAC6 expression and outcome could mean that an increased expression of HDAC6 is not required for aggressive behavior and/or increased expression does not cause treatment failures on current therapeutic regimens. The latter result would predict that treating patients with HDACIs would be of limited clinical utility.
Discussion
We have previously shown that HDAC inhibition using a broad HDAC inhibitor (TSA) increases Ku70 acetylation, induces Bax dissociation from Ku70, and triggers Bax-dependent cell death [4,5]. Although our results have indicated that CBP acetylates Ku70 in NB cells, it is not clear which HDACs deacetylate cytosolic Ku70 to protect cells from Bax-induced death or nuclear Ku70. This must be established to guide the selection of HDACIs to test this therapeutic mechanism in NB treatment. Some recent studies have shown that HDAC1 and HDAC8 are important in NB tumorigenesis [19,20], which raises the possibility of their involvement and underscores the importance of establishing which HDACs deacetylate Ku70 to precisely target it therapeutically. Here, we provide evidence demonstrating that HDAC6 forms a complex with cytoplasmic Ku70 and that inhibition of HDAC6 activity, either by depleting endogenous HDAC6 or by using HDAC6-specific inhibitors, increases Ku70 acetylation triggering Bax-dependent cell death. We have also shown that HDAC6 is found in tumors from NB patients, underscoring the potential importance of this HDAC as a therapeutic target in the treatment of NB.
HDAC6 is a class IIb HDAC containing two catalytic domains [21,22]. HDAC6 is mainly localized in the cytoplasm and has been associated with many cell functions including tubulin stabilization, cell motility, and regulation of the binding between Hsp90 and its cochaperone [23]. A high level of HDAC6 has been associated with ovarian cancer [24] and breast cancer [25], but it is unclear whether HDAC6 plays a role in tumor development or tumor maintenance. The role of HDAC6 in cell survival is highlighted by its involvement in aggresome, an alternate pathway that regulates protein degradation in cells. It has been shown that HDAC6 inhibitor enhances the effect of proteosome inhibitor in killing cancer cells, resulting in the reduction of cell viability, mainly because, when the proteosome pathway is blocked, cells degrade unfolded proteins using the aggresome pathway, but if both pathways are blocked, it will trigger cell death [26].
Although HDAC6 has been identified in complex isolated from various cellular contexts [27], three direct deacetylation substrates have been reported, including tubulin [28], cortactin [29], and Hsp90 [30]. Our results show that Ku70 is another substrate of HDAC6. Although we do not have direct in vitro evidence (using purified HDAC6 and purified acetylated Ku70) showing that acetylated Ku70 is a direct substrate of HDAC6, our results show that blocking HDAC6 activity using tubacin increases Ku70 acetylation and that tubacin treatment increases NB cell death, which is blocked by the Ku70 deacetylation mutant. These results are consistent with our proposed model in which Ku70 acetylation is regulated by HDAC6 and that blocking HDAC6 activity will result in Ku70 acetylation triggering Bax-dependent cell death.
We have shown previously that Ku70 is required for the survival of some NB cells because knocking down of Ku70 by siRNA triggers Bax-dependent cell death [4]. Thus, for the NB cells to survive, Ku70 must be kept unacetylated. Currently, it is not clear how the deacetylation activity of HDAC6 is regulated in cells. Although we have shown that HDAC6 is expressed in NB tumor samples, its expression is not correlated with any clinical outcome variables, such as stages or patient survival. Much work is needed to elucidate how HDAC6 is regulated in cells. This information may provide addition therapeutic targets to suppress HDAC6 activity in cells.
Although currently there is no HDAC6-specific inhibitor approved by the FDA or in clinical trials, recent development of more potent and more specific HDAC6 inhibitors is encouraging [31,32]. Using HDAC6-specific inhibitors in the treatment of NB is beneficial because it is not known whether simultaneous inhibition of class I and II HDACs by current FDA-approved HDAC inhibitor, SAHA, may have other effects other than the specific targets.
Our results show the feasibility of targeting a single HDAC to therapeutically engage the Ku70-Bax complex in NB. Whether it is due to differing HDAC expression or due to the overlap between HDAC activities in processing Ku70 as a substrate in the cytoplasm or in the nucleus will require more selective agents. Knowing the identity of the deacetylases that deacetylate Ku70 in the cytoplasm is of fundamental importance in understanding how Ku70 acetylation is regulated in NB.
Acknowledgments
The authors thank Stuart L. Schreiber and Ralph Mazitschek for providing tubacin.
Footnotes
This work was supported in part by the National Institutes of Health (grant DK067102 to R.P.S.K.), the Janette Ferrantino Hematology Research Fund (V.P.C.), the Ravitz Foundation (V.P.C.), Hope Street Kids Foundation (C.S.), and the A. Alfred Taubman Medical Research Institute (V.P.C., R.P.S.K., and A.W.O.). This work used the Sequencing Core of the Michigan Diabetes Research and Training Center, which was funded by National Institute of Diabetes and Digestive and Kidney Diseases (grant NIH5P60 DK20572).
References
- 1.Ho PT, Estroff JA, Kozakewich H, Shamberger RC, Lillehei CW, Grier HE, Diller L. Prenatal detection of neuroblastoma: a ten-year experience from the Dana-Farber Cancer Institute and Children's Hospital. Pediatrics. 1993;92:358–364. [PubMed] [Google Scholar]
- 2.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 3.Attiyeh EF, London WB, Mosse YP, Wang Q, Winter C, Khazi D, McGrady PW, Seeger RC, Look AT, Shimada H, et al. Chromosome 1p and 11q deletions and outcome in neuroblastoma. N Engl J Med. 2005;353:2243–2253. doi: 10.1056/NEJMoa052399. [DOI] [PubMed] [Google Scholar]
- 4.Subramanian C, Jarzembowski JA, Opipari AW, Jr, Castle VP, Kwok RP. CREB-binding protein is a mediator of neuroblastoma cell death induced by the histone deacetylase inhibitor trichostatin A. Neoplasia. 2007;9:495–503. doi: 10.1593/neo.07262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subramanian C, Opipari AW, Jr, Bian X, Castle VP, Kwok RP. Ku70 acetylation mediates neuroblastoma cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci USA. 2005;102:4842–4847. doi: 10.1073/pnas.0408351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Gennaro E, Bruzzese F, Caraglia M, Abruzzese A, Budillon A. Acetylation of proteins as novel target for antitumor therapy: review article. Amino Acids. 2004;26:435–441. doi: 10.1007/s00726-004-0087-3. [DOI] [PubMed] [Google Scholar]
- 7.de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases, (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ajamian F, Salminen A, Reeben M. Selective regulation of class I and class II histone deacetylases expression by inhibitors of histone deacetylases in cultured mouse neural cells. Neurosci Lett. 2004;365:64–68. doi: 10.1016/j.neulet.2004.04.087. [DOI] [PubMed] [Google Scholar]
- 9.De los Santos M, Zambrano A, Aranda A. Combined effects of retinoic acid and histone deacetylase inhibitors on human neuroblastoma SH-SY5Y cells. Mol Cancer Ther. 2007;6:1425–1432. doi: 10.1158/1535-7163.MCT-06-0623. [DOI] [PubMed] [Google Scholar]
- 10.McPake CR, Tillman DM, Poquette CA, George EO, Houghton JA, Harris LC. Bax is an important determinant of chemosensitivity in pediatric tumor cell lines independent of Bcl-2 expression and p53 status. Oncol Res. 1998;10:235–244. [PubMed] [Google Scholar]
- 11.Castle VP, Heidelberger KP, Bromberg J, Ou X, Dole M, Nunez G. Expression of the apoptosis-suppressing protein bcl-2, in neuroblastoma is associated with unfavorable histology and N-myc amplification. Am J Pathol. 1993;143:1543–1550. [PMC free article] [PubMed] [Google Scholar]
- 12.Dole MG, Jasty R, Cooper MJ, Thompson CB, Nunez G, Castle VP. Bcl-xL is expressed in neuroblastoma cells and modulates chemotherapyinduced apoptosis. Cancer Res. 1995;55:2576–2582. [PubMed] [Google Scholar]
- 13.Teitz T, Wei T, Valentine MB, Vanin EF, Grenet J, Valentine VA, Behm FG, Look AT, Lahti JM, Kidd VJ. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med. 2000;6:529–535. doi: 10.1038/75007. [DOI] [PubMed] [Google Scholar]
- 14.Poulaki V, Mitsiades N, Romero ME, Tsokos M. Fas-mediated apoptosis in neuroblastoma requires mitochondrial activation and is inhibited by FLICE inhibitor protein and Bcl-2. Cancer Res. 2001;61:4864–4872. [PubMed] [Google Scholar]
- 15.Featherstone C, Jackson SP. Ku, a DNA repair protein with multiple cellular functions? Mutat Res. 1999;434:3–15. doi: 10.1016/s0921-8777(99)00006-3. [DOI] [PubMed] [Google Scholar]
- 16.Cohen HY, Lavu S, Bitterman KJ, Hekking B, Imahiyerobo TA, Miller C, Frye R, Ploegh H, Kessler BM, Sinclair DA. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell. 2004);13:627–638. doi: 10.1016/s1097-2765(04)00094-2. [DOI] [PubMed] [Google Scholar]
- 17.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 18.Sawada M, Hayes P, Matsuyama S. Cytoprotective membrane-permeable peptides designed from the Bax-binding domain of Ku70. Nat Cell Biol. 2003;5:352–357. doi: 10.1038/ncb955. [DOI] [PubMed] [Google Scholar]
- 19.Keshelava N, Davicioni E, Wan Z, Ji L, Sposto R, Triche TJ, Reynolds CP. Histone deacetylase 1 gene expression and sensitization of multidrug-resistant neuroblastoma cell lines to cytotoxic agents by depsipeptide. J Natl Cancer Inst. 2007;99:1107–1119. doi: 10.1093/jnci/djm044. [DOI] [PubMed] [Google Scholar]
- 20.Oehme I, Deubzer HE, Wegener D, Pickert D, Linke JP, Hero B, Kopp-Schneider A, Westermann F, Ulrich SM, von Deimling A, et al. Histone deacetylase 8 in neuroblastoma tumorigenesis. Clin Cancer Res. 2009;15:91–99. doi: 10.1158/1078-0432.CCR-08-0684. [DOI] [PubMed] [Google Scholar]
- 21.Grozinger CM, Hassig CA, Schreiber SL. Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc Natl Acad Sci USA. 1999;96:4868–4873. doi: 10.1073/pnas.96.9.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verdel A, Khochbin S. Identification of a new family of higher eukaryotic histone deacetylases. Coordinate expression of differentiation-dependent chromatin modifiers. 1999;Biol Chem 274:2440–2445. doi: 10.1074/jbc.274.4.2440. [DOI] [PubMed] [Google Scholar]
- 23.Lee YS, Lim KH, Guo X, Kawaguchi Y, Gao Y, Barrientos T, Ordentlich P, Wang XF, Counter CM, Yao TP. The cytoplasmic deacetylase HDAC6 is required for efficient oncogenic tumorigenesis. Cancer Res. 2008;68:7561–7569. doi: 10.1158/0008-5472.CAN-08-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bazzaro M, Lin Z, Santillan A, Lee MK, Wang MC, Chan KC, Bristow RE, Mazitschek R, Bradner J, Roden RB. Ubiquitin proteasome system stress underlies synergistic killing of ovarian cancer cells by bortezomib and a novel HDAC6 inhibitor. Clin Cancer Res. 2008;14:7340–7347. doi: 10.1158/1078-0432.CCR-08-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z, Yamashita H, Toyama T, Sugiura H, Omoto Y, Ando Y, Mita K, Hamaguchi M, Hayashi S, Iwase H. HDAC6 expression is correlated with better survival in breast cancer. Clin Cancer Res. 2004;10:6962–6968. doi: 10.1158/1078-0432.CCR-04-0455. [DOI] [PubMed] [Google Scholar]
- 26.Aldana-Masangkay GI, Sakamoto KM. The role of HDAC6 in cancer. J Biomed Biotechnol. 2011;2011:875824. doi: 10.1155/2011/875824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyault C, Sadoul K, Pabion M, Khochbin S. HDAC6, at the crossroads between cytoskeleton and cell signaling by acetylation and ubiquitination. Oncogene. 2007;26:5468–5476. doi: 10.1038/sj.onc.1210614. [DOI] [PubMed] [Google Scholar]
- 28.Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci USA. 2003;100:4389–4394. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Yuan Z, Zhang Y, Yong S, Salas-Burgos A, Koomen J, Olashaw N, Parsons JT, Yang XJ, Dent SR, et al. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell. 2007;27:197–213. doi: 10.1016/j.molcel.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005;280:26729–26734. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- 31.Butler KV, Kalin J, Brochier C, Vistoli G, Langley B, Kozikowski AP. Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J Am Chem Soc. 2010;132:10842–10846. doi: 10.1021/ja102758v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozikowski AP, Chen Y, Gaysin AM, Savoy DN, Billadeau DD, Kim KH. Chemistry, biology, and QSAR studies of substituted biaryl hydroxamates and mercaptoacetamides as HDAC inhibitors-nanomolar-potency inhibitors of pancreatic cancer cell growth. ChemMedChem. 2008;3:487–501. doi: 10.1002/cmdc.200700314. [DOI] [PMC free article] [PubMed] [Google Scholar]