Abstract
OBJECTIVES
To evaluate time trends in surgical resection rates and operative mortality in older adults diagnosed with locoregional pancreatic cancer and to determine the effect of age on surgical resection rates and 2-year survival after surgical resection.
DESIGN
Retrospective cohort study using data from the Surveillance, Epidemiology, and End Results (SEER) and linked Medicare claims database (1992–2005).
SETTING
Secondary data analysis of population-based tumor registry and linked claims data.
PARTICIPANTS
Medicare beneficiaries aged 66 and older diagnosed with locoregional pancreatic cancer (N = 9,553), followed from date of diagnosis to time of death or censorship.
MEASUREMENTS
Percentage of participants undergoing surgical resection, 30-day operative mortality after resection, and 2-year survival according to age group.
RESULTS
Surgical resection rates increased significantly, from 20% in 1992 to 29% in 2005, whereas 30-day operative mortality rates decreased from 9% to 5%. After controlling for multiple factors, participants were less likely to be resected with older age. Resection was associated with lower hazard of death, regardless of age, with hazard ratios of 0.46, 0.51, 0.47, 0.43, and 0.35 for resected participants younger than 70, 70 to 74, 75 to 79, 80 to 84, and 85 and older respectively compared with unresected participants younger than 70 (P<.001).
CONCLUSION
With older age, fewer people with pancreatic cancer undergo surgical resection, even after controlling for comorbidity and other factors. This study demonstrated increased resection rates over time in all age groups, along with lower surgical mortality rates. Despite previous reports of greater morbidity and mortality after pancreatic resection in older adults, the benefit of resection does not diminish with older age in selected people.
Keywords: age, pancreatic resection, short-term outcomes
Pancreatic cancer is the fourth leading cause of cancer deaths in men and women in the United States.1 The overall annual incidence of pancreatic cancer is approximately 11 cases per 100,000 people, and it increases sharply with age. People aged 20 to 29 have an annual incidence of 0.1 cases of pancreatic cancer per 100,000 population, whereas those aged 80 and older have an annual incidence of 87.2 cases per 100,000 population.2 Mean age at the time of diagnosis is 70.3
Pancreatic resection is the only potentially curative option for people with pancreatic cancer. The 5-year survival after pancreaticoduodenectomy for pancreatic cancer is approximately 15% to 20%.4 In the 1970s and early 1980s, the high morbidity and mortality rates after pancreatic surgery led many surgeons to recommend palliative care for people with pancreatic and other periampullary cancers.5,6 In the last 3 decades, mortality rates after pancreatic resection have dropped to less than 2% at experienced centers,7–11 but complication rates remain high, exceeding 30% in many series.12–14
Initially, pancreatic resection was not commonly performed in people aged 70 and older and rarely, if ever, in people aged 80 and older. As pancreatic surgery became safer and more commonly performed, the indications broadened, and many individual centers began reporting their results after pancreatic resection in older adults.
Some studies from high-volume centers report no difference in mortality15–18 or survival16,19 with older age. Other studies report statistically higher morbidity rates in older adults than in younger people.16,17,20,21 It is important to remember that people undergoing surgery, whatever their age, would be selected for good underlying health. Two population-based studies demonstrated greater inhospital mortality, longer length of stay, and greater likelihood of discharge to a skilled nursing facility with older age.22,23 One of the population-based studies evaluated long-term survival and found shorter long-term survival with older age and greater number of comorbidities.22
The complex nature of pancreatic surgery and the low survival rates after pancreatic resection for pancreatic cancer make decisions regarding such surgery in the elderly population difficult. Previous studies evaluated outcomes in people who underwent resection but did not examine the effect of age on surgical resection in all people with locoregional pancreatic cancer. No population-based studies have evaluated time trends in surgical resection rates and associated operative mortality in older adults. In addition, it is unclear whether the greater morbidity and mortality observed in population-based studies negate the benefit of surgical resection in this population. The goals of the current study were threefold. First, the effect of age on surgical resection in people with early-stage pancreatic cancer was determined. Second, time trends in surgical resection rates and operative mortality for the overall cohort and according to age group were evaluated. Third, the effect of the interaction between age and surgical resection on long-term survival was evaluated to determine whether resection is still of benefit in older adults.
METHODS
The institutional review board at the University of Texas Medical Branch at Galveston approved the study.
Data Source
Data from the Surveillance, Epidemiology, and End Results (SEER)–Medicare linked database were used for the analysis. The SEER tumor registry, sponsored by the National Cancer Institute, is derived from specific geographic areas currently representing 26% of the U.S. population.24
The SEER–Medicare linked database is a linkage of two large population-based sources of data: the SEER tumor registry and the Medicare claims data collected by the Centers for Medicare and Medicaid Services. This data set provides detailed information about elderly adults with cancer.25 Approximately 93% of all people aged 65 and older in SEER are matched with Medicare enrollment and claims files. SEER collects and provides information on participant demographics, cancer prevalence, cancer incidence, stage of disease, first course of therapy, and survival. The Medicare claims data include information on hospital stays, physician services, and hospital outpatient visits. A data use agreement has been signed. The data used in this proposal include participants diagnosed with pancreatic cancer between 1992 and 2005, inclusive, and their Medicare claims through 2007.
Cohort Selection
Using the SEER–Medicare linked data, the cohort was identified using the following inclusion criteria: people with International Classification of Diseases (ICD) for Oncology-3 histology codes consistent with adenocarcinoma (neuroendocrine and acinar cell cancers excluded), people with localized or regional (locoregional) pancreatic cancer based on SEER historic stage, people diagnosed between 1992 and 2005, people with pancreatic cancer as their first primary cancer, people enrolled in Medicare Part A and B not in a health maintenance organization for 12 months before and 3 months after their cancer diagnosis, and people aged 66 and older. People diagnosed at autopsy or according to death certificate only were excluded.
In 2004, SEER changed the coding and abstracting rules for tumor stage, using all information gathered through completion of all surgical procedures in first course of treatment or all information available within 4 months of the date of diagnosis in the absence of disease progression, whichever is longer. The new collaborative staging system represents the aggregate information obtained during the period of diagnosis and examination, not just the initial contact with the person. For example, if diagnostic tests or surgery within 4 months show more-precise extension or more-precise tumor size, this revised information goes in initial stage and is not considered disease progression. The new system may lead to more-accurate staging and a stage migration phenomenon whereby both stages will appear to have better survival. For this reason, localized and regional disease were not analyzed separately but were considered as a group.
Variable Definitions
A participant was considered to have undergone curative resection if the ICD, Ninth Revision, procedure codes for total pancreatectomy, pancreaticoduodenectomy, distal pancreatectomy, or other pancreatic resection (52.6, 52.7, 52.51, 52.52, 52.53, 52.59) were identified in the inpatient Medicare claims files. Participant were considered to have been evaluated by a surgeon if they had surgery or were seen by a surgeon as identified by the Unique Physician Identification Number and Medicare specialty claims codes for general surgeon (specialty claim code 02). Participants who saw a physician with a specialty claim for general practice (01), family practice (08), internal medicine (11), or geriatrics (38) in the year before diagnosis were designated as having a primary care physician.
Participants were divided into five age groups for the purpose of analysis (<70, 70–74, 75–79, 80–84, and ≥85). For time trend analyses examining surgical resection rates and operative mortality, participant age was categorized as younger than 70, 70 to 79, and 80 and older to increase stability of estimates.
The SEER–Medicare claims files do not provide individual-level information on socioeconomic status. As a surrogate, ZIP code–level data from the 2000 census on median income and percentage of participants in the ZIP codes with less than 12 years of education were used. Income and education were analyzed as quartiles in the multivariate models.
To evaluate the effect of participant comorbidities on surgical evaluation, resection, and long-term survival, Klabunde’s modification of the Charlson Comorbidity Index was used.26 Participants were classified as having zero, one, two, or three or more comorbidities.
Statistical Analysis
All statistical analysis was performed using SAS software, version 9.2 (SAS Institute, Inc., Cary, NC). Summary statistics were calculated for the entire cohort of participants, including demographic informaiton, socioeconomic status, tumor characteristics, and surgical resection rates. Time trends were evaluated using a Cochran-Armitage test for trend. Surgical resection and long-term survival were compared between five age groups in univariate and multivariate models. Chi-square analysis was used to compare proportions for all categorical data. The reported chi-square P-values represent an overall test for difference between any of the five groups; pairwise comparisons were not performed. All values are reported as means ± standard deviations. Significance was accepted at the P<.05 level.
Multivariate Analysis: Surgical Evaluation and Surgical Resection
Multivariate logistic regression models were used to determine the adjusted odds ratios (ORs) for surgical resection in each age group to determine the independent effect of age on surgical resection rates. All models controlled for year of diagnosis, sex, race, marital status, socioeconomic status, SEER region, nodal status, whether a participant had a primary care physician, operability of the tumor based on SEER extent of disease codes, and Charlson comorbidity score. Covariates were removed from the model in backward sequential fashion based on Akaike information criteria and the deviance per degree of freedom. Clinically relevant variables were forced into the model even if they did not reach statistical significance.
Survival Analysis
Survival from the time of diagnosis was calculated based on Medicare date of death. All participants were followed for at least 2 years from the time of SEER diagnosis of pancreatic cancer. Although longer follow-up was available in many participants, the survival analysis was truncated at 2 years, with subjects surviving more than 2 years censored at the 2-year time point. A 2-year cutoff was chosen because the cohort was selected through 2005. Therefore, complete 2-year follow-up was available on all participants, whereas complete 5-year follow-up was not available on all participants after 2002, although 5-year survival was also evaluated, censoring participants as alive at the date of last Medicare follow-up, and the results and conclusions were identical. Survival analyses estimated all-cause mortality rather than disease-specific mortality because, although date of death was available, cause of death was not available in SEER–Medicare data for participants dying in 2006 or 2007. Pancreatic cancer–specific survival was also evaluated using a follow-up cut off of December 2005 and censoring participants who died of other causes, and the direction and magnitude of the hazard ratios were similar. These analyses are not reported.
An unadjusted survival analysis was first performed using the Kaplan-Meier method. Survival curves were generated for the entire cohort and compared according to age group, resection status, and other variables of interest. Log-rank tests were used to identify overall differences between groups.
Using Cox proportional hazards regression models, a multivariate survival analysis was performed. All clinically significant variables were put into the initial model. Backward elimination was then used for nonsignificant covariates. Akaike information criteria were used to select the most parsimonious model. The overall model fit was determined using Cox-Snell residuals. The assumption of proportionality for variables of interest was determined by comparing the hazard curves and evaluating Schoenfeld residuals. Type 3 P-values and the individual hazard ratios and 95% confidence intervals (CIs) for each category are presented for all variables of interest. Significance was accepted at the P<.05 level.
After choosing the final model with the main covariates, the effect of the interaction between resection and age group on long-term survival was examined. The interaction term was added to the model, and contrast statements were used to generate hazard ratios and 95% confidence intervals based on age group and resection status.
RESULTS
Overall Cohort
Nine thousand five hundred fifty-three participants with locoregional pancreatic adenocarcinoma met the inclusion criteria. The mean age for the entire cohort was 77.0 ± 7.0. Table 1 shows participant characteristics, vital status, and surgical evaluation and resection for the overall cohort and according to age group. Sixteen percent of participants were younger than 70, 24% were aged 70 to 74, 26% were aged 75 to 79, 19% were aged 80 to 84 years, and 16% were aged 85 and older. Participants were more likely to be female, non-Hispanic white, and married. More than half of the cohort had a Charlson comorbidity score of 0. There were more participants in later years because several SEER registries were added in 2000 or later. Approximately 69% of participants with locoregional pancreatic cancer were evaluated by a surgeon, and 25% (n = 2,393) of participants underwent resection with curative intent. At 2-year follow-up, 1,305 participants (14%) remained alive.
Table 1.
Demographics, Comorbidity, Vital Status, Surgical Evaluation, and Resection for Total Cohort and According to Age Group
| Patient Characteristic | % | P-Value | |||||
|---|---|---|---|---|---|---|---|
| Overall (N = 9,553) | <70 (n = 1,519) | 70–74 (n = 2,308) | 75–79 (n = 2,459) | 80–84 (n = 1,772) | ≥85 (n = 1,495) | ||
| Female | 58 | 50 | 55 | 57 | 60 | 69 | <.001 |
| Race | |||||||
| Non-Hispanic white | 80 | 77 | 78 | 80 | 84 | 82 | <.001 |
| Non-Hispanic black | 9 | 11 | 10 | 9 | 7 | 8 | |
| Hispanic | 5 | 6 | 6 | 5 | 4 | 5 | |
| Other | 6 | 6 | 5 | 6 | 5 | 5 | |
| Marital status | |||||||
| Married | 51 | 65 | 60 | 54 | 45 | 28 | <.001 |
| Widowed | 32 | 14 | 21 | 30 | 42 | 59 | |
| Charlson comorbidity score | |||||||
| 0 | 56 | 61 | 57 | 56 | 55 | 53 | <.001 |
| 1 | 26 | 23 | 26 | 27 | 26 | 26 | |
| 2 | 11 | 10 | 10 | 10 | 11 | 11 | |
| 3 | 7 | 5 | 7 | 7 | 8 | 10 | |
| Alive at 2 years | 14 | 20 | 18 | 14 | 9 | 5 | <.001 |
| Surgical evaluation | 69 | 81 | 78 | 72 | 63 | 45 | <.001 |
| Charlson comorbidity score of those undergoing surgical evaluation | |||||||
| 0 | 71 | 80 | 79 | 73 | 66 | 47 | |
| 1 | 69 | 83 | 79 | 73 | 61 | 41 | |
| 2 | 68 | 85 | 79 | 71 | 57 | 44 | |
| ≥3 | 61 | 73 | 69 | 65 | 58 | 45 | |
| Surgical resection | 25 | 39 | 34 | 27 | 17 | 4 | <.001 |
| Charlson comorbidity score of those undergoing surgical resection | |||||||
| 0 | 28 | 39 | 37 | 30 | 20 | 5 | |
| 1 | 25 | 41 | 33 | 27 | 17 | 4 | |
| 2 | 20 | 37 | 30 | 18 | 11 | 3 | |
| ≥3 | 13 | 27 | 19 | 15 | 10 | 1 | |
| 30-day mortality | 7.4 | 7.0 | 6.9 | 8.1 | 6.8 | 11.5 | .41 |
Older participants were more likely to be female, non-Hispanic white, and widowed (Table 1). The distribution of Charlson comorbidity scores differed between age groups, with older participants being less likely to have no comorbidities. As expected, deaths increased with increasing age.
Time Trends in Resection Rates and Operative Mortality
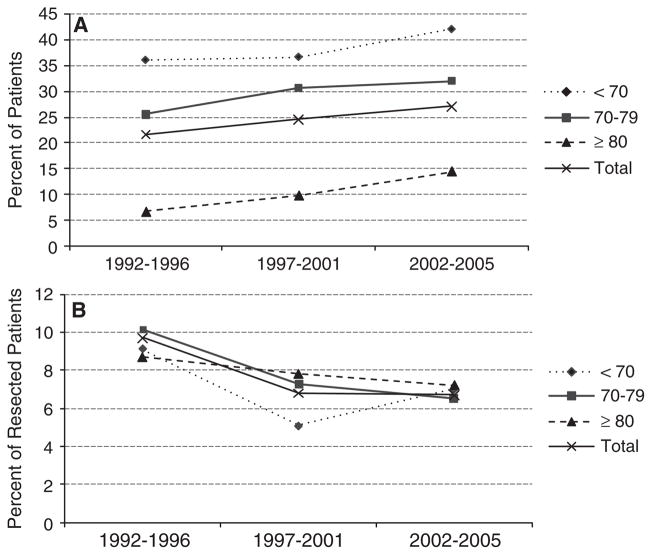
Time trends in rates of surgical resection and 30-day operative mortality were evaluated from 1992 to 2005 for the overall cohort and according to age group. Surgical resection rates for participants with locoregional pancreatic cancer increased from 20% of participants in 1992/93 to 29% in 2004/05 (P<.001). Surgical resection rates significantly increased for all age groups over the study period (Figure 1A). At the same time, 30-day operative mortality rates decreased from 9% of resected participants in 1992/ 93 to 5% in 2004/05 (P = .03). This trend was apparent for each age group but was not statistically significant (Figure 1B). Similar to previous studies, operative mortality increased with increasing age: 30-day mortality increased from 7.0% in participants younger than 70 to 11.5% in participants aged 85 and older (P = .41, Table 1).
Figure 1.
Time trends in rates of (A) surgical resection and (B) 30-day mortality after surgical resection from 1992–1996 to 2002–2005 for the overall cohort and acdording to age group. Surveillance, Epidemiology, and End Results–Medicare linked data.
In multivariate logistic regression analyses controlling for sex, race, marital status, SEER region, Charlson comorbidity score, site of tumor, extent of disease, and population, participants were 11% more likely to be resected with each increasing year of diagnosis (OR = 1.11, 95% CI = 1.09–1.13). For resected participants, 30-day operative mortality decreased 4% with each year of diagnosis, according to logistic regression models controlling for all covariates (OR = 0.96, 95% CI = 0.91–1.01), although the trend was not statistically significant.
Surgical Evaluation and Resection Status According to Age Group
With older age, participants with locoregional pancreatic cancer were less likely to undergo evaluation by a surgeon (81% of participants <70 vs 45% of participants ≥85; P<.001). This was true regardless of comorbidity status. Even in participants with no comorbidities, the percentage of participants evaluated by a surgeon decreased from 80% to 47% with older age (Table 1). In addition to being less likely to see a surgeon, according to univariate analysis, older participants with locoregional pancreatic cancer were less likely to undergo surgical resection. Even in participants with no comorbidities, surgical resection rates decreased from 39% in participants younger than 70 to 5% in participants aged 85 and older (Table 1).
In a multivariate logistic regression analysis controlling for year of diagnosis, race, Charlson comorbidity score, site of tumor, extent of disease, population, and SEER region, older age was an independent negative predictor of surgical resection. Nine thousand one hundred forty-three participants had no missing data for all covariates. Participants aged 70 to 74 were 21% less likely (OR = 0.79, 95% CI = 0.69–0.92), participants aged 75 to 79 were 47% less likely (OR = 0.53, 95% CI = 0.46–0.61), participants aged 80 to 84 were 72% less likely (OR = 0.28, 95% CI = 0.23–0.33), and participants aged 85 and older were 94% less likely than those younger than 70 to undergo surgical resection (OR = 0.06, 95% CI = 0.05–0.08).
Long-Term Survival
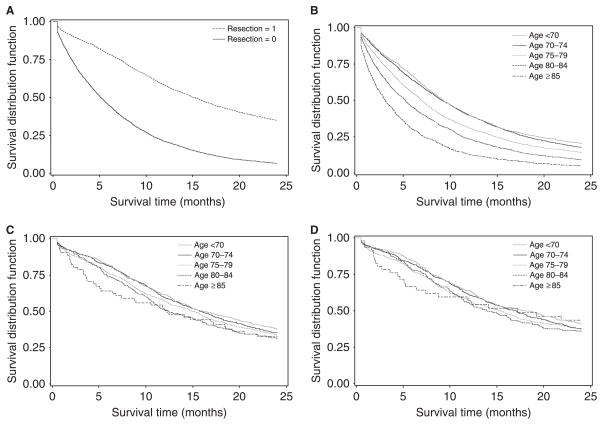
Overall survival, survival according to resection status, and survival according to age group are summarized in Table 2. Overall Kaplan-Meier survival for the entire cohort was 30% at 1 year and 14% at 2 years (median 6.7 months). In the unadjusted survival analysis, surgical resection was associated with longer survival. Two-year survival was 35% in resected participants and 7% in unresected participants (Figure 2A, Table 2, P<.001). Overall survival decreased with increasing age, with 2-year survival rates of 20%, 18%, 14%, 9%, and 5% for each age group, respectively (Figure 2B, Table 2, P<.001).
Table 2.
Unadjusted Kaplan-Meier Survival According to Age and Resection Status for All-Cause Mortality in Older Adults with Locoregional Pancreatic Cancer
| Group (N/n censored) | 1-Year Survival | 2-Year Survival | Median, months | P-Value |
|---|---|---|---|---|
| Overall (9,553/1,305) | 30 | 14 | 6.7 | <.001 |
| Resected (2,393/832) | 58 | 35 | 15.2 | <.001 |
| Unresected (7,160/473) | 21 | 7 | 5.1 | |
| <70 (1,519/310) | 40 | 20 | 9.3 | <.001 |
| 70–74 (2,308/410) | 40 | 18 | 9.2 | |
| 75–79 (2,459/346) | 31 | 14 | 6.9 | |
| 80–84 (1,772/160) | 24 | 9 | 5.2 | |
| ≥85 (1,495/79) | 13 | 5 | 3.1 | |
| Resected participants | ||||
| <70 (589/222) | 61 | 38 | 16.1 | .08 |
| 70–74 (779/275) | 60 | 35 | 15.8 | |
| 75–79 (655/219) | 57 | 33 | 14.9 | |
| 80–84 (309/96) | 51 | 31 | 12.4 | |
| ≥85 (61/20) | 51 | 33 | 12.3 | |
Figure 2.
Unadjusted Kaplan-Meier survival curves showing all-cause mortality over 2 years of follow-up according to (A) surgical resection status, (B) age group, and (C) age group after surgical resection and (D) pancreatic cancer–specific mortality through 2005 according to age group after surgical resection. Surveillance, Epidemiology, and End Results–Medicare linked data.
For resected participants, the unadjusted Kaplan-Meier survival curves were nearly overlapping for participants of all age groups. Survival was not significantly different between the five age groups according to the log-rank test (Figure 2C, Table 2, P = .08).
The multivariate survival analysis controlled for year of diagnosis, race, sex, comorbidity, marital status, income, education, site of tumor, population, and SEER region. In the final parsimonious multivariate Cox proportional hazards survival model, year of diagnosis (P = .004), age group (P<.001), surgical resection (P<.001), education (P< .001), Charlson score (P<.001), nodal status (P<.001), marital status (P<.001), and SEER region (P = .001) were independent predictors of survival. To determine whether older age negated the benefit of surgical resection, an age group–by-resection interaction term was added to the model. This interaction was not significant (P = .11), indicating that the benefit of surgical resection did not significantly change with age. Table 3 shows the hazard ratios and 95% CIs for resected and unresected participants in each age group. Hazard ratios for resected participants were similar across age groups. Resected participants younger than 70 were 54% less likely, resected participants aged 70 to 74 were 49% less likely, resected participants aged 75 to 79 were 53% less likely, resected participants aged 80 to 84 were 57% less likely, and resected participants aged 85 and older were 65% less likely than unresected participants younger than 70 to die of pancreatic cancer.
Table 3.
Hazard of Death According to Resection Status and Age
| Group | HR (95% Confidence Interval) |
|---|---|
| Unresected | |
| <70 (reference group)* | 1.00 |
| 70–74 | 1.11 (0.94–1.30) |
| 75–79 | 1.01 (0.85–1.19) |
| 80–84 | 0.93 (0.76–1.13) |
| ≥85 | 0.76 (0.54–1.06) |
| Resected | |
| <70 | 0.46 (0.41–0.53) |
| 70–74 | 0.51 (0.46–0.58) |
| 75–79 | 0.47 (0.41–0.53) |
| 80–84 | 0.43 (0.37–0.50) |
| ≥85 | 0.35 (0.26–0.48) |
All hazard ratios (HRs) are compared with unresected participants <70. Model controls for year of diagnosis, education, Charlson score, marital status, node, and Surveillance, Epidemiology, and End Results region.
DISCUSSION
This study demonstrates that older age is an independent predictor of surgical resection in people with locoregional pancreatic cancer. After controlling for all covariates, age was negatively associated with surgical resection, with odds of resection decreasing from 0.79 in participants aged 70 to 74 to 0.06 in participants aged 85 and older, compared with participants younger than 70, although this study also demonstrates that resection rates have increased over time for all older adults, with decreasing operative mortality rates in the same time period. Moreover, in participants who underwent pancreatic resection for early-stage pancreatic cancer, the benefit of surgical resection did not decrease with increasing age. Regardless of age group, participants undergoing surgical resection were less than half as likely to die as the youngest group of unresected participants.
Prior studies evaluating the influence of age on outcomes after pancreatic resection did not include a comparison group of unresected participants to examine how age influences selection for surgery. The current study is the first to examine these issues, demonstrating that age is still used as a criterion for resection of locoregional pancreatic cancer, with fewer elderly adults undergoing surgical evaluation and resection after controlling for participant comorbidity and other factors. Even in people with no comorbidities, surgical evaluation rates decreased from 80% in participants younger than 70 to 47% in participants aged 85 and older. Similarly, surgical resection rates in participants with no comorbidities was 39% in participants younger than 70 to 5% in participants aged 85 and older. These data suggest that surgical evaluation rates and resection rates can be improved across age groups, with a goal of 100% surgical evaluation in people of all ages with no comorbidities.
With regard to operative morbidity and mortality, these data are consistent with two previously published population-based studies.22,23 The first study, using the Nationwide Inpatient Sample from 1994 to 2003, demonstrated that inhospital mortality was 6.7% in people aged 65 to 69 and 15.5% in those aged 80 and older. Length of stay increased from a mean of 17 days in the youngest group to 20 days in the oldest group. For participants surviving surgery, discharge to a skilled nursing facility increased from 6% in participants aged 65 to 69 to 25% in participants aged 80 and older.22 A second study using Texas Hospital discharge data from 1999 to 2005 showed similar findings, with in-hospital mortality increasing from 5.8% for participants aged 60 to 69 to 11.4% for participants aged 80 and older. Discharge to a SNF was 3% for participants younger than 60 and 38% for those aged 80 and older, and length of stay increased from a median of 11 days to 15 days, respectively.23 The current study, using a different population-based cohort, confirms these findings, with in-hospital mortality of 7.0% in participants younger than 70 and 11.5% in participants aged 85 and older. Although not statistically significant, probably because of the smaller number of participants and older age distribution of the cohort, this observed higher mortality in older participants is similar to that found in the two previously discussed studies. In addition, the Texas cohort included participants with benign disease who were more often younger, perhaps widening the mortality difference between age groups. This study differs from the previous studies in that it has the ability to evaluate 30-day mortality and not only in-hospital mortality.
The median survival rates reported in the current study of 12 to 16 months in resected participants are similar to those in previous population-based studies.3,22,27 These population-based rates differ significantly from recent single-institution studies, which report a median survival after resection of 19 to 24 months.14,28 This reflects the fact that single-institution data may not be representative of actual practice in the general population. Single-institution data come from high-volume centers with significant experience. These centers have lower operative morbidity and mortality. In addition, the published data are often part of trials for adjuvant therapy in which all participants are receiving adjuvant therapy, which is not the case in this cohort. This highlights the need for population-based studies.
The age-specific long-term survival estimates confirm previous results also using SEER-Medicare data.22 Minor differences may be attributed to different censoring. That study found that survival at 5 years decreased from 16.4% for participants aged 65 to 69 undergoing pancreatectomy for pancreatic cancer to 11.3% for participants aged 80 and older. When extrapolated from that study’s 5-year survival curves, 2-year survival was approximately 38% (median ~ 16 months) in the group aged 65 to 69 and 38% in those aged 80 and older (median ~ 14 months) with no comorbidities and decreased to approximately 22% in those aged 80 and older (median ~ 12 months) with two or more comorbidities.22 The current study reports 2-year survival rates of 38% for participants younger than 70 and 33% for participants aged 85 and older, with lower median survival in the older participants. The fact that people aged 80 and older have greater operative mortality primarily explains this, and these early deaths decrease their median survival, although 2-year survival, although slightly lower in octogenarians, is clinically and statistically similar between the groups. In addition, older adults without pancreatic cancer would be expected to have lower median survival than their younger counterparts, which can also partly explain the shorter median survival.
The current study has several limitations. Because Medicare data were used, all participants were aged 66 and older. The influence of age on outcomes might be underestimated because the youngest group (reference group, aged 66–70) represents the approximate mean age at presentation for pancreatic cancer.1 Only 2-year survival was reported because all participants in the study were followed for at least 2 years, although when 5-year survival (not reported) was evaluated, the conclusions were identical and the results similar to previous population-based reports.
As with all observational studies, selection bias limited the current study. The use of multivariate statistics can control for comorbidities and other factors, but the assessment of comorbidities in claims data may not be accurate or complete, limiting the ability to adequately risk adjust for case-mix differences across age groups.29 In addition, factors such as frailty, exercise tolerance, and social support in older adults cannot be measured using administrative data. Furthermore, the reasons for lack of surgical evaluation or resection cannot be addressed, and it is possible that many older adults refuse such care.
Older adults are also more likely to die before undergoing surgical evaluation or resection. In this study, participants who died within 3 months of diagnosis were older (mean age 79.6) than the total sample, and only approximately 10% of these participants underwent surgical resection. However, restricting analyses to participants surviving longer than 3 months after diagnosis would underestimate operative mortality rates for older adults. In participants who died within 3 months of diagnosis, 30-day operative mortality rates after surgical resection ranged from 66% for adults younger than 70 to 53% for adults aged 80 and older. Sensitivity analyses excluding participants who died within 3 months of diagnosis were conducted, and results were substantively equivalent.
Survival analyses estimated all-cause mortality rather than disease-specific mortality because, although date of death was available, cause of death was not available in SEER–Medicare data for people dying in 2006 or 2007. Operative mortality and overall mortality from other causes increase with age. Because whether surgery was still of benefit despite the greater operative and overall mortality observed in older adults was being asked, it was felt that it was critical to account for noncancer deaths in this analysis because they are a significant factor in the decision-making process regarding the risks and benefits of surgery. In addition, the percentage of people with pancreatic cancer dying of other causes is approximately 10%. Pancreatic cancer–specific survival was also evaluated using a follow-up cut-off of December 2005 and censoring people who died of other causes, and the direction and magnitude of the hazard ratios were similar. These analyses are not reported. In sum, despite the above limitations, in carefully selected people, the benefit of surgical resection does not decrease with increasing age, despite the greater morbidity and mortality associated with surgical resection.
This study has several important implications for older adults with pancreatic cancer. The complex nature of pancreatic surgery and the low survival rates after pancreatic resection for pancreatic cancer make decisions regarding surgery in the elderly population difficult. These data indicate the need for greater rates of surgical evaluation and surgical resection in older adults with locoregional pancreatic cancer. It is possible that surgical resection rates are not maximized in older adults, as evidenced by the low rates of evaluation and resection even in people with no other medical problems. Furthermore, increasing resection rates without increasing operative mortality over the last decade suggest the same. This study presents important additional information to provide when counseling older adults considering pancreatic resection for locoregional pancreatic cancer. It is important that they understand their greater risk of mortality, complications, and need for skilled nursing care after pancreatic surgery when making this decision, but it is equally important that they understand the benefit of surgical resection and that this benefit is not significantly diminished with increasing age despite the greater short-term complications.
Acknowledgments
This research was supported in part by the Dennis W. Jahnigen Career Development Scholars Award, National Institutes of Health (NIH) Prevention, Control, and Population Sciences Career Development Award (K07CA130983-01A1) and NIH Established Investigator Award in Cancer Prevention and Control (K05CA134923).
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute SEER Program under Contract N01-PC-35136 awarded to the Northern California Cancer Center, Contract N01-PC-35139 awarded to the University of Southern California, and Contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention National Program of Cancer Registries, under agreement U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s), and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended, nor should it be inferred. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc.; and the SEER Program tumor registries in the creation of the SEER–Medicare database.
Sponsor’s Role: The sponsor was not involved in the design, methods, data analysis, or preparation of the manuscript.
Footnotes
Conflict of Interest: The authors have no financial or any other kind of personal conflicts of interest to report.
Author Contributions: Riall: study concept and design, data analysis, interpretation of data, and manuscript preparation. Sheffield: data analysis, interpretation of data, and manuscript preparation. Kuo: acquisition of data, data analysis, and interpretation of data. Townsend and Goodwin: study concept and design, interpretation of data, and critical review of manuscript.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed September 2, 2008.];Medscape [on-line] Available at http://www.medscape.com/viewarticle/537123_3.
- 3.Riall TS, Nealon WH, Goodwin JS, et al. Pancreatic cancer in the general population: Improvements in survival over the last decade. J Gastrointest Surg. 2006;10:1212–1224. doi: 10.1016/j.gassur.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Riall TS, Cameron JL, Lillemoe KD, et al. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 2006;140:764–772. doi: 10.1016/j.surg.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Crile G., Jr The advantages of bypass operations over radical pancreatoduodenectomy in the treatment of pancreatic carcinoma. Surg Gynecol Obstet. 1970;130:1049–1053. [PubMed] [Google Scholar]
- 6.Shapiro TM. Adenocarcinoma of the pancreas: A statistical analysis of biliary bypass vs Whipple resection in good risk patients. Ann Surg. 1975;182:715–721. doi: 10.1097/00000658-197512000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68–73. doi: 10.1016/s0002-9610(05)80406-4. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy EP, Rosato EL, Sauter PK, et al. Initiation of a critical pathway for pancreaticoduodenectomy at an academic institution–the first step in multi-disciplinary team building. J Am Coll Surg. 2007;204:917–924. doi: 10.1016/j.jamcollsurg.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 9.Porter GA, Pisters PW, Mansyur C, et al. Cost and utilization impact of a clinical pathway for patients undergoing pancreaticoduodenectomy. Ann Surg Oncol. 2000;7:484–489. doi: 10.1007/s10434-000-0484-0. [DOI] [PubMed] [Google Scholar]
- 10.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: Results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 11.Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg. 1990;211:447–458. doi: 10.1097/00000658-199004000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balcom JH, Rattner DW, Warshaw AL, et al. Ten-year experience with 733 pancreatic resections: Changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391–398. doi: 10.1001/archsurg.136.4.391. [DOI] [PubMed] [Google Scholar]
- 13.Emick DM, Riall TS, Cameron JL, et al. Hospital readmission after pancreaticoduodenectomy. J Gastrointest Surg. 2006;10:1243–1253. doi: 10.1016/j.gassur.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Winter JM, Cameron JL, Campbell KA, et al. 1,423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199–1211. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Fong Y, Blumgart LH, Fortner JG, et al. Pancreatic or liver resection for malignancy is safe and effective for the elderly. Ann Surg. 1995;222:426–437. doi: 10.1097/00000658-199522240-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makary MA, Winter JM, Cameron JL, et al. Pancreaticoduodenectomy in the very elderly. J Gastrointest Surg. 2006;10:347–356. doi: 10.1016/j.gassur.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Scurtu R, Bachellier P, Oussoultzoglou E, et al. Outcome after pancreaticoduodenectomy for cancer in elderly patients. J Gastrointest Surg. 2006;10:813–822. doi: 10.1016/j.gassur.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Sohn TA, Yeo CJ, Cameron JL, et al. Should pancreaticoduodenectomy be performed in octogenarians? J Gastrointest Surg. 1998;2:207–216. doi: 10.1016/s1091-255x(98)80014-0. [DOI] [PubMed] [Google Scholar]
- 19.Bathe OF, Caldera H, Hamilton KL, et al. Diminished benefit from resection of cancer of the head of the pancreas in patients of advanced age. J Surg Oncol. 2001;77:115–122. doi: 10.1002/jso.1081. [DOI] [PubMed] [Google Scholar]
- 20.Bathe OF, Levi D, Caldera H, et al. Radical resection of periampullary tumors in the elderly: Evaluation of long-term results. World J Surg. 2000;24:353–358. doi: 10.1007/s002689910056. [DOI] [PubMed] [Google Scholar]
- 21.Brozzetti S, Mazzoni G, Miccini M, et al. Surgical treatment of pancreatic head carcinoma in elderly patients. Arch Surg. 2006;141:137–142. doi: 10.1001/archsurg.141.2.137. [DOI] [PubMed] [Google Scholar]
- 22.Finlayson E, Fan Z, Birkmeyer JD. Outcomes in octogenarians undergoing high-risk cancer operation: A national study. J Am Coll Surg. 2007;205:729–734. doi: 10.1016/j.jamcollsurg.2007.06.307. [DOI] [PubMed] [Google Scholar]
- 23.Riall TS, Reddy DM, Nealon WH, et al. The effect of age on short-term outcomes after pancreatic resection: A population-based study. Ann Surg. 2008;248:459–467. doi: 10.1097/SLA.0b013e318185e1b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Cancer Institute. [Accessed October 26, 2009.];Surveillance, Epidemiology, and End Results [online] Available at http://seer.cancer.gov.
- 25.National Cancer Institute. [Accessed October 26, 2009.];Health Services and Economics. SEER-Medicare: A Brief Description of the SEER-Medicare Database [on-line] Available at http://healthservices.cancer.gov/seermedicare/overview.
- 26.Baldwin LM, Klabunde CN, Green P, et al. In search of the perfect comorbidity measure for use with administrative claims data: Does it exist? Med Care. 2006;44:745–753. doi: 10.1097/01.mlr.0000223475.70440.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cress RD, Yin D, Clarke L, et al. Survival among patients with adenocarcinoma of the pancreas: A population-based study. Cancer Cause Control. 2006;17:430–409. doi: 10.1007/s10552-005-0539-4. [DOI] [PubMed] [Google Scholar]
- 28.Neoptolemos JP, Stockton DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: A randomized controlled trial. JAMA. 2010;304:1073–1081. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 29.Fisher ES, Whaley FS, Krushat WM, et al. The accuracy of Medicare’s hospital claims data: Progress has been made, but problems remain. Am J Public Health. 1992;82:243–248. doi: 10.2105/ajph.82.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]