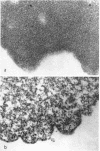
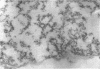
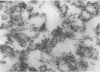
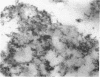
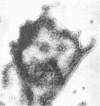
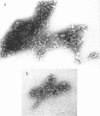
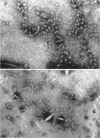
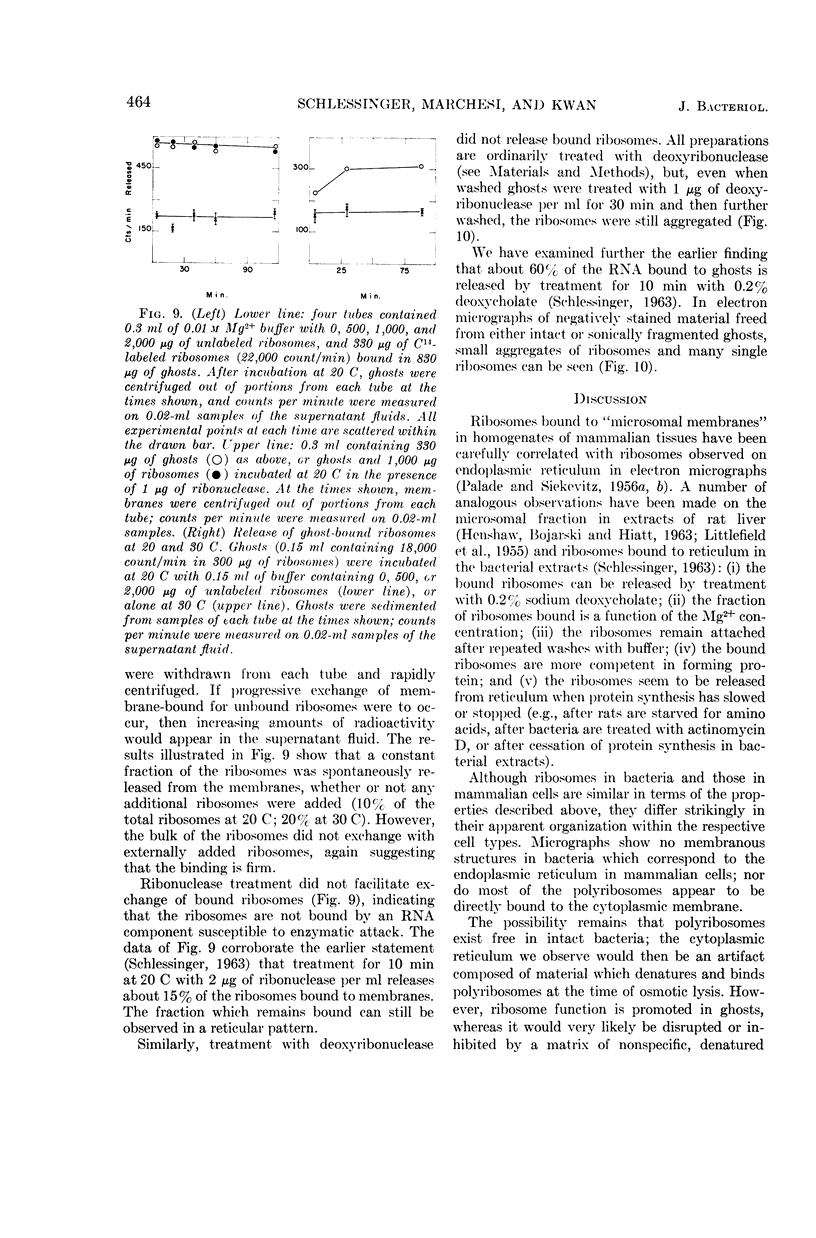
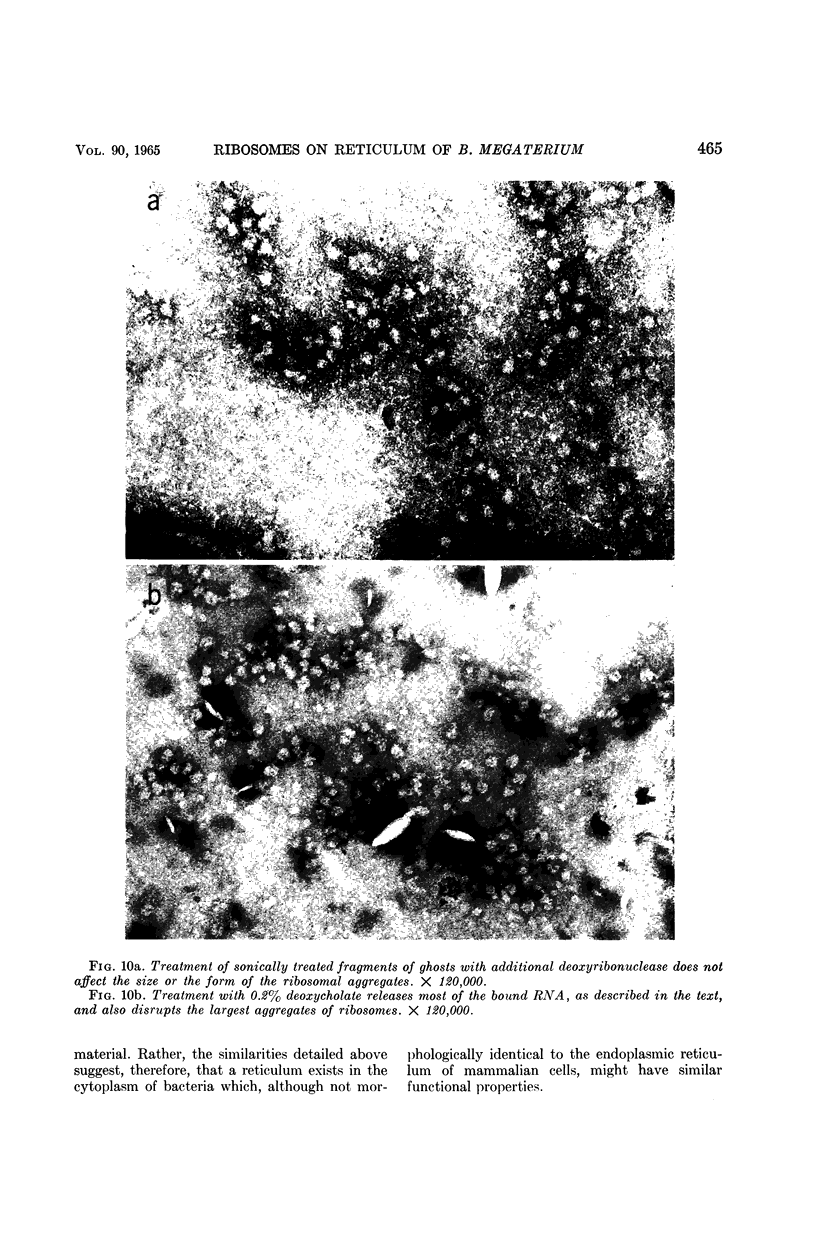
Abstract
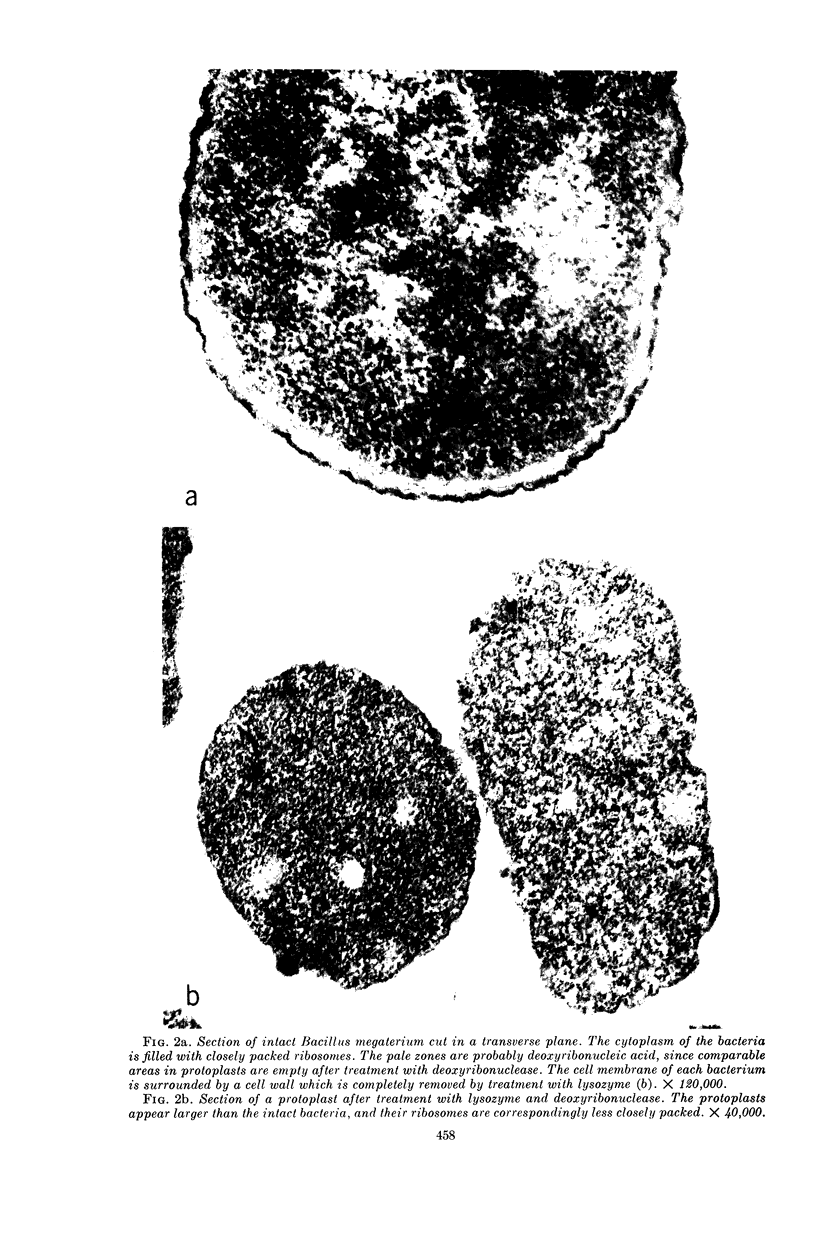
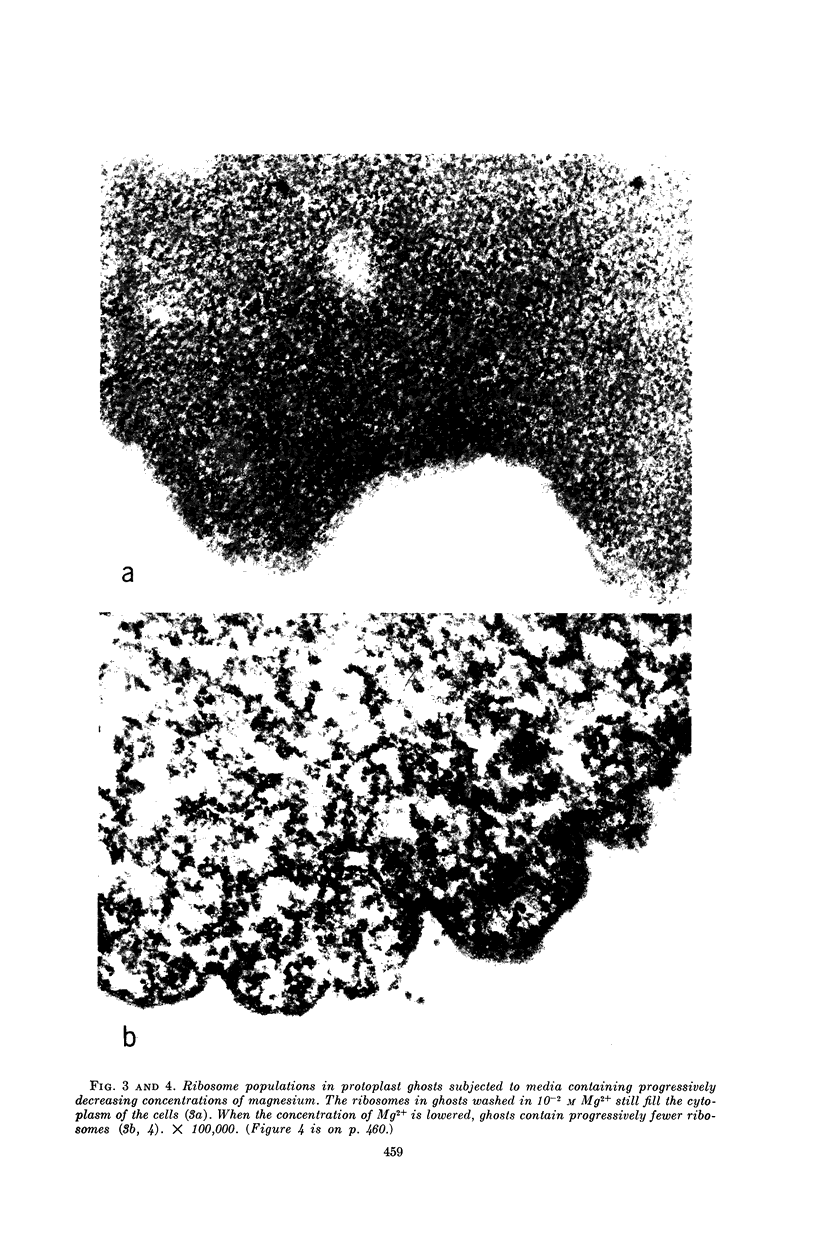
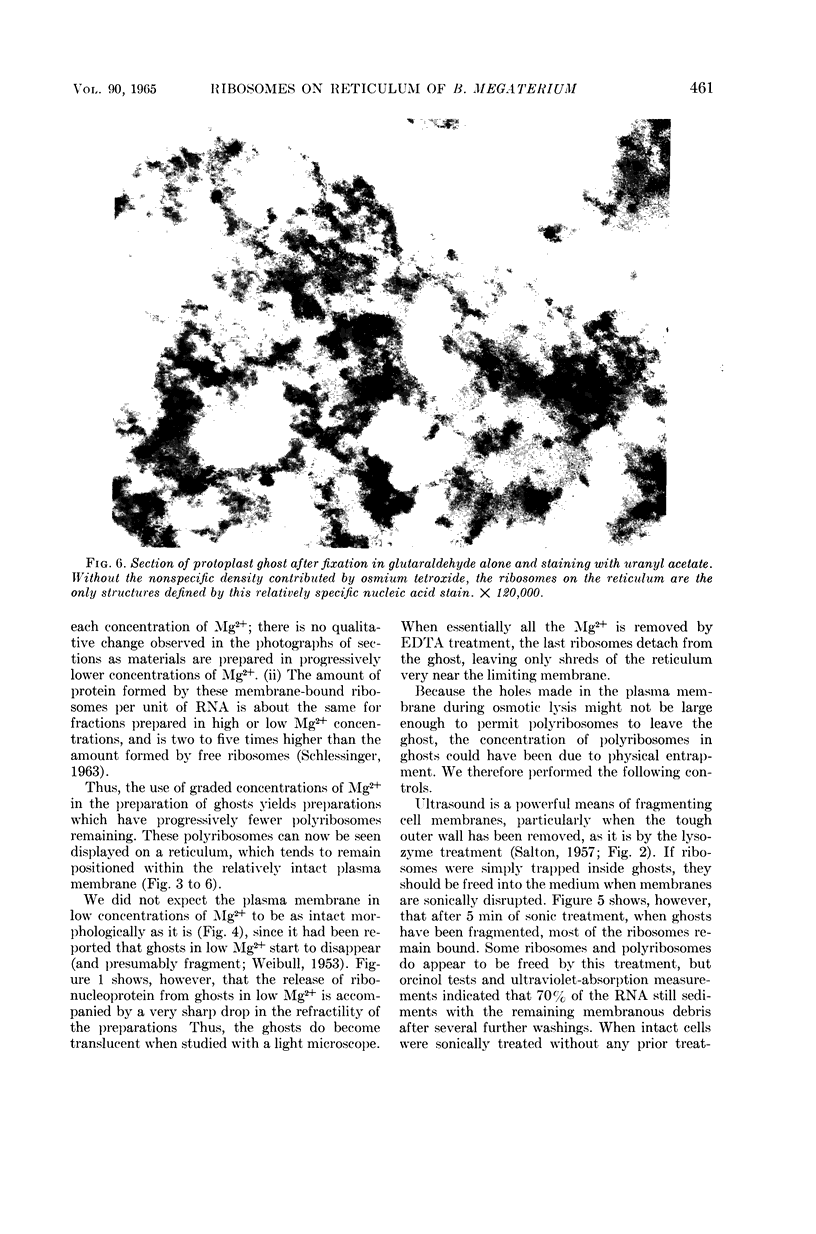
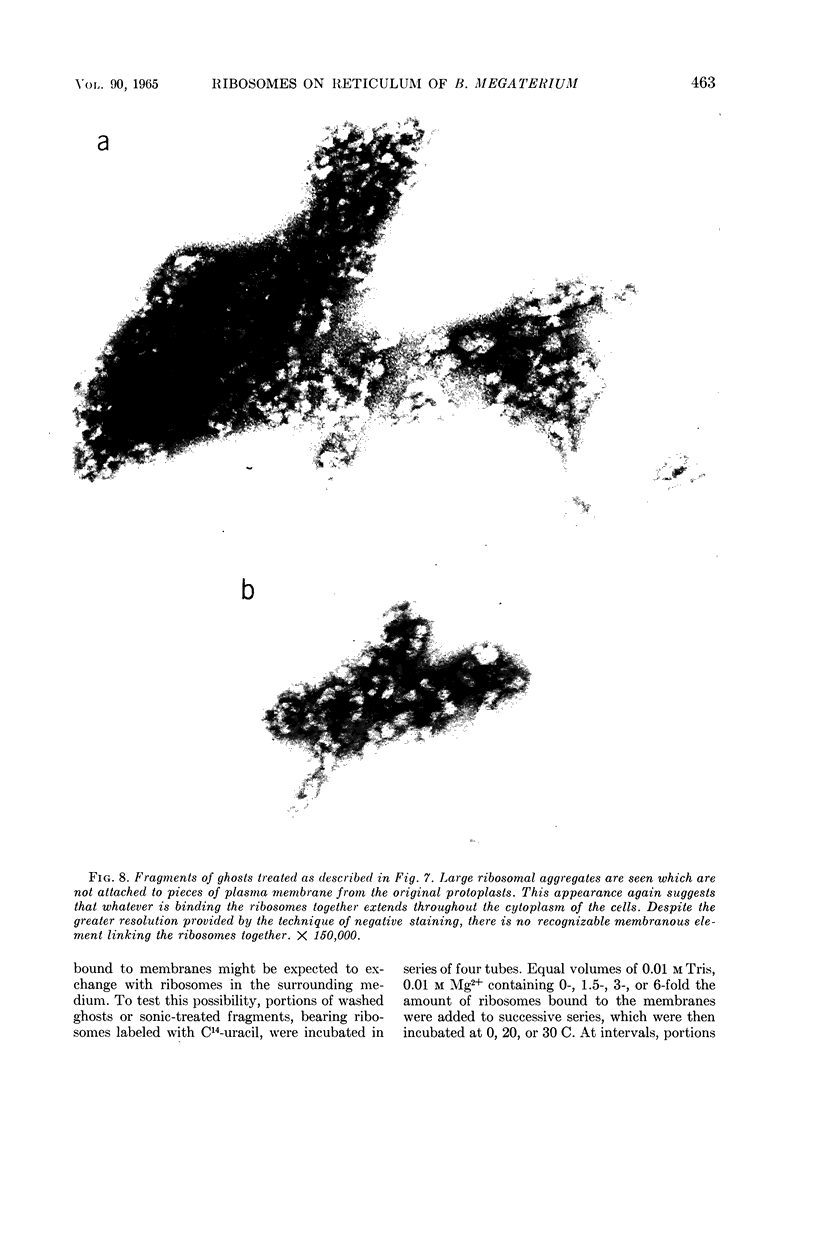
Schlessinger, David (Washington University School of Medicine, St. Louis, Mo.), Vincent T. Marchesi, and Benjamin C. K. Kwan. Binding of ribosomes to cytoplasmic reticulum of Bacillus megaterium. J. Bacteriol. 90:456–466. 1965.—As many as 60% of the cellular ribosomes are bound to membrane “ghosts” in lysozyme lysates in 0.02 m Mg2+. Bound ribosomes labeled with C14-uracil do not exchange with added unlabeled ribosomes, even after disruption of the cell membrane by sonic treatment. Electron micrographs of thin sections of ghosts, or of fragments produced by sonic disruption of protoplasts, indicate that the ribosomes are distributed on a reticular matrix which extends throughout the cytoplasm. The binding of ribosomes to this matrix is insensitive to ribonuclease or deoxyribonuclease, and has many other features in common with the binding of ribonucleoprotein to the membranous elements of the mammalian microsomal fraction, though the reticulum does not appear to be membranous. Thus, functioning ribosomes may be bound to a cytoplasmic structure in all cell types.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A., NIELSEN L., THAEMERT J. RAPIDLY SYNTHESIZED RIBONUCLEIC ACID IN MEMBRANE GHOSTS FROM STREPTOCOCCUS FECALIS PROTOPLASTS. Biochim Biophys Acta. 1964 Feb 17;80:325–337. doi: 10.1016/0926-6550(64)90104-5. [DOI] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. POLYRIBOSOMES IN PROTOPLASTS OF BACILLUS MEGATERIUM. Can J Microbiol. 1964 Feb;10:92–94. doi: 10.1139/m64-014. [DOI] [PubMed] [Google Scholar]
- GLAUERT A. M., GLAUERT R. H. Araldite as an embedding medium for electron microscopy. J Biophys Biochem Cytol. 1958 Mar 25;4(2):191–194. doi: 10.1083/jcb.4.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENDLER R. W., BANFIELD W. G., TANI J., KUFFEL ON THE CYTOLOGICAL UNIT FOR PROTEIN SYNTHESIS IN VIVO IN E. COLI. III. ELECTRON MICROSCOPIC AND ULTRACENTRIFUGAL EXAMINATION OF INTACT CELLS AND FRACTIONS. Biochim Biophys Acta. 1964 Feb 17;80:307–314. [PubMed] [Google Scholar]
- HENSHAW E. C., BOJARSKI T. B., HIATT H. H. PROTEIN SYNTHESIS BY FREE AND BOUND RAT LIVER RIBOSOMES IN VIVO AND IN VITRO. J Mol Biol. 1963 Aug;7:122–129. doi: 10.1016/s0022-2836(63)80041-8. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W., KELLER E. B., GROSS J., ZAMECNIK P. C. Studies on cytoplasmic ribonucleoprotein particles from the liver of the rat. J Biol Chem. 1955 Nov;217(1):111–123. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLONIG G. A modified procedure for lead staining of thin sections. J Biophys Biochem Cytol. 1961 Dec;11:736–739. doi: 10.1083/jcb.11.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALADE G. E., SIEKEVITZ P. Liver microsomes; an integrated morphological and biochemical study. J Biophys Biochem Cytol. 1956 Mar 25;2(2):171–200. doi: 10.1083/jcb.2.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALADE G. E., SIEKEVITZ P. Pancreatic microsomes; an integrated morphological and biochemical study. J Biophys Biochem Cytol. 1956 Nov 25;2(6):671–690. doi: 10.1083/jcb.2.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALTON M. R. The properties of lysozyme and its action on microorganisms. Bacteriol Rev. 1957 Jun;21(2):82–100. doi: 10.1128/br.21.2.82-100.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLESSINGER D. PROTEIN SYNTHESIS BY POLYRIBOSOMES ON PROTOPLAST MEMBRANES OF B. MEGATERIUM. J Mol Biol. 1963 Nov;7:569–582. doi: 10.1016/s0022-2836(63)80103-5. [DOI] [PubMed] [Google Scholar]
- WEIBULL C. The isolation of protoplasts from Bacillus megaterium by controlled treatment with lysozyme. J Bacteriol. 1953 Dec;66(6):688–695. doi: 10.1128/jb.66.6.688-695.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]