Abstract
We observed the structure of collagen fibrils in rat tail tendons after treatment with NKISK and cathepsin G. NKISK is a pentapeptide that has been previously shown to bind fibronectin, while cathepsin G is a serine protease that cleaves fibronectin but not type I collagen. In tendons treated with NKISK, fibrils were seen to extensively dissociate into smaller-diameter subfibrils. These subfibrils were homogeneous in diameter with an average diameter of 26.3 ± 5.8 nm. Similar, although less extensive, dissociation into subfibrils was found in tendons treated with cathepsin G. The average diameter of these subfibrils was 24.8 ± 4.9 nm. The ability of NKISK and cathepsin G to release subfibrils at physiological pH without harsh denaturants may enhance the study of the subfibrillar structure of collagen fibrils.
Keywords: collagen fibrils, subfibrils, microfibrils, NKISK, cathepsin G, electron microscopy
Introduction
Collagen is a common structural component of the extracellular matrix (ECM) and provides resistance to tensile force in connective tissues. A single collagen monomer consists of a triple helix formed by three polypeptide chains ∼300 nm in length and 1.5 nm in diameter. Collagen monomers are then aggregated in an organized head-to-tail fashion into a structure called a collagen fibril. These collagen fibrils can be seen with an electron microscope and exhibit a 67 nm D-period banded appearance due to staggered gaps between the heads and tails of the molecules in each row [1]. The lengths of collagen fibrils are difficult to determine in tissues because they move in and out of the planes of tissues and so cannot be followed microscopically from end to end. Fibril diameters, however, are readily measured; they vary depending on the type of tissue and even the location within the tissue. Rat tail tendon is made up of mostly type I collagen and its collagen fibril diameters are heterogeneous, ranging from 30 to 300 nm with an average diameter of 150 ± 10.9 nm [1,2].
The current literature on the subfibrillar organization of collagen generally agrees that collagen fibrils contain smaller units often termed microfibrils, which spiral within the fibril in a right-handed fashion and at a constant tilt angle of 15–17° [3–8]. Investigation of collagen microfibrils has been carried out using a variety of methods including freeze-fracture, freeze-etching, atomic force microscopy, X-ray diffraction, electron tomography, electron density mapping and transmission electron microscopy [5,6,9–13]. Microfibrils have been visualized after treatment with urea, glycerol or guanidine HCL, and even in native collagen [5,9,10,14,15]. Many investigators have found the diameter of these microfibrils to be ∼3.8–4 nm which supports the hypothesis that these microfibrils consist of five monomers of collagen [3,9,10,16–18]. However, others such as Meyers et al. [19] found 17–25 nm subfibrils in the synovium, while Kajikawa et al. [20] have shown the presence of two different microfibril sizes of 5 and 15–20 nm in the skin.
The previous discovery by Schmidt et al. that the pentapeptide NKISK competitively inhibits fibronectin from binding decorin, small leucine-rich proteoglycans on the surface of collagen, led to our laboratory's subsequent studies of effects of NKISK on tendon lengthening [21,22]. Our previous studies have found that in vivo injections of NKISK caused rat patellar tendons to lengthen significantly [22]. During recent scanning electron microscopy (SEM) studies of NKISK-treated rat tail tendons, we serendipitously observed that NKISK caused collagen fibrils to reveal a subfibrillar organization. Relationship of NKISK with fibronectin led us to also test treatments with cathepsin G, a protease that cleaves fibronectin as well as other ECM glycoproteins. We report our observations of tendons after these treatments and hope that this new method of looking at the subfibrillar structure of collagen will be useful to other investigators.
Methods
Tendon treatments
Tendons were obtained from Sprague–Dawley rat cadavers from an unrelated study. A single vertebral segment was obtained from the middle region of the tail, and tendons were pulled out from the distal end. Tendons were cut transversely into thirds and teased apart with microforceps. Four randomly selected tendons were placed into each of three unlabeled tubes containing distilled H2O. The tubes were then randomly assigned to treatment with buffer-only, 25 mM NKISK or 0.4 U ml−1 cathepsin G. NKISK peptide was obtained from the Peptide Synthesis Facility at the University of North Carolina (Chapel Hill, NC, USA). Cathepsin G from human leukocytes was purchased from Sigma-Aldrich (St. Louis, MO, USA). All reactions were made in 0.1 M Tris–HCl, pH 7.5, 0.15 M NaCl, 10 mM CaCl2, 0.05% Brij-35 and 0.01% NaN3. Treatment solutions were added and samples incubated at 37°C for 20 h, shaken at medium speed with an S/P multi-tube vortexer for 4 h at room temperature and incubated for 24 additional hours at 37°C. A similar uncontrolled experiment using 25 mM NKISK and 0.4 U ml−1 cathepsin G was also performed in which after 48 h, samples were shaken for 4 h each day for 3 additional days and stored at −20°C between shaking.
Scanning electron microscopy
Tendons were prepared for SEM by rinsing in 0.15 M sodium phosphate buffer and fixing in 4% paraformaldehyde in 0.15 M sodium phosphate buffer for 1 h at room temperature. The fixed samples were then rinsed with 0.15 M sodium phosphate buffer, pH 7.4, for 10 min and dehydrated by transferring through increasing concentrations of ethanol. Once samples were in 100% anhydrous ethanol, they were then transferred into a critical point dryer. Following the drying procedure, the samples were mounted on 13 mm aluminum planchettes using silver paste and coated with a 10 nm thickness of gold–palladium alloy (60:40) using the Hummer X sputter coater (Anatech USA, Union City, CA, USA). Examination was carried out using a Zeiss Supra 25 FESEM operating at 5 kV using a 10 µm aperture (Carl Zeiss SMT, Inc., Peabody, MA, USA).
Measurements
SEM of the samples was carried out to make general observations. To prevent selection bias, a grid was superimposed on the samples at 67× magnification and the coordinates, where the grid lines intersected over the sample, were used as random sampling points. The grid was created by enabling the graticale option on the microscope and setting the spacing value to 150 units. After the coordinates were selected, each grid line intersection was then zoomed in and examined at 30 280× magnification to determine whether any subfibril dissociation was present. The subfibril diameters in that region were then measured from photos taken at various higher magnifications ranging from 155 050× to 476 680× using ImageJ 1.42q (NIH, Bethesda, MD, USA). Diameters were measured across the length of the subfibrils because these images did not provide cross-sectional cuts. Measurements from 2- and 5-day samples were combined in calculating the mean diameter of the subfibrils.
Statistical methods
Routine descriptive statistics (mean and standard deviation) were calculated for the measurements made in each group. The Fisher exact test was used to determine whether the prevalence of subfibril dissociation of the treated tendons differed from the buffer-only group.
Results
Buffer-only samples
SEM examination of the 48 h control sample (Fig. 1) found collagen fibrils that appeared normal with characteristic D-banding patterns similar to other published SEMs of collagen fibrils. Sampling of 17 graticale intersections to check for regions of collagen dissociation into smaller subfibrils found 0 of 17 coordinates showing dissociation. Observation of random sections beyond those at the grid intersection in the 48 h sample and observation of other independent buffer-only samples did not reveal any regions of dissociation comparable to those seen with NKISK or cathepsin G samples. In a few locations where fibrils were bent or cut, subfibrillar organization could be vaguely discerned (Fig. 1a–c). In these locations, collagen fibrils appeared to be made up of bundles of smaller fibrils.
Fig. 1.
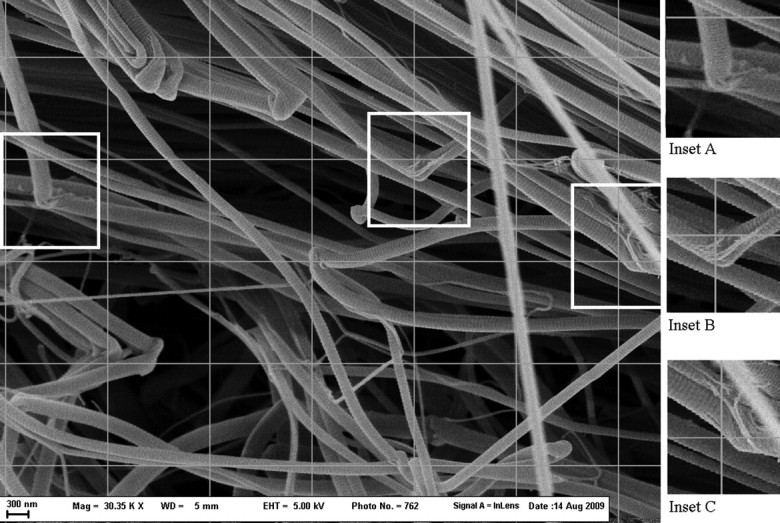
Rat tail tendon in buffer-only for 48 h (30 350× magnification). Normal collagen fibers are shown. This region displays significant bending of fibrils. Subfibrillar structure can be vaguely seen in insets A, B and C at sites where fibrils are bending.
Experimental NKISK treatment samples
Examination of the 48 h NKISK treatment sample (Fig. 2) found many regions with significant fibrillar dissociation into subfibrils interspersed with regions of normal appearing collagen. Sampling of 18 graticale intersections found eight clearly showing collagen fibrils dissociating into smaller subfibrils. The subfibrils appeared to be arranged in the previously described right-handed spiral, and characteristic D-banding patterns can be seen. The prevalence of subfibril dissociation was found to be significantly increased (P < 0.05) with the NKISK treatment relative to the buffer-only tendons. These subfibrils appeared homogenous in diameter and tended to separate from each other creating a web-like appearance. Further NKISK treatment (5 days) found 14 of 14 coordinates to show extensive dissociation into subfibrils. The degree of dissociation was much greater (Figs. 3 and 4) compared to the 48 h treatment and the number of unaffected fibrils was markedly fewer. From a sample size of n = 380, the mean diameter of the subfibrils was found to be 26.3 ± 5.8 nm.
Fig. 2.
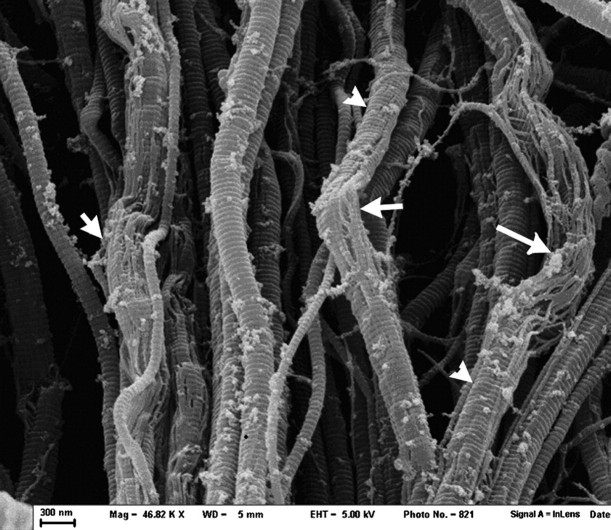
Rat tail tendon treated with 25 mM NKISK for 48 h at (46 820× magnification). In many regions, collagen fibrils can be seen dissociating into subfibrils (arrows), while other regions maintain fibril integrity (arrowheads). Note that treatment with NKISK has resulted in the appearance of unknown debris on the surface of the fibrils.
Fig. 3.
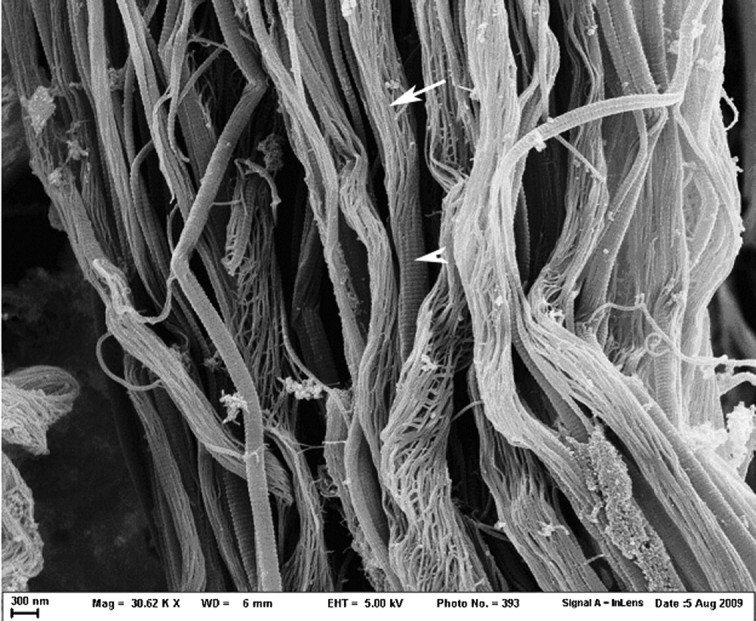
Rat tail tendons treated with 25 mM NKISK for 5 days (30 620× magnification). With longer treatment time, dissociation into subfibrils became much more extensive. Intact fibrils can be seen as well as a markedly dissociated fibrils and fibrils in transition from an intact state (arrowhead) to a dissociated state (arrow). Note that the subfibrils appear to be arranged in a right-handed spiral.
Fig. 4.
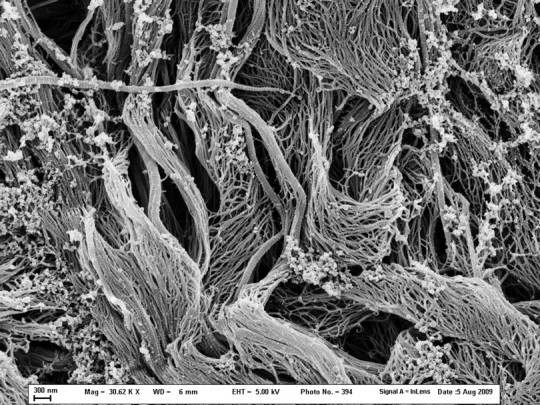
Rat tail tendons treated with 25 mM NKISK for 5 days (30 620× magnification). This image displays an area in which the fibrils have almost entirely dissociated.
Experimental cathepsin G treatment sample
Examination of the 48 h cathepsin G treatment sample found that the extent and prevalence of dissociation into smaller fibrils were less than either of the NKISK treatments with only 1 out of 19 graticale intersections showing fibrillar dissociation. The prevalence of subfibril dissociation was not found to differ between the cathepsin G-treated tendon and the buffer-only tendons. Further cathepsin G treatment for 5 days (Fig. 5) resulted in 13 of 13 examined locations showing extensive dissociation into subfibrils. The degree of dissociation was much greater compared to the 48 h cathepsin G treatment but was not as pervasive as when treated with NKISK. The subfibrils again appear to be arranged in a right-handed spiral with characteristic D-banding patterns. Notably, the banding pattern appears more prominent in subfibrils originating from the surface of fibrils and less prominent in subfibrils from the inside of fibrils (Fig. 6). The 5-day cathepsin G sample showed dissociation occurring in localized segments, whereas the 5-day NKISK sample showed dissociation down much of the length of the fibrils. From a sample size of n = 271, the average diameter of the subfibrils was found to be 24.8 ± 4.9 nm.
Fig. 5.

Rat tail tendons treated with cathepsin G for 5 days (52 590× magnification). Collagen fibrils of small and large diameters can be seen breaking up into subfibrils similar to those seen in NKISK treatments although the dissociation is less extensive.
Fig. 6.
Rat tail tendons treated with cathepsin G for 5 days (266 240× magnification). This is a higher magnification of the central region in Fig. 5, with arrows indicating the greater prominence of the banding pattern is visible on subfibrils originating from the fibril surface. This suggests that the banding seen on SEM is due to materials accreted to the bands at the surface but not the depths of the fibril.
Discussion
Collagen is a very stable protein that is known to form long parallel fibrils that are heterogeneous in diameter. In our experiments, we observed that a subfibrillar organization of collagen fibrils can be readily seen after treatment with NKISK (Figs. 2–4). These subfibrils are homogeneous in diameter and can frequently be seen to spiral in a right-handed fashion as previously described in the literature. The average subfibril diameter of 25–26 nm seen in our experiments is similar to the diameters of the 25 nm filaments found by Hashizume et al. [4], Meyers et al. [19], Kajikawa et al. [20] and Scott [23]. We were able to induce dissociation into separated subfibrils clearly visualized on SEM using a treatment that is mild and at physiological pH. Previously published in vivo experiments with NKISK have shown that its effects on tendon lengthening is time- and concentration-dependent with the most effective in vivo treatment found being four doses of 50 mM NKISK over a period of 4 weeks [22]. This study found a similar time-dependent increase in effectiveness of NKISK in dissociation of collagen fibrils between a 2-day treatment and a 5-day treatment. This time-dependent increase in efficacy may be due to slow diffusion of molecules into the tightly packed collagen fibrils. However, time may not have been the only factor, as the three freeze–thaw cycles during the 5-day treatment may also have contributed to increased fibril dissociation of the NKISK-treated collagen.
It is interesting to note that subfibrillar organization was occasionally seen even in the control sample with tendons in just buffer (Fig. 1), although the subfibrils still appear tightly bundled together. These regions were usually very short and localized and could be found only where collagen fibrils were bent, cut or twisted. These locations were most likely due to the mechanical disruptive force produced from teasing the tendon apart with forceps and subjecting it to the vortexing process. This suggests that mechanical disruption may be important to dissociation of the NKISK and cathepsin G specimens as well, although we did not test this hypothesis.
It may be that these 25-nm subfibrils are simply a standard construction unit of collagen fibrils and such subfibrils aggregate with aging to form the larger fibrils that are normally observed in tendons and ligaments. This idea is supported by research that has found young tendon fibrils to be more homogeneous in diameter with an average diameter of 25 nm and that fibrils increase in size with maturation [24]. The fact that NKISK treatment did not break collagen fibrils up into the 4-nm microfibrils found with freeze-fracture techniques suggests that these 25 nm fibrils are on a different organizational level and are held together by a different force than the 4-nm microfibrils.
Five-day treatment with cathepsin G led to results similar to the 5-day NKISK treatment. The average subfibril diameter found was similar to the subfibril diameter found in the NKISK treatment. However, the dissociation from cathepsin G treatment (Fig. 5) was less extensive than with NKISK treatment in that the regions of dissociation were more localized and shorter in length. It is unclear to us the exact reason why cathepsin G was less effective at causing dissociation than NKISK. We speculate that one reason might be due to NKISK being a smaller molecule than cathepsin G, which diffuses faster and is able to exert a greater effect in a shorter amount of time. Also, NKISK is a pentapeptide that competes with fibronectin, while cathepsin G is an enzyme that exerts its effect by cleaving fibronectin. It is likely that the different rate of dissociation might be a reflection of their different mechanisms of action.
D-banding patterns are visible on subfibrils (Fig. 6), although the banding pattern appears more prominent in subfibrils originating from the surface of fibrils and less prominent in subfibrils from the inside of fibrils. It is possible that much of the D-banding pattern visible on collagen fibrils using SEM is due to surface proteoglycans such as decorin which may be more prevalent on subfibrils from the surface of fibrils and less prevalent on subfibrils from the inside of the fibril.
Concluding remarks
Our study has shown through SEM that the pentapeptide NKISK is an agent that can induce the dissociation of fibrils into subfibrils with an average diameter of 26.3 nm. We believe that treatment with NKISK provides a unique look at subfibrillar structure and will enhance future the study of collagenous tissues.
Funding
This research was supported by the Aileen Stock Research Fund and in part by the NIH T35 DK007863 Fund.
Acknowledgement
The authors gratefully acknowledge the assistance of Mrs Victoria J. Madden in preparing specimens for electron microscopy.
References
- 1.Franchi M, Raspanti M, Dell'Orbo C, Quaranta M, De Pasquale V, Ottani V, Ruggeri A. Different crimp patterns in collagen fibrils relate to the subfibrillar arrangement. Connect. Tissue Res. 2008;49:85–91. doi: 10.1080/03008200801913635. doi:10.1080/03008200801913635. [DOI] [PubMed] [Google Scholar]
- 2.Lavagnino M, Arnoczky S, Frank K, Tian T. Collagen fibril diameter distribution does not reflect changes in the mechanical properties of in vitro stress-deprived tendons. J. Biomech. 2005;38:69–75. doi: 10.1016/j.jbiomech.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 3.Ruggeri A, Benazzo F, Reale E. Collagen fibrils with straight and helicoidal microfibrils: a freeze-fracture and thin section study. J. Ultrastruct. Res. 1979;68:101–108. doi: 10.1016/s0022-5320(79)90146-1. doi:10.1016/S0022-5320(79)90146-1. [DOI] [PubMed] [Google Scholar]
- 4.Hashizume H, Hitomi J, Ushiki T. Growth of collagen fibrils produced by human osteosarcoma cells: high-resolution scanning electron microscopy. Arch. Histol. Cytol. 1999;62:327–335. doi: 10.1679/aohc.62.327. doi:10.1679/aohc.62.327. [DOI] [PubMed] [Google Scholar]
- 5.Kischer C W, Droegemueller W, Shetlar M, Chvapil M, Vining J. Ultrastructural changes in the architecture of collagen in the human cervix treated with urea. Am. J. Pathol. 1980;99:525–538. [PMC free article] [PubMed] [Google Scholar]
- 6.Orgel J P, Irving T C, Miller A, Wess T J. Microfibrillar structure of type I collagen in situ. Proc. Natl. Acad. Sci. 2006;103:9001–9005. doi: 10.1073/pnas.0502718103. doi:10.1073/pnas.0502718103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes D F, Gilpin C J, Baldock C, Ziese U, Koster A J, Kadler K E. Corneal collagen fibril structure in three dimensions: structural insights into fibril assembly, mechanical properties, and tissue organization. Proc. Natl. Acad. Sci. 2001;98:7307–7312. doi: 10.1073/pnas.111150598. doi:10.1073/pnas.111150598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cisneros D A, Hung C, Franz C M, Muller D J. Observing growth steps of collagen self-assembly by time-lapse high-resolution atomic force microscopy. J. Struct. Biol. 2006;154:232–245. doi: 10.1016/j.jsb.2006.02.006. doi:10.1016/j.jsb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Raspanti M, Ottani V, Ruggeri A. Different architectures of the collagen fibril: morphological aspects and functional implications. Int. J. Biol. Macromol. 1989;11:367–371. doi: 10.1016/0141-8130(89)90009-3. doi:10.1016/0141-8130(89)90009-3. [DOI] [PubMed] [Google Scholar]
- 10.Lillie J H, MacCallum D K, Scaletta L J, Occhino J C. Collagen structure: evidence for a helical organization of the collagen fibril. J. Ultrastruct. Res. 1977;58:134–143. doi: 10.1016/s0022-5320(77)90025-9. doi:10.1016/S0022-5320(77)90025-9. [DOI] [PubMed] [Google Scholar]
- 11.Starborg T, Lu Y, Meadows R S, Kadler K E, Holmes D F. Electron microscopy in cell-matrix research. Methods. 2008;45:53–64. doi: 10.1016/j.ymeth.2008.01.004. doi:10.1016/j.ymeth.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Castellani P P, Morocutti M, Franchi M, Ruggeri A, Bigi A, Roveri N. Arrangement of microfibrils in collagen fibrils of tendons in the rat tail: ultrastructural and X-ray diffraction investigation. Cell Tissue Res. 1983;234:735–743. doi: 10.1007/BF00218664. [DOI] [PubMed] [Google Scholar]
- 13.Baselt D R, Revel J P, Baldeschwieler J D. Subfibrillar structure of type I collagen observed by atomic force microscopy. Biophys. J. 1993;65:2644–2655. doi: 10.1016/S0006-3495(93)81329-8. doi:10.1016/S0006-3495(93)81329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen B R. Electron microscope studies on collagen. I. Native collagen fibrils. Z. Zellforsch. Mikrosk. Anat. 1963;5:184–198. doi: 10.1007/BF00320444. doi:10.1007/BF00320444. [DOI] [PubMed] [Google Scholar]
- 15.Leonardi L, Ruggeri A, Roveri N, Bigi A, Reale E. Light microscopy, electron microscopy, and X-ray diffraction analysis of glycerinated collagen fibers. J. Ultrastruct. Res. 1983;85:228–237. doi: 10.1016/s0022-5320(83)90109-0. doi:10.1016/S0022-5320(83)90109-0. [DOI] [PubMed] [Google Scholar]
- 16.Hulmes D J. Building collagen molecules, fibrils, and suprafibrillar structures. J. Struct. Biol. 2002;137:2–10. doi: 10.1006/jsbi.2002.4450. doi:10.1006/jsbi.2002.4450. [DOI] [PubMed] [Google Scholar]
- 17.Smith J W. Molecular pattern in native collagen. Nature. 1968;219:157–158. doi: 10.1038/219157a0. doi:10.1038/219157a0. [DOI] [PubMed] [Google Scholar]
- 18.Kure M, Araki K, Ogata T. Scanning tunneling microscopic study of osmium-impregnated collagen. J. Electron Microsc. 1995;44:207–211. [PubMed] [Google Scholar]
- 19.Meyers D B, Highton T C, Rayns D G. Acid mucopolysaccharides closely associated with collagen fibrils in normal human synovium. J. Ultrastruct. Res. 1969;28:203–213. doi: 10.1016/s0022-5320(69)90080-x. doi:10.1016/S0022-5320(69)90080-X. [DOI] [PubMed] [Google Scholar]
- 20.Kajikawa K, Nakanishi I, Hori I, Matsuda Y, Kondo K. Electron microscopic observations on connective tissues using ruthenium red staining. J. Electron Microsc. 1970;19:347–354. [PubMed] [Google Scholar]
- 21.Schmidt G, Hausser H, Kresse H. Interaction of the small proteoglycan decorin with fibronectin: involvement of the sequence NKISK of the core protein. Biochem. J. 1991;280:411–414. doi: 10.1042/bj2800411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esther R, Creighton R, Draeger R, Weinhold P, Dahners L. Effect of NKISK on tendon lengthening: an in vivo model for various clinically applicable dosing regimens. J. Orthop. Res. 2008;26:971–976. doi: 10.1002/jor.20594. doi:10.1002/jor.20594. [DOI] [PubMed] [Google Scholar]
- 23.Scott J. Proteoglycan: collagen interactions and subfibrillar structure in collagen fibrils. J. Anat. 1999;169:23–35. [PMC free article] [PubMed] [Google Scholar]
- 24.Parry D, Craig A. Growth and development of collagen fibrils in connective tissue. In: Ruggeri A, Motta PM, editors. Ultrastructure of the Connective Tissue Matrix. Boston, MA: Martinus Nijhoff; 1984. pp. 34–64. [Google Scholar]