Abstract
Novick, Richard P. (The Rockefeller Institute, New York, N.Y.), and Mark H. Richmond. Nature and interactions of the genetic elements governing penicillinase synthesis in Staphylococcus aureus. J. Bacteriol. 90:467–480. 1965.—It has been found previously that penicillinase-producing strains of Staphylococcus aureus each harbor an extrachromosomal element, or plasmid, which apparently carries all the genetic information necessary for penicillinase synthesis. These plasmids behave in a manner consistent with their being small chromosomelike structures in that they comprise linkage groups containing several markers and in that they undergo such genetic events as mutation, segregation, and recombination. There is currently no evidence for conjugal cell-cell transfer of the plasmids or for a state of stable integration into the staphylococcal chromosome. A certain amount of variability has been encountered among the penicillinase plasmids harbored by different staphylococcal strains. It has been found that: (i) there are at least three molecular variants of the enzyme itself; (ii) most, but not all, of the penicillinase plasmids carry a genetic determinant of resistance to mercuric ion; (iii) plasmids carried by a very small number of the strains bear a determinant of resistance to erythromycin; (iv) the plasmids determine the fraction of penicillinase excreted into the medium during growth, and this also varies from strain to strain. The penicillinase plasmids appear to behave as integral genetic structures. The entire known linkage group is transduced intact, and is occasionally lost completely as a spontaneous occurrence during the growth of the organisms. Rarely, the plasmid markers dissociate during transduction, resulting in transduced clones which have inherited only a part of the plasmid linkage group. Similarly, dissociation occurs as a spontaneous event during normal growth, also resulting in rare clones which appear to have lost one or more but not all of the plasmid markers. When crosses are performed between two plasmid-harboring strains, a plasmid heterozygote is formed. In most cases, this persists for only one or a few cell divisions before segregating, with or without the formation of recombinant plasmids. In two instances thus far observed, the heterozygote persists as a stable plasmid heterodiploid, in which the continued presence of both plasmids can be readily demonstrated. The fate of the heterozygote, i.e., early segregation or persistence as a heterodiploid, depends upon which particular pair of plasmids is involved in the cross. This observation has led to the hypothesis of a plasmid-linked determinant of plasmid compatibility. A pair of plasmids is considered compatible if it can form a stable heterodiploid, incompatible if it cannot.
Full text
PDF

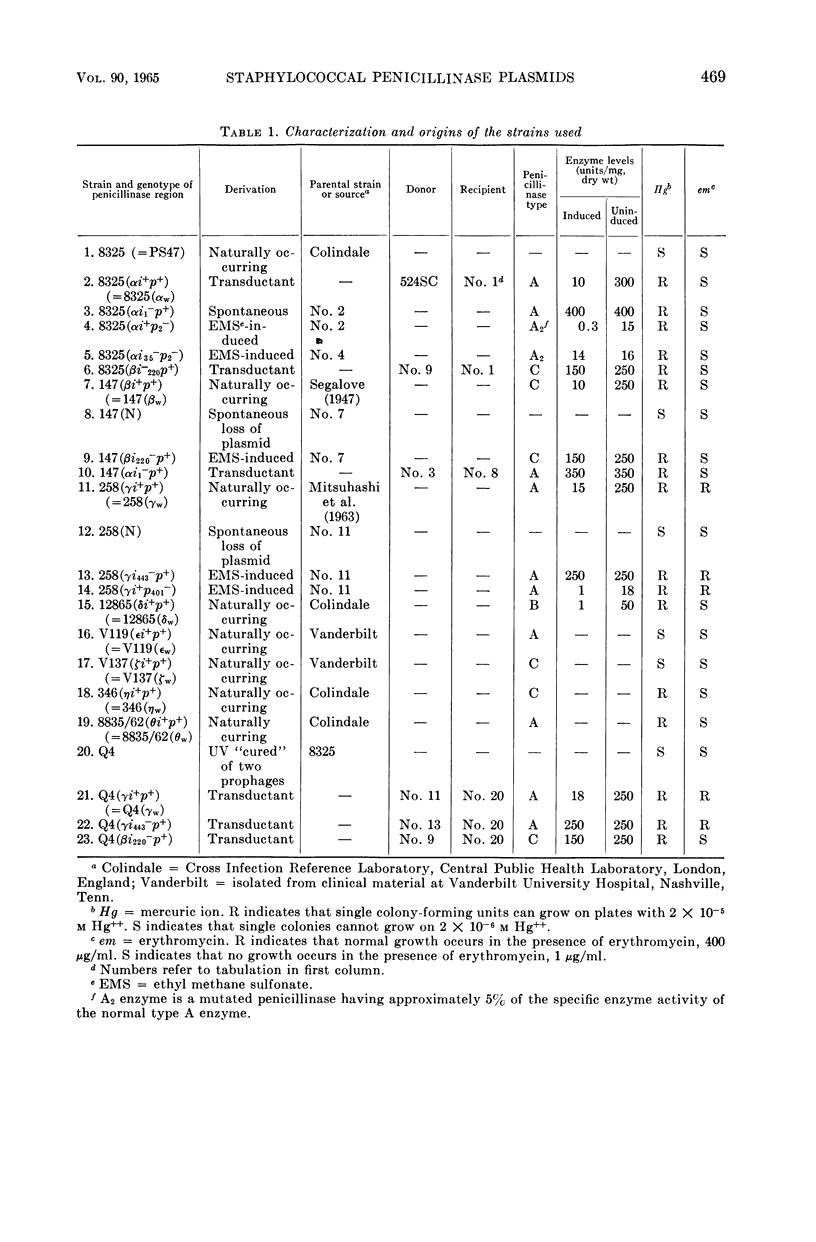






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W. Transduction of chromosomal genes and episomes in Escherichia coli. Virology. 1960 May;11:273–288. doi: 10.1016/0042-6822(60)90066-0. [DOI] [PubMed] [Google Scholar]
- BONDI A., KORNBLUM J., DE SAINT PHALLE M. Isolation of penicillin-susceptible mutants from penicillinase-producing strains of M. pyogenes. Proc Soc Exp Biol Med. 1953 Jul;83(3):527–530. doi: 10.3181/00379727-83-20405. [DOI] [PubMed] [Google Scholar]
- CUZIN F. [Autonomous multiplication of the sexual episome of Escherichia coli K12 n an Hfr strain]. C R Hebd Seances Acad Sci. 1962 Jun 13;254:4211–4213. [PubMed] [Google Scholar]
- Echols H. PROPERTIES OF F' STRAINS OF ESCHERICHIA COLI SUPERINFECTED WITH F-LACTOSE AND F-GALATOSE EPISOMES. J Bacteriol. 1963 Feb;85(2):262–268. doi: 10.1128/jb.85.2.262-268.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAIRBROTHER R. W., PARKER L., EATON B. R. The stability of penicillinase-producting strains of Staphylococcus aureus. J Gen Microbiol. 1954 Apr;10(2):309–316. doi: 10.1099/00221287-10-2-309. [DOI] [PubMed] [Google Scholar]
- GAREN A., ZINDER N. D. Radiological evidence for partial genetic homology between bacteriophage and host bacteria. Virology. 1955 Nov;1(4):347–376. doi: 10.1016/0042-6822(55)90030-1. [DOI] [PubMed] [Google Scholar]
- GARROD L. P. The erythromycin group of antibiotics. Br Med J. 1957 Jul 13;2(5036):57–63. doi: 10.1136/bmj.2.5036.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARMON S. A., BALDWIN J. N. NATURE OF THE DETERMINANT CONTROLLING PENICILLINASE PRODUCTION IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1964 Mar;87:593–597. doi: 10.1128/jb.87.3.593-597.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO H., KONO K., MITSUHASHI S. ELIMINATION OF PENICILLIN RESISTANCE OF STAPHYLOCOCCUS AUREUS BY TREATMENT WITH ACRIFLAVINE. J Bacteriol. 1964 Jul;88:261–262. doi: 10.1128/jb.88.1.261-262.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., ADELBERG E. A. Transfert de caractéres génétiques par incorporation au facteur sexuel d'Escherichia coli. C R Hebd Seances Acad Sci. 1959 Jul 6;249(1):189–191. [PubMed] [Google Scholar]
- KAHN P., HELINSKI D. R. RELATIONSHIP BETWEEN COLICINOGENIC FACTORS E1 AND V AND AN F FACTOR IN ESCHERICHIA COLI. J Bacteriol. 1964 Dec;88:1573–1579. doi: 10.1128/jb.88.6.1573-1579.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAISER A. D., JACOB F. Recombination between related temperate bacteriophages and the genetic control of immunity and prophage localization. Virology. 1957 Dec;4(3):509–521. doi: 10.1016/0042-6822(57)90083-1. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J. Cell genetics and hereditary symbiosis. Physiol Rev. 1952 Oct;32(4):403–430. doi: 10.1152/physrev.1952.32.4.403. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- MAAS R. EXCLUSION OF A FLAC EPISOME BY AN HFR GENE. Proc Natl Acad Sci U S A. 1963 Dec;50:1051–1055. doi: 10.1073/pnas.50.6.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAY J. W., HOUGHTON R. H., PERRET C. J. THE EFFECT OF GROWTH AT ELEVATED TEMPERATURES ON SOME HERITABLE PROPERTIES OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1964 Nov;37:157–169. doi: 10.1099/00221287-37-2-157. [DOI] [PubMed] [Google Scholar]
- MITSUHASHI S., MORIMURA M., KONO K., OSHIMA H. ELIMINATION OF DRUG RESISTANCE OF STAPHYLOCOCCUS AUREUS BY TREATMENT WITH ACRIFLAVINE. J Bacteriol. 1963 Jul;86:162–164. doi: 10.1128/jb.86.1.162-164.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE B. A new screen test and selective medium for the rapid detection of epidemic strains of Staph. aureus. Lancet. 1960 Aug 27;2(7148):453–458. doi: 10.1016/s0140-6736(60)91591-9. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. Staphylococcal penicillinase and the new penicillins. Biochem J. 1962 May;83:229–235. doi: 10.1042/bj0830229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRET C. J. Iodometric assay of penicillinase. Nature. 1954 Nov 27;174(4439):1012–1013. doi: 10.1038/1741012a0. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. DOMINANCE OF THE INDUCIBLE STATE IN STRAINS OF STAPHYLOCOCCUS AUREUS CONTAINING TWO DISTINCT PENICILLINASE PLASMIDS. J Bacteriol. 1965 Aug;90:370–374. doi: 10.1128/jb.90.2.370-374.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHMOND M. H., JOHN M. CO-TRANSDUCTION BY A STAPHYLOCOCCAL PHAGE OF THE GENES RESPONSIBLE FOR PENICILLINASE SYNTHESIS AND RESISTANCE TO MERCURY SALTS. Nature. 1964 Jun 27;202:1360–1361. doi: 10.1038/2021360a0. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H., PARKER M. T., JEVONS M. P., JOHN M. HIGH PENICILLINASE PRODUCTION CORRELATED WITH MULTIPLE ANTIBIOTIC RESISTANCE IN STAPHYLOCOCCUS AUREUS. Lancet. 1964 Feb 8;1(7328):293–296. doi: 10.1016/s0140-6736(64)92407-9. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. WILD-TYPE VARIANTS OF EXOPENICILLINASE FROM STAPHYLOCOCCUS AUREUS. Biochem J. 1965 Mar;94:584–593. doi: 10.1042/bj0940584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS H. J. Variant populations within a hyaluronidase-producing culture of Staphylococcus aureus. J Pathol Bacteriol. 1953 Oct;66(2):545–551. doi: 10.1002/path.1700660226. [DOI] [PubMed] [Google Scholar]
- SCAIFE J., GROSS J. D. Inhibition of multiplication of an Flac factor in Hfr cells of Escherichia coli K-12. Biochem Biophys Res Commun. 1962 May 11;7:403–407. doi: 10.1016/0006-291x(62)90324-8. [DOI] [PubMed] [Google Scholar]
- WATANABE T. Infective heredity of multiple drug resistance in bacteria. Bacteriol Rev. 1963 Mar;27:87–115. doi: 10.1128/br.27.1.87-115.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., NISHIDA H., OGATA C., ARAI T., SATO S. EPISOME-MEDIATED TRANSFER OF DRUG RESISTANCE IN ENTEROBACTERIACEAE. VII. TWO TYPES OF NATURALLY OCCURRING R FACTORS. J Bacteriol. 1964 Sep;88:716–726. doi: 10.1128/jb.88.3.716-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]