Abstract
Objective
To determine the effect of a novel formulation of fluocinonide cream on skin barrier function in subjects with atopic dermatitis.
Design
The authors performed an open-label, investigator-blinded, side-by-side, controlled trial examining skin barrier function before and after a two-week course of a class I, super-potent topical steroid.
Setting
Outpatient university-based dermatology clinic in Portland, Oregon.
Subjects
Twenty-five subjects aged 12 or older with a diagnosis of moderate, severe, or very severe AD were recruited for this study.
Intervention
Fluocinonide 0.1% cream, a novel formulation of a class I super-potent topical steroid, was applied to all affected areas, except a control site, once daily tor two weeks or until clear. The control target site was treated with the vehicle once daily.
Main Outcome Measure(s)
The study’s primary outcome was change in skin barrier function as measured by basal transepidermal water loss (TEWL) in acute lesional skin from baseline as measured at two weeks.
Results
TEWL readings significantly decreased (reflecting improved barrier function) in both the active and control target sites. The active target site decreased 14.35 ± 16 mg/cm2 per hour; 95 percent confidence interval, P<0.001. The control target site decreased 8.75 ± 11.80 mg/cm2 per hour in 25 subjects; 95 per cent confidence interval, P<0.001. Skin electrical capacitance also improved significantly, reflecting improved stratum corneum hydration with therapy. Pruritus, clinical severity, and quality of life scores all showed significant improvement by the end of the study.
Conclusion
The authors have shown that short-term treatment with a novel formulation of 0.1% fluocinonide led to significantly improved barrier function as measured by basal TEWL in subjects with active moderate to severe AD. These data suggest short-term treatment with AD with a super-potent corticosteroid improves skin barrier function.
INTRODUCTION
Topical steroids remain the mainstay of treatment for disease flares in atopic dermatitis (AD) despite the advent of several new topical anti-inflammatory medications.1,2 Despite their widespread use, it is now debated whether the short-term use of topical steroids is detrimental to the skin barrier. Aalto-Korte initially showed that skin barrier function in AD actually improves with the use of topical steroid.3 More recently, Kao and colleagues reported that the use of a super-potent topical steroid applied to normal human skin leads to decreased skin barrier function, specifically reduced stratum corneum integrity and impaired repair capability, after just three days of use.4 With recent genetic studies revealing the importance of the skin barrier in AD pathogenesis, understanding the effects of the short-term use of topical steroids on skin barrier function is critical.5
The authors sought to determine the effects of a novel formulation of a super-potent topical corticosteroid on lesional and non-lesional skin of patients with AD. The authors hypothesized that short-term corticosteroid treatment of inflamed skin improves basal skin barrier function as measured by transepidermal water loss (TEWL) in patients with AD. To test this hypothesis, the authors performed an investigator-blinded, side-by-side, controlled trial examining skin barrier function before and after a two-week course of a class I, super-potent topical steroid. The authors also aimed to determine what effects the novel vehicle formulation may have on barrier function.
METHODS
Study Design Overview
Institutional Review Board approval was obtained for this study, and the study was performed using Good Clinical Practice Guidelines as published by the U.S. Food and Drug Administration (FDA).6 This was an investigator-initiated study and was registered at clinicaltrials.gov prior to subject enrollment (# NCT00819507). Subjects with moderate-to-severe AD were enrolled in an open-label, investigator-blinded, side-by-side study. Two lesional target sites were identified for each subject; one site receiving active medication (active target site), one site receiving the vehicle alone (control target site). The target sites were chosen as the most severely affected areas with symmetrical involvement without active weeping or crusting. Each site had to be at least 45cm2 in size (credit card size) and could not be chosen from the hands, feet, face or genital area. The active and control target sites were assigned by the research coordinator in a random fashion (coin flip), and without the investigator’s knowledge. Although only the target areas were used for outcome measurement, all lesional skin on the body was allowed to be treated with the study cream except the control target site. Subjects were evaluated at baseline and at two weeks.
Population
Twenty-five subjects were recruited from Oregon Health & Science University’s Department of Dermatology clinics. Subjects had to be 12 years of age or older with a diagnosis of AD according to Hanifin-Rajka criteria.7 Subjects had to have an Investigator Global Assessment (IGA) of moderate, severe, or very severe and have failed to achieve adequate disease control despite appropriate topical or systemic therapy. They also had to be a candidate, according to the principal investigator, for a super-potent topical steroid course.
Exclusionary criteria included active skin infection, hypersensitivity to any ingredients in fluocinonide 0.1% cream, previous use of super-potent topical steroids within two weeks of starting study (class I steroid), or current use of systemic therapy, unless on stable doses for at least three months. There was no washout period required for use of class II or less potent topical steroids, representing a typical clinical scenario in which patients use progressively more potent topical steroids to achieve disease control.
Intervention
Fluocinonide 0.1% cream (VANOS™, Medicis Pharmaceutical Corporation, Scottsdale, AZ) is a novel formulation of a class I super-potent topical steroid. It is approved for once-daily use in patients 12 years of age and older for a maximum of two weeks for the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses. The ingredient list for VANOS cream is as follows: Micronized fluocinonide in a cream base of propylene glycol USP, dimethyl isosorbide, glyceryl stearate (and) PEG-100 stearate, glyceryl monostearate NF, purified water USP, carbopol 980 NF, diisopropanolamine, and citric acid USP.
Fluocinonide 0.1% cream was applied to all affected areas, except the control site, once daily for two weeks or until clear. If an area of skin cleared, fluocinonide 0.1% cream was used in the previously affected areas for two days after visual clearance to assure all subclinical inflammation had resolved.
The control target site was treated with the cream vehicle once daily for the same amount of time as the treatment of the active target site.
Concomitant Medications
Systemic therapy (including phototherapy) for AD was allowed only if the subject was on stable doses for at least three months. Topical antipruritics or topical anti-inflammatory medications (topical steroids or topical calcineurin inhibitors) were not permitted during the study, but required no washout. The use of emollients (only Cetaphil™ cream, Aquaphor™, plastibase, or petrolatum) was permitted during the study, but not for eight hours prior to measurements. The use of antibiotics (topical or oral) or antihistamines was permitted during the study. Subjects continued all other oral medications needed for stable medical problems.
Outcomes
Primary
The primary outcome was change in basal transepidermal water loss (TEWL) in acute lesional skin of the active target site from baseline as measured at two weeks.
Secondary
Secondary outcomes included the following: Change in TEWL at the control target site to measure the improvement seen with the vehicle alone; change in skin capacitance from baseline at target sites; change in overall Eczema Area and Severity Index (EASI)8 score; change in pruritus visual analogue score,9 change in quality of life measurement as measured by Dermatology Quality of Life Index (DLQI) instrument,10 and percentage of subjects achieving an IGA of clear, or almost clear.
Procedures
Transepidermal Water Loss (TEWL)
TEWL is a direct measure of the permeability barrier of the stratum corneum, and is the most common measure of stratum corneum barrier permeability in AD studies.11 TEWL was measured using a Tewameter TM 210 (Courage & Khazaka, Cologne, Germany). Standard measurements were made following published guidelines.12 Measurements were performed by two study authors only (ES and PB).
Two measurements per site were performed and averaged. In addition to the two measurements from each of the target and control sites, two measurements from one non-lesional (normal skin) site were made.
Sites were marked on a body map so the areas could be identified at the return visit and repeat measures were taken from the same areas as originally measured. Subjects did not apply any topical therapies eight hours prior to measurements.
Skin Electrical Capacitance
Skin hydration was assessed in target sites by measurements of skin electrical capacitance. This helped monitor emollient compliance and is also a measure of barrier integrity. Measurements were made using a corneometer CM 820 (Courage & Khazaka). The location and number of measurements were the same as for TEWL measurements. Measurements were performed by two study authors only (ES and PB).
Sample Size and Statistics
The authors expected from previous studies that baseline TEWL measurements would measure on average between 30 and 40 mg/cm2 per hour. The authors also predicted that TEWL measurements should decrease to approximately 15–20 mg/cm2 per hour, with a difference in means between baseline and the end of study to be ranging between 10–15 mg/cm2 per hour. Therefore, for 80 percent power to detect a significant decrease in TEWL from baseline, approximately 14–32 subjects were required. The authors therefore chose 25 subjects. With 25 subjects, we would have 80 percent power to detect a mean difference of 8 mg/cm2 per hour between time points. Paired sample t tests were used for continuous variables in the same target sites, independent t tests were used to compare between treatment groups.
RESULTS
The mean age of the subjects was 39 years of age with the youngest being 17 and the oldest 67. There were 19 Caucasian, two African-American, two Asian, one each Hispanic and mixed race subjects enrolled. At baseline, all subjects were diagnosed with moderate-to-severe atopic dermatitis according to Hanifin-Rajka criteria.7 There were no subject drop-outs or protocol violations.
From baseline to the week 2 visit, TEWL readings significantly decreased in both the active and control target sites. The active target site decreased 14.35 ± 16 mg/cm2 per hour; 95 percent confidence interval, P<0.001. The control target site decreased 8.75 ± 11.80 mg/cm2 per hour in 25 subjects; 95% confidence interval, P<0.001. Although the active target site TEWL readings improved more than the control target site, the difference was not statistically significant (P=0.169). The non-lesional TEWL decreased 1.79 ± 4.92 mg/cm2 per hour in 25 subjects; 95% confidence interval, P<0.081. When control site was compared to normal skin changes, there was also a significant difference in the values (P=0.009) (Table 1).
Table 1.
Transepidermal water loss (TEWL) values before and after treatment with 0.1% fluocinonide cream and vehicle. Significant improvements seen in both the active and control sites.
| Site | TEWL at Baseline (mg/cm2) | TEWL at Week 2 Visit (mg/cm2) | Change in TEWL from Baseline to Week 2 visit (mg/cm2) | P value for Change from Baseline to Week 2 visit** |
|---|---|---|---|---|
| Active | 31.33±14.82 | 16.99±8.31 | −14.35±16* | <0.001 |
| Control | 29.18±15.03 | 20.43±12.30 | −8.75±11.80* | <0.001 |
| Non-lesional | 11.84±4.74 | 10.05±3.43 | −1.79±4.92 | 0.081 |
P=0.169, confidence interval 95% for difference in change between active and control sites
confidence interval 95%
Skin electrical capacitance increased from baseline to the week 2 visit. The active target site increased 5.5 ± 9.8 (a.u.); 95 percent confidence interval, P<0.01. The control target site increased 5.3 ± 10.6 (a.u.); 95 percent confidence interval, P<0.021. The non-lesional skin readings increased 2.5 ± 11.5 (a.u.); 95 percent confidence interval, P<0.287 (Table 2).
Table 2.
Stratum corneum hydration as measured by skin capacitance before and after treatment with 0.1% fluocinonide cream and vehicle. Significant improvements in hydration seen in both the active and control sites.
| Site | Capacitance at Baseline (a.u.) | Capacitance at Week 2 Visit (a.u.) | Change in Capacitance from Baseline to Week 2 visit (a.u.) | P value for Change from Baseline to Week 2 Visit** |
|---|---|---|---|---|
| Active | 17.2±3.4 | 22.8±13.0 | 5.5± 9.8* | <0.01 |
| Control | 18.7±10.7 | 23.9±14.2 | 5.3± 10.6* | 0.021 |
| Non-lesional | 28.5±11.0 | 31.0±13.0 | 2.5± 11.5 | 0.287 |
P=0.667, confidence interval 95% for difference in change between active and control sites
confidence interval 95%
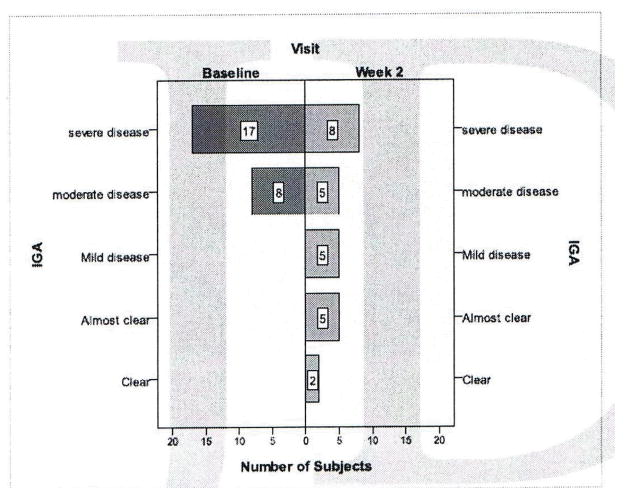
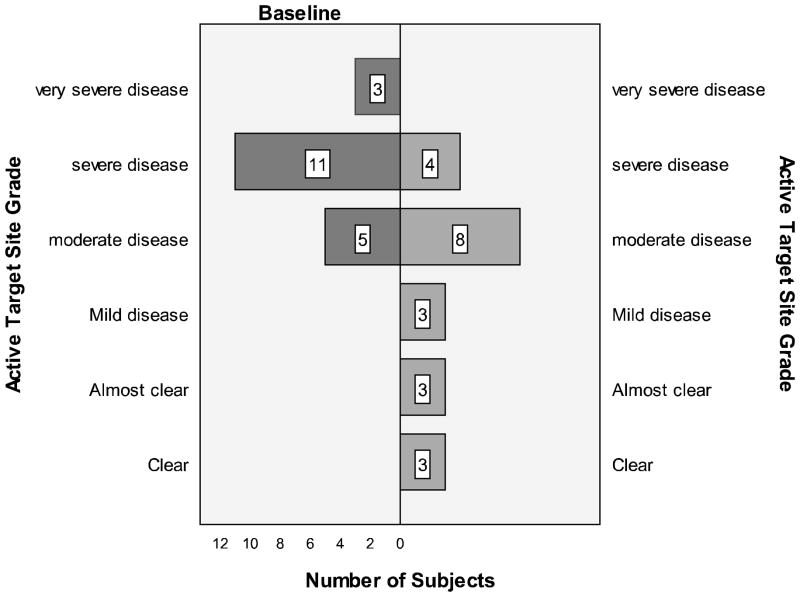
The EASI score improved 6.01 ± 7.90; 95 percent confidence interval, P<0.001, from a baseline average of 13.07 ± 10.9 to the week 2 visit average of 7.06 ± 9.41. Subjects’ IGA improved over the two-week span: At week 2, there were eight severe cases versus 17 at baseline (Figure 3). The active and control targets site investigator scores both improved from baseline at the week 2 visit (Figure 4). Pruritus scale scores decreased 3.5 ± 3.4; 95 percent confidence interval, P<0.001 from baseline to the week 2 visit. The DLQI scores decreased 5.3 ± 6.2; 95 percent confidence interval, P<0.001 from a baseline average of 8.92 ± 6.23 (corresponding to a moderate effect on quality of life) to the week 2 visit average of 3.64 ± 4.24 (corresponding to a small effect on quality of life). Based on IGA, 8 percent of subjects were clear at week 2 and 28 percent were clear or almost clear.
Figure 3.
Change in Investigator Global Assessment (IGA) before and after therapy with fluocinonide cream.
Figure 4.
Improvements seen in overall grading of active target sites with fluocinonide therapy.
During the study, there were no serious adverse events. One subject complained of burning at the active drug site that lasted for the first two days of application and then subsided. The subject described the burning as mild.
DISCUSSION
The authors have shown that short-term treatment with a novel formulation of 0.1% fluocinonide led to significantly improved barrier function as measured by basal TEWL in subjects with active moderate-to-severe AD. Subjects also experienced significantly improved stratum corneum hydration and clinical scores after the two-week treatment period. This improvement in skin barrier function with the active drug therapy appeared greater than seen with the vehicle alone, but this difference did not reach statistical significance. Therefore, the authors were unable to definitely state whether the improvement in skin barrier function was due to the anti-inflammatory effects of the steroid or the vehicle effects. Both components of the therapy are likely having an effect. These data suggest short-term treatment of AD with this super-potent corticosteroid improves skin barrier function.
These data confirm a previous study by Aalto-Korte, who also found significant improvements in lesional TEWL with four to six days of treatment with topical dexamethasone cream.3 On the other hand, these clinical data would appear to conflict with recent studies revealing that short-term treatment with topical steroids may decrease skin barrier integrity and repair.4
There are at least two potential explanations for these seemingly discordant findings. First, the anti-inflammatory effects of topical steroids may outweigh any potential detrimental effects they may have on skin barrier physiology in patients with active lesions of atopic dermatitis. The study by Kao used subjects with normal skin, not active dermatitic lesions. Second, corticosteroid effects may have beneficial effects on basal TEWL while simultaneously exerting negative effects on assays measuring stratum corneum integrity and cohesion. The current study and the study by Aalto-Korte measured basal TEWL without artificial barrier abrogation, whereas studies such as Kao’s used measurements of barrier dysfunction after tape stripping. These tape strip models measure barrier integrity, cohesion, and barrier recovery, but do not measure basal TEWL. Artificial barrier disruption models help to detect more subtle barrier dysfunction and, while scientifically important, have unknown clinical relevance. Changes in basal TEWL are the changes most likely to have short-term clinical relevance, although steroid effects on stratum corneum adhesion and integrity may be important with chronic steroid use.
The improvement in skin barrier function with fluocinonide cream in our study appears to be the result of both reducing skin inflammation and barrier improvement from the vehicle effects. Improvement in skin barrier function likely occurs with the short-term use of most topical steroid preparations in eczematous skin, assuming it is delivered using a vehicle that does not harm the skin barrier. In contrast, the long-term use of topical steroids is known to cause skin atrophy. It is unclear whether steroid molecules differ in their atrophogenic potential, although a study by Gans determined that fluocinonide 0.1% had a lower potential to produce atrophic changes than clobetasol cream or foam.13
Clinical scores significantly improved during the course of the study. Our study had a smaller percentage of subjects reaching clear or almost clear using IGA grading (28%) than that found in the study reported in the fluocinonide 0.1% package insert (59%). This likely reflects the disease severity of the population enrolled in our university-based study. Quality of life scores also improved significantly. Subjects had baseline DLQI scores corresponding to their disease having a “moderate effect” on their life at baseline.14 The mean scores after two weeks of treatment corresponded to the disease having a “small effect.”
In conclusion, the short-term use of fluocinonide 0.1% cream improved skin barrier function as measured by TEWL in subjects with atopic dermatitis and demonstrated significant improvements in clinical and quality of life scores. Although topical steroids may negatively affect the skin barrier with long-term use, they play an important role in the acute control of inflammation and can rapidly restore skin barrier function with short-term use. Further research is needed regarding the effect topical steroids may have on other aspects of skin barrier function such as antimicrobial peptide production and filaggrin gene expression.
Acknowledgments
Eric Simpson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors wish to thank Christine E. Carocci for assistance with proofreading, editing and preparation of this manuscript.
Footnotes
Author Contributions: The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Simpson, Woods. Acquisition of data: Woods, Brown, Simpson. Analysis and interpretation of data: Woods, Brown, Simpson. Drafting of the manuscript: Woods. Critical revision of the manuscript for important intellectual content: Woods, Brown, Simpson. Statistical analysis: Brown, Simpson. Obtained funding: Simpson. Administrative, technical, or material support: Simpson. Study supervision: Simpson.
DISCLOSURES
Funding for this work was provided by Medicis Pharmaceutical Corporation.
The sponsor had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; or in the preparation, review of approval of the manuscript.
Relevant to this manuscript (during the past five years):
Dr. Woods and Peter Brown and Shahana Baig-Lewis have no relevant conflicts of interest to disclose.
Dr. Simpson received a research grant from Medicis Pharmaceuticals for this project.
All Other Relationships (during the past five years):
Dr. Woods and Peter Brown have no relevant conflicts of interest to disclose.
Dr. Simpson has served as consultant to Asubio, Centocor, CombinatoRx, Corgentech, Galderma International, Graceway, Stiefel and Vertex. Dr. Simpson has received honoraria from Aerovance, Connetics, and Stiefel.
References
- 1.Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technology Assessment. 2000;4(37):1–191. [PMC free article] [PubMed] [Google Scholar]
- 2.Hanifin JM, Cooper KD, Ho VC, Kang S, Krafchik BR, Margolis DJ, Schachner LA, Sidbury R, Whitmore SE, Sieck CK, Van Voorhees As. Guidelines of care for atopic dermatitis, developed in accordance with the American Academy of Dermatology (AAD)/American Academy of Dermatology Association “Administrative Regulations for Evidence-based Clinical Practice Guidelines. J Am Acad Dermatol. 2004 March;50(3):391–404. doi: 10.1016/j.jaad.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Aalto-Korte K. Improvement of skin barrier function during treatment of atopic dermatitis. J Am Acad Dermatol. 1995;33(6):969–972. doi: 10.1016/0190-9622(95)90288-0. [DOI] [PubMed] [Google Scholar]
- 4.Kao JS, Fluhr JW, Man MQ, Fowler AJ, Hachem JP, Crumrine D, Ahn SK, Brown BE, Elias PM, Feingold KR. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003 Mar;120(3):456–464. doi: 10.1046/j.1523-1747.2003.12053.x. [DOI] [PubMed] [Google Scholar]
- 5.Palmer CNA, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 6.Federal Register (62 FR 25692) May 9, 1997. [Google Scholar]
- 7.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Dermatovener. 1980;92(Suppl):44–47. [Google Scholar]
- 8.Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The Eczema Area and Severity Index (EASI) assessment of reliability in atopic dermatitis. Exp Dermatol. 2001;10:11–18. doi: 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- 9.McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. 1988;18(4):1007–1019. doi: 10.1017/s0033291700009934. [DOI] [PubMed] [Google Scholar]
- 10.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994 May 19;3:210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 11.Fluhr JW, Feingold KR, Elias PM. Transepidermal water loss reflects permeability barrier status: validation in human and rodent in vivo and ex vivo models. Exp Dermatol. 2006;15:483–492. doi: 10.1111/j.1600-0625.2006.00437.x. [DOI] [PubMed] [Google Scholar]
- 12.Pinnagoda J, Tupker RA, Agner T, Serup J. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164–178. doi: 10.1111/j.1600-0536.1990.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 13.Gans EH, Sadiq I, Stoudemayer T, Stoudemayer M, Kligman AM. In vivo determination of the skin atrophy potential of the super-high-potency topical corticosteroid fluocinonide 0.1% cream compared with clobetasol propionate 0. 05% cream and foam, and a vehicle. J Drugs Dermatol. 2008 Jan;7(1):28–32. [PubMed] [Google Scholar]
- 14.Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: What do dermatology life quality index scores mean? J Invest Dermatol. 2005 Oct;125(4):659–664. doi: 10.1111/j.0022-202X.2005.23621.x. [DOI] [PubMed] [Google Scholar]