Abstract
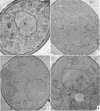
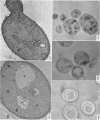
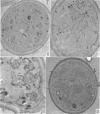
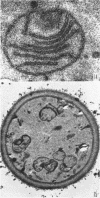
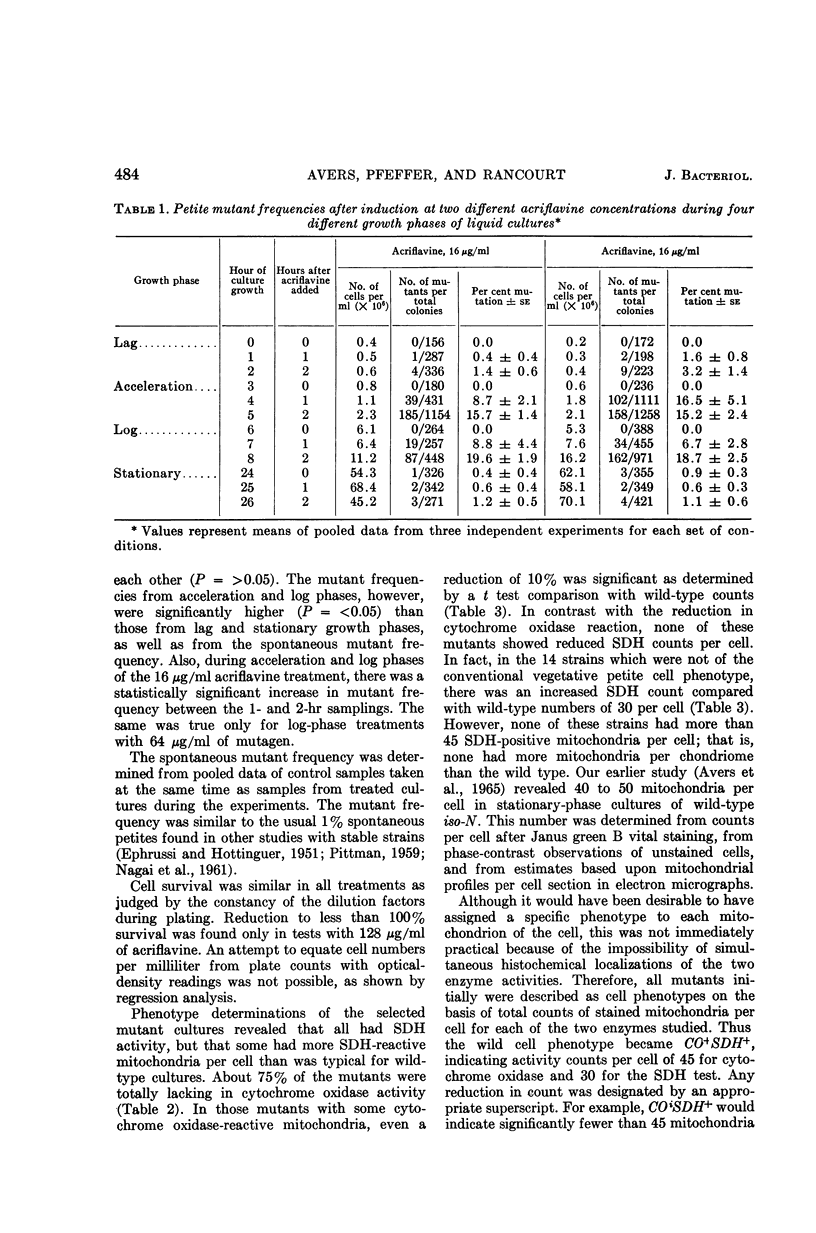
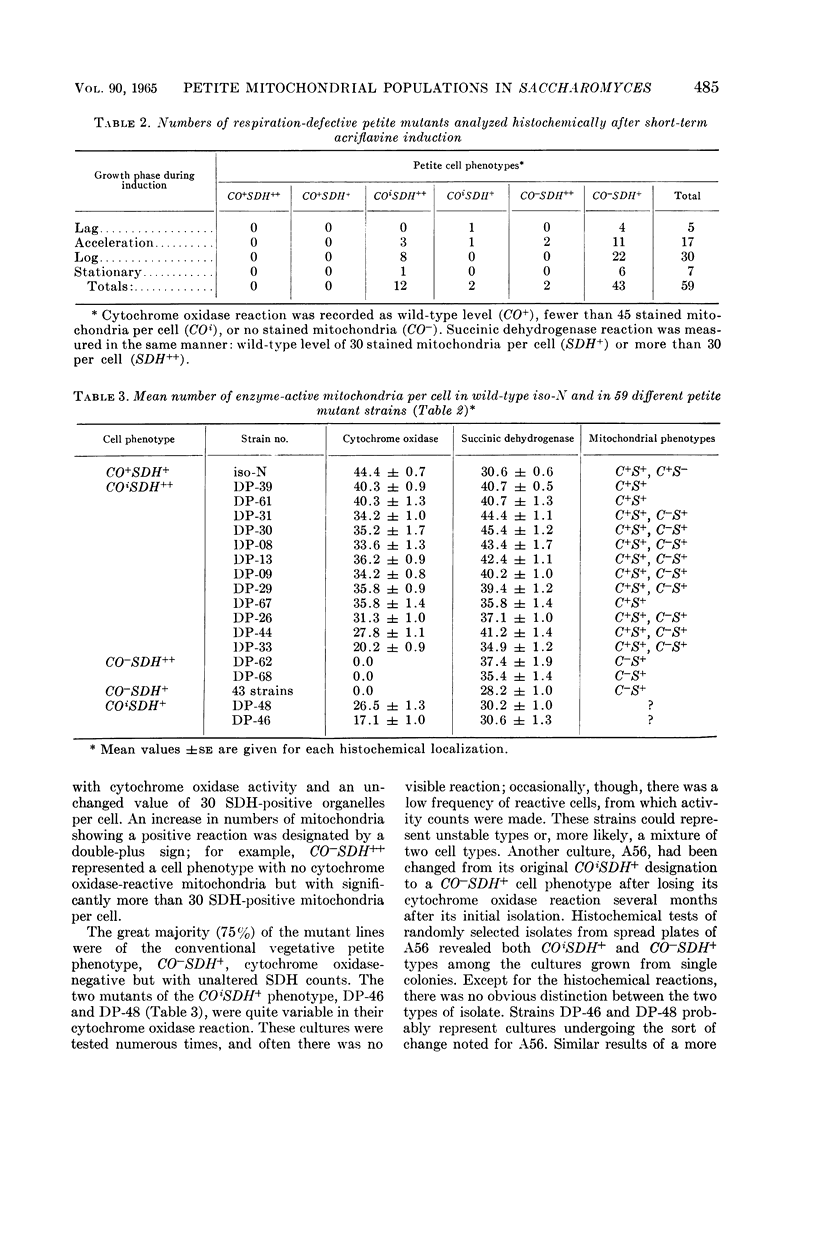
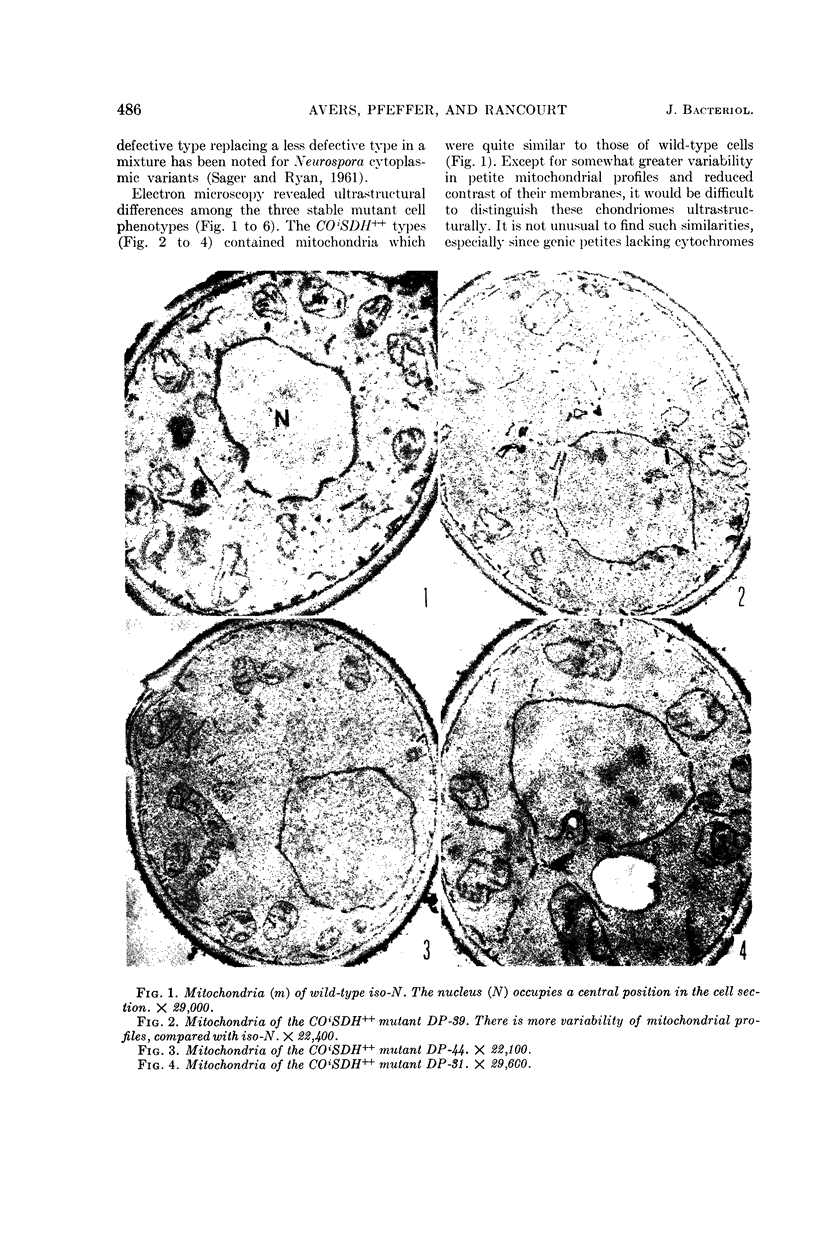
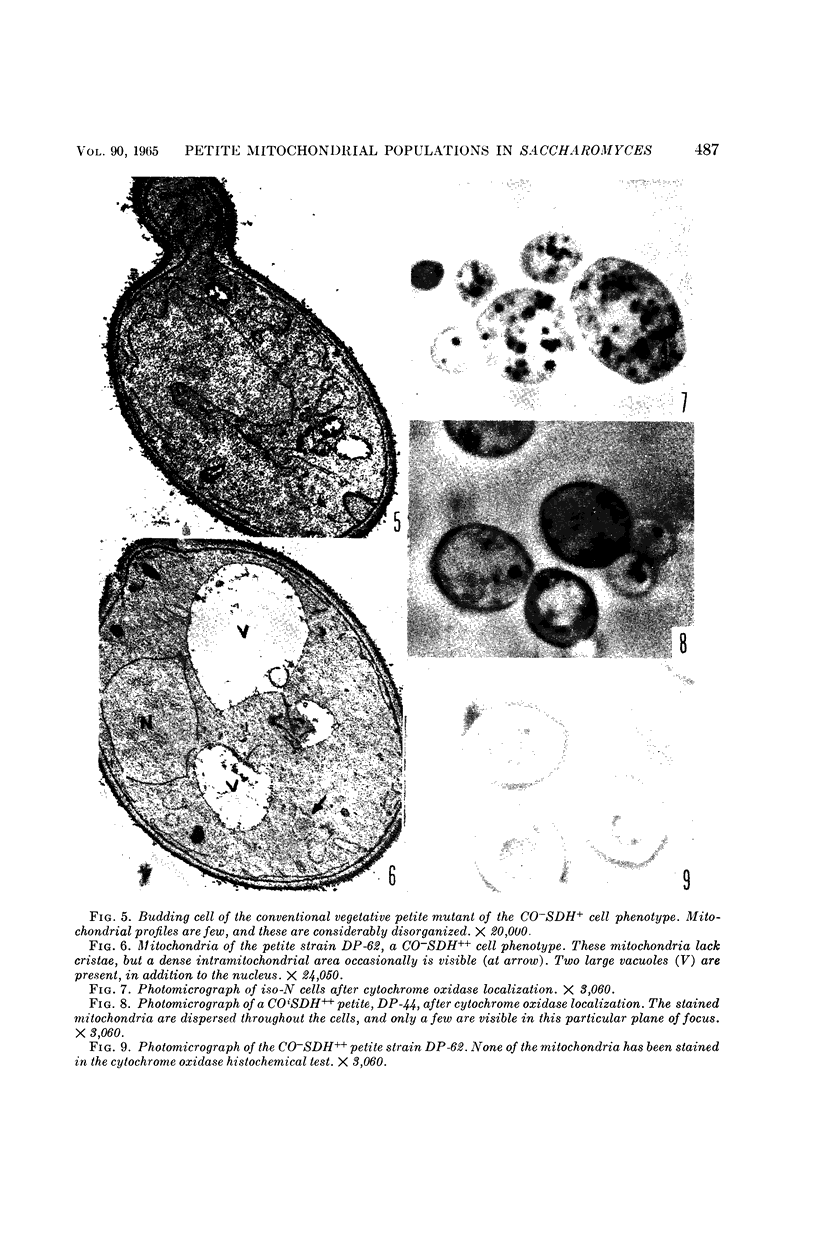
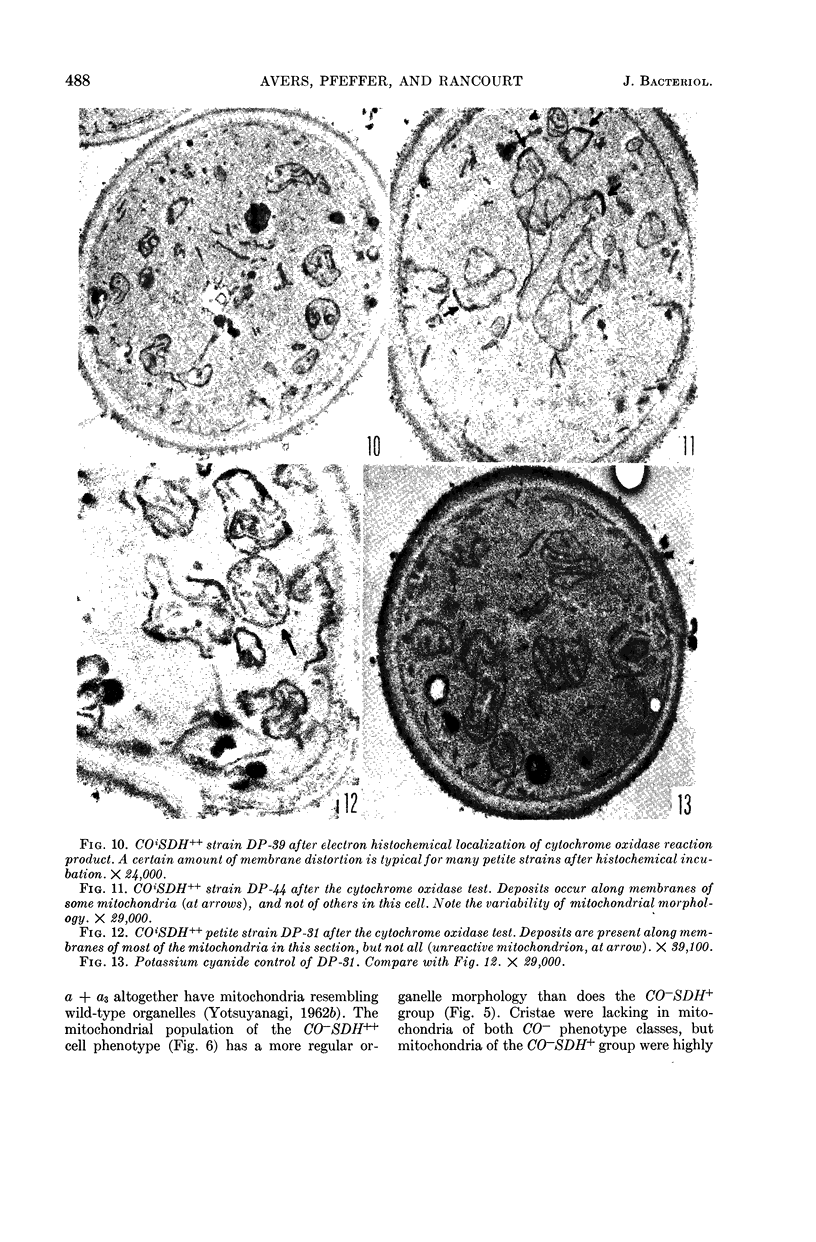
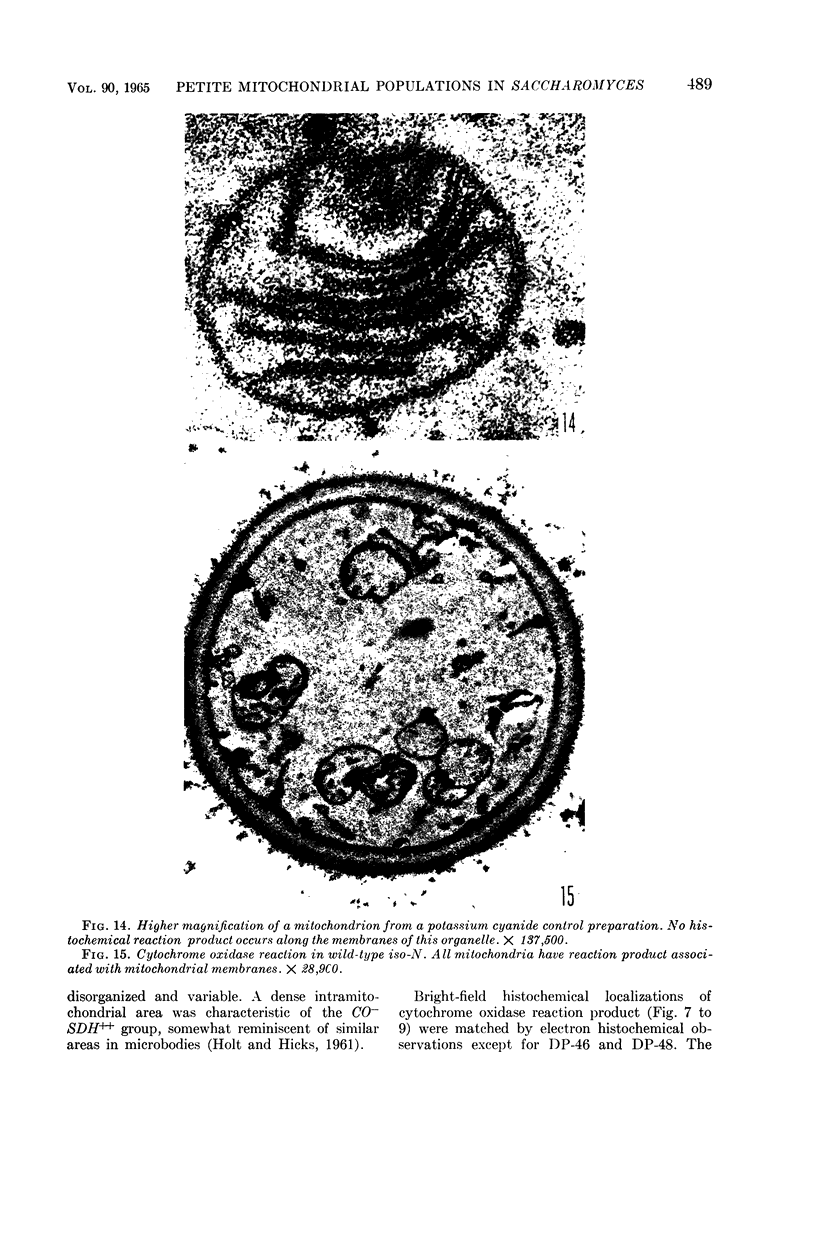
Avers, Charlotte J. (Rutgers, The State University, New Brunswick, N. J.), Cynthia R. Pfeffer, and Martha W. Rancourt. Acriflavine induction of different kinds of “petite” mitochondrial populations in Saccharomyces cerevisiae. J. Bacteriol. 90:481–494. 1965.—Mutant frequencies induced by 1 or 2 hr in 16 and 64 μg/ml of acriflavine were significantly higher during acceleration and log-phase exposures than during lag or stationary phases. From these induced petites, 59 colonies were selected at random and established in pure culture. All strains were analyzed histochemically for mitochondrial cytochrome oxidase and succinic dehydrogenase (SDH) reactions. On the basis of counts of stained mitochondria per cell obtained by light microscopy, four different cell phenotypes were recognized among the mutant strains: (i) reduced cytochrome oxidase, wild-type SDH; (ii) reduced cytochrome oxidase, high SDH; (iii) absent cytochrome oxidase, high SDH; and (iv) absent cytochrome oxidase, wild-type SDH. The last group was the most common, characterizing 43 of the 59 strains. Electron microscopy showed differences in mitochondrial ultrastructure for the various cell phenotype classes. Electron histochemical localizations showed cytochrome oxidase reaction product only on mitochondrial membranes of respiration-competent cells. Both reactive and unreactive mitochondria occurred in the same cell in mutants with partial respiratory competence. Different mitochondrial subpopulation mixtures characterized the mutant strains, many of which had at least two kinds of respiratory-competent types per chondriome. The diverse chondriomes comprised a stable feature of the mutants, since they have been maintained unchanged during serial transfer for more than 1 year in culture. Together with earlier reports of at least two kinds of mitochondria in wild-type cells, the evidence indicated that mitochondria were capable of regulating some portion of their phenotype. The recognition of mitochondrial phenotypes was proposed as an initial step in a formal analysis of organelle heredity.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHUN E. H., VAUGHAN M. H., Jr, RICH A. THE ISOLATION AND CHARACTERIZATION OF DNA ASSOCIATED WITH CHLOROPLAST PREPARATIONS. J Mol Biol. 1963 Aug;7:130–141. doi: 10.1016/s0022-2836(63)80042-x. [DOI] [PubMed] [Google Scholar]
- EPHRUSSI B., HOTTINGUER H. On an unstable cell state in yeast. Cold Spring Harb Symp Quant Biol. 1951;16:75–85. doi: 10.1101/sqb.1951.016.01.007. [DOI] [PubMed] [Google Scholar]
- GIBOR A., GRANICK S. PLASTIDS AND MITOCHONDRIA: INHERITABLE SYSTEMS. Science. 1964 Aug 14;145(3633):890–897. doi: 10.1126/science.145.3635.890. [DOI] [PubMed] [Google Scholar]
- GREENAWALT J. W., ROSSI C. S., LEHNINGER A. L. EFFECT OF ACTIVE ACCUMULATION OF CALCIUM AND PHOSPHATE IONS ON THE STRUCTURE OF RAT LIVER MITOCHONDRIA. J Cell Biol. 1964 Oct;23:21–38. doi: 10.1083/jcb.23.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLT S. J., HICKS R. M. The localization of acid phosphatase in rat liver cells as revealed by combined cytochemical staining and electron microscopy. J Biophys Biochem Cytol. 1961 Oct;11:47–66. doi: 10.1083/jcb.11.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAEPELIN G. NORMALISIERUNG DES ATMUNGSDEFEKTES BEI HEFE. RUECKFUEHRUNG STABILISIERTER RD-MUTANTEN IN VOLL ATMUNGSFAEHIGE NORMALZELLEN. Arch Mikrobiol. 1964 Jun 2;48:299–305. [PubMed] [Google Scholar]
- LUCK D. J., REICH E. DNA IN MITOCHONDRIA OF NEUROSPORA CRASSA. Proc Natl Acad Sci U S A. 1964 Oct;52:931–938. doi: 10.1073/pnas.52.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLONIG G. A modified procedure for lead staining of thin sections. J Biophys Biochem Cytol. 1961 Dec;11:736–739. doi: 10.1083/jcb.11.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGAI S. Induction of the respiration-deficient mutation in yeast by various synthetic dyes. Science. 1959 Oct 30;130(3383):1188–1189. doi: 10.1126/science.130.3383.1188-a. [DOI] [PubMed] [Google Scholar]
- NAGAI S., YANAGISHIMA N., NAGAI H. Advances in the study of respiration-deficient (RD) mutation in yeast and other microorganisms. Bacteriol Rev. 1961 Dec;25:404–426. doi: 10.1128/br.25.4.404-426.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASS S., NASS M. M. INTRAMITOCHONDRIAL FIBERS WITH DNA CHARACTERISTICS. II. ENZYMATIC AND OTHER HYDROLYTIC TREATMENTS. J Cell Biol. 1963 Dec;19:613–629. doi: 10.1083/jcb.19.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGAWA K., BARRNETT R. J. ELECTRON HISTOCHEMICAL EXAMINATION OF OXIDATIVE ENZYMES AND MITOCHONDRIA. Nature. 1964 Aug 15;203:724–726. doi: 10.1038/203724a0. [DOI] [PubMed] [Google Scholar]
- ORGEL A., BRENNER S. Mutagenesis of bacteriophage T4 by acridines. J Mol Biol. 1961 Dec;3:762–768. doi: 10.1016/s0022-2836(61)80081-8. [DOI] [PubMed] [Google Scholar]
- RIS H. Ultrastructure and molecular organization of genetic systems. Can J Genet Cytol. 1961 Jun;3:95–120. doi: 10.1139/g61-015. [DOI] [PubMed] [Google Scholar]
- SAGER R., ISHIDA M. R. CHLOROPLAST DNA IN CHLAMYDOMONAS. Proc Natl Acad Sci U S A. 1963 Oct;50:725–730. doi: 10.1073/pnas.50.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHATZ G. THE ISOLATION OF POSSIBLE MITOCHONDRIAL PRECURSOR STRUCTURES FROM AEROBICALLY GROWN BAKER'S YEAST. Biochem Biophys Res Commun. 1963 Aug 20;12:448–451. doi: 10.1016/0006-291x(63)90313-9. [DOI] [PubMed] [Google Scholar]
- SEDAR A. W., BURDE R. M. LOCALIZATION OF THE SUCCINIC DEHYDROGENASE SYSTEM IN ESCHERICHIA COLI USING COMBINED TECHNIQUES OF CYTOCHEMISTRY AND ELECTRON MICROSCOPY. J Cell Biol. 1965 Feb;24:285–295. doi: 10.1083/jcb.24.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHERMAN F., SLONIMSKI P. P. RESPIRATION-DEFICIENT MUTANTS OF YEAST. II. BIOCHEMISTRY. Biochim Biophys Acta. 1964 Jul 15;90:1–15. doi: 10.1016/0304-4165(64)90113-8. [DOI] [PubMed] [Google Scholar]
- SYLVEN B., TOBIAS C. A., MALMGREN H., OTTOSON R., THORELL B. Cyclic variations in the peptidase and catheptic activities of yeast cultures synchronized with respect to cell multiplication. Exp Cell Res. 1959 Jan;16(1):75–87. doi: 10.1016/0014-4827(59)90197-1. [DOI] [PubMed] [Google Scholar]
- Stinson H T. Extranuclear Barriers to Interspecific Hybridization between Oenothera Hookeri and Oenothera Argillicola. Genetics. 1960 Jul;45(7):819–838. doi: 10.1093/genetics/45.7.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOTSUYANAGI Y. [Study of yeast mitochondria. I. Variations in mitochondrial ultrastructure during the aerobic growth cycle]. J Ultrastruct Res. 1962 Aug;7:121–140. doi: 10.1016/s0022-5320(62)80031-8. [DOI] [PubMed] [Google Scholar]
- YOTSUYANAGI Y. [Study of yeast mitochondria. II. Mitochondria of respiration-deficient mutants]. J Ultrastruct Res. 1962 Aug;7:141–158. doi: 10.1016/s0022-5320(62)80032-x. [DOI] [PubMed] [Google Scholar]