Abstract
The Akt activation inhibitor triciribine and the farnesyltransferase inhibitor tipifarnib have modest to little activity in clinical trials when used as single agents. In this manuscript pre-clinical data demonstrate that the combination is more effective than single agents both in cultured cells and in vivo. Combination index data analysis demonstrates that this combination is highly synergistic at inhibiting anchorage-dependent growth of breast cancer cells. This synergistic interaction is also observed with structurally unrelated inhibitors of Akt (MK-2206) and farnesyltransferase (FTI-2153). The triciribine/tipifarnib synergistic effects are seen with several cancer cell lines including those from breast, leukemia, multiple myeloma and lung tumors with different genetic alterations such as K-Ras, B-Raf, PI3K, p53 and pRb mutations, PTEN, pRB and Ink4a deletions and ErbB receptor overexpression. Furthermore, the combination is synergistic at inhibiting anchorage-independent growth and at inducing apoptosis in breast cancer cells. The combination is also more effective at inhibiting the Akt/mTOR/S6 kinase pathway. In an ErbB2-driven breast tumor transgenic mouse model the combination, but not single agent, treatment with triciribine and tipifarnib induces significant breast tumor regression. Our findings warrant further investigation of the combination of farnesyltransferase and Akt inhibitors.
Introduction
Accumulation of genetic alterations during oncogenesis leads to deregulation of complex signal transduction pathways, which in turn results in several hallmarks of cancer such as uncontrolled cell division, resistance to apoptosis, angiogenesis and metastasis (1, 2). Among the signal transduction pathways most often found deregulated in cancer cells are those triggered by receptor tyrosine kinases (RTKs) such as receptors for EGF, PDGF, IGF-1 and VEGF (3-5). These receptors activate complex and highly integrated signaling cascades that often culminate in the nucleus to regulate gene transcription. For example, binding of growth factors to their RTKs induces receptor dimerization and cross receptor tyrosine auto-phosphorylation, which leads to the recruitment of several key signaling proteins such as the GTP/GDP exchange factor m-SOS1 that activates the small GTPase Ras. Ras in turn can trigger a multitude of signaling cascades including the Raf/Mek/Erk1/2 kinase cascade and the Ral GDS/Ral A/B pathway (6-9). Activated RTKs can also recruit PI3K which can activate several pathways including those mediated by Akt (see more below) (10). Other pathways activated by RTKs involve non-receptor tyrosine kinases such as JAK and Src which stimulate pathways mediated by signal transducers and activators of transcription such as STAT3 (11, 12). While these RTK-driven pathways are highly regulated in normal cells, they are often found persistently hyper-activated in cancer cells. For example, aberrant activation, overexpression and/or mutations in RTKs, Ras, PI3K, Akt, Ral GDS and/or STAT3 are common occurrences in cancer cells, and consequently the pathways mediated by these proteins are found aberrantly activated (1). This prompted the development of inhibitors of these pathways as novel therapeutic agents, many of which are presently in clinical trials (6, 9, 13-20). Unfortunately, as single agents they often show little to no clinical efficacy, suggesting that inhibiting single targets in complex signal transduction pathways that contain multiple oncogenic lesions may not be sufficient, and that combination of small molecules that target several pathways may be more effective at treating cancer (3-5, 21, 22).
One of the major pathways targeted for anti-cancer drug discovery is the PI3K-Akt pathway (10, 15-18). Stimulation of RTKs activates PI3K which converts phosphatidylinositol bisphosphate (PIP2) to PIP3. Akt and PDK1 are then recruited to the plasma membrane through binding of their PH domains to PIP3. PDK1 then phosphorylates Akt on Thr308, and full activation of Akt occurs when Ser473 is phosphorylated by kinases such as the TORC2 complex (23, 24). Activated Akt then phosphorylates a multitude of substrates involved in several hallmarks of cancer such as evasion of apoptosis, angiogenesis and metastasis (10). We have identified triciribine (TCN/TCN-P), a small molecule inhibitor of the activation of all 3 isoforms of Akt: Akt1, Akt2 and Akt3 (25). TCN-P inhibits Akt activation by binding to the PH domain of Akt, blocking its membrane binding and subsequent phosphorylation (26). We and others have performed clinical trials in both solid tumors (27) and leukemia (28) and found that TCN-P is able to inhibit P-Akt levels by comparing biopsies before and during treatment (27). Unfortunately, no major clinical responses were observed, suggesting that inhibition of Akt activation alone is not sufficient and that combination therapy is warranted.
Many small G-proteins that are involved in oncogenesis require post-translational modifications with the lipids farnesyl or geranylgeranyl for their cancer-causing activity (29, 30). Farnesyltransferase (FTase) catalyzes the transfer of farnesyl from farnesylpyrophosphate to the thiol of cysteines at the carboxyl termini of proteins that end with the consensus sequence C-A-A-X where C= cysteine, A=any aliphatic amino acid and X=any amino acid but preferably methionine or serine (30). Geranylgeranyltransferase I (GGTase I) transfers geranylgeranyl from geranylgeranyl-pyrophosphate to the thiol of C-A-A-X cysteine but prefers X to be leucine (30). Because farnesylated small G-proteins such as Ras and Rheb and geranylgeranylated small G-proteins such as Rho and Ral require these post-translational modifications for mediating malignant transformation, FTase and GGTase I inhibitors (FTIs and GGTIs) are being evaluated as potential anti-cancer drugs (29, 31-35). Pre-clinical studies demonstrated that FTIs interfere with cell cycle progression mainly during mitosis whereas GGTIs induce cell cycle arrest at the G1 phase. Furthermore, whereas FTIs have been shown to disrupt the Rheb/mTOR/S6Kinase pathway, GGTIs induce the accumulation of the CDK inhibitors p21waf1 and p27Kip1, inhibit CDK activity, and induce pRb hypophosphorylation (36-47). While several FTIs have been investigated in the clinic extensively, only one GGTI has entered phase I clinical trials recently (48, 49). Unfortunately, as single agents, FTIs such as tipifarnib have shown no clinical benefit in phase III clinical trials in metastatic colorectal and pancreatic cancer patients (50, 51). However, the combination of tipifarnib with doxorubicin and cyclophosphamide in phase II trials appears to benefit patients with locally advanced breast cancer, increasing the pathologic complete response rate (52). In this manuscript we provide preclinical data that demonstrate in cultured human breast cancer cells that the combination of the Akt activation inhibitor TCN and the FTI tipifarnib is synergistic at inhibiting anchorage-dependent and -independent growth and inducing apoptosis. Furthermore, in an ErbB2-driven breast cancer transgenic animal model, the combination, but not single agent treatment, causes significant breast tumor regression.
Methods
Cell lines and small molecule inhibitors
Cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA). TCN and TCN-P were obtained from NCI (Bethesda, MD). FTI-2153 was synthesized as described previously (53). Tipifarnib was obtained from Johnson and Johnson Pharmaceutical (Raritan, NJ). MK-2206 was purchased from Chemietek (Indianapolis, IN). AG1024 was purchased from EMD Chemicals (Gibbstown, NJ). All drugs for cell culture were dissolved in DMSO (Sigma).
Western blot analyses
SDS-PAGE and western blotting were performed as previously described (39). Primary antibody sources were as follows: P-Akt (Ser473), P-ERK1/2 (Thr202/Tyr204), ERK1/2, P-4EBP1 (Thr37/46), 4EPB1, P-p70S6K (Thr389) and p70S6K were from Cell Signaling Technology (Danvers, MA); HDJ-2 was from Labvision/Thermo Fisher Scientific (Fremont, CA); Rap1 and Akt 1/2 were from Santa Cruz Biotechnology (Santa Cruz, CA); β-actin and α-tubulin were from Sigma (St. Louis, MO). All secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
MTT assays
Cells were cultured in 96-well plates and allowed to attach overnight. The medium was then replaced with medium containing drugs as indicated for 72 hours before incubating with 1 mg/ml thiazolyl blue tetrazolium bromide (Sigma) for 3 hours. The medium was then removed and MTT crystals were solubilized in DMSO (Sigma). Plates were read on a µQuant microplate reader using KC4 data analysis software (Bio-Tek Instruments). The 540 nm absorbance of vehicle-treated wells was used to define 100% proliferation. Each condition was performed in replicates of eight wells.
Synergy analysis
The effects of drug combinations were evaluated with Calcusyn software (Biosoft; Cambridge, UK). This software uses the Chou-Talalay combination index method, which is based upon the median-effect equation, itself a derivation from the mass-action law (54). For this analysis, Drug1 was combined with Drug2 at a constant ratio determined by (IC50)Drug1/(IC50)Drug2. We entered the resulting proliferation data, along with the data obtained from single drug treatments, into Calcusyn to determine a combination index value (CI) for each combination point, which quantitatively defines additivity (CI=1), synergy (CI<1), and antagonism (CI>1). The resulting values were used to construct a plot of CI values over a range of fractions affected (Fa-CI plot) (54).
Active caspase-3 staining
After drug treatment, cells were trypsinized, washed, and fixed using BD Cytofix/Cytoperm™ Fixation/Permeabilization Solution Kit from BD Biosciences (San Jose, CA). Cells were stained with FITC-conjugated antibody to the active form of caspase-3 (BD Biosciences) according to the manufacturer's recommended protocol. Samples were analyzed using a FACSCalibur flow cytometer (BD; Franklin Lakes, NJ).
Soft agar assay
Cells were seeded in regular growth medium containing 0.3% agar (Sigma) and drug added at the indicated concentrations, as previously described (39). Colonies were allowed to grow for three weeks before 1 mg/ml MTT (Sigma) solution was added overnight to facilitate counts. Colonies were scored and counted according to size, as previously described (39).
Mouse Experimental Procedures
MMTV/neu transgenic mice [FVB/N-Tg(MMTVneu)202Mul/J] were purchased from the Jackson Laboratory (Bar Harbor, ME) and bred to produce multiple litters in order to maintain the colony. Female mice were palpated once each week for mammary gland tumor nodules. At the time of tumor onset, orthogonal measurements were taken 2-3 times per week and the tumor volume calculated using the formula: V= (L2W)/2, where L= length and W= width with width defined as the larger measurement. Drug treatment was initiated when the tumors grew to about 200-2000 mm3 and treatment lasted up to for 14 days. The treatment groups were: a) vehicle, b) TCN-P, at a dose of 20 mpk per day in 1.5% sodium bicarbonate (Thermo Fisher) w/v in water, pH 8.0, administered in 100μL intraperitoneal injections once daily, c) tipifarnib, at a dose of 10mpk per day in 20% 2-hydroxypropyl-β-cyclodextrin, was administered by a s.c. minipump (Alzet, Cupertino, CA), releasing at a rate of 0.5 μL per hour for 14 days, or d) a combination of the two treatments. There was no evidence of gross toxicity in the drug-treated animals as measured by weight loss. All methods involving mice were approved by the Institutional Animal Care and Use Committee of the University of South Florida.
To analyze the effect of each treatment, we calculated the percent change in volume of each tumor during treatment. The percent change in volume was calculated based on the tumor volume on the last day of treatment (VT) relative to that on the day of initiation of treatment (VI). The average percent change in tumor volume was then calculated for each treatment group.
Statistical Analysis
For statistical analysis, percent volume change of 44 tumors was compared among the four treatment groups (vehicle, TCN-P, tipifarnib, and combination treatments) using a generalized linear model (Proc GLM in SAS V9.2). When differences were detected Dunnett-Hsu test was performed to adjust for multiple comparisons and to examine if the combination treatment is more effective than single agent treatment.
Pre- and Post-Treatment Tumor Biopsies and Western Blot Analyses
Incisional biopsies were taken from the same tumor before treatment and after seven days of treatment with vehicle, tipifarnib, TCN-P or the combination. Tumors were homogenized with a PowerGen 125 Tissue Homogenizer (Thermo Fisher) in T-PER Tissue Protein Extraction Reagent (Thermo Fisher) supplemented with 1X Complete Protease Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN), 4 mM sodium orthovanadate (Sigma), 10 mM p-nitrophenyl phosphate (Sigma) and 0.5 mM phenylmethanesulfonyl fluoride (Sigma). Homogenates were used in SDS-PAGE and western blotting as described previously (39).
Results
The Akt activation inhibitor TCN and the FTI tipifarnib synergize to inhibit the proliferation of human breast cancer cells
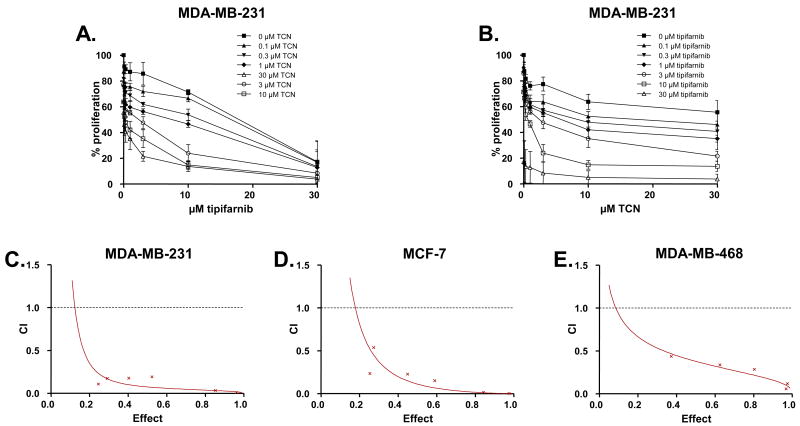
To determine the effects of combination treatment of the Akt inhibitor TCN and the FTI tipifarnib on human breast tumor cell proliferation, we first treated MDA-MB-231 cells with various concentrations of TCN and tipifarnib alone and in combination and monitored cell proliferation by MTT assay as described under Methods. Figures 1A and 1B show that tipifarnib or TCN alone caused a concentration-dependent inhibition of proliferation with IC50 values of 18 μM (tipifarnib) and greater than 30 μM (TCN). Furthermore, Figure 1B shows that treatment of MDA-MB-231 with fixed concentrations of tipifarnib decreased the IC50 values of TCN from greater than 30 μM (in the absence of tipifarnib) to 6 μM (in the presence of 1 μM tipifarnib) and 0.5 μM (in the presence of 10 μM tipifarnib). Therefore, tipifarnib sensitized MDA-MB-231 cells to TCN. Similarly, as shown in Figure 1A, TCN sensitized these cells to tipifarnib treatments, decreasing the IC50 values of tipifarnib from 18 μM in the absence of TCN, to 8 μM and 0.2 μM in the presence of 1 μM and 10 μM TCN, respectively. These data suggest that the two compounds, TCN and tipifarnib, may synergize to inhibit anchorage-dependent growth. To confirm this synergism, we treated cells with a combination of TCN and tipifarnib in a constant ratio to one another and used Calcusyn software to generate Fa-CI plots as described under Methods. Figure 1C and Supplemental Table S3 show that all the experimental points have CI (Combination Index) values of less than 1. The resulting Fa-CI plot curve falls well below 1 for the effect range, demonstrating that the combination of TCN and tipifarnib is highly synergistic in MDA-MB-231 breast cancer cells.
Figure 1. TCN and tipifarnib synergize to inhibit anchorage-dependent growth of human breast cancer cells.
A and B, MDA-MB-231 cells, were plated in 96 well plates, treated for 72 hours with various concentrations of TCN and tipifarnib either alone or in combination and processed for MTT staining as described under Methods. Each data point is the average of 8 wells each from three independent experiments. Error bars indicate standard error. C (MDA-MB-231), D (MCF-7) and E (MDA-MB-468) cells were plated, treated and processed as in A and B, and combination index analysis to determine synergy (defined as CI values below 1) was performed using CalcuSyn software as described in Methods. The graphs display CI values versus the fraction inhibited by each combination treatment (Effect). The Effect ranges from 0 (no inhibition) to 1 (complete inhibition). The data are representative of three independent experiments.
We next determined whether this synergistic interaction between tipifarnib and TCN is unique to MDA-MB-231 cells or whether it can also occur in other human breast cancer cell lines as well as cell lines from other cancer types. To this end, we treated two additional breast cancer cell lines, (MCF-7 and MDA-MB-468), one lung (A549), one leukemia (MV4-11) and one multiple myeloma (U266) cancer cell lines, with TCN and tipifarnib, alone and in combination, monitored cell proliferation by MTT assay, and analyzed the data for synergy as described under the Methods section. Figures 1D and 1E show the resulting Fa-CI plots, and demonstrate that in both MCF-7 (Figure 1D) and MDA-MB-468 (Figure 1E) cells, the combination treatment of TCN and tipifarnib resulted in synergistic inhibition of anchorage-dependent proliferation (also see Supplemental Table S3). A synergistic interaction was also observed with A-549, MV4-11 and U266 (see Supplemental Figure S1), suggesting that the combination is effective in a variety of human tumor types.
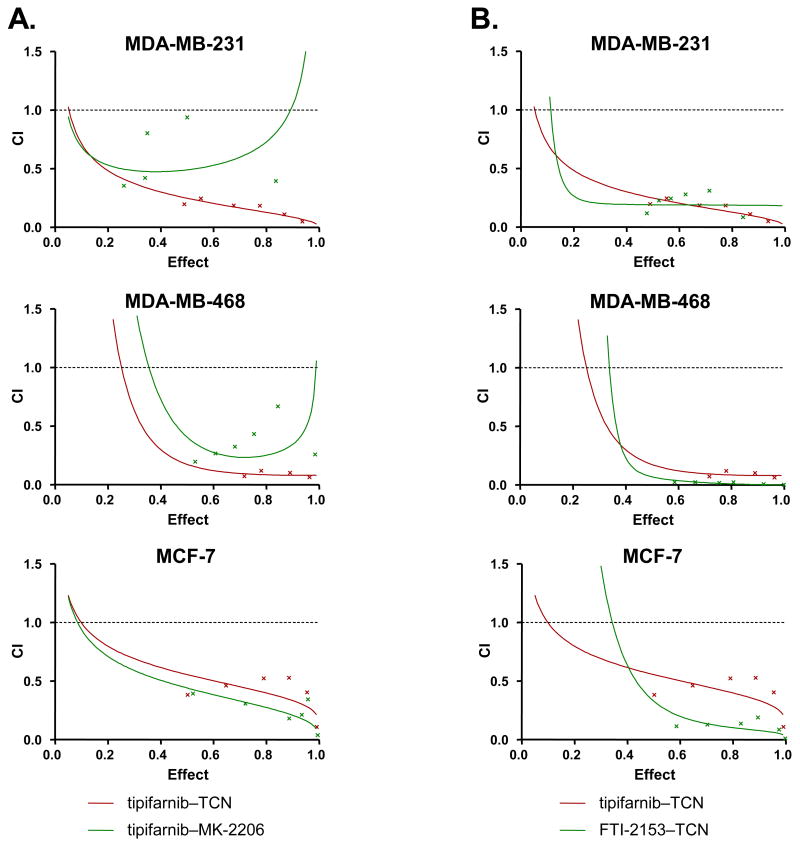
We next determined whether this synergistic interaction also occurs with inhibitors of Akt and farnesyltransferase that are structurally unrelated to TCN and tipifarnib. To this end, we first treated MDA-MB-231, MDA-MB-468 and MCF-7 cells, with the Akt inhibitor MK-2206 (55) or TCN in combination with tipifarnib as described under the Methods section. In all three cell lines the MK-2206/tipifarnib combination was more effective than the single agent treatment (see Supplemental Figure S2). To determine if the combination is synergistic, we co-treated the cells with MK-2206 and tipifarnib in a constant ratio to one another and generated Fa-CI plots. Figure 2A and Supplemental Table S3 show that in MDA-MB-231, MDA-MB-468 and MCF-7 cells, the combination of MK-2206 and tipifarnib was synergistic. We next treated MDA-MB-231, MDA-MB-468 and MCF-7 cells with the farnesyltransferase inhibitor FTI-2153 (53) in combination with the Akt activation inhibitor TCN and found this combination to also be more effective than single agent treatment (Supplemental Figure S2) and synergistic (Figure 2B) in all three cell lines. Thus, the synergistic interactions seen are not unique to TCN and tipifarnib and can also occur with other structurally unrelated inhibitors of Akt and farnesyltransferase.
Figure 2. The Akt inhibitors (TCN and MK-2206) and the FTase inhibitors (FTI-2153 and tipifarnib) synergize to inhibit the proliferation of breast cancer cells.
MDA-MB-231, MDA-MB-468 and MCF-7 cells were plated, treated and processed as described under Figure 1 with the following combinations: A, MK-2206 and tipifarnib (green) or TCN and tipifarnib (red); B, FTI-2153 and TCN (green) or tipifarnib and TCN (red). The data are representative of three independent experiments.
TCN and tipifarnib combination treatment is more effective at inhibiting signal transduction pathways in human cancer cells
Several aberrant growth factor signal transduction pathways that rely on farnesylated small G-proteins and Akt result in the persistent activation of the mTOR kinase (see introduction). Therefore, we determined the effect of TCN and tipifarnib on the persistent activation of these pathways. To this end, we treated MDA-MB-231 cells with TCN and tipifarnib either alone or in combination and processed the cells for western blotting as described under Methods. Figure 3A shows that treatment of the cells with the FTI tipifarnib alone inhibited the prenylation of the exclusively farnesylated HDJ2 protein without affecting the prenylation of the exclusively geranylgeranylated Rap1 protein, whereas treatment with the Akt activation inhibitor TCN alone resulted in decreased phosphorylation of Akt, demonstrating that both drugs inhibited their targets. As single agents, TCN and tipifarnib partially decreased the phosphorylation levels of the mTOR kinase substrate p70-S6K. When used in combination, the effects on the phosphorylation levels of p70-S6K were more pronounced (Figure 3A). Furthermore, the combination also decreased but only slightly the phosphorylation levels of 4EBP1 (Figure 3 D). In contrast, the combination did not decrease the levels of P-Erk1/2 (Figure 3 D).
Figure 3. Effects of tipifarnib and TCN alone and in combination on prenylation and signal transduction pathways in breast cancer cells.

(A, B and C) MDA-MB-231, MCF-7 and MDA-MB-468 cells were plated, treated for 48 hours with tipifarnib and TCN either alone or in combination and processed for Western blotting as described under Methods. (D) MDA-MB-231 cells were treated and processed as in (A); in addition, the cells were treated with AG1024 either alone or in combination with tipifarnib (last 2 lanes) The data are representative of two independent experiments.
We next determined whether TCN and tipifarnib inhibit their targets and subsequent signaling in the other human cancer cell lines where they synergize to inhibit proliferation. To this end, MDA-MB-468, MCF-7, A-549, MV-4-11 and U266 cells were treated with TCN and tipifarnib alone and in combination and processed for western blotting as in Figure 3A. Figures 3B-3C show that TCN decreased the levels of P-Akt and tipifarnib inhibited HDJ2 farnesylation without affecting the geranylgeranylation of Rap1 in all the cell lines. As seen in MDA-MB-231 cells, the combination of TCN and tipifarnib was more effective than the single agent treatment in decreasing the phosphorylation levels of the mTOR substrate p70-S6K in the MDA-MB-468 and MCF-7 breast cancer cell lines (Figures 3B-3C). Similar results were also obtained with A-549, MV-4-11 and U266 cell lines (data not shown).
With regards to the effect of the inhibitors on P-Akt levels, it is important to point out that in MDA-MB-231, but not in MDA-MB-468 and MCF-7 cells, tipifarnib increased P-Akt levels, and that co-treatment with TCN prevented tipifarnib from increasing P-Akt levels. A possible explanation is that inhibition of the farnesylated protein Rheb results in inhibition of mTORC1 which in turn inhibits the phosphorylation of IRS-1 by S6K, relieving the feed back loop previously proposed for rapamycin (58). To determine if this is the case here, we treated MDA-MB-231 cells with tipifarnib and the IGF-1R tyrosine kinase inhibitor AG1024 either alone or in combination. Figure 3 D shows that AG1024 inhibited the phosphorylation of Erk1/2 indicating that the drug treatment worked. However, AG1024 did not prevent tipifarnib from increasing P-Akt levels suggesting that the above-mentioned mechanism is unlikely. It is also important to note that in some cell lines the combination was more effective at decreasing the levels of P-Akt; and that with some treatments the total levels of Akt and S6K were also decreased.
TCN and tipifarnib synergize to inhibit anchorage-independent tumor cell growth and to induce apoptosis
We next determined whether TCN and tipifarnib combination treatment is more effective than single agent treatment toward inhibiting the anchorage-independent growth of MDA-MB-231, MDA-MB-468 and MCF-7 breast cancer cells in soft agar. Cells plated in soft agar were treated with TCN and tipifarnib alone or in combination. Colony numbers were counted after three weeks of treatment and analyzed for synergy using Calcusyn as described under Methods. Figure 4A and Supplemental Table S3 show that TCN and tipifarnib synergistically inhibited colony formation in all three breast cancer cell lines as demonstrated by the fact that all experimental CI values are less than 1. We next determined whether the treatment with TCN and tipifarnib induces apoptosis and whether the combination is more effective than single agent treatment. To this end, MDA-MB-231 and MDA-MB-468 cells treated with TCN and tipifarnib alone and in combination were stained for active caspase-3 and analyzed with flow cytometry as described under Methods (MCF-7 cells do not express caspase-3 (56)). Figure 4B shows that the percentage of MDA-MB-231 cells with active caspase-3 increased from 5.4% in the vehicle-treated cells to 15.1% in the TCN-treated cells and 12.4% in the tipifarnib-treated cells. When the cells were treated with the combination, the percentage of cells with active caspase-3 increased to 44.9%, a 3 to 4-fold increase over either drug alone. Similarly, in MDA-MB-468 cells, the percentages of cells with active caspase-3 in the control, tipifarnib, TCN and the combination treated cells were 4.1, 9.3, 22.1 and 47.7 %, respectively. These results suggest that TCN and tipifarnib also synergize to activate caspase-3 and induce apoptosis.
Figure 4. TCN and tipifarnib synergize to inhibit anchorage-independent growth and to induce apoptosis.
A, MDA-MB-231, MDA-MB-468 and MCF-7 cells were plated in soft agar with various concentration of TCN and tipifarnib alone or in combination. After 21 days, colonies were counted. The combination index analysis to determine synergy was performed using CalcuSyn software as described under Methods. B, MDA-MB-231 and MDA-MB-468 cells were plated and treated with either vehicle, TCN and tipifarnib either alone or in combination for 72 hours and processed for active caspase-3 staining as described under Methods.
The combination of TCN-P and tipifarnib causes significant breast tumor regression in MMTV-ErbB2 transgenic mice
Figures 1-4 strongly suggest that the Akt activation inhibitor TCN and the FTI tipifarnib in combination are more effective at inhibiting signaling, anchorage-dependent and -independent tumor cell proliferation, as well as at inducing apoptosis in cultured breast cancer cells. We next determined whether this combination is also more efficacious against breast tumors in in vivo settings. To this end, we used a MMTV-Her2/Neu/ErbB2 transgenic mouse model (57). In this model, mice spontaneously develop mammary gland tumors. As described in the Methods section, breast tumors were measured beginning at the time of tumor onset and treatment with vehicle, tipifarnib, TCN-P, or the TCN-P/tipifarnib combination began when tumor volumes reached about 200-2000 mm3. A wide range of tumor volumes was used to ensure that responses were not volume-dependent.
Figure 5A depicts representative tumor growth curves from animals treated either with vehicle, each drug alone or in combination. The tumor from the vehicle treated mouse continued to grow and the tumors treated with either TCN-P or tipifarnib alone changed in size minimally, whereas the tumor from the mouse treated with the combination experienced significant regression as evident from a large decrease in tumor volume (Figure 5A). Figure 5B shows the average percent change for each treatment group. Supplemental Table S1 shows the percent change in tumor volume of each tumor for a total of 44 tumors. The percent change was calculated from the tumor volume on the last day of treatment (VT) relative to the volume on the day of initiation of treatment (VI), as described in Methods. All tumors from mice treated with vehicle increased in size with an average percent change in tumor volume of 62.9 (+/- 18.8) % (Figures 5B and Supplemental Table S1). In contrast, tumors from mice treated with the TCN-P/tipifarnib combination regressed with an average decrease in tumor volume of -39.4 (+/-6.7) %. The tumors from mice treated with either TCN-P or tipifarnib as single agents had an average percent change in tumor volume of -3 (+/- 9.9) % for TCN-P and 1.6 (+/- 9.2) % for tipifarnib. There was a significant difference of percent volume change observed among treatment groups with statistical significance (p < 10-4). To be conservative, even after adjusting for multiple comparison using Dunnett-Hsu test, significant difference was still detected between the combination treatment group and TCN-P (p = 0.03), Tipifarnib (p = 0.004), and the vehicle groups (p < 10-4). Thus, the combination treatment of TCN-P and tipifarnib is significantly more effective than single agent treatment groups and causes breast tumor regression in vivo.
Figure 5. TCN and tipifarnib combination treatment causes significant tumor regression in the ErbB2-driven breast cancer transgenic mouse model.

A. Representative breast tumor growth curves from mice treated with either vehicle, tipifarnib (10 mpk) and TCN-P (20 mpk) alone or in combination. Treatment was initiated at day 0 and continued for 14 days unless otherwise noted (see Supplemental Table S1). B, Average percent change in volume of tumors from mice treated either with vehicle, tipifarnib and TCN-P alone or in combination. C. Mice were treated as in A. for 7 days. Pre- and post (at day 7)-treatment biopsies were taken from the same tumor and processed for western blotting. Mouse treatments, tumor volume measurements and western blotting were as described under Methods.
To determine if tipifarnib and TCN-P reached their targets in vivo, we performed incisional biopsies from the same tumor before treatment (pre) and after seven days of treatment (post) with vehicle, tipifarnib, TCN-P or the combination, and processed the tumors for western blotting as described under the Methods section. Figure 5C shows that neither HDJ-2 farnesylation nor Akt phosphorylation were inhibited in tumors from mice treated with vehicle control. In contrast, treatment with tipifarnib inhibited HDJ-2 farnesylation in tumors from all 4 mice treated (2 with tipifarnib as single agent and 2 with the combination of tipifarnib and TCN-P). Furthermore, TCN-P treatment resulted in decreased Akt phosphorylation levels in 3 out of the 4 tumors from the mice treated (1 with TCN-P as a single agent and 2 with the combination of TCN-P with tipifarnib). In one of the mice treated with TCN-P alone, tumor levels of phosphorylated Akt increased but so did the tumor levels of total Akt (with out effect on the loading control alpha tubulin) (Figure 5C).
Discussion
The uncontrolled proliferation and resistance to apoptosis as well as the angiogenic and metastatic character of cancer cells are believed to be due to deregulated signal transduction pathways that are the results of multiple genetic lesions (see Introduction). The multitude of persistently activated signal transduction pathways in cancer cells allow them to survive under the pressure of single agent treatments. Furthermore, interfering with persistently activated pathways to which tumor cells are not addicted may not be effective. Human clinical trials have taught us that single agent treatments rarely result in clinical benefits to cancer patients, suggesting that combination therapy may be necessary for effective treatment of tumors with multiple oncogenic lesions. In the present study we have found that the Akt activation inhibitor TCN and the FTI tipifarnib synergize to inhibit proliferation of human breast cancer cells. Similar synergistic effects were also observed with leukemia (MV-4-11), multiple myeloma (U266) and lung (A-549) tumor cells. These synergistic effects are not unique to TCN and were also observed with the combination of tipifarnib with another structurally unrelated Akt inhibitor, MK-2206. Similarly, synergy was also seen with the combination of TCN with another structurally unrelated FTI, FTI-2153, suggesting that the synergistic effects are due at least in part to inhibition of Akt and farnesyltransferase. In addition to the effects on anchorage-dependent cell growth, the combination of TCN and tipifarnib was also synergistic at inhibiting anchorage-independent growth in soft agar. The combination was more effective at inducing apoptosis than the single agents alone. Finally, the combination was also much more effective than single agent treatment in vivo in the ErbB2-driven breast cancer transgenic mouse model. In this model, the combination of tipifarnib and TCN induced significant breast tumor regression.
Tumors from breast cancer patients often overexpress members of the ErbB family of RTKs such as EGFR and ErbB2, and this is associated with poor prognosis, resistance to chemotherapy, and shorter survival time (3-5, 52). Overexpression of ErbB family RTKs results in persistent activation of downstream signaling pathways such as those mediated by hyperphosphorylation of Akt, Erk 1/2 and STAT3 (1, 2). We found that treatment with TCN alone completely inhibited the levels of P-Akt in MDA-MB-231 cells. However, in the other two breast cancer cell lines, MDA-MB-468 and MCF-7, TCN alone partially inhibited P-Akt levels. In these two cell lines, combination treatment with TCN and tipifarnib was more effective at inhibiting the levels of P-Akt, suggesting that farnesylated proteins need to be inhibited for efficient inhibition of P-Akt levels in MDA-MD-468 and in MCF-7, but not in MDA-MB-231. Considering that Akt phosphorylation is believed to be dependent on Akt recruitment to the membrane, and that TCN inhibits such recruitment (26), these results also suggest that under the pressure of TCN treatment, some breast cancer cells may overcome the effects of TCN by harboring farnesylation-dependent pathways capable of phosphorylating Akt. However, the synergistic effects on tumor cell growth and apoptosis can not be explained solely by this effect on P-Akt levels since, at least in MDA-MB-231, TCN by itself abolished P-Akt levels but synergy with tipifarnib was still seen. It is also important to point out that in MDA-MB-231 cells, tipifarnib treatment alone resulted in an increase in P-Akt levels. This is similar to the previously reported increase in P-Akt levels following treatment with the mTORC1 inhibitor rapamycin (58). A possible explanation is that inhibition of the farnesylated protein Rheb results in inhibition of mTORC1 which in turn inhibits the phosphorylation of IRS-1 by S6K, relieving the feed back loop previously proposed for rapamycin (58). However, the IGF-1R tyrosine kinase inhibitor AG1024 did not prevent tipifarnib from increasing the levels of P-Akt suggesting that this mechanism is not involved. Whether other feed back loops with other RTKs are involved is not known.
TCN inhibition of Akt activation (26) is anticipated to result in the activation of the Rheb GAP, TSC 1/2, which in turn would inhibit Rheb activation, leading to the inhibition of mTORC1 phosphorylation of S6 Kinase (41-47). Furthermore, inhibition of Rheb farnesylation by tipifarnib is also anticipated to inhibit mTORC1-mediated phosphorylation of S6 Kinase (41-47). In all three breast cancer cell lines, the inhibition of P-S6 Kinase is only partial and requires combination treatment for a more complete inhibition. This suggests that neither inhibition of Rheb farnesylation nor prevention of the Akt-dependent inhibition of TCS 1/2 is sufficient to fully inactivate mTORC1 from phosphorylating S6 Kinase. While these chemical biology studies are intriguing and suggest this combination approach is required to fully inactivate this pivotal signaling pathway, further studies are required to confirm that the synergistic effect of TCN and tipifarnib on tumor growth and survival is mediated at least in part by inhibition of mTORC1 phosphorylation of S6 Kinase.
An important finding of this study is that the combination of TCN and tipifarnib is synergistic in human cancer cell lines with a variety of genetic alterations. For example, the levels of expression of ErbB family members are different among these cell lines, with MDA-MB-468 and MDA-MB-231 cells expressing high levels of ErbB1 and ErbB3, and MCF-7 expressing high levels of ErbB3 and ErbB4 (Supplemental Table S2). In addition, while MDA-MB-231 and A-549 cells harbor K-Ras mutations, the other 4 cell lines have wild type K-Ras. Furthermore, MDA-MB-468 has a deleted PTEN, whereas the other 5 cell lines have wild type PTEN. While MDA-MB-231 and U266 cells have B-Raf mutations, the other 4 cell lines have wild type B-Raf (For other mutations in PI3K, p53, pRb and Ink4a please refer to Supplemental Table S2). Despite these differences, the combination of TCN and tipifarnib was synergistic at inhibiting proliferation of all 6 cell lines evaluated. The fact that several tumor types with multiple genetic lesions are sensitive to this combination treatment suggests that the dual targeting of the Akt/Rheb/mTOR pathway, a signaling node pivotal to tumor growth and survival, may be an effective approach to chemotherapy.
In summary, our studies have identified a combination treatment that appears to be highly effective at inhibiting tumor growth both in cultured cancer cells as well as in an ErbB2-driven breast cancer transgenic mouse model. This combination treatment may have wide spread use since the synergistic effect does not depend on specific genetic/oncogenic lesions harbored by the tumor cell lines and was observed in human cancer cell lines of various lineage, including breast, leukemia, multiple myeloma and lung. Thus, inhibition of farnesyltransferase coupled with inhibition of Akt activation is a novel drug combination treatment approach with a broad application for cancer therapy. Further pre-clinical studies are warranted to confirm and validate this approach prior to clinical trials.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Norbert Berndt and Dr. Hua Yang (Moffitt Cancer Center) for their careful reading of the manuscript and suggestions. This work has been supported in part by the Flow Cytometry Core Facility of the Moffitt Cancer Center, a comprehensive cancer center designated by the National Cancer Institute. This work was partially funded by NCI grant CA118210.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Grunert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol. 2003;4:657–65. doi: 10.1038/nrm1175. [DOI] [PubMed] [Google Scholar]
- 3.Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology. 2009;77:400–10. doi: 10.1159/000279388. [DOI] [PubMed] [Google Scholar]
- 4.Pennell NA, Lynch TJ., Jr Combined inhibition of the VEGFR and EGFR signaling pathways in the treatment of NSCLC. Oncologist. 2009;14:399–411. doi: 10.1634/theoncologist.2008-0276. [DOI] [PubMed] [Google Scholar]
- 5.Tortora G, Ciardiello F, Gasparini G. Combined targeting of EGFR-dependent and VEGF-dependent pathways: rationale, preclinical studies and clinical applications. Nat Clin Pract Oncol. 2008;5:521–30. doi: 10.1038/ncponc1161. [DOI] [PubMed] [Google Scholar]
- 6.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 7.Bodemann BO, White MA. Ral GTPases and cancer: linchpin support of the tumorigenic platform. Nat Rev Cancer. 2008;8:133–40. doi: 10.1038/nrc2296. [DOI] [PubMed] [Google Scholar]
- 8.Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–31. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 2009;283:125–34. doi: 10.1016/j.canlet.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Dillon RL, Muller WJ. Distinct biological roles for the akt family in mammary tumor progression. Cancer Res. 2010;70:4260–4. doi: 10.1158/0008-5472.CAN-10-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yue P, Turkson J. Targeting STAT3 in cancer: how successful are we? Expert Opin Investig Drugs. 2009;18:45–56. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarez RH, Valero V, Hortobagyi GN. Emerging targeted therapies for breast cancer. J Clin Oncol. 2010;28:3366–79. doi: 10.1200/JCO.2009.25.4011. [DOI] [PubMed] [Google Scholar]
- 14.Yeh JJ, Der CJ. Targeting signal transduction in pancreatic cancer treatment. Expert Opin Ther Targets. 2007;11:673–94. doi: 10.1517/14728222.11.5.673. [DOI] [PubMed] [Google Scholar]
- 15.Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8:187–98. doi: 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- 16.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–62. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 17.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 18.Workman P, Clarke PA, Raynaud FI, van Montfort RL. Drugging the PI3 kinome: from chemical tools to drugs in the clinic. Cancer Res. 2010;70:2146–57. doi: 10.1158/0008-5472.CAN-09-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Call JA, Eckhardt SG, Camidge DR. Targeted manipulation of apoptosis in cancer treatment. Lancet Oncol. 2008;9:1002–11. doi: 10.1016/S1470-2045(08)70209-2. [DOI] [PubMed] [Google Scholar]
- 20.Pratilas CA, Solit DB. Therapeutic strategies for targeting BRAF in human cancer. Rev Recent Clin Trials. 2007;2:121–34. doi: 10.2174/157488707780599393. [DOI] [PubMed] [Google Scholar]
- 21.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–75. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 22.Rodon J, Perez J, Kurzrock R. Combining targeted therapies: practical issues for bench and bedside. Oncologist. 2010;15:37–50. doi: 10.1634/theoncologist.2009-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alessi DR, James SR, Downes CP, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–9. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 24.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 25.Yang L, Dan HC, Sun M, et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–9. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- 26.Berndt N, Yang H, Trinczek B, et al. The Akt activation inhibitor TCN-P inhibits Akt phosphorylation by binding to the PH domain of Akt and blocking its recruitment to the plasma membrane. Cell Death Differ. 2010 doi: 10.1038/cdd.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garrett CR, Coppola D, Wenham RM, et al. Phase I pharmacokinetic and pharmacodynamic study of triciribine phosphate monohydrate, a small-molecule inhibitor of AKT phosphorylation, in adult subjects with solid tumors containing activated AKT. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravandi FM, Sampath D, Plunkett W, et al. Phase I Study of the Akt-Inhibitor Triciribine Phosphate Monohydrate in Patients With Advanced Hematologic Malignancy. Blood (ASH Annual Meeting Abstracts) 2008;112:2987. [Google Scholar]
- 29.Sebti SM. Protein farnesylation: implications for normal physiology, malignant transformation, and cancer therapy. Cancer Cell. 2005;7:297–300. doi: 10.1016/j.ccr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–69. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 31.Sebti SM, Der CJ. Opinion: Searching for the elusive targets of farnesyltransferase inhibitors. Nat Rev Cancer. 2003;3:945–51. doi: 10.1038/nrc1234. [DOI] [PubMed] [Google Scholar]
- 32.Gibbs RA, Zahn TJ, Sebolt-Leopold JS. Non-peptidic prenyltransferase inhibitors: diverse structural classes and surprising anti-cancer mechanisms. Curr Med Chem. 2001;8:1437–65. doi: 10.2174/0929867013372111. [DOI] [PubMed] [Google Scholar]
- 33.Cox AD, Der CJ. Farnesyltransferase inhibitors: promises and realities. Curr Opin Pharmacol. 2002;2:388–93. doi: 10.1016/s1471-4892(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 34.Lane KT, Beese LS. Thematic review series: lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I. J Lipid Res. 2006;47:681–99. doi: 10.1194/jlr.R600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Sjogren AK, Andersson KM, Liu M, et al. GGTase-I deficiency reduces tumor formation and improves survival in mice with K-RAS-induced lung cancer. J Clin Invest. 2007;117:1294–304. doi: 10.1172/JCI30868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crespo NC, Delarue F, Ohkanda J, Carrico D, Hamilton AD, Sebti SM. The farnesyltransferase inhibitor, FTI-2153, inhibits bipolar spindle formation during mitosis independently of transformation and Ras and p53 mutation status. Cell Death Differ. 2002;9:702–9. doi: 10.1038/sj.cdd.4401023. [DOI] [PubMed] [Google Scholar]
- 37.Vogt A, Sun J, Qian Y, Hamilton AD, Sebti SM. The geranylgeranyltransferase-I inhibitor GGTI-298 arrests human tumor cells in G0/G1 and induces p21(WAF1/CIP1/SDI1) in a p53-independent manner. J Biol Chem. 1997;272:27224–9. doi: 10.1074/jbc.272.43.27224. [DOI] [PubMed] [Google Scholar]
- 38.Sun J, Qian Y, Chen Z, Marfurt J, Hamilton AD, Sebti SM. The geranylgeranyltransferase I inhibitor GGTI-298 induces hypophosphorylation of retinoblastoma and partner switching of cyclin-dependent kinase inhibitors. A potential mechanism for GGTI-298 antitumor activity. J Biol Chem. 1999;274:6930–4. doi: 10.1074/jbc.274.11.6930. [DOI] [PubMed] [Google Scholar]
- 39.Falsetti SC, Wang DA, Peng H, et al. Geranylgeranyltransferase I inhibitors target RalB to inhibit anchorage-dependent growth and induce apoptosis and RalA to inhibit anchorage-independent growth. Mol Cell Biol. 2007;27:8003–14. doi: 10.1128/MCB.00057-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kazi A, Carie A, Blaskovich MA, et al. Blockade of protein geranylgeranylation inhibits Cdk2-dependent p27Kip1 phosphorylation on Thr187 and accumulates p27Kip1 in the nucleus: implications for breast cancer therapy. Mol Cell Biol. 2009;29:2254–63. doi: 10.1128/MCB.01029-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Law BK, Norgaard P, Moses HL. Farnesyltransferase inhibitor induces rapid growth arrest and blocks p70s6k activation by multiple stimuli. J Biol Chem. 2000;275:10796–801. doi: 10.1074/jbc.275.15.10796. [DOI] [PubMed] [Google Scholar]
- 42.Basso AD, Mirza A, Liu G, Long BJ, Bishop WR, Kirschmeier P. The farnesyl transferase inhibitor (FTI) SCH66336 (lonafarnib) inhibits Rheb farnesylation and mTOR signaling. Role in FTI enhancement of taxane and tamoxifen anti-tumor activity. J Biol Chem. 2005;280:31101–8. doi: 10.1074/jbc.M503763200. [DOI] [PubMed] [Google Scholar]
- 43.Gau CL, Kato-Stankiewicz J, Jiang C, Miyamoto S, Guo L, Tamanoi F. Farnesyltransferase inhibitors reverse altered growth and distribution of actin filaments in Tsc-deficient cells via inhibition of both rapamycin-sensitive and -insensitive pathways. Mol Cancer Ther. 2005;4:918–26. doi: 10.1158/1535-7163.MCT-04-0347. [DOI] [PubMed] [Google Scholar]
- 44.Mavrakis KJ, Zhu H, Silva RL, et al. Tumorigenic activity and therapeutic inhibition of Rheb GTPase. Genes Dev. 2008;22:2178–88. doi: 10.1101/gad.1690808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang K, Coppola D, Crespo NC, et al. The phosphoinositide 3-OH kinase/AKT2 pathway as a critical target for farnesyltransferase inhibitor-induced apoptosis. Mol Cell Biol. 2000;20:139–48. doi: 10.1128/mcb.20.1.139-148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oh SH, Jin Q, Kim ES, Khuri FR, Lee HY. Insulin-like growth factor-I receptor signaling pathway induces resistance to the apoptotic activities of SCH66336 (lonafarnib) through Akt/mammalian target of rapamycin-mediated increases in survivin expression. Clin Cancer Res. 2008;14:1581–9. doi: 10.1158/1078-0432.CCR-07-0952. [DOI] [PubMed] [Google Scholar]
- 47.Castro AF, Rebhun JF, Clark GJ, Quilliam LA. Rheb binds tuberous sclerosis complex 2 (TSC2) and promotes S6 kinase activation in a rapamycin- and farnesylation-dependent manner. J Biol Chem. 2003;278:32493–6. doi: 10.1074/jbc.C300226200. [DOI] [PubMed] [Google Scholar]
- 48.Dwyer PJ, Gallagher M, Nguyen B, Waddell MJ, Chiorean EG. Phase I accelerated dose-escalating safety and pharmacokinetic (PK) study of GGTI-2418, a novel geranylgeranyltransferase I inhibitor with refractory solid tumors [abstract F01]. Ann Oncol; 8th International Symposium on Targeted Anticancer Therapies; 2010 Mar 4-6; Bethesda, MD. 2010. p. ii42. [Google Scholar]
- 49.Rowinsky EK. Lately, it occurs to me what a long, strange trip it's been for the farnesyltransferase inhibitors. J Clin Oncol. 2006;24:2981–4. doi: 10.1200/JCO.2006.05.9808. [DOI] [PubMed] [Google Scholar]
- 50.Van Cutsem E, van de Velde H, Karasek P, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22:1430–8. doi: 10.1200/JCO.2004.10.112. [DOI] [PubMed] [Google Scholar]
- 51.Rao S, Cunningham D, de Gramont A, et al. Phase III double-blind placebo-controlled study of farnesyl transferase inhibitor R115777 in patients with refractory advanced colorectal cancer. J Clin Oncol. 2004;22:3950–7. doi: 10.1200/JCO.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 52.Sparano JA, Moulder S, Kazi A, et al. Phase II trial of tipifarnib plus neoadjuvant doxorubicin-cyclophosphamide in patients with clinical stage IIB-IIIC breast cancer. Clin Cancer Res. 2009;15:2942–8. doi: 10.1158/1078-0432.CCR-08-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun J, Blaskovich MA, Knowles D, et al. Antitumor efficacy of a novel class of non-thiol-containing peptidomimetic inhibitors of farnesyltransferase and geranylgeranyltransferase I: combination therapy with the cytotoxic agents cisplatin, Taxol, and gemcitabine. Cancer Res. 1999;59:4919–26. [PubMed] [Google Scholar]
- 54.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 55.Hirai H, Sootome H, Nakatsuru Y, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–67. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 56.Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357–60. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- 57.Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54:105–15. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- 58.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.