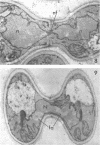
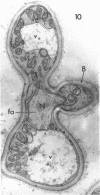
Abstract
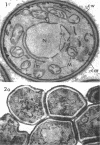
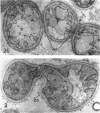
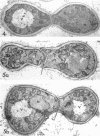
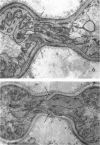
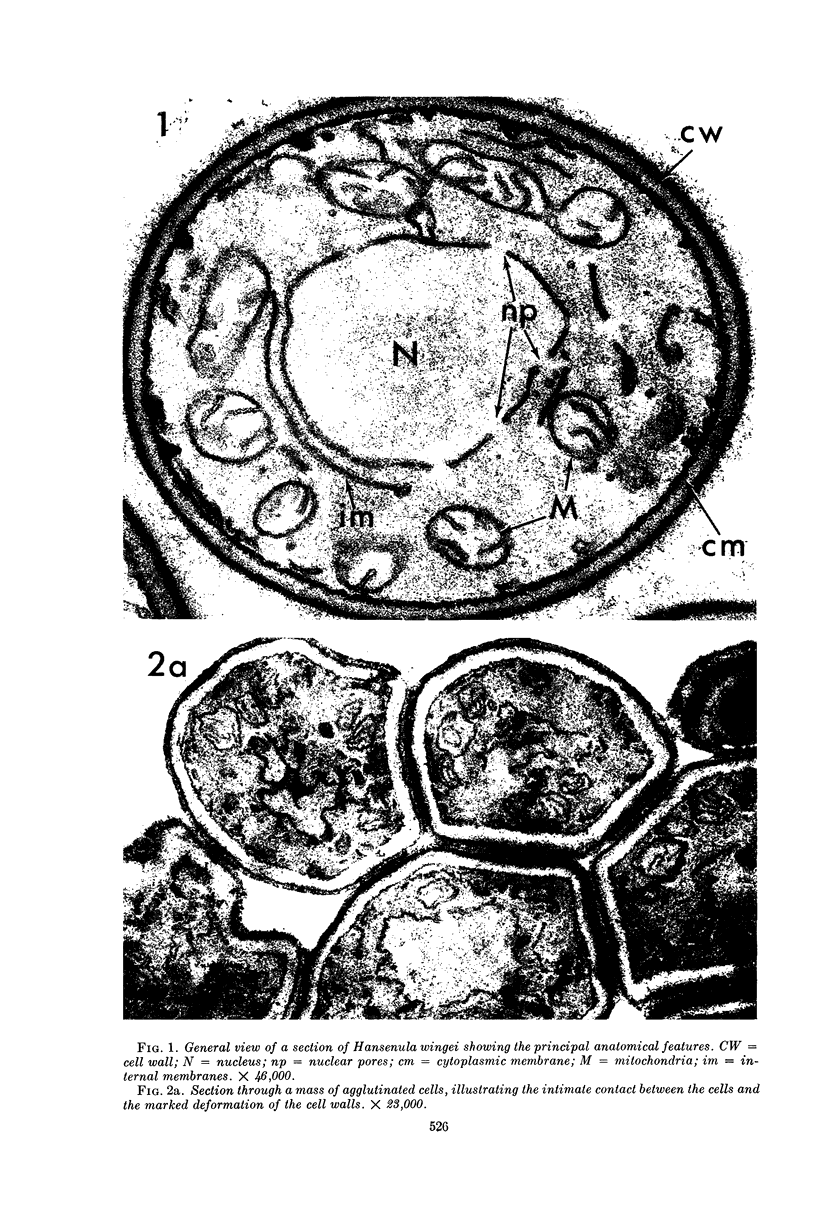
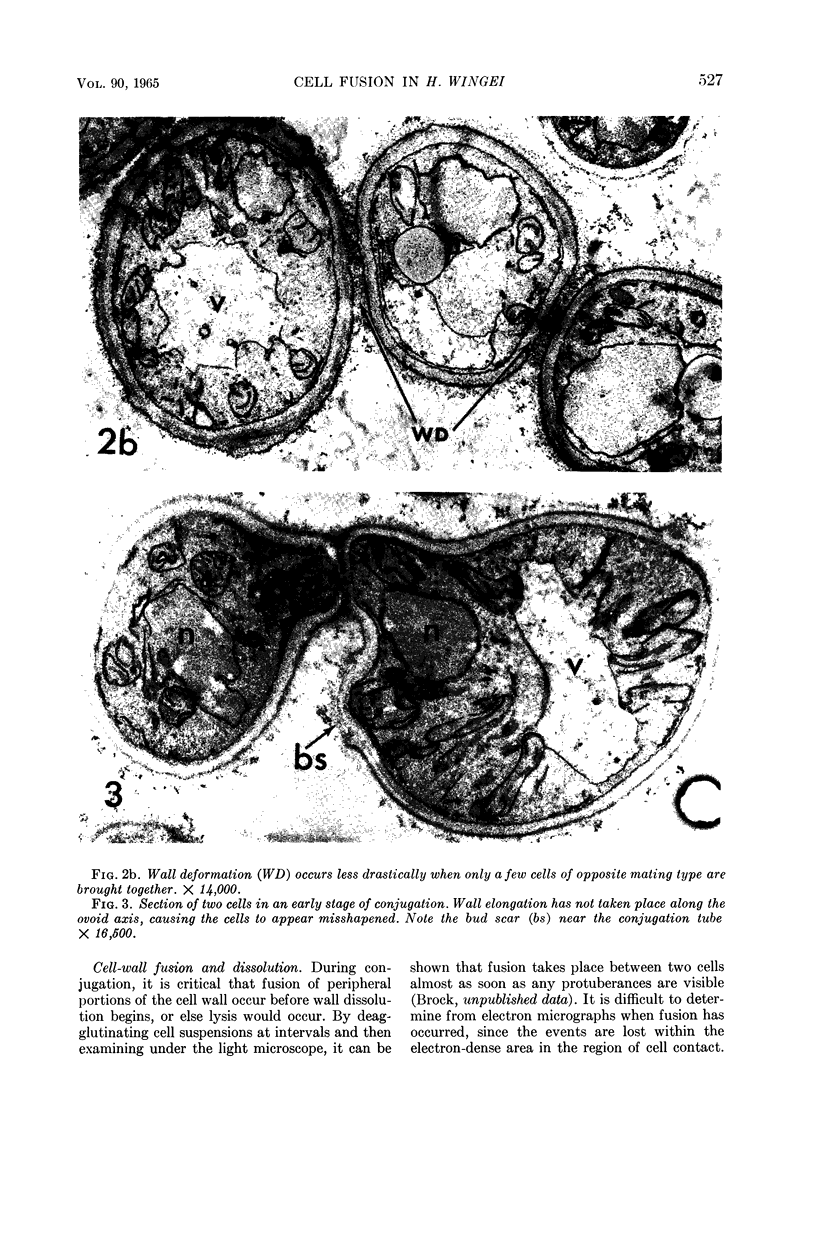
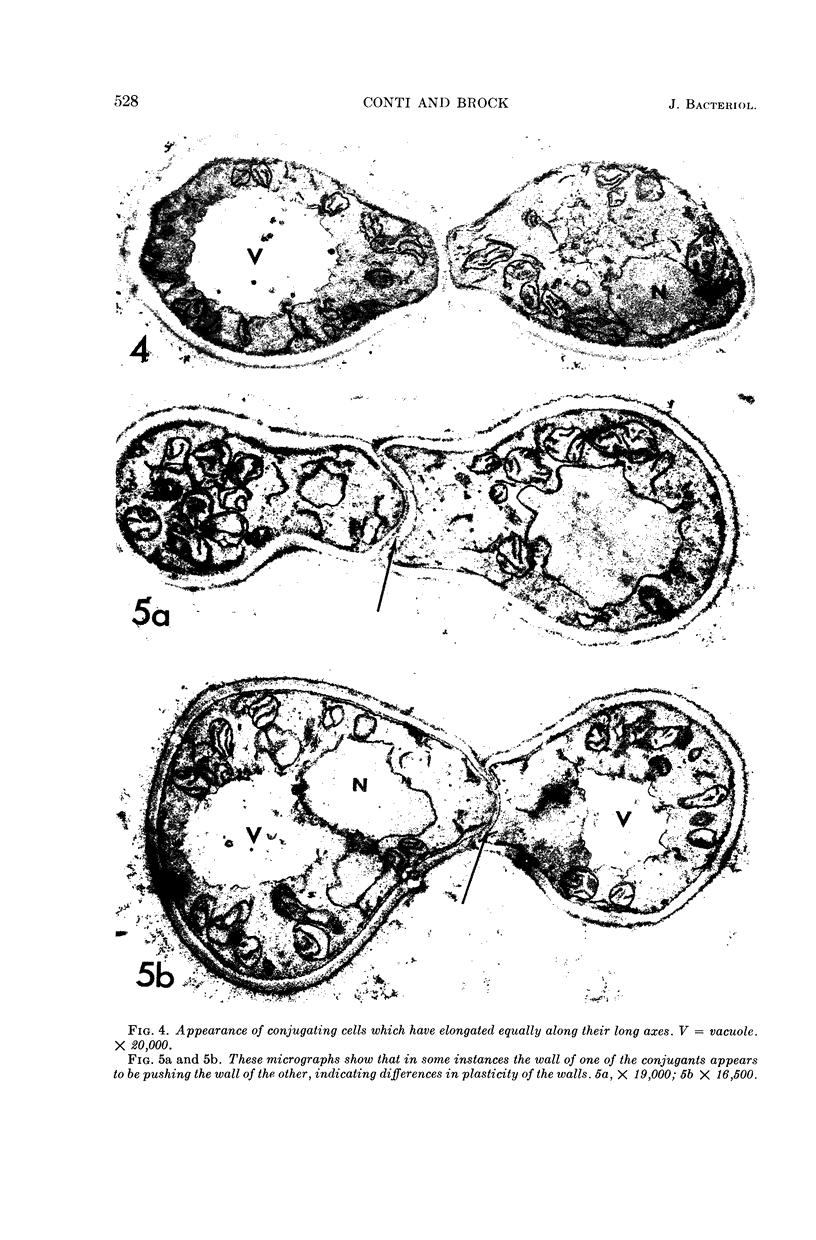
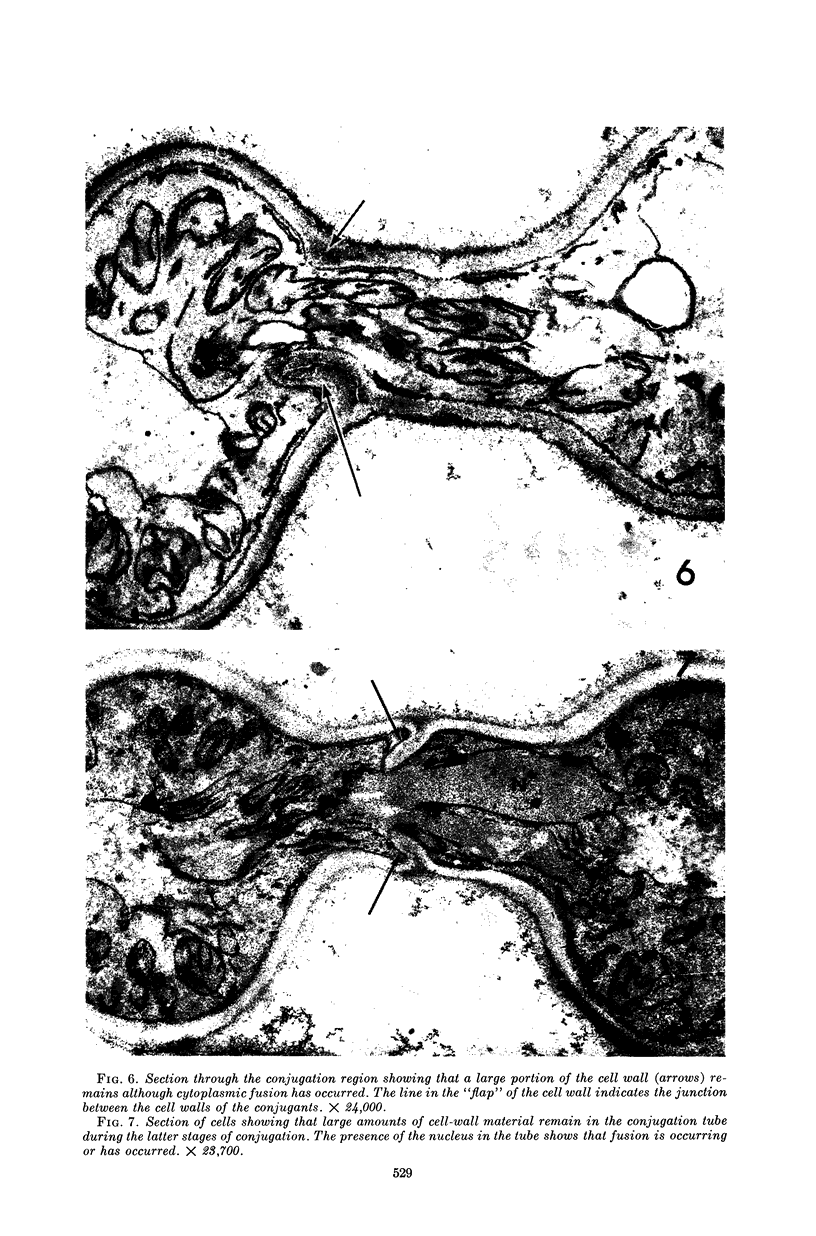
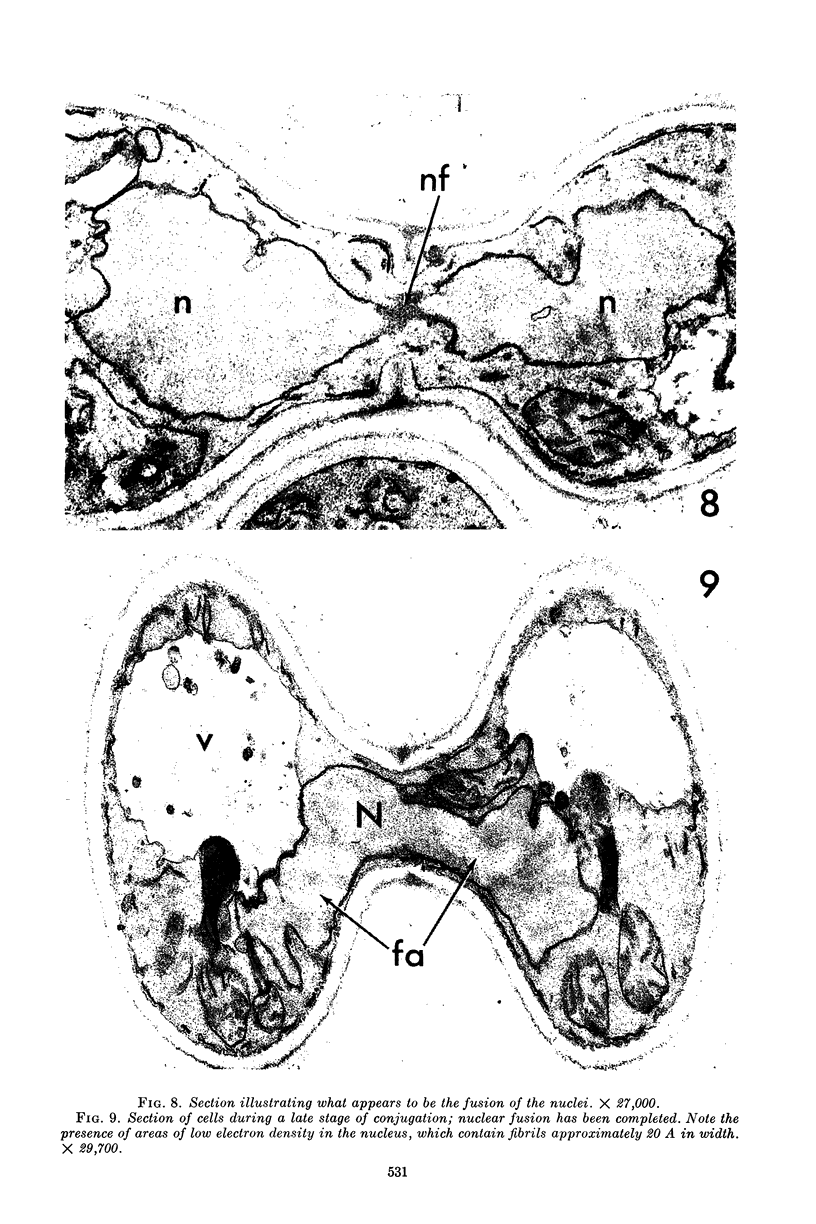
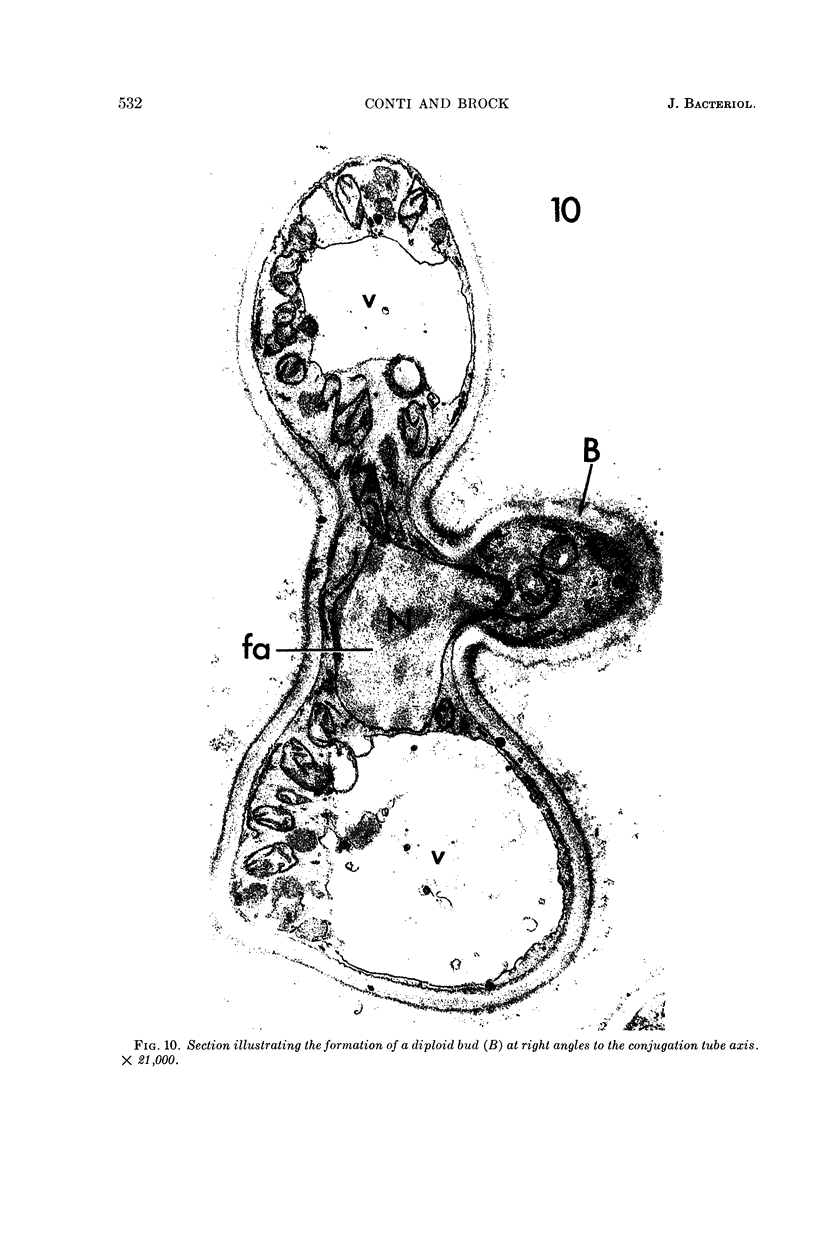
Conti, S. F. (Dartmouth Medical School, Hanover, N.H.), and T. D. Brock. Electron microscopy of cell fusion in conjugating Hansenula wingei. J. Bacteriol. 90:524–533. 1965.—The heterothallic yeast Hansenula wingei is a favorable organism for the study of the process of cell fusion, since strong agglutination of cells of the two mating types ensures a high percentage of cell fusions. The initial agglutination reaction results in cell-wall deformation, so that the walls in the region of contact are tightly appressed over an extensive area. The fusion process is initiated when the walls of two cells elongate, and this elongation seems to be restricted to the region where the cells touch. Occasionally, one cell is seen to push in the wall of the other, but in many cases both cells elongate equally, as would be expected in an isogamous organism. The precise disposition of the elongating wall probably reflects the manner in which the cells initially become associated in the agglutinated cell clump. Soon after wall elongation begins, cell-wall fusion occurs along the margin of contact. Only after fusion is complete is the wall separating the two cells dissolved away. If wall dissolution begins at one edge of the conjugation tube, a flap is formed in which can be seen the remnants of the fused walls. Alternatively, dissolution can begin at the center of the conjugation tube, proceeding towards the outside. Conjugating cells are uninucleate, and the nuclei are large and frequently lobed or elongated. After the conjugation tube is formed, the nuclei migrate towards the center, and fusion occurs only over a small region where the nuclear membranes come in contact. After nuclear fusion, the first diploid bud forms from the conjugation tube and at right angles to the tube axis. The diploid nucleus then migrates into this bud. Frequently, in the later stages of conjugation, a large vacuole develops in each of the original cells. All of the above events will occur in a medium devoid of a nitrogen source and in which vegetative budding will not occur.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCK T. D. Mating reaction in the yeast Hansenula wingei; preliminary observations and quantitation. J Bacteriol. 1958 Jun;75(6):697–701. doi: 10.1128/jb.75.6.697-701.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK T. D. Physiology of the conjugation process in the yeast Hansenula wingei. J Gen Microbiol. 1961 Nov;26:487–497. doi: 10.1099/00221287-26-3-487. [DOI] [PubMed] [Google Scholar]
- CONTI S. F., NAYLOR H. B. Electron microscopy of ultrathin sections of Schizosaccharomyces octosporus. II. Morphological and cytological changes preceding ascospore formation. J Bacteriol. 1960 Mar;79:331–340. doi: 10.1128/jb.79.3.331-340.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLEN W. L., BARBER R., MCCONKEY H. M., GRANT G. A. Isolation of beta-dihydroequilin and alpha-dihydroequilenin from the urine of pregnant mares. Nature. 1956 Apr 21;177(4512):753–753. doi: 10.1038/177753a0. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO T., GERHARDT P., CONTI S. F., NAYLOR H. B. Studies on the fine structure of microorganisms. V. Morphogenesis of nuclear and membrane structures during ascospore formation in yeast. J Biophys Biochem Cytol. 1960 Apr;7:305–310. doi: 10.1083/jcb.7.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THYAGARAJAN T. R., CONTI S. F., NAYLOR H. B. Electron microscopy of Rhodotorula glutinis. J Bacteriol. 1962 Feb;83:381–394. doi: 10.1128/jb.83.2.381-394.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]