Abstract
Background
The ability to construct biologically meaningful gene networks and modules is critical for contemporary systems biology. Though recent studies have demonstrated the power of using gene modules to shed light on the functioning of complex biological systems, most modules in these networks have shown little association with meaningful biological function. We have devised a method which directly incorporates gene ontology (GO) annotation in construction of gene modules in order to gain better functional association.
Results
We have devised a method, Semantic Similarity-Integrated approach for Modularization (SSIM) that integrates various gene-gene pairwise similarity values, including information obtained from gene expression, protein-protein interactions and GO annotations, in the construction of modules using affinity propagation clustering. We demonstrated the performance of the proposed method using data from two complex biological responses: 1. the osmotic shock response in Saccharomyces cerevisiae, and 2. the prion-induced pathogenic mouse model. In comparison with two previously reported algorithms, modules identified by SSIM showed significantly stronger association with biological functions.
Conclusions
The incorporation of semantic similarity based on GO annotation with gene expression and protein-protein interaction data can greatly enhance the functional relevance of inferred gene modules. In addition, the SSIM approach can also reveal the hierarchical structure of gene modules to gain a broader functional view of the biological system. Hence, the proposed method can facilitate comprehensive and in-depth analysis of high throughput experimental data at the gene network level.
Background
High throughput technologies to accurately profile the transcriptome have been the major thrust of modern systems biology [1,2]. Based on the premise that co-expressed genes and/or closely interconnected proteins are more likely to be related to a particular biological function, researchers have made an effort to identify groups of genes, called modules [3], to gain a better understanding of the biological system of interest. Early attempts in building such gene modules depended primarily on the co-expression property of genes [4-8]. Combining gene expression with protein-protein interaction data greatly expanded the modules and in some cases enhanced the functional association of modules [9-15]. Although gene expression and protein interaction information have been used in constructing gene modules, these approaches have not taken advantage of the vast amount of knowledge accumulated about gene annotation/function. Therefore, these approaches would not be expected to be maximally effective in constructing modules with strong association to biological functions [16,17].
Recently, the concept of semantic similarity, which was developed for and used in the field of natural language processing [18-20] has been applied to analyze gene ontology terms and used to predict and confirm protein functions and interactions [21-24]. Wang et al. [25] developed a Gene Ontology (GO) [26] structure-based measure to quantify semantic similarity between individual terms as well as genes and showed the advantage of using semantic similarity in organizing complex biological terms.
We postulated that the direct incorporation of semantic similarity based on GO annotation could significantly enhance the construction of biologically meaningful gene modules which have strong associations with known biological functions. We have therefore developed SSIM (Semantic Similarity-Integrated approach for Modularization), to integrate various gene-gene pairwise relationships including similarity measures based on GO biological process (BP) annotation of genes, gene expression pattern, and protein-protein interaction information. The integrated information is then used to group genes into modules using affinity propagation [27]. Affinity propagation is a clustering method that does not use pre-selected centers for clustering, instead it generates exemplars that best represent a group of data points, in this case genes, by considering all similarities between pairs of data points and testing all data points as potential exemplars. A group of data points that have the same exemplar can then be considered to be in a cluster. The modules generated by SSIM are found to be more significantly and specifically associated with biological functions than the results obtained from two methods, Module Analysis via Topology of Interactions and Similarity Sets (MATISSE) [12] and Interaction Component Models for Gene modules (ICMg) [14].
Results and discussion
MATISSE was originally evaluated using the expression information of 1990 osmotic stress-associated genes in yeast and ~69,000 yeast protein interactions. ICMg was tested with a slightly smaller set of osmotic stress-associated genes and using a different set of protein interaction data (see methods). MATISSE also adapted a strategy to generate connected modules by including information from genes that are not in the original selected gene set, referred to back nodes [12]. ICMg focused on the information within the set of selected genes only.
To compare and evaluate the performance of SSIM, we analyzed the same datasets used in MATISSE and ICMg studies (MATISSE dataset and ICMg dataset). Since MATISSE and ICMg used probabilistic approaches to optimize the construction of gene modules, the inferred connections and number of modules could vary in each run. To obtain the number of modules for comparison, MATISSE was executed 20 times as described [14], which yielded the median number of gene modules of 24 for both datasets. ICMg was then also executed 20 times with a fixed number of modules at 24. For SSIM, we adjusted the preference value to obtain the same number of modules (see methods).
Semantic similarity-integrated approach for modularization (SSIM) generates functionally relevant gene modules
GO enrichment analysis with statistical testing such as Fisher exact test was the most commonly used approach to evaluate the functional association of individual gene modules. A low p-value between a gene module and a GO term would imply a strong association of the module with the specific biological function represented by the term. Since SSIM integrates semantic similarity of GO BP (biological process) terms in gene module construction, it is expected to have a better GO enrichment performance compared to other methods. Therefore a different annotation scheme, MIPS FunCat [28], which is independent of GO, was also used to evaluate the functional associations of gene modules.
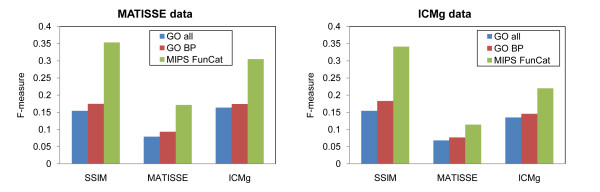
For a given significance level, the number of modules enriched with at least one annotation term and the number of annotation terms enriched in at least one module are referred as specificity and sensitivity [13]. For each method, the sensitivity and specificity were calculated and summarized into a measure of functional enrichment, an F-measure defined as F = 2 × Sensitivity × Specificity/(Sensitivity + Specificity) [13]. Note that the ratio of modules enriched with at least one annotation term (i.e. specificity) might be also expressed as precision. The results from SSIM showed better functional enrichment significance (F-measure) for MIPS FunCat annotations (as well as GO and GO BP terms) than other methods (Figure 1 and Additional File 1). ICMg gave comparable results to SSIM, whereas MATISSE showed lower performances in terms of both sensitivity and specificity. This could be due to a constraint on the size of the modules (a default parameter with no more than 100 genes per module was used in this study, but it is adjustable by user) and the addition of back nodes (genes that were not in the initial set of genes but were later included to make connected gene modules) in MATISSE.
Figure 1.
Functional enrichment analysis results. The functional enrichment performance of each method was evaluated using three different annotation datasets, all GO terms, GO BP terms and MIPS FunCat terms. The results were summarised using F-measure (Y-axis, see text). Results for MATISSE and ICMg were obtained using the mean values of 20 runs.
To further evaluate the overall functional enrichment performance from the three different methods, semantic similarity between the terms and the shortest paths of the terms to the root (e.g. GO:0008150, biological process) were investigated (see methods). If enriched terms were closely related to each other (coherency) and were far away from the root in GO hierarchy (depth), they may reveal more specific and detailed biological functions. Enriched GO terms associated with the modules from SSIM have higher average semantic coherency, which measures how enriched GO terms are coherent in terms of semantic similarity, and comparable depth relative to other methods (see method and Additional file 2).
SSIM produces modules with strong functional association and high expression homogeneity
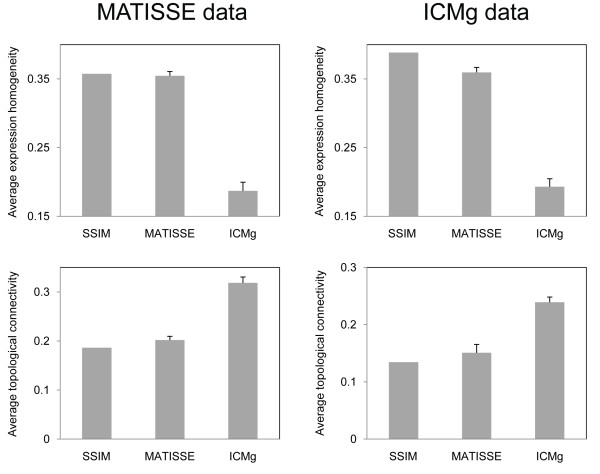
We also investigated the homogeneity of gene expression profiles and topological connectivity of modules generated by three different methods using average Pearson correlation as well as average clustering coefficient [29] of genes within the same module. As shown in Figure 2, for both datasets, SSIM and MATISSE yielded modules with similar levels of expression homogeneity and topological connectivity while ICMg produced modules composed of densely connected genes with poorly co-expressed profiles.
Figure 2.
Expression homogeneity and topological connectivity of modules. Expression homogeneity and topological connectivity of a module were calculated using average Pearson correlation among genes in the module and average clustering coefficient of the network generated by the genes, respectively. Average expression homogeneity and connectivity over all modules were shown for each method. For MATISSE and ICMg, mean and standard deviation over 20 runs were taken.
This finding also suggests the possibility of using SSIM as a tool to explore gene regulatory networks since genes with similar expression profiles are commonly co-regulated [4,7,30-33]. Notably, the study conducted by Ulitsky and Shamir [12] indicates that modules generated by random sampling of genes with sufficient network connectivity could give favorable topological properties and functional enrichment results, but with much lower expression homogeneity. This implies that significant GO enrichment results for gene modules might be obtained by chance if we just considered topological connectivity of genes; thus, additional criteria such as gene expression homogeneity must be used to ensure the reliability of functional association for gene modules.
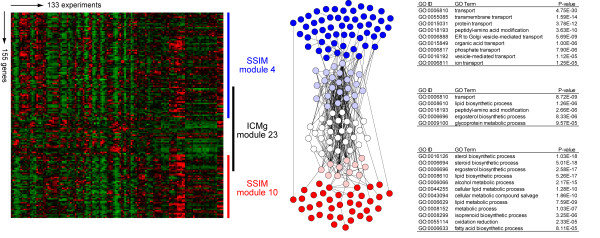
For a given number of modules, the SSIM approach seems to generate gene modules with better functional association and higher correlations in their expression homogeneity. For example, one of the ICMg modules (ICMg module 23) shared a large portion of genes with two modules generated by SSIM (SSIM module 4 and 10) (see methods and Additional File 3, Table S1). The GO terms enriched in the ICMg module 23 implicated two biological functions, "transport" and "lipid biosynthetic process" while in SSIM, the two functions were separated into two different modules, module 4 and 10, with much higher functional association (lower p-value) and expression homogeneity (Figure 3 and Additional file 3, Table S2 and S4).
Figure 3.
Comparison between modules identified by ICMg and SSIM. One of the modules identified by ICMg method (ICMg module) shares a number of genes with two separate modules identified by SSIM (SSIM module A and B). Expression profiles of genes in three modules were shown on the left and protein-protein interaction network was shown in the middle. Genes shared by ICMg and SSIM modules are indicated by pink and light blue nodes, and ones exclusively belong to ICMg, SSIM module A and SSIM module B are depicted by white, red and blue nodes, respectively. In the right panel, enriched GO BP terms (p < 1 × 10-5) and their uncorrected p-values for each module are summarized.
SSIM can be used to reveal the hierarchical structure of gene modules
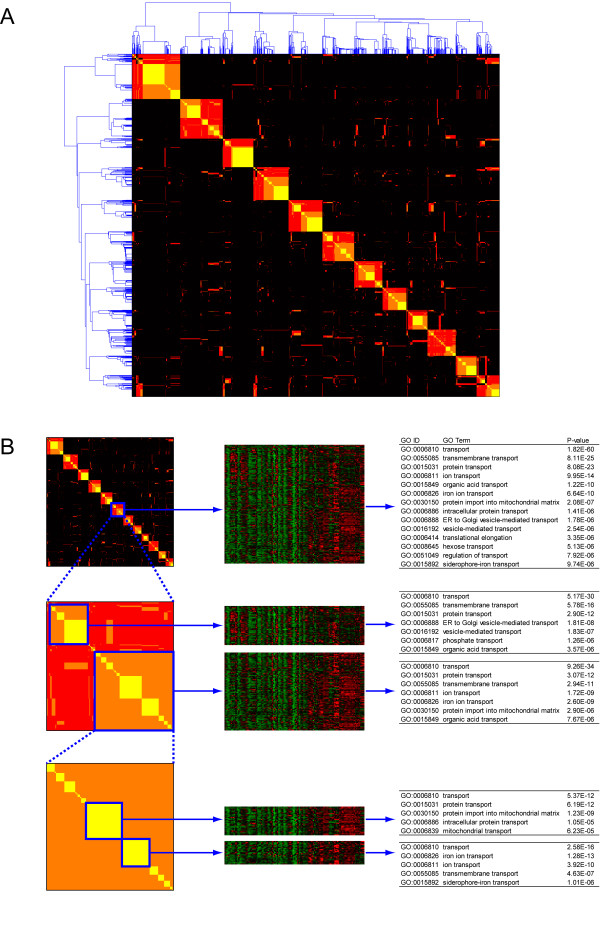
To compare the efficiency of constructing gene modules with different algorithms, we have used a fixed number of modules (24 modules in this study). In SSIM, a larger preference value of affinity propagation [27] means that every gene is more likely to be an exemplar of a module (module center), which would produce a large number of modules composed of highly similar genes in terms of integrated similarity and allow us to view the system in detail. A smaller preference value would generate fewer modules which offer a broader, less detailed overview of the system. This implies that SSIM can also be used as a tool to generate a functional hierarchy of gene modules by virtue of using semantic similarity. As an example, we applied the SSIM approach to a MATISSE dataset with a wide range of preference values and chose three sets of modularization results with 12, 18 and 37 modules (see Additional file 4) to illustrate the hierarchical structure of gene module generated by SSIM. Figure 4A shows that large-size modules obtained from a smaller preference value were hierarchically decomposed into smaller modules using a larger preference value. As an example of the hierarchical structure of gene modules generated with SSIM using different preference values, one of the module obtained from the "12-module set" representing various "transport" functions is split into two groups, based on the membership of "18-module set", showing slightly different expression profiles. One of the two groups from the "18-module set" is further divided into two smaller groups based on "37-module set", which further stratify the "transport" function into protein and ion transport (Figure 4B).
Figure 4.
Hierarchical structure of modules generated by SSIM approach. Using different preference values, three sets of modules (12, 18 and 37 modules) were obtained (see Additional file 4) and the membership of genes over the three module sets was expressed as a matrix form. When gene i and j belong to the same module and the membership is conserved in any one, two and three sets, the element of matrix in ith row and jth column is set to 1 (red, least conserved over the sets of modules), 2 (orange) and 3 (yellow, most conserved), respectively. If two genes are not in the same module, the corresponding element in the matrix has a zero value (black). Hierarchical clustering result of the matrix was shown in (A). Most orange and yellow squares are subsets of red squares along the diagonal, which means that a large module is hierarchically split into several smaller modules according to the change of preference values. (B) As an example, the decomposition of a large module (red square) identified in "12-module set" into smaller modules obtainable in "18-" and "37-module set" is shown with the expression profiles of genes and enriched GO BP terms. A module representing general transport function (12-module set) is stratified into modules specifically related to protein and iron ion transport (37-module set).
Extension of the module identified by SSIM approach
Suppose that we have information about a set of genes U and thereby choose a subset V as genes of interest to be used to construct gene modules. The remaining genes (VC), where V ∪VC = U and V ∩VC = ø, are generally not considered in constructing gene modules. However, the genes in VC might have many interactions with the genes in gene set V and potentially provide important information in representing the biological function of the constructed modules. Such information can be incorporated by including these genes in VC using a module extension procedure (see methods). As an example, one of the modules identified by SSIM (using the MATISSE dataset) had 72 genes representing functions related to "cell cycle". The addition of 55 neighboring interconnected genes from V that were not in the initial gene list VC increased the significance level of enriched GO BP terms and the average clustering coefficient from 0.0648 to 0.2934 (see Additional File 5).
SSIM can be used to reveal biological processes involved in complex disease
The recently described gene networks involved in prion disease model was a significant development in the advance of systems biology [34]. It demonstrated the value of constructing gene networks to reveal biological processes involved in this complex disease. The study analyzed gene expression profiling results from several different experimental conditions and time points for prion disease animal model. A set of roughly 300 differentially expressed genes (DEGs) that were common to different experimental conditions (mouse strains and prion types) was identified from these analyses. These DEGs were first classified by GO annotations, then hand-curated and assigned into different gene networks based on functional enrichment results and prior knowledge of the genes. Additional non-differentially expressed genes were also included to complete and illustrate the functionality of the networks. It was surprising to find that the pathogenesis of prion disease can be largely explained by only four major networks: PrPSc replication and accumulation, microglial and astrocytic activation, synaptic degeneration, and neuronal cell death [34]. Genes in these four networks are involved in proteolysis and lipid metabolism for PrPSc replication and accumulation network, immune responses for microglial and astrocytic activation network, mitochondrial dysfunction and apoptosis for neuronal cell death network, and transcription-related function, intracellular signal transduction and ion transport-related function for synaptic degeneration network.
To test the feasibility of using SSIM in complex disease analysis, the same set of genes used in the prion network construction was fed into SSIM. A total of 16 gene modules were obtained and they could largely subsume into the four larger manually generated networks previously described (see Table 1 and Additional File 6). For example, modules 1 and 3 contain a total of 36 genes that are highly associated with lipid metabolism and proteolysis based on GO BP enrichment result, which almost completely recapitulate the key nodes involved in PrPSc accumulation network. In addition to the modules related directly to the four reported networks, SSIM also suggested processes involved in tissue remodeling such as actin cytoskeleton organization (GO:0030036), angiogenesis (GO:0001525), multicellular organismal development (GO:0007275) and cell differentiation (GO:0030154) might also be involved in the progression of prion disease. We will conduct more detailed network analyses with SSIM using prion disease datasets including the comparison between different incubation times, host strains, and infectious agents, since this preliminary study agrees well with the manually constructed networks.
Table 1.
Similarity between modules identified by SSIM and four major prion subnetworks
| Module | Genes in the module | PrPSc accumulation | Microglial activation | Neuronal cell death | Synaptic degeneration |
|---|---|---|---|---|---|
| 1 | 21 | 16 (76%) | 5 (24%) | 2 (10%) | 0 (0%) |
| 2 | 27 | 7 (26%) | 21 (78%) | 1 (4%) | 1 (4%) |
| 3 | 15 | 12 (80%) | 0 (0%) | 3 (20%) | 0 (0%) |
| 4 | 37 | 5 (14%) | 5 (14%) | 10 (27%) | 20 (54%) |
| 5 | 42 | 16 (38%) | 14 (33%) | 13 (31%) | 4 (10%) |
| 6 | 33 | 1 (3%) | 1 (3%) | 12 (36%) | 24 (73%) |
| 7 | 34 | 5 (15%) | 22 (65%) | 9 (26%) | 2 (6%) |
| 8 | 37 | 6 (16%) | 0 (0%) | 9 (24%) | 24 (65%) |
| 9 | 49 | 2 (4%) | 7 (14%) | 37 (76%) | 6 (12%) |
| 10 | 41 | 19 (46%) | 19 (46%) | 8 (20%) | 2 (5%) |
| 11 | 39 | 7 (18%) | 29 (74%) | 5 (13%) | 4 (10%) |
| 12 | 43 | 2 (5%) | 13 (30%) | 18 (42%) | 12 (28%) |
| 13 | 46 | 19 (41%) | 38 (83%) | 2 (4%) | 0 (0%) |
| 14 | 57 | 5 (9%) | 36 (63%) | 17 (30%) | 7 (12%) |
| 15 | 67 | 5 (7%) | 2 (3%) | 23 (34%) | 49 (73%) |
| 16 | 60 | 5 (8%) | 0 (0%) | 2 (3%) | 54 (90%) |
For each module, the number and the proportion of genes corresponding to prion subnetworks are shown. Since some genes are involved in multiple subnetworks, the row-wise summation of proportions may exceed 100%.
Conclusions
In this report, we have proposed and demonstrated a new approach, Semantic Similarity-Integrated approach for Modularization (SSIM), for inferring biologically meaningful gene modules. The SSIM method integrates various gene-gene pairwise similarity information obtained from gene expression profiling results, protein interactions and GO annotations to construct gene modules. We showed that gene modules generated by SSIM gave higher specificity with stronger association with biological functions based on the assessment of GO and MIPS annotation terms.
Since SSIM is based on pairwise similarity values of genes, there is room for further improvement by integrating additional quantitative similarity measures. For example, GO semantic similarity of other categories (molecular function (MF) and cellular component (CC)) or newly developed semantic similarity measures [35-37] can also be used. In addition, different clustering algorithms can also be used to enhance the construction of gene modules. The evaluation and comparison between various gene pairwise similarities and clustering methods will be interesting to explore in the future.
We have also demonstrated the ability to use SSIM to uncover the hierarchical structure of gene modules, i.e. hierarchical association and dissociation of modules at different levels of functional detail. This would allow us to gain a systematic understanding of a given biological system. When some genes are excluded from the set of selected genes (often differentially expressed genes) due to marginal expression changes or for other reasons, the module extension procedure adapted in SSIM can incorporate them and increase the functional interpretability of gene modules without sacrificing the statistical significance of functional association of gene modules. Although a similar approach has been used in other studies [12,15], the effect of network extension on functional association of gene modules has not been evaluated carefully in those methods.
Even though tools to construct gene modules have been developed, they have been mainly applied in datasets from relatively simple model organisms [4,5,9,11,13,14]. The SSIM performed well not only with the dataset from yeast, but also with a much more complicated dataset, the prion disease mouse model. Gene modules from SSIM effectively recaptured the manually constructed key networks described in prion disease, and also revealed new processes that might also be involved in the disease development. Our results suggest the SSIM approach can quickly infer gene modules with coherent biological meaning and thereby accelerate systems biology studies in complex diseases.
Methods
Reference datasets and methods
We used 133 expression profiles representing the osmotic shock response of Saccharomyces cerevisiae in various conditions [38]. Ulitsky and Shamir [12] selected 1990 genes and curated ~69,000 protein interactions for MATISSE. Parkkinen and Kaski [14] used 1771 genes and ~10,000 interactions for ICMg. While 1771 genes are a subset of 1990 genes, ~10,000 interactions are not exactly a subset of ~69,000 interactions since they used a different approach to curate yeast protein interaction data. Both of the datasets were used to compare and evaluate the performance of all three different methods. We used implementations of MATISSE (Java software) and ICMg (R-package) with their default parameters. These datasets (MATISSE data and ICMg data) and implementations were downloaded at http://acgt.cs.tau.ac.il/matisse and http://www.cis.hut.fi/projects/mi/software/ICMg, respectively.
Similarity measures
Building gene modules can be viewed as the grouping genes of interest according to their pairwise similarity values. Pearson correlation between each pair of genes (gene i and gene j), was used as expression similarity, eij. From the global protein-protein interaction network, pairwise topological similarity (tij) was obtained using topological overlap matrix [39] which was initially developed to identify gene modules in E. Coli metabolism. It reaches the maximum value of 1 when there is a direct connection between two genes and has the value between 0 and 1 when two genes are not linked but share some direct neighbors. If there is no direct connection between two genes and no direct neighbors shared by them, tij has the minimum value of 0. GO semantic similarity between two genes (gij) was computed by the metric proposed by Wang et al. [25] which took the characteristics of GO hierarchy into account and overcame drawbacks of previous semantic similarity measures [18-20]. gij has the value of 0 for two genes with no similarity and 1 for genes having identical GO annotations. Although GO semantic similarity can be computed for three categories of GO terms, biological process (BP), molecular function (MF) and cellular components (CC), we used the similarity of GO BP terms in this study. An overview of GO semantic similarity can be found in Pesquita et al. [40].
To combine three similarity measures, they were assumed to be independent of each other, which is probably a good first approximation since they were derived from different information sources. First, for expression similarity, the empirical cumulative density function, F(E) could be estimated from all eij values, where E = {eij | i = 1,..., n-1, j = i+1,..., n, and n = total number of genes of interest} and the probability of having the similarity less than or equal to eij, Pr(E≤eij) could be obtained from F(eij). With this method, a large eij value from highly similar pair of genes has a high probability - close to one. The probabilities for tij, Pr(T≤tij) and gij, Pr(G≤gij) could be obtained similarly to eij. Next, the joint probability was calculated by simply multiplying three probabilities in accord with the independence assumption, i.e. Pr(E≤eij, T≤tij, G≤gij) = Pr(E≤eij)·Pr(T≤tij)·Pr(G≤gij). This is then used as a composite similarity measure.
Clustering method
Affinity propagation is a clustering algorithm using the concept of message-passing [27]. It was modeled using a factor graph [41] with two types of messages between data points (in this study the points were genes) that were derived from the max-sum algorithm in the factor graph. First consider all data points as potential cluster centers and then find high quality cluster centers and their members by updating messages. The detailed description of the method can be found in [27,42]. Since affinity propagation takes non-positive real-valued similarities as input, the combined similarity was converted into Sij = Pr(E≤eij, T≤tij, G≤gij)-1, Sij∈[-1,0]. The only parameter affecting the number of clusters is the "preference" which represents how "preferable" each data point is as a cluster center. We used a globally shared preference for all genes and found a proper value for the comparison study in order to produce the same number of modules as other methods, so that the comparisons could be made. We used authors' MATLAB implementations of affinity propagation http://www.psi.toronto.edu/affinitypropagation.
Gene ontology enrichment analysis
Gene ontology data were downloaded from GO consortium (http://www.geneontology.org, OBO v1.2 format) and MIPS FTP site (ftp://ftpmips.gsf.de/catalogue/annotation_data, FunCat v2.1 last modified on 5/25/2008). Yeast gene association with gene ontology and gene information data were obtained from the NCBI repository on 2/17/2010 and 9/5/2010, respectively. P-value of enriched annotation term was obtained by one-sided Fisher exact test without multiple testing corrections. The number of enriched annotation terms in at least one module at a given significance level (i.e. p < 1 × 10-4) and the number of modules enriched with at least one annotation term at the level were counted and used for computing sensitivity and specificity, respectively. In Additional File 1, these numbers were expressed as functions of p-values (p = [10-1, 10-2,..., 10-20]).
Coherence and depth of enriched gene ontology terms
For each method used in the comparison study, enriched GO BP terms in ith module having uncorrected p-values less than 1 × 10-5 were chosen. All pairwise semantic similarities between the terms, gBP,jk (j = 1,..., mi-1 and k = j+1,... mi, where mi = the number of chosen terms from ith module) were calculated by Wang's approach [25] and the depth of each term, dBP,j (j = 1,... mi) was obtained using the shortest path to the root term (GO:0008150 biological process) in GO hierarchy. We could obtain representative coherency and depth of enriched GO BP terms for the ith module by GBP,i = 2/mi(mi-1)·∑j∑k gBP,jk and DBP,i = 1/mi·∑j dBP,j, respectively (i = 1,..., total number of modules identified by the method). Mean values of GBP and DBP over all modules were defined as average coherency and depth of enriched GO terms and shown in Additional File 2.
Comparison of the modules identified by different methods
For MATISSE and ICMg, due to their probabilistic nature, it is not possible to make a unique assignment of genes into modules. Instead, the most likely assignment can be identified using consensus matrix of which element at ith row and jth column represents how many times gene i and gene j are grouped into the same module among 20 runs. Using hierarchical clustering of the consensus matrix, the assignment of genes into 24 modules was obtained and used for the comparison between ICMg module and SSIM modules (see text and Additional File 3).
Extension of a module
A module identified by SSIM can be extended according to the following procedure.
Step 1. find neighbor genes which have direct interactions with more than one gene in the module, but are not in the selected gene set. Sort them in descending order based on the number of interactions with genes in the module
Step 2. for the top scoring neighbor gene, GO semantic similarities between the gene and all genes in the module were calculated
Step 3. if the mean value of the similarities computed in Step 2 is larger than the mean semantic similarity among genes in the module, the neighbor gene is added to the module
Step 4. go back to Step 2 until the genes are all classified
This procedure imposes a priority to the neighbor genes having many connections with genes in the module (Step 1) and minimizes the involvement of irrelevant neighbor genes by increasing average semantic similarity of the module at every addition of a relevant neighbor gene (Step 3). Although we only used semantic similarity between the neighbor gene and genes in the module due to the limitation of expression profiles, expression similarity can be also added to Step 2 and 3 when gene expression of neighbors are available.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JHC, KW and DJG co-conceived the study. JHC developed the method and performed data analyses. JHC and KW wrote the initial manuscript, DJG edited the manuscript. All of the authors read and approved the final manuscript.
Supplementary Material
Additional enrichment results. The number of enriched modules, number of annotation terms and F-measures (GO, GO BP and MIPS FunCat) were shown as functions of p-value. Results for MATISSE and ICMg were obtained using the mean and standard deviation values of 20 runs.
Coherency and depth of significantly enriched GO BP terms. Average expression coherency and depth of significantly enriched GO BP terms in the modules identified by different methods were calculated as described in the method section. Large coherency and depth values mean that GO BP terms enriched in the same module are semantically similar and associated with specific functions, respectively. For MATISSE and ICMg, mean and standard deviation over 20 runs were taken.
Summary of the modules identified by three different methods. The assignments of 1990 genes in MATISSE data into 24 modules identified by SSIM, MATISSE and ICMg were summarized in Table S1 (see methods). GO BP enrichment results of the modules obtained from SSIM, MATISSE and ICMg were also shown in Table S2, S3 and S4, respectively.
Results of SSIM method over a wide range of preference values. The number of modules and average expression, topological and semantic similarities of the modules were expressed as functions of preference.
An example of the extension of a module
An application of SSIM to prion network datasets
Contributor Information
Ji-Hoon Cho, Email: jcho@systemsbiology.org.
Kai Wang, Email: kwang@systemsbiology.org.
David J Galas, Email: dgalas@systemsbiology.org.
Acknowledgements
We would like to thank Drs. Paul Shannon, Inyoul Lee and Nikita Sakhanenko for their help with computation and comments on the manuscript. This work was supported by the University of Luxembourg-ISB Program, research subcontract from Georgetown University (RX 4222-802-ISB) and research contracts from the Department of Defense (W911SR-07-C-0101 and HDTRA 1-08-C-0023).
References
- Hood L, Heath JR, Phelps ME, Lin B. Systems biology and new technologies enable predictive and preventative medicine. Science. 2004;306:640–643. doi: 10.1126/science.1104635. [DOI] [PubMed] [Google Scholar]
- Wang K, Lee I, Carlson G, Hood L, Galas D. Systems biology and the discovery of diagnostic biomarkers. Dis Markers. 2010;28:199–207. doi: 10.3233/DMA-2010-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH, Hopfield JJ, Leibler S, Murray AW. From molecular to modular cell biology. Nature. 1999;402:C47–52. doi: 10.1038/35011540. [DOI] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman PT, Sherlock G, Zhang MQ, Iyer VR, Anders K, Eisen MB, Brown PO, Botstein D, Futcher B. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol Biol Cell. 1998;9:3273–3297. doi: 10.1091/mbc.9.12.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon U, Barkai N, Notterman DA, Gish K, Ybarra S, Mack D, Levine AJ. Broad patterns of gene expression revealed by clustering analysis of tumor and normal colon tissues probed by oligonucleotide arrays. Proc Natl Acad Sci USA. 1999;96:6745–6750. doi: 10.1073/pnas.96.12.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JC, Trent JM, Staudt LM, Hudson J Jr, Boguski MS. et al. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- Sharan R, Maron-Katz A, Shamir R. CLICK and EXPANDER: a system for clustering and visualizing gene expression data. Bioinformatics. 2003;19:1787–1799. doi: 10.1093/bioinformatics/btg232. [DOI] [PubMed] [Google Scholar]
- Ge H, Liu Z, Church GM, Vidal M. Correlation between transcriptome and interactome mapping data from Saccharomyces cerevisiae. Nat Genet. 2001;29:482–486. doi: 10.1038/ng776. [DOI] [PubMed] [Google Scholar]
- Ideker T, Ozier O, Schwikowski B, Siegel AF. Discovering regulatory and signalling circuits in molecular interaction networks. Bioinformatics. 2002;18(Suppl 1):S233–240. doi: 10.1093/bioinformatics/18.suppl_1.S233. [DOI] [PubMed] [Google Scholar]
- Shiga M, Takigawa I, Mamitsuka H. Annotating gene function by combining expression data with a modular gene network. Bioinformatics. 2007;23:i468–478. doi: 10.1093/bioinformatics/btm173. [DOI] [PubMed] [Google Scholar]
- Ulitsky I, Shamir R. Identification of functional modules using network topology and high-throughput data. BMC Syst Biol. 2007;1:8. doi: 10.1186/1752-0509-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitsky I, Shamir R. Identifying functional modules using expression profiles and confidence-scored protein interactions. Bioinformatics. 2009;25:1158–1164. doi: 10.1093/bioinformatics/btp118. [DOI] [PubMed] [Google Scholar]
- Parkkinen JA, Kaski S. Searching for functional gene modules with interaction component models. BMC Syst Biol. 2010;4:4. doi: 10.1186/1752-0509-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Chen Y, Li S, Li Y. Identification of responsive gene modules by network-based gene clustering and extending: application to inflammation and angiogenesis. BMC Syst Biol. 2010;4:47. doi: 10.1186/1752-0509-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer LJ, Kruglyak S, Yooseph S. Exploring expression data: identification and analysis of coexpressed genes. Genome Res. 1999;9:1106–1115. doi: 10.1101/gr.9.11.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhang J. In search of the biological significance of modular structures in protein networks. PLoS Comput Biol. 2007;3:e107. doi: 10.1371/journal.pcbi.0030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Conrath D. Semantic similarity based on corpus statistics and lexical taxonomy. Proceedings of International Conference Research on Computational Linguistics (ROCLING X); Taiwan. 1997.
- Lin D. An information-theoretic definition of similarity. Proceedings of the Fifteenth International Conference on Machine Learning; USA. 1998.
- Resnik P. Semantic similarity in a taxonomy: an information-based measure and its application to problems of ambiguity in natural language. J Artificial Intelligence Res. 1999;11:95–130. [Google Scholar]
- Lee PH, Lee D. Modularized learning of genetic interaction networks from biological annotations and mRNA expression data. Bioinformatics. 2005;21:2739–2747. doi: 10.1093/bioinformatics/bti406. [DOI] [PubMed] [Google Scholar]
- Wu X, Zhu L, Guo J, Zhang DY, Lin K. Prediction of yeast protein-protein interaction network: insights from the Gene Ontology and annotations. Nucleic Acids Res. 2006;34:2137–2150. doi: 10.1093/nar/gkl219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Liu R, Shriver CD, Hu H, Liebman MN. Assessing semantic similarity measures for the characterization of human regulatory pathways. Bioinformatics. 2006;22:967–973. doi: 10.1093/bioinformatics/btl042. [DOI] [PubMed] [Google Scholar]
- Cho YR, Shi L, Ramanathan M, Zhang A. A probabilistic framework to predict protein function from interaction data integrated with semantic knowledge. BMC Bioinformatics. 2008;9:382. doi: 10.1186/1471-2105-9-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JZ, Du Z, Payattakool R, Yu PS, Chen CF. A new method to measure the semantic similarity of GO terms. Bioinformatics. 2007;23:1274–1281. doi: 10.1093/bioinformatics/btm087. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT. et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey BJ, Dueck D. Clustering by passing messages between data points. Science. 2007;315:972–976. doi: 10.1126/science.1136800. [DOI] [PubMed] [Google Scholar]
- Ruepp A, Zollner A, Maier D, Albermann K, Hani J, Mokrejs M, Tetko I, Guldener U, Mannhaupt G, Munsterkotter M, Mewes HW. The FunCat, a functional annotation scheme for systematic classification of proteins from whole genomes. Nucleic Acids Res. 2004;32:5539–5545. doi: 10.1093/nar/gkh894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabasi AL, Oltvai ZN. Network biology: understanding the cell's functional organization. Nat Rev Genet. 2004;5:101–113. doi: 10.1038/nrg1272. [DOI] [PubMed] [Google Scholar]
- Lee TI, Rinaldi NJ, Robert F, Odom DT, Bar-Joseph Z, Gerber GK, Hannett NM, Harbison CT, Thompson CM, Simon I. et al. Transcriptional regulatory networks in Saccharomyces cerevisiae. Science. 2002;298:799–804. doi: 10.1126/science.1075090. [DOI] [PubMed] [Google Scholar]
- Segal E, Shapira M, Regev A, Pe'er D, Botstein D, Koller D, Friedman N. Module networks: identifying regulatory modules and their condition-specific regulators from gene expression data. Nat Genet. 2003;34:166–176. doi: 10.1038/ng1165. [DOI] [PubMed] [Google Scholar]
- Stuart JM, Segal E, Koller D, Kim SK. A gene-coexpression network for global discovery of conserved genetic modules. Science. 2003;302:249–255. doi: 10.1126/science.1087447. [DOI] [PubMed] [Google Scholar]
- Lee SI, Dudley AM, Drubin D, Silver PA, Krogan NJ, Pe'er D, Koller D. Learning a prior on regulatory potential from eQTL data. PLoS Genet. 2009;5:e1000358. doi: 10.1371/journal.pgen.1000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D, Lee IY, Yoo H, Gehlenborg N, Cho JH, Petritis B, Baxter D, Pitstick R, Young R, Spicer D. et al. A systems approach to prion disease. Mol Syst Biol. 2009;5:252. doi: 10.1038/msb.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhou X, Zhu J, Zhou C, Guo Z. Revealing and avoiding bias in semantic similarity scores for protein pairs. BMC Bioinformatics. 2010;11:290. doi: 10.1186/1471-2105-11-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabderrahmane S, Smail-Tabbone M, Poch O, Napoli A, Devignes MD. IntelliGO: a new vector-based semantic similarity measure including annotation origin. BMC Bioinformatics. 2010;11:588. doi: 10.1186/1471-2105-11-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Bader GD. An improved method for scoring protein-protein interactions using semantic similarity within the gene ontology. BMC Bioinformatics. 2010;11:562. doi: 10.1186/1471-2105-11-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke SM, Herskowitz I. Unique and redundant roles for HOG MAPK pathway components as revealed by whole-genome expression analysis. Mol Biol Cell. 2004;15:532–542. doi: 10.1091/mbc.E03-07-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravasz E, Somera AL, Mongru DA, Oltvai ZN, Barabasi AL. Hierarchical organization of modularity in metabolic networks. Science. 2002;297:1551–1555. doi: 10.1126/science.1073374. [DOI] [PubMed] [Google Scholar]
- Pesquita C, Faria D, Falcao AO, Lord P, Couto FM. Semantic similarity in biomedical ontologies. PLoS Comput Biol. 2009;5:e1000443. doi: 10.1371/journal.pcbi.1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kschischang FR, Frey BJ, Loeliger H-A. Factor graphs and the sum-product algorithm. IEEE Trans Inf Theory. 2001;47:498–519. doi: 10.1109/18.910572. [DOI] [Google Scholar]
- Dueck D. PhD thesis. University of Toronto, Department of electrical & computer engineering; 2009. Affinity propagation: clustering data by passing messages. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional enrichment results. The number of enriched modules, number of annotation terms and F-measures (GO, GO BP and MIPS FunCat) were shown as functions of p-value. Results for MATISSE and ICMg were obtained using the mean and standard deviation values of 20 runs.
Coherency and depth of significantly enriched GO BP terms. Average expression coherency and depth of significantly enriched GO BP terms in the modules identified by different methods were calculated as described in the method section. Large coherency and depth values mean that GO BP terms enriched in the same module are semantically similar and associated with specific functions, respectively. For MATISSE and ICMg, mean and standard deviation over 20 runs were taken.
Summary of the modules identified by three different methods. The assignments of 1990 genes in MATISSE data into 24 modules identified by SSIM, MATISSE and ICMg were summarized in Table S1 (see methods). GO BP enrichment results of the modules obtained from SSIM, MATISSE and ICMg were also shown in Table S2, S3 and S4, respectively.
Results of SSIM method over a wide range of preference values. The number of modules and average expression, topological and semantic similarities of the modules were expressed as functions of preference.
An example of the extension of a module
An application of SSIM to prion network datasets