Abstract
Purpose
To elucidate the incidence of cytochrome P450 1B1 (CYP1B1) and myocillin (MYOC) mutations in Korean patients with primary congenital glaucoma (PCG).
Methods
Genomic DNA was collected from peripheral blood of 85 unrelated Korean patients who were diagnosed as having PCG by standard ophthalmological examinations and screened for mutations in the CYP1B1 and MYOC genes by using bi-directional sequencing.
Results
Among 85 patients with PCG, 22 patients (22/85; 25.9%) had either one (n=11) or two (n=11) mutant alleles of the CYP1B1 gene. Among 11 different CYP1B1 mutations identified, a frameshift mutation (c.970_971dupAT; p.T325SfsX104) was the most frequent mutant allele (6/33; 18.2%) while p.G329S and p.V419Gfs11X were novel. In the MYOC gene, two variants of unknown significance (p.L228S and p.E240G) were identified in two PCG patients (2/85; 2.4%), respectively. No patient had mutations in both genes.
Conclusions
Although CYP1B1 mutations are major causes of PCG in Korea, ~70% of PCG patients have neither CYP1B1 nor MYOC mutations suggesting a high degree of genetic heterogeneity. Furthermore, the fact that 11 out of 22 patients had only one mutant allele in the CYP1B1 gene necessitates further investigation for other genetic backgrounds underlying PCG.
Introduction
Primary congenital glaucoma (PCG; OMIM 231300) is a rare but severe form of glaucoma, which usually manifests within the first year of life [1]. It is characterized by high ocular pressure (IOP) resulting from an obstruction of aqueous outflow from the anterior segment of the eye, and is thought to be the result of an anatomic defect in the trabecular meshwork and anterior chamber [2]. Increased IOP causes irreversible damage to the optic nerve and can lead to blindness if untreated. Affected children typically present with photophobia, epiphora, corneal clouding, and enlargement of globe or cornea. PCG occurs in both familial and sporadic patterns [3]. Inheritance in familial cases is usually autosomal recessive. Incidence of PCG is geographically and ethnically variable, with the lowest incidence (1:10,000) in the Western population and higher incidence in inbred populations, such as the Gypsy subpopulation of Slovakia (1:1,250) [4]. Three loci have been mapped for PCG (gene symbol GLC3), GLC3A (2p21; OMIM 231300), GLC3B (1p36.2; OMIM 600975), and GLC3C (14q24.3). The gene associated with GLC3A, cytochrome P450, family 1, subfamily B, polypeptide 1 (CYP1B1; OMIM 601771), has been implicated in the pathogenesis of PCG. Physiologic studies have confirmed that mutations in CYP1B1 can cause disease; however, the pathway by which CYP1B1 affects development of the anterior chamber of the eye is unknown. The proportion of PCG patients whose disease is due to CYP1B1 mutations is generally high, but varies among populations, ranging from 100% in Slovakian Roma to ~10% in Mexico [4,5]. No responsible gene has yet been identified at the GLC3B and GLC3C loci [6].
Of particular interest, the myocilin gene (MYOC; OMIM 601652), the first open angle glaucoma gene, was initially reported to interact with CYP1B1 through a digenic mechanism, leading to juvenile open angle glaucoma [7]. However, MYOC has recently been implicated in the pathogenesis of some cases of PCG, either independently or in association with CYP1B1 [8,9].
Herein, we screened both the CYP1B1 and MYOC genes in 1 familial and 84 sporadic cases of PCG to identify the underlying genetic mutations in a Korean population.
Methods
Subjects
The study protocol adhered to the tenets of the Declaration of Helsinki and informed consent was obtained from patients or their responsible guardians. We conducted a prospective multi-institutional collaborative study from September, 2008 to February, 2010. A total of 85 unrelated PCG patients were recruited from seven hospitals in South Korea. Of the 85 cases, only one case was familial and the rest were sporadic. Criteria for PCG diagnosis included IOP ≥21 mmHg in at least one eye; megalocornea; corneal edema/clouding/ opacity; and glaucomatous optic nerve head damage when examination was possible. Corroborating features included symptoms of epiphora and photophobia. Patients with other ocular or systemic anomalies were excluded.
To determine whether sequence variants identified were polymorphic in Korean population, we used a panel of DNA from 105 to 200 unrelated Korean individuals who attended the clinic for conditions other than glaucoma.
Genetic analyses of CYP1B1 and MYOC genes
Blood samples were taken from affected subjects and their parents or relatives when possible. According to the manufacturer’s instructions, genomic DNA was isolated from peripheral blood leukocytes using the Wizard genomic DNA purification kit (Promega, Madison, WI). Using the primers designed by the authors (Table 1 and Table 2), entire coding exons and flanking intronic sequences of CYP1B1 and MYOC were amplified by polymerase chain reaction (PCR). Using the BigDye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems, Foster City, CA), cycle sequencing of CYP1B1 and MYOC was performed on the ABI 3100 Genetic Analyzer (Applied Biosystems).
Table 1. PCR and sequencing primers for CYP1B1 gene analysis.
| Primer name | Primer sequence (5'→3') | Size (bp) |
|---|---|---|
| CYP1B1 1F | TCTCCAGAGAGTCAGCTCCG | 786 |
| CYP1B1 1R | GGGTCGTCGTGGCTGTAG | |
| CYP1B1 2F | ATGGCTTTCGGCCACTACT | 787 |
| CYP1B1 2R | GATCTTGGTTTTGAGGGGTG | |
| CYP1B1 3F | AGTGAGAAATTAGGAAGCTGTTTTAGA | 594 |
| CYP1B1 3R | GCCAGGATGGAGATGAAGAG | |
| CYP1B1 4F | CCCAAGGACACTGTGGTTTT | 498 |
| CYP1B1 4R | AACGCTAATTGAGAAGCAGCA |
Annealing temperature, 60 °C for all primer pairs.
Table 2. PCR and sequencing primers for MYOC gene analysis.
| Primer name | Primer sequence (5'→3') | Size (bp) |
|---|---|---|
| MYOC 1F | CTCTGTCTTCCCCCATGAAG | 462 |
| MYOC 1R | AGCCTGGTCCAAGGTCAAT | |
| MYOC 2F | AGGCCATGTCAGTCATCCAT | 478 |
| MYOC 2R | GCGCCTGTAGCAGGTCACTA | |
| MYOC 3F | GCAGCCTATTTAAATGTCATCCT | 310 |
| MYOC 3R | TGGGTGGGCATTTACCCTAT | |
| MYOC 4F | TCCGCATGATCATTGTCTGT | 467 |
| MYOC 4R | ACCCCAAGAATACGGGAACT | |
| MYOC 5F | ACTCGGGGAGCCTCTATTTC | 461 |
| MYOC 5R | CTCCAGGGGGTTGTAGTCAA | |
| MYOC 6F | CCCAGAGAATCTGGAACTCG | 478 |
| MYOC 6R | CGCCCTCAGACTACAATTCC |
Annealing temperature, 60 °C for all primer pairs.
The Sequencher program (Gene Codes Corp., Ann Arbor, MI) was used for analysis of sequence variations with reference to the wild type sequence. Variations were described according to guidelines established by the Human Genome Variation Society (HGVS); the ‘A’ of the ATG codon for translation initiation was numbered +1 and the 1st methionine was numbered +1 (CYP1B1; NP_000095.2, MYOC; NP_000252.1). We referred to the CYP1B1 and MYOC mutation database, as well as the literature, to determine whether a detected variation was novel or known [10]. When a novel sequence variant was identified, 400 ethnically matched normal control chromosomes were tested for the presence of the variant.
To assess the extent of conservation of a novel variation in CYP1B1 thought to be associated with disease, the deduced amino acid sequence was assessed by aligning the protein sequences of different mammalian species and of related CYP1B1 family members using ClustalW2 software (European Bioinformatics Institute, Hinxton, UK).
Results
Mutational analysis of CYP1B1
Eleven different mutations in 22 sporadic cases were identified by direct sequencing of the CYP1B1 gene in 85 PCG probands (Table 3). In total, three subjects were homozygous for a CYP1B1 mutation and 8 patients were compound heterozygous. Another 11 patients were heterozygous for a CYP1B1 mutation. Among 11 different mutations, two novel variations, including a missense variation and a 2-bp deletion (p.G329S and p.V419Gfs11X), were identified. The p.G329S variation occurs in a highly conserved residue (Figure 1) and p.V419Gfs11X causes a frameshift, resulting in a premature termination codon at residue 429 (Figure 2). These two variations were not found in 400 normal chromosomes. The remaining 9 mutations identified in our cohort have been reported previously. They included the following missense changes: c.985G>T (p.V320L), c.988_989delinsTT (p.A330F), c.1090G>A (p.V364M), c.1103G>A (p.R368H), c.1169G>A (p.R390H), and c.1331G>A (p.R444Q), along with three deleterious mutations, including c. 55C>T (p.Q19X), c.243C>G (p.T81X), and a two base pair duplication in exon 2 (c.970_971dupAT; p.T325SfsX104). p.T325SfsX104 was the most frequent allele (18.2%, 6/33) and exhibited compound heterozygosity with p.T81X, p.G329S, p.R390H, and p.R444Q, along with one case of homozygosity (Table 3).
Table 3. CYP1B1 mutations identidied in Korean probands with PCG.
| Patients | Nucleotide change | Amino acid change | Hetero-/homozygous |
|---|---|---|---|
| PCG 11 |
c.[243C>G]+[1090G>A] |
p.[Y81X]+[V364M] |
Compound heterozygous |
| PCG 17 |
c.[1090G>A]+[1090G>A] |
p.[V364M]+[V364M] |
Homozygous |
| PCG 18 |
c.[958G>T]+? |
p.[V320L]+[?] |
Heterozygous |
| PCG 20 |
c.[1090G>A]+? |
p.[V364M]+[?] |
Heterozygous |
| PCG 37 |
c.[958G>T]+? |
p.[V320L]+[?] |
Heterozygous |
| PCG 40 |
c.[970_971dupAT]+[985G>A] |
p.[T325SfsX104]+[G329S] |
Compound heterozygous |
| PCG 45 |
c.[55C>T]+[1103G>A] |
p.[Q19X]+[R368H] |
Compound heterozygous |
| PCG 46 |
c.1090G>A+? |
p.[V364M]+[?] |
Heterozygous |
| PCG 49 |
c.[243C>G]+[970_971dupAT] |
p.[Y81X]+[T325Sfs104X] |
Compound heterozygous |
| PCG 53 |
c.[958G>T]+? |
p.[V320L]+[?] |
Heterozygous |
| PCG 54 |
c.[988_989delGCinsTT]+[1256_1257delTG] |
p.[A330F]+[V419GfsX11] |
Compound heterozygous |
| PCG 55 |
c.[958G>T]+? |
p.[V320L]+[?] |
Heterozygous |
| PCG 6 |
c.[970_971dupAT]+[970_971dupAT] |
p.[T325SfsX104]+[T325SfsX104] |
Homozygous |
| PCG 69 |
c.[988_989delGCinsTT]+[1331G>A] |
p.[A330F]+[R444Q] |
Compound heterozygous |
| PCG 72 |
c.[988_989delGCinsTT]+? |
p.[A330F]+[?] |
Heterozygous |
| PCG 73 |
c.[958G>T]+? |
p.[V320L]+[?] |
Heterozygous |
| PCG 74 |
c.[988_989delGCinsTT]+? |
p.[A330F]+[?] |
Heterozygous |
| PCG 75 |
c.[970_971dupAT]+[1331G>A] |
p.[T325SfsX104]+[R444Q] |
Compound heterozygous |
| PCG 78 |
c.[970_971dupAT]+[1169G>A] |
p.[T325SfsX104]+[R390H] |
Compound heterozygous |
| PCG 84 |
c.[988_989delGCinsTT]+? |
p.[A330F]+[?] |
Heterozygous |
| PCG 86 |
c.[985G>A]+[985G>A] |
p.[G329S]+[G329S] |
Homozygous |
| PCG 100 | c.[985G>A]+[?] | p.[G329S]+[?] | Heterozygous |
*Reported as a causative mutation for hepatocellular adenoma. Bold lettering is used to represent novel mutations identified in this study.
Figure 1.
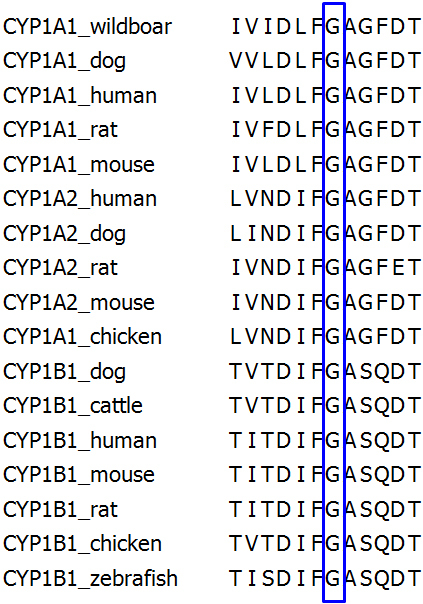
Conservation of p.Gly329 residues (numeration according to human CYP1B1, as shown by protein alignment of several CYP1B1 orthologs and other CYP family members, using ClustalW2.
Figure 2.
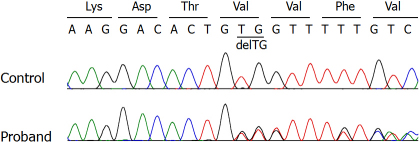
Direct sequencing of the CYP1B1 gene. p.V419GfsX11 is a novel deletion mutation detected in a patient in the compound heterozygous state.
Eleven probands had only one identifiable mutant allele. The mutations found in the heterozygous state were p.V320L, p. A330F, p.V364M, and p.G329S.
In addition to 11 different mutations, 7 previously reported polymorphisms, c.-13C>T, c.142C>G (p.R48G), c.319C>G (p.L107V), c.355G>T (p.A119S), c.729G>C (p.V243V), c.1294G>C (p.V432L), and c.1347T>C (p. D449D) were detected (Table 4).
Table 4. Single nucleotide polymorphisms of CYP1B1 identified in Korean probands with PCG.
| Location | Nucleotide change | Amino acid change | Allele frequency (%) | Reference SNP number | |
|---|---|---|---|---|---|
| Intron 1 |
5′UTR-13C>T |
NA |
C 130/170 (76.5) |
T 40/170 (23.5) |
rs2617266 |
| Exon 2 |
c.142C>G |
p.R48G |
C 130/170 (76.5) |
G 40/170 (23.5) |
rs10012 |
| Exon 2 |
c.319C>G |
p.L107V |
C 166/170 (97.6) |
G 4/170 (2.4) |
rs56339482 |
| Exon 2 |
c.355G>T |
p.A119S |
G 129/170 (75.9) |
T 41/170 (24.1) |
rs1056827 |
| Exon 2 |
c.729G>C |
p.V243V |
G 165/170 (97.1) |
C 5/170 (2.9) |
rs9341249 |
| Exon 3 |
c.1294G>C |
p.V432L |
G 23/170 (13.5) |
C 147/170 (86.5) |
rs1056836 |
| Exon 3 | c.1347T>C | p.D449D | T 27/170 (15.9) | C 143/170 (84.1) | rs1056537 |
Abbreviations: SNP, single nucleotide polymorphism; UTR, untranslated region; NA, not applicable.
Mutational analysis of MYOC
Due to DNA availability, direct sequencing of MYOC was performed for 79 out of 85 patients. As a result, 2 patients were shown to harbor possible novel mutations in MYOC: c.683T>C (p.L228S) and c.719A>G (p.E240G), respectively. Neither of the two variations was found in 400 normal chromosomes. Five previously described polymorphisms, including c.34G>C (p.G12R), c.227G>A (p.R76K), c.624C>G (p.D208E), c.730+35G>A, and c.1058C>T (p.T353I) were identified (Table 5). In addition, two novel synonymous variations, c.864C>T (p.I288I) and c.1110G>A (p.P370P), were each found once in heterozigosity (0.6%, 1/158). These two variations were not found in 400 normal chromosomes.
Table 5. Single nucleotide polymorphisms of MYOC in korean patients with PCG.
|
Location |
Nucleotide change |
Amino acid change |
Allele frequency (%) |
Reference SNP number |
|||
|---|---|---|---|---|---|---|---|
| PCG (n=79) | Control (n=200) | ||||||
| Exon1 |
c.34G>C |
p.G12R |
G 2/158 (1.3) |
C 156/158 (98.7) |
G 395/400 (98.8) |
C 5/400 (1.3) |
Rare polymorphism [32,33], |
| Exon1 |
c.227G>A |
p.R76K |
G 8/158 (5.1) |
A 150/158 (94.9) |
G 383/400 (95.8) |
A 17/400 (4.3) |
rs2234926 |
| Exon2 |
c.624C>G |
p.D208E |
C 2/158 (1.3) |
G 156/158 (98.7) |
C 391/400 (97.8) |
G 9/400 (2.3) |
rs2234927 |
| Intron2 |
IVS2+35G>A |
NA |
G 10/158 (6.3) |
A 148/158 (93.7) |
G 71/400 (17.8) |
A 329/400 (82.3) |
rs2032555 |
| Exon3 |
c.864C>T |
p.I288I |
C 1/158 (0.6) |
T 157/158 (99.4) |
C 400/400 (100.0) |
T 0/400 (0.0) |
This study |
| Exon3 |
c.1058C>T |
p.T353I |
C 1/158 (0.6) |
T 157/158 (99.4) |
C 399/400 (99.8) |
T 1/400 (0.3) |
Rare polymorphism [33,36,38], |
| Exon3 | c.1110G>A | p.P370P | G 1/158 (0.6) | A 157/158 (99.4) | G 400/400(100.0) | A 0/400 (0.0) | This study |
Abbreviation: SNP, single nucleotide polymorphism; NA, not applicable. Bold lettering is used to represent novel variations in this study.
Discussion
Herein, we report on mutation screening of the CYP1B and MYOC genes in 85 and 79 PCG cases, respectively. CYP1B1 screening revealed that about 26% of 85 patients had at least one mutation, although half of them carried only one mutant allele with the other mutation unidentified. Homozygisity of the mutant allele was seen in only three cases and compound heterozygosity in eight cases. Consistent with other CYP1B1 mutation spectrum studies from populations where consanguinity is uncommon, we observed a high degree of allelic heterogeneity and compound heterozygosity. To the best of our knowledge, of 11 different CYP1B1 mutations identified in the 85 probands, two are novel.
p.G329S has been previously reported as a pathologic mutation for hepatocellular carcinoma [11]; however, in this study, it was reported as a new causative mutation for PCG in a case of compound heterozygosity with p.T325SfsX104 along with one each case of homozygosity and heterozygosity. p.G329S occurs at an amino acid position that is highly conserved among other species and CYP1B1 family members (Figure 1).
Among all of the mutations, p.T325SfsX104 was most frequently found (6/33 mutant alleles). It is interesting to note that this disease-causing mutation has only been described in a single Japanese patient with PCG [12]. We speculated that p.T325SfsX104 might be recurrent among Korean individuals with PCG, possibly with a founder effect.
Although the presence of heterozygous CYP1B1 mutations in 11 PCG patients does not match a typical recessive pattern of inheritance in PCG, heterozygous CYP1B1 mutations have been documented [13-17]. Mutations such as p.T81N, p.Q229K, p.R368H, and p.R469W have been described in PCG patients in the heterozygous state [13,15-18]. The mutations found in the heterozygous state in this study were p.V320L, p.A330F, p.V364M, and p.G329S. As described above, p.G329S is a novel mutation. Since p.V320L, p.A330F, and V364M, have not been reported in heterozygous state [12,19-22], additionally, we performed population screening involving 210 control chromosomes. As a result, p.A330F and p.V364M were not found in any normal chromosome and p.V320L was occurred in less than 1% (2/210). As only the coding region of CYP1B1 was sequenced, we thought that it might be due to mutations in (1) the CYP1B1 promoter or other non-coding regions; (2) genes linked to other PCG loci, such as GLC3B and GLC3C; (3) other glaucoma genes such as MYOC, resulting in digenic inheritance; or (4) some other unknown genes causing glaucoma. The presence of double heterozygous variants, CYP1B1 and MYOC, has recently been described in one PCG case; however, the role of possible digenism in disease causation is yet to be established [8,23]. PCG-causing mutations in latent transforming growth factor beta binding protein 2 (LTBP2; OMIM 602091) have recently been identified in Pakistani, European Gypsy, and Iranian patients [24,25]. LTBP2 lies very close to GLC3C on chromosome 14, but is not strictly within the locus, as originally defined [26]. As such, it is not clear whether LTBP2 is the PCG-associated gene within GLC3C, or whether the gene within this locus remains unknown and LTBP2 defines a fourth locus for PCG. Observation of unrelated PCG cases with a heterozygous mutation also raises the possibility that the mutation might be a dominant cause of PCG [16].
Due to ethnic differences and geographical variations, the prevalence of CYP1B1 mutations varies in different patient populations, from ~10% in Mexico [5], to 20% in Indonesia [27], Australia [28], China [29], and Japan [19]; around 40% in Turkish patients [13]; approximately 50% in Brazil [20] and France [16]; and about 100% in consanguineous Saudi Arabian [30] and Slovakian Gypsy [4] patients. Although direct comparison between these studies is difficult, it should be noted that the proportion of CYP1B1 mutations accounting for PCG in our population is similar to those in Japan and China. These data also illustrate that the contribution of defects in this gene varies significantly among human populations, which highlights the need for analysis of large groups of PCG from different ethnic backgrounds to ascertain the role of this gene in a specific population.
Direct sequencing of the coding region of MYOC was performed in 79 of all PCG probands. As a result, two variants of unknown significance (p.L228S and p.E240G) were identified in two PCG patients (2/79; 2.5%). These variations lead to replacement of leucine by serine at codon 228 (p.L228S) and glutamic acid by glycine at codon 240 (p.E240G), respectively, which involve a highly conserved region among other species (Figure 3). Neither p.L228S nor p.E240G was found in 400 control chromosomes, suggesting that these two variations are possible mutations. In one previous study, MYOC mutations accounted for 2.6% (3/116) of Chinese patients with PCG [29].
Figure 3.
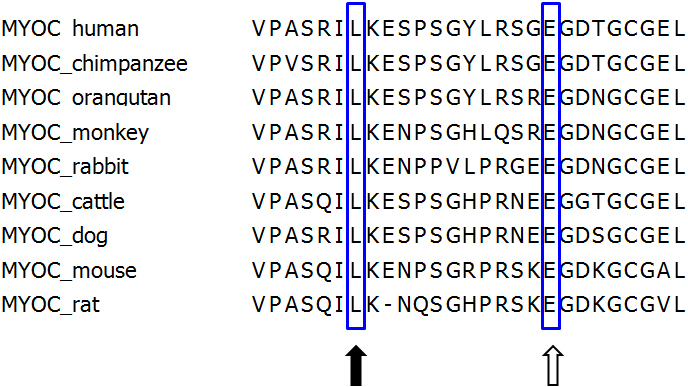
Alignment of MYOC peptide sequences in human and other species. The CLUSTAL W (2.0) computer program was used for multiple alignment of amino acid sequences. The position of mutated amino acids in the PCG patient reported in this study is boxed. Note that the p.L228S and p.E240G mutations occurred at highly conserved positions of the amino acids (filled arrow; p.Leu228, open arrow; p.Glu240).
Of the polymorphisms identified, p.G12R and p.T353I have been previously reported as possible POAG causing mutations; however, they were found in control subjects in this study (Table 5). p.G12R, which has been reported as a possible disease-causing mutation in a sporadic northern Chinese case of POAG [31], was found in control subjects in a few studies [32,33]. p.T353I was found in a Korean family with POAG [34], a Japanese patient with POAG [35], seventeen Chinese individuals with POAG [31-33,36], and one Indian patient with juvenile-onset POAG [37]. However, this change has been also detected in normal individuals [33,36,38]. Therefore, p.G12R and p.T353I are rare polymorphisms rather than disease-causing mutations; however, it remains possible that they affect the risk of POAG.
To the best of our knowledge, this is the first report on molecular genetic analysis of PCG in the Korean population. One fourth of Korean PCG probands were found to have at least one CYP1B1 mutation and half of the patients with CYP1B1 mutations had only one mutant allele. We observed a high degree of allelic heterogeneity in our cohort with CYP1B1 mutations. Only two patients carried possible MYOC mutations. These results suggest that CYP1B1 should be regarded as a potential primary cause of PCG in Korea. However, due to the relatively low contribution of CYP1B1 to Korean PCG, it is suggested that other genetic factors remain to be identified and that further work is needed to identify the causative genes.
Acknowledgments
This study was supported by the heart to heart foundation. Drs. Chang-Seok Ki and Changwon Kee contributed equally to the conduct of this research and are to be considered co-corresponding authors.
References
- 1.Sarfarazi M, Stoilov I. Molecular genetics of primary congenital glaucoma. Eye (Lond) 2000;14(Pt 3B):422–8. doi: 10.1038/eye.2000.126. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DR. The development of the trabecular meshwork and its abnormality in primary infantile glaucoma. Trans Am Ophthalmol Soc. 1981;79:458–85. [PMC free article] [PubMed] [Google Scholar]
- 3.Sarfarazi M, Stoilov I, Schenkman JB. Genetics and biochemistry of primary congenital glaucoma. Ophthalmol Clin North Am. 2003;16:543–54. doi: 10.1016/s0896-1549(03)00062-2. vi. [DOI] [PubMed] [Google Scholar]
- 4.Plásilová M, Stoilov I, Sarfarazi M, Kadasi L, Ferakova E, Ferak V. Identification of a single ancestral CYP1B1 mutation in Slovak Gypsies (Roms) affected with primary congenital glaucoma. J Med Genet. 1999;36:290–4. [PMC free article] [PubMed] [Google Scholar]
- 5.Zenteno JC, Hernandez-Merino E, Mejia-Lopez H, Matias-Florentino M, Michel N, Elizondo-Olascoaga C, Korder-Ortega V, Casab-Rueda H, Garcia-Ortiz JE. Contribution of CYP1B1 mutations and founder effect to primary congenital glaucoma in Mexico. J Glaucoma. 2008;17:189–92. doi: 10.1097/IJG.0b013e31815678c3. [DOI] [PubMed] [Google Scholar]
- 6.Akarsu AN, Turacli ME, Aktan SG, Barsoum-Homsy M, Chevrette L, Sayli BS, Sarfarazi M. A second locus (GLC3B) for primary congenital glaucoma (Buphthalmos) maps to the 1p36 region. Hum Mol Genet. 1996;5:1199–203. doi: 10.1093/hmg/5.8.1199. [DOI] [PubMed] [Google Scholar]
- 7.Vincent AL, Billingsley G, Buys Y, Levin AV, Priston M, Trope G, Williams-Lyn D, Heon E. Digenic inheritance of early-onset glaucoma: CYP1B1, a potential modifier gene. Am J Hum Genet. 2002;70:448–60. doi: 10.1086/338709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaur K, Reddy AB, Mukhopadhyay A, Mandal AK, Hasnain SE, Ray K, Thomas R, Balasubramanian D, Chakrabarti S. Myocilin gene implicated in primary congenital glaucoma. Clin Genet. 2005;67:335–40. doi: 10.1111/j.1399-0004.2005.00411.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhuo YH, Wang M, Wei YT, Huang YL, Ge J. Analysis of MYOC gene mutation in a Chinese glaucoma family with primary open-angle glaucoma and primary congenital glaucoma. Chin Med J (Engl) 2006;119:1210–4. [PubMed] [Google Scholar]
- 10.Human Gene Mutation Database http://www.hgmd.cf.ac.uk/ac/index.php
- 11.Jeannot E, Poussin K, Chiche L, Bacq Y, Sturm N, Scoazec JY, Buffet C, Van Nhieu JT, Bellanne-Chantelot C, de Toma C, Laurent-Puig P, Bioulac-Sage P, Zucman-Rossi J. Association of CYP1B1 germ line mutations with hepatocyte nuclear factor 1alpha-mutated hepatocellular adenoma. Cancer Res. 2007;67:2611–6. doi: 10.1158/0008-5472.CAN-06-3947. [DOI] [PubMed] [Google Scholar]
- 12.Ohtake Y, Kubota R, Tanino T, Miyata H, Mashima Y. Novel compound heterozygous mutations in the cytochrome P4501B1 gene (CYP1B1) in a Japanese patient with primary congenital glaucoma. Ophthalmic Genet. 2000;21:191–3. [PubMed] [Google Scholar]
- 13.Bagiyeva S, Marfany G, Gonzalez-Angulo O, Gonzalez-Duarte R. Mutational screening of CYP1B1 in Turkish PCG families and functional analyses of newly detected mutations. Mol Vis. 2007;13:1458–68. [PubMed] [Google Scholar]
- 14.Campos-Mollo E, Lopez-Garrido MP, Blanco-Marchite C, Garcia-Feijoo J, Peralta J, Belmonte-Martinez J, Ayuso C, Escribano J. CYP1B1 mutations in Spanish patients with primary congenital glaucoma: phenotypic and functional variability. Mol Vis. 2009;15:417–31. [PMC free article] [PubMed] [Google Scholar]
- 15.Chavarria-Soley G, Michels-Rautenstrauss K, Pasutto F, Flikier D, Flikier P, Cirak S, Bejjani B, Winters DL, Lewis RA, Mardin C, Reis A, Rautenstrauss B. Primary congenital glaucoma and Rieger's anomaly: extended haplotypes reveal founder effects for eight distinct CYP1B1 mutations. Mol Vis. 2006;12:523–31. [PubMed] [Google Scholar]
- 16.Colomb E, Kaplan J, Garchon HJ. Novel cytochrome P450 1B1 (CYP1B1) mutations in patients with primary congenital glaucoma in France. Hum Mutat. 2003;22:496. doi: 10.1002/humu.9197. [DOI] [PubMed] [Google Scholar]
- 17.Reddy AB, Kaur K, Mandal AK, Panicker SG, Thomas R, Hasnain SE, Balasubramanian D, Chakrabarti S. Mutation spectrum of the CYP1B1 gene in Indian primary congenital glaucoma patients. Mol Vis. 2004;10:696–702. [PubMed] [Google Scholar]
- 18.Hollander DA, Sarfarazi M, Stoilov I, Wood IS, Fredrick DR, Alvarado JA. Genotype and phenotype correlations in congenital glaucoma. Trans Am Ophthalmol Soc. 2006;104:183–95. [PMC free article] [PubMed] [Google Scholar]
- 19.Mashima Y, Suzuki Y, Sergeev Y, Ohtake Y, Tanino T, Kimura I, Miyata H, Aihara M, Tanihara H, Inatani M, Azuma N, Iwata T, Araie M. Novel cytochrome P4501B1 (CYP1B1) gene mutations in Japanese patients with primary congenital glaucoma. Invest Ophthalmol Vis Sci. 2001;42:2211–6. [PubMed] [Google Scholar]
- 20.Stoilov IR, Costa VP, Vasconcellos JP, Melo MB, Betinjane AJ, Carani JC, Oltrogge EV, Sarfarazi M. Molecular genetics of primary congenital glaucoma in Brazil. Invest Ophthalmol Vis Sci. 2002;43:1820–7. [PubMed] [Google Scholar]
- 21.Curry SM, Daou AG, Hermanns P, Molinari A, Lewis RA, Bejjani BA. Cytochrome P4501B1 mutations cause only part of primary congenital glaucoma in Ecuador. Ophthalmic Genet. 2004;25:3–9. doi: 10.1076/opge.25.1.3.28999. [DOI] [PubMed] [Google Scholar]
- 22.Ohtake Y, Tanino T, Suzuki Y, Miyata H, Taomoto M, Azuma N, Tanihara H, Araie M, Mashima Y. Phenotype of cytochrome P4501B1 gene (CYP1B1) mutations in Japanese patients with primary congenital glaucoma. Br J Ophthalmol. 2003;87:302–4. doi: 10.1136/bjo.87.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakrabarti S, Kaur K, Komatireddy S, Acharya M, Devi KR, Mukhopadhyay A, Mandal AK, Hasnain SE, Chandrasekhar G, Thomas R, Ray K. Gln48His is the prevalent myocilin mutation in primary open angle and primary congenital glaucoma phenotypes in India. Mol Vis. 2005;11:111–3. [PubMed] [Google Scholar]
- 24.Ali M, McKibbin M, Booth A, Parry DA, Jain P, Riazuddin SA, Hejtmancik JF, Khan SN, Firasat S, Shires M, Gilmour DF, Towns K, Murphy AL, Azmanov D, Tournev I, Cherninkova S, Jafri H, Raashid Y, Toomes C, Craig J, Mackey DA, Kalaydjieva L, Riazuddin S, Inglehearn CF. Null mutations in LTBP2 cause primary congenital glaucoma. Am J Hum Genet. 2009;84:664–71. doi: 10.1016/j.ajhg.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narooie-Nejad M, Paylakhi SH, Shojaee S, Fazlali Z, Rezaei Kanavi M, Nilforushan N, Yazdani S, Babrzadeh F, Suri F, Ronaghi M, Elahi E, Paisan-Ruiz C. Loss of function mutations in the gene encoding latent transforming growth factor beta binding protein 2, LTBP2, cause primary congenital glaucoma. Hum Mol Genet. 2009;18:3969–77. doi: 10.1093/hmg/ddp338. [DOI] [PubMed] [Google Scholar]
- 26.Stoilov I, Sarfarazi M. The third genetic locus (GLC3C) for primary congenital glaucoma (PCG) maps to chromosome 14q24.3. ARVO Annual Meeting; 2002 May 5–10; Ft. Lauderdale, FL. [Google Scholar]
- 27.Sitorus R, Ardjo SM, Lorenz B, Preising M. CYP1B1 gene analysis in primary congenital glaucoma in Indonesian and European patients. J Med Genet. 2003;40:e9. doi: 10.1136/jmg.40.1.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimasi DP, Hewitt AW, Straga T, Pater J, MacKinnon JR, Elder JE, Casey T, Mackey DA, Craig JE. Prevalence of CYP1B1 mutations in Australian patients with primary congenital glaucoma. Clin Genet. 2007;72:255–60. doi: 10.1111/j.1399-0004.2007.00864.x. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Jiang D, Yu L, Katz B, Zhang K, Wan B, Sun X. CYP1B1 and MYOC mutations in 116 Chinese patients with primary congenital glaucoma. Arch Ophthalmol. 2008;126:1443–7. doi: 10.1001/archopht.126.10.1443. [DOI] [PubMed] [Google Scholar]
- 30.Bejjani BA, Lewis RA, Tomey KF, Anderson KL, Dueker DK, Jabak M, Astle WF, Otterud B, Leppert M, Lupski JR. Mutations in CYP1B1, the gene for cytochrome P4501B1, are the predominant cause of primary congenital glaucoma in Saudi Arabia. Am J Hum Genet. 1998;62:325–33. doi: 10.1086/301725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia LY, Tam PO, Chiang SW, Ding N, Chen LJ, Yam GH, Pang CP, Wang NL. Multiple gene polymorphisms analysis revealed a different profile of genetic polymorphisms of primary open-angle glaucoma in northern Chinese. Mol Vis. 2009;15:89–98. [PMC free article] [PubMed] [Google Scholar]
- 32.Lam DS, Leung YF, Chua JK, Baum L, Fan DS, Choy KW, Pang CP. Truncations in the TIGR gene in individuals with and without primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2000;41:1386–91. [PubMed] [Google Scholar]
- 33.Pang CP, Leung YF, Fan B, Baum L, Tong WC, Lee WS, Chua JK, Fan DS, Liu Y, Lam DS. TIGR/MYOC gene sequence alterations in individuals with and without primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2002;43:3231–5. [PubMed] [Google Scholar]
- 34.Yoon SJ, Kim HS, Moon JI, Lim JM, Joo CK. Mutations of the TIGR/MYOC gene in primary open-angle glaucoma in Korea. Am J Hum Genet. 1999;64:1775–8. doi: 10.1086/302407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fingert JH, Heon E, Liebmann JM, Yamamoto T, Craig JE, Rait J, Kawase K, Hoh ST, Buys YM, Dickinson J, Hockey RR, Williams-Lyn D, Trope G, Kitazawa Y, Ritch R, Mackey DA, Alward WL, Sheffield VC, Stone EM. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8:899–905. doi: 10.1093/hmg/8.5.899. [DOI] [PubMed] [Google Scholar]
- 36.Fan BJ, Wang DY, Fan DS, Tam PO, Lam DS, Tham CC, Lam CY, Lau TC, Pang CP. SNPs and interaction analyses of myocilin, optineurin, and apolipoprotein E in primary open angle glaucoma patients. Mol Vis. 2005;11:625–31. [PubMed] [Google Scholar]
- 37.Bhattacharjee A, Acharya M, Mukhopadhyay A, Mookherjee S, Banerjee D, Bandopadhyay AK, Thakur SK, Sen A, Ray K. Myocilin variants in Indian patients with open-angle glaucoma. Arch Ophthalmol. 2007;125:823–9. doi: 10.1001/archopht.125.6.823. [DOI] [PubMed] [Google Scholar]
- 38.Aung T, Yong VH, Chew PT, Seah SK, Gazzard G, Foster PJ, Vithana EN. Molecular analysis of the myocilin gene in Chinese subjects with chronic primary-angle closure glaucoma. Invest Ophthalmol Vis Sci. 2005;46:1303–6. doi: 10.1167/iovs.04-1163. [DOI] [PubMed] [Google Scholar]