Abstract
C-terminal Src kinase-homologous kinase (CHK) exerts its tumor suppressor function by phosphorylating the C-terminal regulatory tyrosine of the Src-family kinases (SFKs). The phosphorylation suppresses their activity and oncogenic action. In addition to phosphorylating SFKs, CHK also performs non-SFK related functions by phosphorylating other cellular protein substrates. To define these non-SFK related functions of CHK, we used the `kinase substrate tracking and elucidation' method to search for its potential physiological substrates in rat brain cytosol. Our search revealed β-synuclein as a potential CHK substrate, and Y127 in β-synuclein as the preferential phosphorylation site. Using peptides derived from β-synuclein and positional scanning combinatorial peptide library screening, we defined the optimal substrate phosphorylation sequence recognized by the CHK active site to be E-x-[Φ/E/D]-Y-Φ-x-Φ, where Φ and x represent hydrophobic residues and any residue, respectively. Besides β-synuclein, cellular proteins containing motifs resembling this sequence are potential CHK substrates. Intriguingly, the CHK-optimal substrate phosphorylation sequence bears little resemblance to the C-terminal tail sequence of SFKs, indicating that interactions between the CHK active site and the local determinants near the C-terminal regulatory tyrosine of SFKs play only a minor role in governing specific phosphorylation of SFKs by CHK. Our results imply that recognition of SFKs by CHK is mainly governed by interactions between motifs located distally from the active site of CHK and determinants spatially separate from the C-terminal regulatory tyrosine in SFKs. Thus, besides assisting in the identification of potential CHK physiological substrates, our findings shed new light on how CHK recognizes SFKs and other protein substrates.
Keywords: Src-family kinases, Csk, Csk-homologous kinase, substrate specificity determinants, positional peptide library screen, β-synuclein
Protein kinases relay transmission of specific cellular signaling pathways mainly by selectively phosphorylating unique subsets of cellular proteins. Although a protein substrate contains many serine, threonine and tyrosine residues, its regulatory protein kinase selects only one or a few of these residues as the phosphorylation sites. A kinase recognizes its protein substrates by two mechanisms: (i) co-localization of the kinase with its protein substrates, and (ii) binding of the active site of the kinase to specific structural determinants near the site of phosphorylation in the protein substrates (reviewed in (1)). Thus, there are two types of structural determinants in a protein substrate that govern its selective phosphorylation by the protein kinase. The first type, referred to as distal docking determinants are motifs that bind to the interaction domains in the kinase that are distally separated from the active site. For example, the PxxP motifs (where x stands for any amino acid) at both the N- and C-terminal portions of the scaffolding protein p130CAS act as the distal docking determinants that bind to the SH3 domain of its regulatory kinase c-Src (2, 3). The binding facilitates c-Src phosphorylation of p130CAS by co-localizing the two proteins in cells. The second type of determinant, the consensus phosphorylation sequence, contain the amino acid residues located near the phosphorylation site that interact with unique structural features within and nearby the active site of the kinase. For example, the c-Src active site recognizes the E/D-E/D-I-Y-E/G-x-F as its optimal phosphorylation sequence (4). The “DIYDVP” motif containing Y253 of p130CAS, which conforms to the c-Src optimal phosphorylation sequence, directs the active site of c-Src to selectively phosphorylate Y253 (2, 3). Thus, defining the distal docking determinant and the optimal phosphorylation sequence that are recognized by a protein kinase can facilitate the pursuit and identification of its potential protein substrates. In this manuscript, we present our findings using two biochemical approaches to define the optimal substrate phosphorylation sequence of C-terminal Src kinase-homologous kinase (CHK).
Both C-terminal Src kinase (Csk) and its homologous kinase (CHK) are major endogenous inhibitors of Src-family kinases (SFKs) (reviewed in (5–8)). Over-expression and aberrant activation of SFKs contribute to the formation and disease progression of many forms of cancer. In normal cells, both Csk and CHK function as tumour suppressors by constraining the SFKs in the inactive state. They suppress the activity of SFKs by selectively phosphorylating their conserved C-terminal regulatory tyrosine. Upon phosphorylation, SFKs adopt the inactive conformation stabilized in part by the binding of the phosphorylated C-terminal tail tyrosine to the SH2 domain. Although much is known about the structural basis of Csk inhibition of SFKs ((9) and reviewed in (10)), how CHK specifically recognizes the C-terminal regulatory tyrosine of SFKs as the target phosphorylation site remains to be elucidated.
To date, SFKs are the only known physiological substrates of CHK. However, there is a growing body of evidence suggesting that CHK is capable of phosphorylating other cellular proteins to perform non-SFK-related cellular functions. For example, overexpression of CHK enhanced activation of the MAP kinase signaling pathway and induced neurite outgrowth of the rat pheochromocytoma 12 (PC12) cells upon nerve growth factor stimulation (11, 12). Furthermore, CHK expression enhanced activation of the MAP kinase signaling pathway even in SFY−/− cells lacking SFKs (12), suggesting that the activation was governed by an SFK-independent mechanism. Relevant to these findings, CHK overexpression could induce tyrosine phosphorylation of the tyrosine-protein phosphatase non-receptor substrate 1 (SHPS-1) in PC12 cells (13). The authors provided data suggesting that CHK binds to SHPS-1 and enhances its phosphorylation at specific tyrosine residues. In addition, CHK was known to localize and phosphorylate some unknown cellular proteins in the nucleus of HeLa cells (14). Identifying the physiological substrates of CHK in cytosol and nucleus is an avenue to decipher the non-SFK-related functions of CHK.
CHK is expressed at a high level in brain cells and it is present predominantly in the cytosolic fraction of rat brain lysate ((15) and reviewed in (16)). In this study, we adopted the “kinase substrate tracking and elucidation” (KESTREL) method to search for and identify potential physiological substrates of CHK in rat brain cytosol extract (17, 18). This method has so far been successfully used to identify new substrates for at least eight different protein kinases (18–22). Using this method, we identified β-synuclein as a potential CHK substrate. Further analyses revealed that CHK preferentially phosphorylates Y127 in the C-terminal region of β-synuclein in vitro and in transfected HEK293T cells. Although α-synuclein and β-synuclein exhibit a high degree of sequence homology, α-synuclein is a much poorer substrate of CHK. Further studies with peptide analogs derived from β-synuclein allowed us to define several residues in its sequence as the substrate specificity determinants recognized by CHK. Using the combinatorial library approach, we define more completely the substrate specificity determinants in peptide substrates recognized by CHK active site.
Results from both KESTREL and peptide library studies allowed us to define the consensus target phosphorylation sequence of CHK. The CHK-optimal peptide, which contains this sequence was found to be an efficient CHK substrate, confirming that the determinants identified by the two approaches govern efficient phosphorylation of the protein and peptide substrates by CHK. Thus, cellular proteins containing motifs conforming to this consensus target phosphorylation sequence are potential physiological substrates of CHK.
MATERIALS AND METHODS
Materials
Recombinant α-synuclein was a gift from Drs Chi Pham and Roberto Cappai (Department of Pathology, The University of Melbourne), the expression and purification of α-synuclein were described by Pham et al.(23). The recombinant baculovirus for the expression of wild type CHK and CHK-His6 were generated by the BacPAK baculovirus expression system (Clontech) as described previously (15, 24). The CHK-His6 mutant contains a “GSGS” linker and a polyhistidine tag of the sequence (GSGSHHHHHH) attached to the C-terminus of the rat CHK sequence. The recombinant kinase-dead mutant of Lyn kinase ([K275M]Lyn), containing the K275M mutation at the ATP-binding site, was generated as described in our previous report (25). The β-synuclein plasmid was from OriGene Technology, Inc. (Cat. number: TC300202). Polyclonal anti-CHK antibodies were raised against a GST fusion protein containing a C-terminal segment of rat CHK (15). The monoclonal anti-Src antibody (MAb327) specifically recognizing an epitope in the SH3 domain of human c-Src was a gift from Dr D.J. Fujita (University of Calgary, Canada). Rabbit polyclonal anti-pY416 phosphospecific antibody (also known as anti-Src[pY-418]) was from Biosource International. The mouse monoclonal anti-phosphotyrosine antibody (Clone PY69) was from BD Biosciences. Anti-α-synuclein antibody was raised to the C-terminal region of human α-synuclein (residues 116–131); anti-β-synuclein antibody was generated against the C-terminal domain of human β-synuclein (residues 99–113) (26). The combinatorial peptide library was synthesized by Anaspec, Inc. All other synthetic peptides were synthesized using [N-(9-flourenyl)methoxycarbonyl] (Fmoc)-based chemistry with the model CEM automated microwave peptide synthesizer (North Carolina, USA). The stock solution of pervanadate used for treatment of cells was freshly prepared prior to each experiment by incubation of the sodium vanadate solution (10 mM) with H2O2 (10 mM) for 15 min at 23 °C. The recombinant wild type CHK and [D311A]CHK expressed in the infected Sf9 cells were purified by sequential column chromatography as described previously (15). The recombinant CHK-His6 was purified to over 90% purity as described previously (24). Recombinant Csk, expressed in Sf9 cells, was purified by sequential column chromatography as described in our previous report (25). Recombinant 14-3-3β was a gift from Dr. Amardeep Dhillon of the Department of Biochemistry and Molecular Biology, University of Melbourne. Native human hsp90 purified from HeLa cells was from Enzo Life Science. Generation of the pGEX-6p-3-β-synuclein plasmid and the pGEX-6p-3-β-synuclein mutant plasmids and expression of recombinant GST-β-synuclein and its mutants are described in supplemental information.
Comparison of the rates of CHK phosphorylation of [K275M]Lyn and β-synuclein
The reaction mixture (25 μl) contained: (i) Assay Buffer (20 mM Tris-HCl, pH 7.0, 10 mM MgCl2, 1 mM MnCl2 and Na3VO4) (ii) 100 μM [γ-32P]ATP, (iii) 0.11 μM CHK and (iv) 0 – 2.2 μM [K275M]Lyn or β-synuclein. The phosphorylation reaction was allowed to proceed at 30 °C for 30 min. The reaction was terminated by the addition of 10 μl of 5×SDS-Sample Buffer. The samples were separated by SDS-PAGE and the gel subjected to autoradiography. Protein bands corresponding to [K275M]Lyn and β-synuclein were excised. The amount of 32P incorporated in the two proteins was determined by scintillation counting. The catalytic activity of CHK was expressed as picomole phosphate incorporated in the substrate protein per min.
Phosphorylation of α- and β-synucleins by CHK
The reaction mixture (25 μl) contained: (i) Assay Buffer, (ii) 100 μM [γ-32P]ATP, (iii) 0.11 μM CHK. (iv) 0 – 2.2 μM α- or β-synucleins. Phosphorylation of the synucleins by CHK was carried out at 30 °C for 30 min; 10 μl of 5× SDS-Sample Buffer was added to each reaction mixture to terminate the reaction. The samples were separated by SDS-PAGE and the gel subjected to autoradiography. Protein bands corresponding to α- or β-synucleins were excised. The amount of 32P incorporated into the protein substrate was assessed by scintillation counting. The enzymatic activity of CHK was expressed as picomole phosphate incorporated in the protein substrate per min.
Phosphorylation of peptides derived from α- and β-synucleins by CHK
To compare the efficiencies of CHK phosphorylation of peptides derived from α- and β-synucleins, CHK (0.11 μM) was incubated with 0 – 320 μM synuclein peptides in the presence of Assay Buffer and 100 μM [γ-32P]ATP. The phosphorylation reaction was performed at 30 °C for 30 min and terminated by adding 20 μl acetic acid. Aliquots of the reaction mixture were spotted onto p81 filter paper squares. The paper squares were washed extensively with 4×400 ml of 5% (v/v) phosphoric acid. The amount of phosphate incorporated into the peptide was quantitated by scintillation counting.
Search for CHK protein substrates in rat brain cytosolic extract by KESTREL
Cytosolic extract prepared from 20 rat brains (Supplemental Information), was used to search for CHK substrates. To minimize background signals due to phosphorylation of proteins by the endogenous kinases in the extract, we partially separated proteins in the extract by DEAE anion-exchange chromatography. Bound proteins were eluted using a 0 – 1M NaCl gradient in Ion Exchange Column Buffer and collected in 8-ml fractions. A 10-μl aliquot from each fraction was incubated with KESTREL Assay Buffer ((20 mM Tris-HCl, pH 7.0, 10 mM MnCl2 and Na3VO4), 5 μM [γ- 32P]ATP (specific radioactivity ≥ 10,000 cpm/pmol) at 30 °C for 5 min in the absence or presence of 1 μg of active recombinant CHK in a final volume of 25 μl. The reactions were terminated by addition of 5 × SDS-Sample Buffer prior to SDS-PAGE. 32P-labelled proteins in the stained SDS-gel were detected by autoradiography. Fractions containing the CHK phosphorylated proteins were pooled, dialysed against the Ion Exchange Column Buffer, and subject to Mono-Q anion-exchange column chromatography. To eliminate the interference of the background signal resulting from protein phosphorylation by the endogenous kinases, an aliquot (50 μl out of 1 ml) of each column fraction was subjected to heat treatment at 60 °C for 15 min to inactivate the endogenous kinases. 10 μl of the heat-treated sample from each fraction was used for screening for CHK phosphorylated protein as described in the previous paragraph. A number of CHK-phosphorylated proteins were detected in several Mono-Q fractions (Fig. 2C). Hence, proteins in these fractions were further separated based on their molecular sizes by SDS-PAGE in gels of different percentage of acrylamide (Fig. 2D and E). 15% SDS-PAGE was used to separate low molecular weight proteins, whereas the high molecular weight proteins were separated by 7.5% SDS-PAGE. Bands containing the CHK phosphorylated proteins were excised, digested with trypsin and analysed by nanoLC/MS/MS as detailed in the next section.
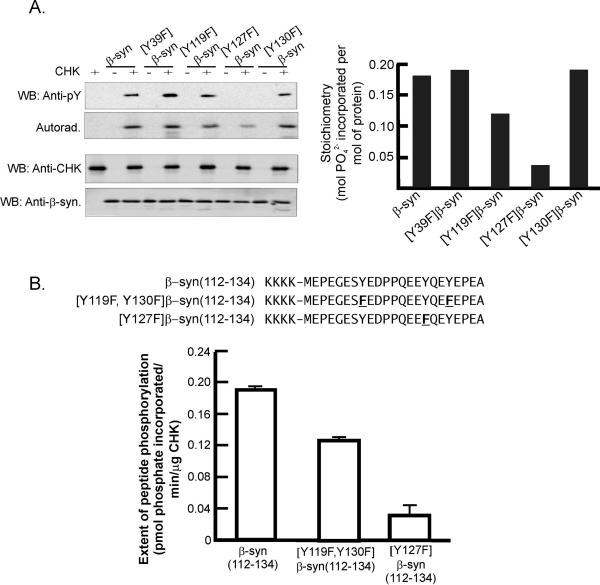
Figure 2. Identification of the tyrosine residues in β-synuclein phosphorylated by CHK.
(A) Autoradiogram showing phosphorylation of recombinant β-synuclein (β-syn) and its mutants ([Y39F] β-syn, [Y119F] β-syn, [Y127F] β-syn and [Y130F] β-syn) by CHK. Briefly, CHK (0.64 – 0.68 μM) was incubated with β-synuclein or its mutants (0.9 – 1.1 μM) and [γ-32P]ATP at 30 °C for 30 min. The extent of phosphorylation of β-synuclein and its mutants was monitored by Western blotting with an anti-phosphotyrosine antibody and autoradiography (left-hand panels). The gel slices corresponding to β-synuclein and its mutants were excised to determine the stoichiometry of phosphorylation (right-hand panel). The experiment was repeated four times (n =4) and they all gave similar patterns of difference in the extent of phosphorylation of β-synuclein and its mutants. The data from one of the repeat experiments are shown.
(B) CHK phosphorylation of β-syn(112–134) derived from the segment corresponding to residues 112–134 of β-synuclein and its substitution analogs, [Y119F, Y130F]β-syn(112–134) and [Y127F]β-syn(112–134). The four lysines at the N-terminal region were added to facilitate solubility and binding to the P81 phosphocellulose paper squares used in the assay. The tyrosine residues replaced by phenylalanine in the analogs are underlined (upper panel).
Preparation of CHK-phosphorylated proteins for mass spectrometry analysis
The protein bands containing the CHK-phosphorylated proteins in the “+CHK” lanes and those at the corresponding locations in the “−CHK” lanes of the Coomassie-blue stained gel were excised and macerated. To destain the proteins, the gel pieces from each excised band were incubated with 600 μl of 1:1 (v/v) 100 mM NH4HCO3/acetonitrile with agitation for 30 min. The gel pieces were then washed twice for 15 min with 200 μl of 1:1 (v/v) solution of 100 mM NH4HCO3/acetonitrile. The gel pieces were then dehydrated with 200 μl of 100% acetonitrile and air dried for 5 min. Proteins in the gel pieces were reduced with 200 μl of freshly prepared 10 mM DTT in 50 mM NH4HCO3 for 1 h at 4 °C. Reduced proteins were subsequently alkylated by incubation of the gel pieces with 20 mM iodoacetamide in 50 mM NH4HCO3 for 1 h at room temperature in the dark. After reduction and alkylation, gel pieces from each protein band were washed twice with 200 μl of 1:1 (v/v) solution of 100 mM NH4HCO3/acetonitrile for 15 min, followed by dehydration with 200 μl of 100% acetonitrile and air dried for 5 min. Dry gel pieces were rehydrated with 40 μl of 50 mM NH4HCO3 containing 250 ng of trypsin (Sigma) and then incubated at 37 °C overnight. Tryptic peptides were extracted from the gel pieces, separated and analyzed by liquid chromatography-tandem MS (LC-MS/MS) using an Agilent 1100 dual pump, nano-LC system linked to an Agilent 1100 ion trap XCT Plus mass spectrometer fitted with an Agilent HPLC-Chip CUBE source. Peptides were injected onto a 40 nl Zorbax 300SB-C18 trapping column at 4 μl/min, and then separated by switching the trap column in-line with the separation column (Zorbaz 300SB-C18, 43 mm × 0.075 mm) and running an 8 min gradient of 5% to 50% acetonitrile/ 0.1% formic acid at 400 nl/min. MS/MS spectra were analyzed using the Mascot search engine (28).
Investigation of the CHK phosphorylation of Flag-tagged β-synuclein in HEK293T cells
The synthetic Flag-β-synuclein and Flag-[Y127F]β-synuclein genes in pMA-T vector were ordered from GENEART. The genes encode Flag-β-synuclein and Flag-[Y127F]β-synuclein proteins with the Flag tag attached at the N-terminus. These genes were then subcloned into pcDNA3 mammalian cell expression vector via the EcoRI and BamHI restriction sites. HEK293T cells were transfected with pcDNA3-CHK, pcDNA3-Flag-β-synuclein or pcDNA3- Flag-[Y127F]β-synuclein plasmids individually. To examine if CHK can induce tyrosine phosphorylation of Flag-β-synuclein or its mutant, HEK293T cells were co-transfected with pcDNA3-CHK and pcDNA3- Flag-β-synuclein plasmids or pcDNA3-CHK and pcDNA3- Flag-[Y127F]β-synuclein plasmids. Prior to addition to HEK293T cells, the plasmids were mixed with FuGENE® HD transfection kit (Roche) at ratio 1 : 4 (1 μg DNA to 6 μl FuGENE® HD, 0.5 μg per DNA construct per well) for 20 min at room temperature. The DNA-FuGENE® HD complex solution was added dropwise to cells (~3×105 cells per well). The transfected HEK293T cells were maintained in DME medium containing 10% (v/v) heat-inactivated fetal bovine serum, 2 mM glutamine and 100 μg/ml penicillin, and incubated in a humidified atmosphere of 10% CO2 at 37°C. The transfected cells were lysed at 48 h after transfection with 120 μl of Lysis Buffer (50 mM Tris, 150 mM NaCl, 1% Triton-X-100, 50 mM NaF, 2 mM MgCl2, 1 mM Na3VO4, 25 μg/ml leupeptin and 25 μg/ml aprotinin) per well at 4°C. To prevent dephosphorylation of the recombinant β-synuclein by the endogenous protein tyrosine phosphatases, the transfected cells were treated with 30 μM of freshly prepared pervanadate for 20 min prior to cell lysis. After clarification by centrifugation (13.000×g for 5 min at 4°C), 33 μl of each lysate sample was then diluted with 17 μl of Dilution Buffer (Lysis Buffer and 5 × SDS-Sample Buffer at the ratio of 1:4). Samples were then heat-denatured at 95 °C for 5 min. Expression of recombinant CHK, Flag-β-synuclein and Flag-[Y127F]β-synuclein was confirmed by Western blotting.
For immunoprecipitation, 7 μl of the anti-Flag (M2) antibody-conjugated agarose beads (Sigma) were added to the cell lysate. After incubation for 3 h at 4°C, the recombinant Flag-β-synuclein and its mutant were isolated from the crude cell lysate by immunoprecipitation. The immune complexes were washed 3 times in ice-cold Wash Buffer (0.5% Tween-20 in PBS) and centrifuged (3,000 × g for 5 min at 4°C). The immune complexes were resuspended in 50 μl of Dilution Buffer. Samples were then heat-denatured at 95°C for 5 min prior to analysis by Western blotting.
Defining the substrate specificity determinants of CHK by positional combinatorial peptide library screening
The CHK phosphorylation site motif was determined using a miniaturized positional scanning peptide library method essentially as described (29). The peptide library is similar in sequence to the one reported previously for analysis of protein Ser/Thr kinase specificity, except that a tyrosine residue replaces the serine-threonin residues at the phosphoacceptor position. The library consisted of 198 peptides having the general sequence G-A-X-X-X-X-X-Y-X-X-X-X-A-G-K-K(biotin), where K(biotin) was ε-biotinamidohexanoyl lysine. In each peptide, eight of the X positions were an approximately equimolar mixture of 18 proteogenic amino acids (all but tyrosine and cysteine), and the remaining position was fixed as one of the 20 unmodified amino acids, phosphothreonine or phosphotyrosine. Peptides (50 μM) were incubated for 2 h at 30 °C in 1,536-well plates with either 30 μg/ml (run 1) or 7.5 μg/ml (run 2) of purified CHK-His6 in 2 μl per well and 50 mM Tris, pH 7.5, 10 mM MgCl2, 1 mM MnCl2, 1 mM DTT, 0.1% Tween-20, 50 μM ATP (30 μCi/ml [γ-33P]ATP). After incubation, 200 nl aliquots were spotted onto streptavidin-coated membrane (Promega), which was washed as described (30), dried and exposed to a phosphor screen. Spot intensities were quantified using QuantityOne software. Data were normalized as done previously so that the average value within a position is 1; values greater than 1 therefore indicate positively selected residues.
Synthesis and purification of peptides
All synthetic peptides were synthesized by Fmoc [N-(-9-fluorenyl)methoxycarbonyl]–based solid phase peptide synthesis chemistry on a CEM Liberty automated microwave peptide synthesizer (North Carolina, USA). At the completion of synthesis, peptides were cleaved from the resin by treatment with TFA/triisopropylsilane/water (95:2.5:2.5) and isolated by precipitation with cold diethyl ether. Crude peptides were purified on an Agilent Zorbax C18 reversed-phase HPLC column in 0.1% TFA/water buffer with a linear acetonitrile gradient. The molecular weight of all peptides was confirmed by electrospray mass spectrometry on an Agilent QTOF LC/MS instrument and peptide purity (>95%) was confirmed by analytical reversed-phase HPLC.
RESULTS
Identification of β-synuclein as a potential CHK substrate
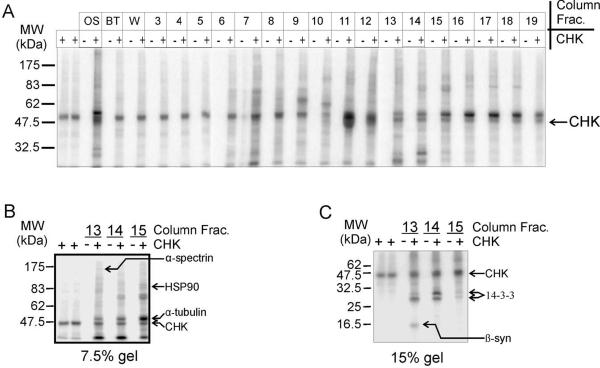
As CHK was reported to reside predominantly in the cytoplasm in cultured cancer cells and brain cells (15, 31), we predicted that some of the physiological substrates of CHK are cytosolic proteins. Thus, we chose rat brain cytosolic extract to search for new CHK substrates. SFig. 1A depicts the scheme used to search for potential CHK substrates in rat brain cytosolic extract. Proteins in the extract were first partially separated by DEAE anion exchange column chromatography. Column fractions containing cellular proteins that were preferentially phosphorylated recombinant CHK (SFig. 1B) were combined, dialyzed, and further purified by Mono-Q anion exchange column chromatography. As shown in Fig. 1A, several prominent signals reflecting preferential phosphorylation of cellular proteins by CHK were detected in Mono-Q fractions 13–15, of which some were from proteins migrating near the dye front of a 10% polyacrylamide gel. We therefore separated proteins in these fractions by 7.5% and 15% polyacrylamide gels (Fig. 1B and C, SFig. 2). The bands corresponding to the CHK phosphorylated proteins were excised and digested with trypsin. The digests were analyzed by nanoLC/MS/MS followed by MASCOT search analysis. The results showed that these protein bands contained β-synuclein, α-tubulin, α-spectrin, 14-3-3, or HSP90 (Fig. 1B and C, SFig. 2, and STable 1). It is noteworthy that some of these protein bands were not well separated from other protein bands. Thus, it is possible that the proteins identified are just cellular proteins migrating very closely with the CHK phosphorylated proteins in SDS-PAGE. Among them, the band containing β-synuclein appeared to be better separated from other protein bands (SFig. 2C). Furthermore, Western blot analysis using the anti-β-synuclein and anti-α-synuclein antibodies indicated the presence of β-synuclein but not α-synuclein in the protein band (data not shown). To further separate proteins in the β-synuclein-containing Mono-Q column fractions (fractions 13–15), we pooled these fractions and subjected them to hydroxyapatite column chromatography. A 16 kDa protein was found to be preferentially phosphorylated by CHK (data not shown). The band corresponding to this 16 kDa protein was excised and its identity was confirmed to be β-synuclein by mass spectrometry (data not shown).
Figure 1. Identification of β-synuclein, α-tubulin, α-spectrin, 14-3-3 and HSP90 as potential protein substrates of CHK.
(A) Autoradiogram showing the profile of CHK phosphorylated proteins in the Mono-Q anion exchange column fractions. 10-μl from each fraction was heat-treated (60 °C for 15 min) prior to incubation with [γ-32P]ATP at 30 °C for 5 min in the presence (+) and absence (−) of CHK (0.8 μM) in a total volume of 25 μl. Fractions 13–15 contained several proteins that were preferentially phosphorylated by CHK.
(B) and (C) Autoradiograms showing preferential phosphorylation of proteins of different molecular sizes by CHK in the selected fractions (fraction number 13–15) of the Mono-Q column step after SDS-PAGE in 7.5% and 15% polyacrylamide gels. The gel slices containing the CHK-phosphorylated proteins in the (+CHK) lane and the those in the corresponding locations in the (−CHK) lane were excised (SFig. 2), digested with trypsin and then analyzed by mass spectrometry. The proteins identified include β-synuclein, α-spectrin, α-tubulin, 14-3-3, HSP90 and CHK (SFig. 2, Table S1). It is noteworthy that recombinant CHK can undergo autophosphorylation in vitro (24). The radioactive band of ~50 kDa in the (+CHK) lanes corresponds to the autophosphorylated CHK.
CHK preferentially phosphorylates recombinant β-synuclein at Tyrosine-127
To further verify β-synuclein as a preferential substrate of CHK, we examined if CHK can directly phosphorylate purified recombinant β-synuclein, 14-3-3β and Hsp90 in vitro. SFig. 4A shows that CHK could directly phosphorylate all three protein substrates in vitro. Among them, CHK phosphorylated 14-3-3β at a negligible level; the rate of CHK phosphorylation of β-synuclein was ~23-fold higher than that of hsp90 (SFig. 4B). Taken together, the results support β-synuclein as a preferential substrate of CHK.
The β-synuclein sequence contains four tyrosines – Y39, Y119, Y127 and Y130. To determine which of the four tyrosines is/are phosphorylated by CHK, we generated β-synuclein mutants with each of the tyrosines mutated to phenylalanine and compared the rates of their phosphorylation by CHK. As illustrated in Fig. 2A, the Y39F mutation had no effect on β-synuclein phosphorylation by CHK, indicating that Y39 was not targeted by CHK. In contrast, β-synuclein phosphorylation was reduced by mutation of any of the other three tyrosines with the greatest reduction induced by the Y127F mutation. These results suggest that CHK can phosphorylate β-synuclein at Y119, Y127 and Y130. Among them, Y127 is the preferential target.
Since Y119, Y127 and Y130 reside in the C-terminal region, the results shown in Fig. 2A imply that the C-terminal region of β-synuclein contains structural features directing CHK to preferentially phosphorylate Y127. To examine this, we synthesized three synthetic peptides derived from this region of β-synuclein and compared the efficiencies of their phosphorylation by CHK. These peptides include: (i) β-syn(112–134), which contains all three tyrosines in this region; (ii) [Y119F, Y130F]β-syn(112–134), which contains Y127 as the only tyrosine; and (iii) [Y127F] β-syn(112–134), of which Y127 is replaced by phenylalanine (Fig. 2B). Substitution of Y119 and Y130 with phenylalanine results in a 30% reduction in CHK phosphorylation rate while the Y127F mutation caused an 84% reduction in CHK phosphorylation rate (Fig. 2B). From these results, we conclude that the local structure surrounding Y127 in the C-terminal region of β-synuclein contains determinants directing its preferential phosphorylation by CHK.
Comparison of CHK phosphorylation of α- and β-synuclein reveals α-synuclein as a poor CHK substrate
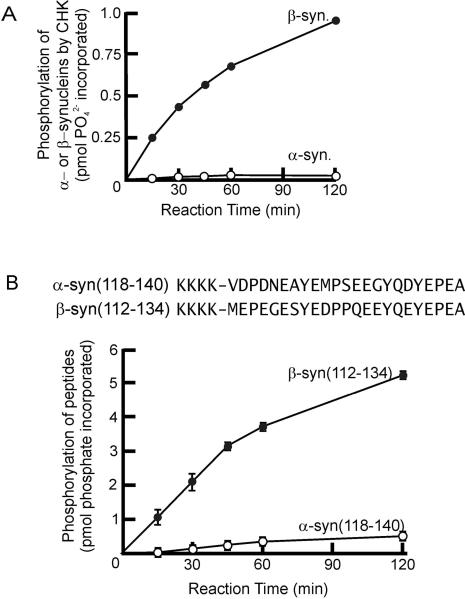
Among the three members of the synuclein family, α-synuclein and β-synuclein show the highest degree of sequence homology (~60% sequence identity). The amino acid sequences of α- and β-synucleins exhibit 33% sequence homology in their acidic C-terminal regions. For this reason, it is logical to examine whether α-synuclein is also phosphorylated by CHK. Fig. 3A shows that CHK phosphorylation of β-synuclein was significantly higher than that of α-synuclein. After a reaction time of 120 min, CHK catalyzed incorporation of ~ 0.9 pmol phosphate to β-synuclein but only 0.024 pmol of phosphate to α-synuclein. Thus, despite both synucleins sharing considerable sequence homology, α-synuclein is a much poorer substrate of CHK compared to β-synuclein. More importantly, the data lend further support to the proposal that β-synuclein contains unique structural features directing CHK to preferentially phosphorylate Y127. Presumably, these structural features reside near Y127 in the β-synuclein sequence. This was confirmed by our demonstration that CHK phosphorylated β-syn(112–134) peptide more efficiently (~10-fold higher) than α-syn(117–140) peptide (Fig. 3B).
Figure 3. Comparison of the rates of CHK phosphorylation of α- and β-synucleins.
(A) Time course of phosphorylation of α- and β-synucleins (0.56 μM) by CHK (0.04 μM).
(B) Upper panel: The sequences of the β-syn(112–134) and α-syn(118–140) peptides derived from the C-terminal region of α- and β-synucleins. The four lysines at the N-terminus of the peptides were added to improve solubility and to facilitate binding of the peptide to phosphocellulose P81 filter paper used in the kinase activity assay. Lower panel: Time course of phosphorylation of β-syn(112–136) and α-syn(118–140) peptides by CHK. The reaction mixture contained CHK (0.11 μM), β-syn(112–134) or α-syn(118–140) (20 μM) and [γ-32P]ATP at 30 °C in a final volume of 25 μl.
D121, P123 and E126 in the β-synuclein sequence contain structural features directing efficient phosphorylation of Y127 by CHK
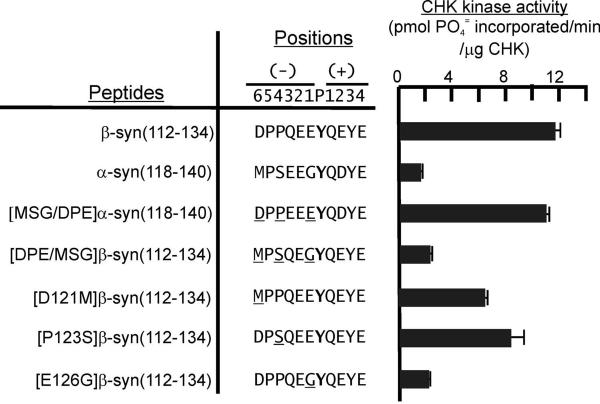
Comparison of the sequences of the C-terminal regions of α- and β-synucleins revealed D121, P123 and E126 in the β-synuclein sequence and M127. S129 and G132 in the α-synuclein sequence as the dissimilar residues. To evaluate the role of D121, P123 and E126 in directing preferential phosphorylation of β-synuclein by CHK, five more β-synuclein peptides analogues were generated and the rates of their phosphorylation by CHK were compared (Fig. 4). When G132, S129 and M127 of the α-syn(118–140) were replaced by the corresponding residues in the β-synuclein sequence, the resultant [MSG/DFE]α-syn(118–140) peptide was phosphorylated by CHK with activity as high as that of the β-syn(112–134). E126, P123 and D121 of the β-syn(112–134) were replaced by the corresponding residues in α-synuclein to generate the [DFG/MSG]β-syn(112–134) peptide. CHK phosphorylated this peptide with a rate lower (~20-fold lower) than that of β-syn(112–143) peptide. These results confirm that D121, P123 and E126 in the β-synuclein sequence govern preferential phosphorylation of β-synuclein by CHK. To further define the role of each of these residues, the rates of CHK phosphorylation of single residue substitution peptide analogues of β-syn(112–134) were compared. The results shown in Fig. 4 indicate that the D121M, P123S and E126G substitutions induce reduction in the efficiency of CHK phosphorylation of the β-syn(112–134) analogues. Taken together, our results suggest that E126, P123 and D121 participate in directing CHK to preferentially phosphorylate Y127.
Figure 4. Comparison of the rates of phosphorylation of β-syn(112–134), α-syn(118–140) and their substitution analogs by CHK.
- β-syn(112–134): KKKK-MEPEGESYEDPPQEEYQEYEPEA;
- α-syn(118–140): KKKK-VDPDNEAYEMPSEEGYQDYEPEA;
- [MSG/DPE] α-syn(118–140): KKKK-VDPDNEAYEDPPEEEYQDYEPEA;
- [DPE/MSG] β-syn(112–134): KKKK-MEPEGESYEMPSQEGYQEYEPEA;
- [D121M]β-syn(112–134); KKKK-MEPEGESYEMPPQEEYQEYEPEA;
- [P123S]β-syn(112–134): KKKK-MEPEGESYEDPSQEEYQEYEPEA;
- [E126G]β-syn(112–134): KKKK-MEPEGESYEDPPQEGYQEYEPEA. Only the residues from the P−6 to P+4 positions of the peptides are presented.
In order to determine whether the secondary structure of these peptides has any input into preferential phosphorylation of Y127 by CHK, we performed circular dichroism analyses to monitor the secondary structure contents of SFK C-terminal peptide, α-syn(118–140), β-syn(112–134) and the peptide analogs. The CD spectra reveal the typical profiles of random coil conformation (SFig. 6). Our data indicate that β-syn(112–134) do not need to adopt a specific secondary structure in aqueous solution. In spite of this finding, it is unclear if β-syn(112–134) adopts a specific secondary structure when it is bound to the CHK active site.
CHK phosphorylates β-synuclein and a Src-family kinase with comparable efficiencies
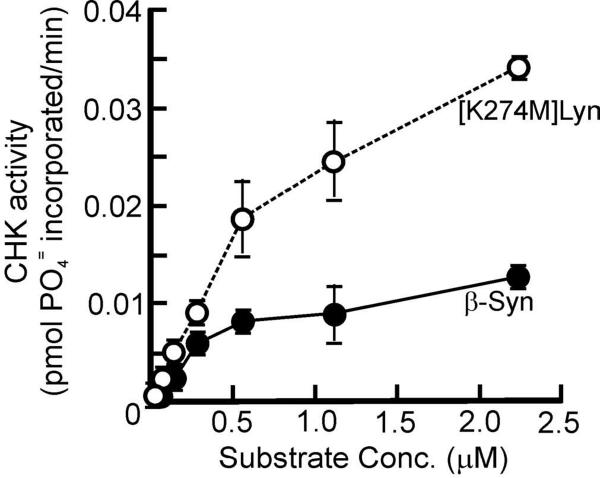
Since Src-family tyrosine kinases are physiological substrates of CHK, we compared the efficiencies of CHK phosphorylation of β-synuclein and the kinase-dead mutant of the Src-family kinase Lyn, [K274M]Lyn. As this Lyn mutant lacks catalytic activity, its use as the CHK substrate eliminates the complication of additional tyrosine phosphorylation due to autophosphorylation. As shown in Fig. 5, at low substrate concentrations (35 – 280 nM), CHK phosphorylated both proteins at similar rates. At higher substrate concentrations (560 nM − 2.24 M), the rate of CHK phosphorylation of [K274M]Lyn was ~2.8-fold higher than that of CHK phosphorylation of β-synuclein. Nevertheless, the similar rates of CHK phosphorylation of both proteins at low substrate concentrations further confirm that β-synuclein contains structural determinants directing its efficient phosphorylation by CHK. To compare the contributions of determinants in the local structures around the phosphorylation sites in SFKs and β-synuclein to the efficiencies of their phosphorylation by CHK, we performed kinetic analyses of CHK phosphorylation of the SFK C-terminal peptide and β-syn(112–134). The kinetic data shown in Table 1 indicate that CHK phosphorylated both peptides with similar rates.
Figure 5. Comparison of the rates of CHK phosphorylation of β-synuclein and [K274M]Lyn mutant.
CHK (0.11 μM) was incubated at 30 °C for 30 min with varying concentrations (35 nM − 2.24 μM) of [K274M]Lyn or GST-β-synuclein. CHK activities at different concentrations of [K274M]Lyn and GST-β-synuclein are presented.
Table 1. Kinetic Parameters of Phosphorylation of Peptide Substrates by CHK.
Kinetic parameters of phosphorylation of the four peptide substrates by CHK-His6. For the phosphorylation reactions, CHK-His6 (0.2 μM) was used to phosphorylate the substrate peptides at 0–400 μM. The initial velocities of CHK-His6 were plotted against the substrate peptide concentrations. The data points were transformed to generate Lineweaver-Burk plots (Fig. S6), which yielded the kinetic parameters KM and kcat.
| PEPTIDE SUBSTRATES | SEQUENCES | KM (μM) | kcat (S−1) | kcat/KM (μM−1S−1) |
|---|---|---|---|---|
| SFK C-terminal Peptide | KKDPEERPTFEYLQSFTATEPQYQPGENL | 255.0 ± 7.8 | 0.13 ± 0.003 | 5.1×10−4 |
| β-syn(112–134) | KKKK–MEPEGESYEDPPQEEYEYEPEA | 253 ± 18.5 | 0.17 ± 0.01 | 6.7×10−4 |
| CHK-optimal peptide | KKK–GESFEDQDEGIYWNVGPEA | 103.1 ± 3.8 | 1.45 ± 0.05 | 140×10−4 |
| [D9A]CHK-optimal peptide | KKK–GESFEAQDEGIYWNVGPEA | 114.5 ± 5.6 | 1.27 ± 0.06 | 111×10−4 |
CHK phosphorylates β-synuclein in transfected HEK293T cells
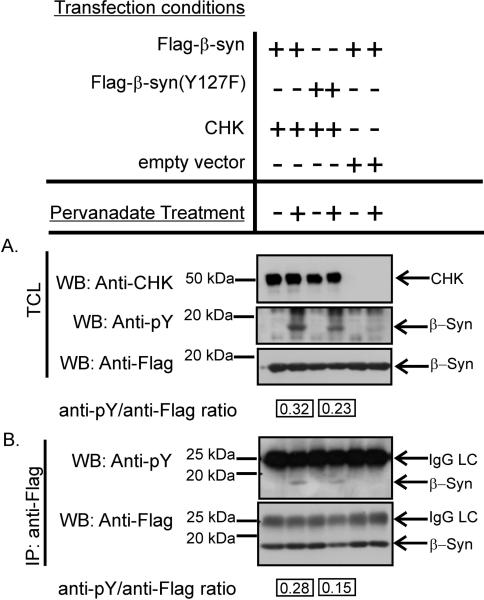
To examine if CHK can phosphorylate β-synuclein in cells, we expressed recombinant CHK, Flag-β-synuclein and Flag-[Y127F]β-synuclein in HEK293T cells. As shown in Fig. 6, CHK co-expression induced tyrosine phosphorylation of both Flag-β-synuclein and Flag-[Y127F]β-synuclein only in cells pretreated with pervanadate to suppress the endogenous phosphatase activity. Furthermore, replacement of the Y127 in Flag-β-synuclein with phenylalanine reduced the level of CHK-induced phosphorylation. The results are in agreement with results of our in vitro studies shown in Fig. 2 that CHK phosphorylates Y127 and one or more other tyrosines in β-synuclein. The requirement of pervanadate treatment to detect tyrosine phosphorylation of β-synuclein indicates that it is readily dephosphorylated by endogenous phosphatases in cells.
Figure 6. Demonstration of phosphorylation of β-synuclein by CHK in transfected HEK293T cells.
Recombinant CHK, Flag-β-synuclein and its mutant were expressed in HEK293T cells by transfection. The transfection conditions were listed above the panels. The transfected cells with and without treatment with pervanadate were lysed. A. Western blot analyses of tyrosine phosphorylation of Flag-β-synuclein in total cell lysate (TCL) of the transfected using anti-phosphotyrosine (anti-pY), anti-Flag and anti-CHK antibodies. B. Western blot analysis of the anti-Flag immunoprecipitates from the TCL using antiphosphotyrosine (anti-pY) and anti-Flag antibodies. IP: immunoprecipitation; WB: Western blotting. The anti-pY and anti-Flag signals associated with the recombinant β-synuclein and its mutant on the Western blots were quantitated by densitometry. The ratios of ant-pY and anti-Flag signals in lane #2 and lane #4 in the Western blots shown in panels A and B are shown.
Determination of the optimal target phosphorylation sequence for CHK by combinatorial peptide library screening
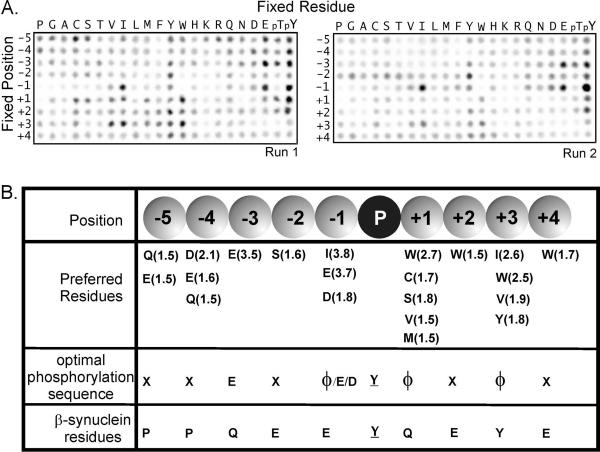
The data presented in Fig. 4 suggests that CHK recognizes the amino acid residues at p−6, p−4 and p−1 position (Footnote) of the β-syn(122–134) peptide as substrate specificity determinants. To more completely identify residues surrounding the site of phosphorylation that influence phosphorylation, we used purified recombinant CHK to screen a positional scanning peptide library previously used to characterize Eph and Axl family receptor tyrosine kinases (32, 33). Fig. 7 shows the results of the screen. CHK appears to be most selective at the p−1 position, where it prefers both acidic (primarily E) and hydrophobic (primarily I) residues. CHK also displayed strong preferences for E at the p−3 position and for hydrophobic residues at the P+3 position. Weaker preferences were also seen at several positions, including hydrophobic residues at P+1 (quantitative preferences at all positions are summarized in Fig. 7B). The results from the naïve peptide library screen agree well with the data obtained from the study of the α- and β-synuclein peptide analogs (Fig. 4), which support the primary importance of a glutamate residue at position p−1. The β-synuclein sequence also includes a CHK-preferred residue (Y) at the P+3 position. The presence of CHK-preferred residues at the p−1 and P+3 positions of the β-synuclein sequence likely contributes to preferential phosphorylation of rat brain β-synuclein by CHK in vitro. Thus, the data in Fig. 4 and Fig. 7 together define the CHK preferred residues in the p−3 to the P+3 positions as E-X-[Φ/E/D]-Y-Φ-X-Φ, where Y is the phosphorylation site and Φ represents hydrophobic amino acid residues.
Figure 7. Defining the CHK-optimal phosphorylation sequence by positional scanning combinatorial peptide library.
(A) CHK was used to phosphorylate a 198-component peptide library in which each peptide had the general sequence G-A-X-X-X-X-X-Y-X-X-X-X-A-G-K-K(biotin). Spot intensities reflect the extent of phosphorylation of peptides having the indicated residue fixed at the indicated position relative to the central tyrosine residue. The results from two runs performed on separate days and with different CHK-His6 concentrations (30 μ/ml for Run 1; 7.5 μ/ml for Run 2) are shown.
(B) A summary of residues positively selected at each position. Values in parenthesis show normalized quantified spot intensities. Only residues with selectivity values of 1.5 or greater are shown. Note that the high signal for peptides with fixed tyrosine residues independent of position is likely to be an artifact due to the presence of two phosphorylatable residues in those peptides.
A synthetic peptide containing selected residues at p−3 to P+3 positions is an efficient peptide substrate of CHK
Based upon the results shown in Fig. 4 and Fig. 7, the CHK-optimal peptide (KKKGESFEDQDEGIYWNVGPEA) was designed. This peptide contains part of the β-syn(112–134) sequence and residues that are preferred by CHK at the p−3 (E), p−1 (I), P+1 (W) and P+3 (V) defined by both the KESTREL and combinatorial peptide library studies. As shown in Table 1 and SFig. 6, the CHK-optimal peptide was phosphorylated with an efficiency higher than those of β-syn(112–134) and SFK-C-terminal peptide. CHK phosphorylated the CHK-optimal peptide with a slightly lower Km and a kcat (1.45 s−1) approximately ten-fold higher than those of β-syn(112–134) and SFK-C-terminal peptide. The data indicate that the CHK-optimal peptide contains determinants that facilitate binding to the active site of CHK and catalysis of the phosphorylation reaction.
Results of analyses of CHK phosphorylation of α-syn(118–140), β-syn(112–134) and their analogs reveal that D121 at the p−6 position of β-syn(112–134) is recognized by CHK as a substrate specificity determinant. Since the combinatorial peptide library screen only covers the p−5 to P+4 residues, further analysis is needed to establish p−6 aspartate as a specificity determinant recognized by CHK. To this end, we generated the [D9A]CHK-optimal peptide to examine if and how substitution of the aspartate at the p−6 position by alanine affects CHK phosphorylation of the CHK-optimal peptide. Our analysis shows that the substitution only resulted in a small increase in KM and a slight reduction of kcat. Thus, the aspartate at the p−6 position plays only a minor role or no role in directing efficient phosphorylation of CHK-optimal peptide by CHK. For this reason, we do not include the p−6 aspartate in the optimal substrate phosphorylation sequence (E-x-[Φ/E/D]-Y-Φ-x-Φ) of CHK.
DISCUSSION
Clinical implications of β-synuclein as a potential substrate of CHK
β-Synuclein is a cytoplasmic protein located predominantly in the presynaptic nerve terminals. Its exact physiological function remains unclear. Recently, two mutations of β-synuclein have been identified as risk factors for development of a Parkinson's disease related neurodegenerative disease Dementia with Lewy Bodies (DLB) (34). A valine to methionine substitution at position 70 (V70M) of β-synuclein was found in one sporadic case of DLB in Japan, while a proline to histidine substitution at position 123 (P123H) was identified in a DLB case in Seattle. Furthermore, transgenic mice overexpressing the [P123H] β-synuclein mutant develop progressive neurodegeneration (35). The P123H mutation occurred at the conserved Pro-123 in the highly conserved C-terminal region of β-synuclein. Relevant to this, our result showed that CHK specifically phosphorylated β-synuclein at Y127, which is only four residues from Pro-123 in the C-terminal region. Thus, it will be worthwhile in future studies to establish if β-synuclein is a physiological substrate of CHK. If it is indeed a physiological substrate, studies to examine if and how tyrosine phosphorylation contributes to and/or modulates the neurotoxicity of [P123H] β-synuclein should be conducted.
How does CHK recognize its substrates?
There are more than twenty tyrosine residues in a Src-family kinase such as c-Src, and yet only the C-terminal regulatory tyrosine is selected by CHK as the phosphorylation site (15). How might the C-terminal tail sequences of SFKs contribute to their selective phosphorylation by CHK? It is noteworthy that the CHK-optimal phosphorylation sequence [E-x-Φ/E/D-Y-Φ-x-Φ] shares little similarity to the conserved sequences EPQYQPGENL and EGQYQQQP found in the C-terminal tail of most Src-family kinases. We predict that the local structure around the C-terminal regulatory tyrosine of SFKs plays only a minor role in directing CHK to recognize SFKs as the substrates. This implies that CHK employs predominantly the docking based mechanism to recognize SFKs as its substrates. The mechanism involves binding of interaction domain(s) located distally from the active site of CHK to one or more distal docking motifs in SFKs. A full understanding of the structural basis underpinning CHK recognition of SFKs requires identification of the interaction domain(s) in CHK and the distal docking motifs in SFKs.
The crystal structure of CSK/c-Src solved by Levinson et al. provides insights into how CHK recognizes SFKs (9). In this structure, CSK and c-Src make extensive ionic and hydrophobic interactions at an interface formed by five basic residues residing in the αD-helix and αF-αG loop of the CSK kinase domain and a motif comprising residues 504–525 near the C-terminal regulatory region of the c-Src kinase domain (reviewed in (10)). Using surface plasmon resonance, Levinson et al. demonstrated direct binding of CSK to c-Src with the dissociation constant (KD) of the CSK/c-Src complex ranging between 4 – 64 μM (9). The crystal structure suggests that the binding of CSK to c-Src is mainly attributed to the aforementioned interactions at the CSK/c-Src interface. Since the sequences of the αD-helix and αF-αG loop of CSK and CHK are highly conserved, it is logical to predict that the homologous motifs in the αD-helix and αF-αG loop of CHK are involved in binding to the motif containing residues 504 – 525 of c-Src.
We have previously demonstrated that CHK but not CSK can tightly bind to the SFK kinase domain to form a stable CHK-SFK complex that could be isolated by co-immunoprecipitation (15, 25). We recently used surface plasmon resonance to quantitatively compare the kinetics of CHK and CSK binding to SFKs. Our results showed that CHK binds to a SFK with a dissociation constant two to three orders of magnitude lower than that of CSK binding to c-Src (unpublished observations), confirming our previous observation that CHK binds to SFKs with a much higher affinity than CSK. Based upon these observations, we hypothesized that an unknown motif as well as the αD-helix and αF-αG loop are involved in mediating CHK to tightly bind to SFKs and directing the CHK active site to selectively phosphorylate the C-terminal regulatory tyrosine of SFKs. Defining this unknown motif in CHK and the distal docking determinant in SFKs targeted by this CHK motif is the major focus of our current investigation.
For protein substrates with phosphorylation sites exhibiting conformity to the CHK-optimal phosphorylation sequence, their recognition by CHK are governed by interactions between the active site and the phosphorylation sites as well as those between the CHK interaction domains with the distal docking motifs of the protein substrates. We predict that CHK first binds to the distal docking motifs of a protein substrate. Once bound, determinants near the phosphorylation site direct CHK active site to phosphorylate the protein.
Nevertheless, the discovery of the optimal phosphorylation sequence of CHK reported in this manuscript is the first step towards elucidation of the structural basis of substrate recognition by CHK. The next step should be definition of the distal docking motifs recognized by CHK (referred to as the CHK-docking motifs) in SFKs and other protein substrates. Once they have been defined, knowledge of the CHK optimal phosphorylation sequence and the CHK-docking motifs will improve the reliability of the search for potential physiological substrates of CHK by informatics approaches.
We recently reported the procedures for high yield expression and purification of recombinant CHK-His6. Using size exclusion chromatography and sedimentation velocity studies, we found that CHK-His6 is monomeric in aqueous solution (24). Thus, our recombinant CHK-His6 is suitable for structural analysis by X-ray crystallography. We have initiated crystallization trials of CHK-His6 with and without the presence of the CHK-optimal peptide. Elucidation of the crystal structure of CHK-His6 complexed with the peptide will reveal the structural basis of recognition of the CHK-optimal phosphorylation sequence by the CHK active site.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to thank Ryan Mills for his help in the circular dichroism analysis, Aainaa Roslee and Daisy Lio for their assistance in enzyme purification and kinetic analysis. We are grateful to Dr. Donald Fujita for his gift of Mab327 c-Src antibody, Drs Roberto Capai and Chi Pham for their gift of purified α-synuclein, Dr. Amardeep Dhillon for the gift of recombinant 14-3-3β and Dr. Lewis Cantley for the peptide library. K.K. Ia was supported by scholarships of the Faculty of Medicine, Dentistry and Health Sciences of the University of Melbourne.
The work described in this manuscript was supported by project grants from the NHMRC of Australia (566743 to H.-C. Cheng and J.G. Culvenor), Cancer Council Victoria (to H.C. Cheng and H.-J. Zhu) and the U.S. NIH (R01 GM079498 and R21 CA147993 to B. E. Turk). A.W. Purcell is supported by a Senior Research Fellowship from NHMRC of Australia.
Footnotes
FOOTNOTES AND ABBREVIATIONS positions N-terminal to the phosphorylation site in a protein/peptide substrate are numbered P−1, P−2, P−3, etc., while those C-terminal to the phosphorylation site are referred to as P+1, P+2, P+3, etc.
SUPPORTING INFORMATION AVAILABLE This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Ubersax JA, Ferrell JE., Jr. Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol. 2007;8:530–541. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg GS, Alexander DB, Pellicena P, Zhang ZY, Tsuda H, Miller WT. Src phosphorylates Cas on tyrosine 253 to promote migration of transformed cells. J Biol Chem. 2003;278:46533–46540. doi: 10.1074/jbc.M307526200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pellicena P, Miller WT. Processive phosphorylation of p130Cas by Src depends on SH3-polyproline interactions. J Biol Chem. 2001;276:28190–28196. doi: 10.1074/jbc.M100055200. [DOI] [PubMed] [Google Scholar]
- 4.Songyang Z, Cantley LC. Recognition and specificity in protein tyrosine kinase-mediated signalling. Trends Biochem Sci. 1995;20:470–475. doi: 10.1016/s0968-0004(00)89103-3. [DOI] [PubMed] [Google Scholar]
- 5.Cheng H-C, Chong YP, Ia KK, Tan O, Mulhern TD. CSK-homologous kinase. UCSD-Nature Molecule Pages. 2006 doi:10.1038/mp.a000705.01. [Google Scholar]
- 6.Chong YP, Ia KK, Mulhern TD, Cheng HC. Endogenous and synthetic inhibitors of the Src-family protein tyrosine kinases. Biochim Biophys Acta. 2005;1754:210–220. doi: 10.1016/j.bbapap.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Chong YP, Mulhern TD, Cheng HC. C-terminal Src kinase (CSK) and CSK-homologous kinase (CHK)--endogenous negative regulators of Src-family protein kinases. Growth Factors. 2005;23:233–244. doi: 10.1080/08977190500178877. [DOI] [PubMed] [Google Scholar]
- 8.Ingley E. Src-family kinase: regulation of their activity, level and identification of new pathways. Biochim Biophys Acta. 2008;1784:56–65. doi: 10.1016/j.bbapap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Levinson NM, Seeliger MA, Cole PA, Kuriyan J. Structural basis for the recognition of c-Src by its inactivator Csk. Cell. 2008;134:124–134. doi: 10.1016/j.cell.2008.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ia KK, Mills RD, Hossain MI, Chan K-C, Jarasrassamee B, Jorissen RN, Cheng H-C. Structural elements and allosteric mechanisms governing regulation and catalysis of Csk-family kinases and their inhibition of Src-family kinases. Growth Factors. 2010;28:329–350. doi: 10.3109/08977194.2010.484424. [DOI] [PubMed] [Google Scholar]
- 11.Kuo SS, Armanini MP, Phillips HS, Caras IW. Csk and BatK show opposite temporal expression in the rat CNS: consistent with its late expression in development, BatK induces differentiation of PC12 cells. Eur J Neurosci. 1997;9:2383–2393. doi: 10.1111/j.1460-9568.1997.tb01655.x. [DOI] [PubMed] [Google Scholar]
- 12.Zagozdzon R, Kaminski R, Fu Y, Fu W, Bougeret C, Avraham HK. Csk homologous kinase (CHK), unlike Csk, enhances MAPK activation via Ras-mediated signaling in a Src-independent manner. Cell Signal. 2006;18:871–881. doi: 10.1016/j.cellsig.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Mitsuhashi H, Futai E, Sasagawa N, Hayashi Y, Nishino I, Ishiura S. Csk-homologous kinase interacts with SHPS-1 and enhances neurite outgrowth of PC12 cells. J Neurochem. 2008;105:101–112. doi: 10.1111/j.1471-4159.2007.05121.x. [DOI] [PubMed] [Google Scholar]
- 14.Hamaguchi I, Yamaguchi N, Suda J, Iwama A, Hirao A, Hashiyama M, Aizawa S, Suda T. Analysis of CSK homologous kinase (CHK/HYL) in hematopoiesis by utilizing gene knockout mice. Biochem Biophys Res Commun. 1996;224:172–179. doi: 10.1006/bbrc.1996.1003. [DOI] [PubMed] [Google Scholar]
- 15.Chong YP, Mulhern TD, Zhu HJ, Fujita DJ, Bjorge JD, Tantiongco JP, Sotirellis N, Lio DS, Scholz G, Cheng HC. A novel non-catalytic mechanism employed by the C-terminal Src-homologous kinase to inhibit Src-family kinase activity. J Biol Chem. 2004;279:20752–20766. doi: 10.1074/jbc.M309865200. [DOI] [PubMed] [Google Scholar]
- 16.Cheng H-C, Chong YP, Ia KK, Tan O. a., Mulhern TD. Csk homologous kinase. UCSD-Nature Molecule Pages. 2006 doi:10.1038/mp.a000705.01. [Google Scholar]
- 17.Cohen P, Knebel A. KESTREL: a powerful method for identifying the physiological substrates of protein kinases. Biochem J. 2006;393:1–6. doi: 10.1042/BJ20051545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knebel A, Morrice N, Cohen P. A novel method to identify protein kinase substrates: eEF2 kinase is phosphorylated and inhibited by SAPK4/p38delta. EMBO J. 2001;20:4360–4369. doi: 10.1093/emboj/20.16.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaleel M, Nichols RJ, Deak M, Campbell DG, Gillardon F, Knebel A, Alessi DR. LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson's disease mutants affect kinase activity. Biochem J. 2007;405:307–317. doi: 10.1042/BJ20070209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray JT, Campbell DG, Morrice N, Auld GC, Shpiro N, Marquez R, Peggie M, Bain J, Bloomberg GB, Grahammer F, Lang F, Wulff P, Kuhl D, Cohen P. Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. Biochem J. 2004;384:477–488. doi: 10.1042/BJ20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray JT, Campbell DG, Peggie M, Mora A, Cohen P. Identification of filamin C as a new physiological substrate of PKBalpha using KESTREL. Biochem J. 2004;384:489–494. doi: 10.1042/BJ20041058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng C, Knebel A, Morrice NA, Li X, Barringer K, Li J, Jakes S, Werneburg B, Wang L. Pim kinase substrate identification and specificity. J Biochem. 2007;141:353–362. doi: 10.1093/jb/mvm040. [DOI] [PubMed] [Google Scholar]
- 23.Pham CL, Leong SL, Ali FE, Kenche VB, Hill AF, Gras SL, Barnham KJ, Cappai R. Dopamine and the dopamine oxidation product 5,6-dihydroxylindole promote distinct on-pathway and off-pathway aggregation of alpha-synuclein in a pH-dependent manner. J Mol Biol. 2009;387:771–785. doi: 10.1016/j.jmb.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Chan KC, Lio DS, Dobson RCJ, Jarasrassamee B, Hossain MI, Roslee AK, Ia KK, Perugini MA, Cheng H-C. Development of the Procedures for High Yield Expression and Rapid Purification of Active Recombinant Csk-Homologous Kinase (CHK) – Comparison of the catalytic activities of CHK and CSK. Protein Expr Purif. 2010;74:139–147. doi: 10.1016/j.pep.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Chong YP, Chan AS, Chan KC, Williamson NA, Lerner EC, Smithgall TE, Bjorge JD, Fujita DJ, Purcell AW, Scholz G, Mulhern TD, Cheng HC. C-terminal Src kinase-homologous kinase (CHK), a unique inhibitor inactivating multiple active conformations of Src family tyrosine kinases. J Biol Chem. 2006;281:32988–32999. doi: 10.1074/jbc.M602951200. [DOI] [PubMed] [Google Scholar]
- 26.Culvenor JG, McLean CA, Cutt S, Campbell BC, Maher F, Jakala P, Hartmann T, Beyreuther K, Masters CL, Li QX. Non-Abeta component of Alzheimer's disease amyloid (NAC) revisited. NAC and alpha-synuclein are not associated with Abeta amyloid. Am J Pathol. 1999;155:1173–1181. doi: 10.1016/s0002-9440(10)65220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulhern TD, To C, Cheng HC. 1H, 13C and 15N chemical shift assignments of the SH2 domain of the Csk homologous kinase. J Biomol NMR. 2002;24:363–364. doi: 10.1023/a:1021657406480. [DOI] [PubMed] [Google Scholar]
- 28.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 29.Mok J, Kim PM, Lam HY, Piccirillo S, Zhou X, Jeschke GR, Sheridan DL, Parker SA, Desai V, Jwa M, Cameroni E, Niu H, Good M, Remenyi A, Ma JL, Sheu YJ, Sassi HE, Sopko R, Chan CS, De Virgilio C, Hollingsworth NM, Lim WA, Stern DF, Stillman B, Andrews BJ, Gerstein MB, Snyder M, Turk BE. Deciphering protein kinase specificity through large-scale analysis of yeast phosphorylation site motifs. Sci Signal. 2010;3:ra 12. doi: 10.1126/scisignal.2000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutti JE, Jarrell ET, Chang JD, Abbott DW, Storz P, Toker A, Cantley LC, Turk BE. A rapid method for determining protein kinase phosphorylation specificity. Nat Methods. 2004;1:27–29. doi: 10.1038/nmeth708. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama Y, Kawana A, Igarashi A, Yamaguchi N. Involvement of the N-terminal unique domain of Chk tyrosine kinase in Chk-induced tyrosine phosphorylation in the nucleus. Exp Cell Res. 2006;312:2252–2263. doi: 10.1016/j.yexcr.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 32.Davis TL, Walker JR, Allali-Hassani A, Parker SA, Turk BE, Dhe-Paganon S. Structural recognition of an optimized substrate for the ephrin family of receptor tyrosine kinases. FEBS J. 2009;276:4395–4404. doi: 10.1111/j.1742-4658.2009.07147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang X, Finerty P, Jr., Walker JR, Butler-Cole C, Vedadi M, Schapira M, Parker SA, Turk BE, Thompson DA, Dhe-Paganon S. Structural insights into the inhibited states of the Mer receptor tyrosine kinase. J Struct Biol. 2009;165:88–96. doi: 10.1016/j.jsb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohtake H, Limprasert P, Fan Y, Onodera O, Kakita A, Takahashi H, Bonner LT, Tsuang DW, Murray IV, Lee VM, Trojanowski JQ, Ishikawa A, Idezuka J, Murata M, Toda T, Bird TD, Leverenz JB, Tsuji S, La Spada AR. Beta-synuclein gene alterations in dementia with Lewy bodies. Neurology. 2004;63:805–811. doi: 10.1212/01.wnl.0000139870.14385.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujita M, Sugama S, Sekiyama K, Sekigawa A, Tsukui T, Nakai M, Waragai M, Takenouchi T, Takamatsu Y, Wei J, Rockenstein E, Laspada AR, Masliah E, Inoue S, Hashimoto M. A beta-synuclein mutation linked to dementia produces neurodegeneration when expressed in mouse brain. Nat Commun. 1:110. doi: 10.1038/ncomms1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.