Abstract
The first transannulation of 1,2,3-triazoles with terminal alkynes into pyrroles is reported. The reaction proceeds in the presence of Rh2(oct)4/AgOCOCF3 binary catalyst system providing a straightforward approach to 1,2,4-trisubstituted pyrroles in good to excellent yields.
Transition metal-catalyzed transannulation is an emerging tool for the interconversion of various heterocyclic cores. Thus, triazoles can be exploited as a convenient precursory platform for the formation of diverse heterocyclic systems. We found that N-fused pyridotriazoles1 can undergo the Rh-catalyzed transannulation with alkynes2 and nitriles3 to produce indolizines and imidazopyridines, respectively.4 Likewise, it has been also shown that the Rh-catalyzed transannulation of N-tosyltriazoles 1 with nitriles, proceeding via intermediacy of 2 (a), can be used for the construction of imidazoles (eq. 1).5 Further developments of the transannulation concept include recently reported Rh-,6 as well as Pd-7 and Ni8-catalyzed transformations.
Thus, Murakami has recently disclosed8c the Ni/AlPh3-catalyzed transannulation of 1 with internal alkynes into the tetrasubstituted pyrroles (b). The reaction was proposed to proceed via azanickelacycle 5.9 Importantly, attempted by both groups (a, b) transannulation of triazoles 1 with terminal alkynes was unsuccessful.5,8c Herein, we report the first transannulation of N-tosyltriazoles 1 with terminal alkynes in the presence of Rh2(oct)4/AgOCOCF3 binary catalyst system (c). This reaction is efficient with electron-rich arylacetylenes and tolerates a wide range of substituents R at the triazole ring to produce trisubstituted pyrroles 4 (eq. 1).
 |
(eq. 1) |
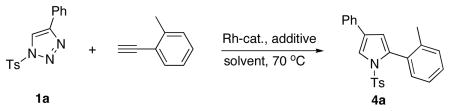
First, we attempted the transannulation of triazole 1a with o-tolylacetylene in the presence of several Rh catalysts and various additives (Table 1). Thus, attempted reactions in the presence of Rh2(OAc)4/AgOCOCF3 and Rh2(pfb)4/AgOCOCF3 combinations were unsuccessful (Table 1, entries 1, 2).
Table 1.
Optimization of the Transannulation Reaction of Triazole 1aa
 | ||||
|---|---|---|---|---|
| no. | catalyst (2.5 mol %) | Lewis acid (5.0 mol %) | solvent 0.06 M | yieldb (%) |
| 1 | Rh2(OAc)4 | AgOCOCF3 | toluene | NR |
| 2 | Rh2(pfb)4 | AgOCOCF3 | toluene | NR |
| 3 | Rh2(dosp)4 | Al(OTf)3 | toluene | 63 |
| 4 | Rh2(oct)4 | AgOCOCF3 | toluene | 53 |
| 5 | Rh2(oct)4 | AgOCOCF3 | hexane | 67 |
| 6 | Rh2(oct)4 | - | hexane | NRc |
| 7 | Rh2(oct)4 | AgOTf | hexane | NR |
| 8 | Rh2(oct)4 | CsF | hexane | NR |
| 9 | Rh2(oct)4 | Zn(OTf)2 | hexane | NR |
| 10 | Rh2(oct)4 | Y(OTf)3 | hexane | NR |
| 11 | Rh2(oct)4 | AgOCOCF3 | DCM | NR |
| 12 | Rh2(oct)4 | AgOCOCF3 | DCE | NR |
| 13 | Rh2(oct)4 | AgOCOCF3 | THF | NR |
| 14 | Rh2(oct)4 | CF3SO3H | hexane | Dec.d |
| 15 | Rh2(oct)4 | CF3CO2H | hexane | Dec. |
All reactions were performed at 70 °C for 12 h with 0.2 mmol of 1a and 0.3 mmol of alkyne;
Isolated yield;
NR = No Reaction;
Dec. = Decomposition.
Gratifyingly, it was found that employment of the Rh2(dosp)4/Al(OTf)3 combination afforded the desired product 4a in 63% yield (entry 3). Use of Rh2(oct)4 gave a slightly lower yield (53%) of the product (entry 4). Further improvement of the yield (67%) was achieved in the presence of Rh2(oct)4 and AgOCOCF3 in hexane (entry 5). The reaction did not proceed without Lewis acid additive at all (entry 6). Further screening of Lewis (Brønsted) acid additives and solvents did not reveal more efficient conditions for this reaction (entries 7–15).
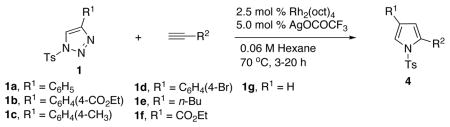
With the optimized conditions in hand, the generality of the transannulation reaction of triazoles 1 with terminal alkynes was examined (Table 2). It was found that these conditions were very efficient for a transannulation of a wide range of N-tosyl-1,2,3-triazoles with electron-rich alkynes. Thus, reaction of 4-phenyltriazole 1a with a number of terminal arylalkynes (and alkenylalkyne) proceeded smoothly to give the trisubstituted pyrrole derivatives 4a-h in good to excellent yields (entries 1–8). Triazole 1b, possessing an electron-deficient aryl ring (entries 9–15), as well as the p-tolyl containing triazole 1c (entries 16–20), were equally efficient in this reaction. Bromophenyl derivative 1d proved to be an excellent substrate for the transannulation reaction, producing pyrroles 4u, v in 99 and 86% yields (entries 21–22). It was also found that triazoles, possessing alkyl- (1e) and ester (1f) groups at the C-4 position of the ring, as well as C-4 unsubstituted triazole 1g, could also participate in the transannulation reaction with alkynes, though the yields of pyrrole were moderate (entries 23–26).
Table 2.
Synthesis of Pyrroles via Rh-Catalyzed Transannulation of N-Tosyltriazoles with Terminal Alkynesa
 | ||
|---|---|---|
| no. | product | yield (%)b |
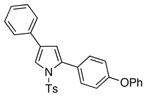
| 1 |
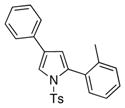 4a 4a
|
67 |
| 2 |
 4b 4b
|
97 |
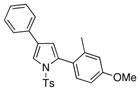
| 3 |
 4c 4c
|
79 |
| 4 |
 4d 4d
|
51 |
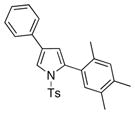
| 5 |
 4e 4e
|
52 |
| 6 |
 4f 4f
|
52 |
| 7 |
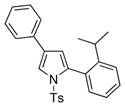 4g 4g
|
54 |
| 8 |
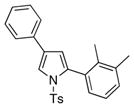 4h 4h
|
66 |
| 9 |
 4i 4i
|
74 |
| 10 |
 4j 4j
|
94 |
| 11 |
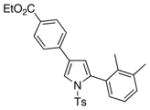 4k 4k
|
58 |
| 12 |
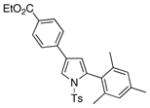 4l 4l
|
72 |
| 13 |
 4m 4m
|
46 |
| 14 |
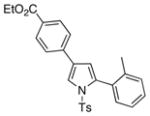 4n 4n
|
65 |
| 15 |
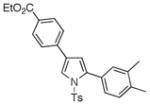 4o 4o
|
60 |
| 16 |
 4p 4p
|
81 |
| 17 |
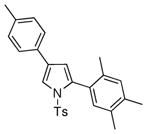 4q 4q
|
77 |
| 18 |
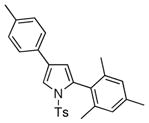 4r 4r
|
87 |
| 19 |
 4s 4s
|
54 |
| 20 |
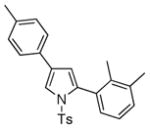 4t 4t
|
70 |
| 21 |
 4u 4u
|
99 |
| 22 |
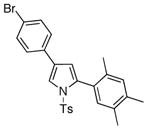 4v 4v
|
86 |
| 23 |
 4w 4w
|
44 |
| 24 |
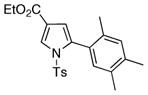 4x 4x
|
57 |
| 25 |
 4y 4y
|
46 |
| 26 |
 4z 4z
|
54 |
All reactions were performed in 0.2 mmol scale;
Isolated yield; For more information see: Supporting Information (SI).
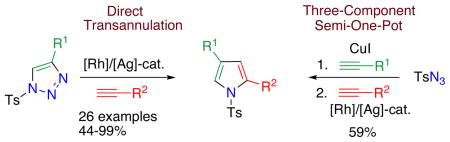
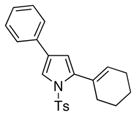
It deserves mentioning that this transannulation reaction was efficient with various electron-rich arylalkynes, possessing alkyl, methoxy, phenoxy, and isopropyl groups. Cyclohexenylacetylene was also competent in this reaction.10
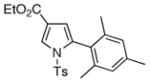
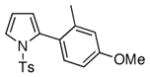
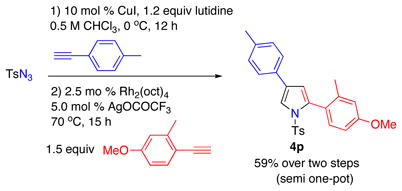
The synthetic utility of this transannulation approach was further highlighted by a three-component semi-one-pot synthesis of pyrrole 4p from tosylazide and two different terminal alkynes. First, tosyl azide was allowed to react with p-tolylacetylene in the presence of 10% CuI, 1.2 equiv of 2,6-lutidine in 0.5 M CHCl3 at 0 °C for 12 h.11 The reaction mixture, containing a newly formed N-tosyltriazole, was filtered through a short pad of silica, evaporated, and used as crude in the subsequent Rh-catalyzed transannulation reaction with 1-ethynyl-2-methyl-4-methoxybenzene to produce pyrrole 4p in 59 % overall yield (eq. 2).
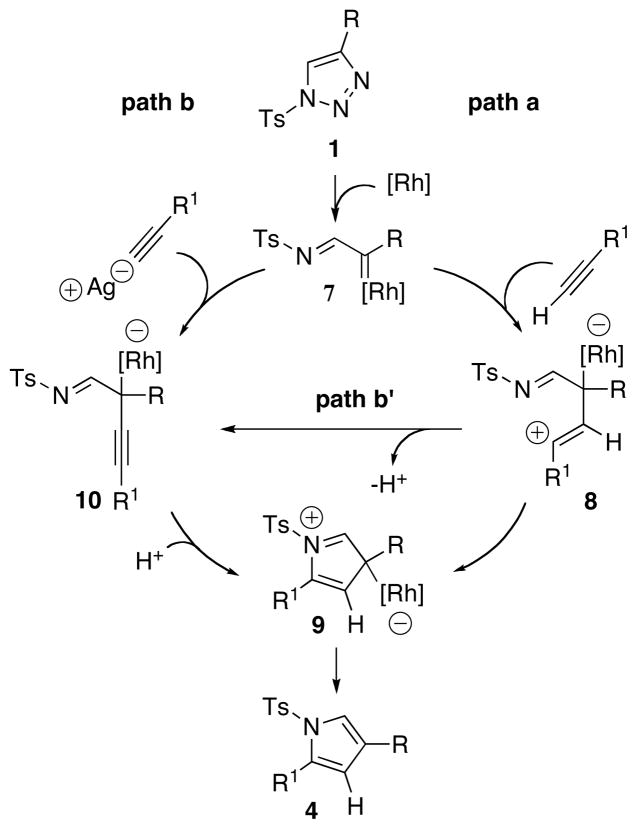
We propose the following plausible mechanism for this transannulation reaction (Scheme 1). Upon treatment with Rh2(oct)4, triazole 1,12 generates the Rh-iminocarbene 7.5 A direct nucleophlic attack of the terminal alkyne at the latter produces ylide 8 (path a),13 which upon cyclization forms a cyclic zwitterionic species 9. Elimination of the Rh catalyst from 9 forms the reaction product 4. On the other hand, the in-situ generated silver acetylide may attack 7 to form a Rh-containing propargylimine species 10 (path b). Alternatively, 10 may arise via a proton loss from 8 (path b′). Proton-assisted 5-endo-trig cyclization of 10 would afford the cyclic intermediate 9.
Scheme 1.
Proposed Mechanism for the Transannulation of N-tosyltriazoles with Terminal Alkynes
 |
(eq. 2) |
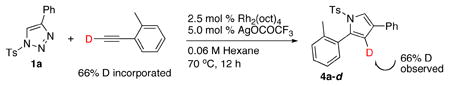
In order to gain further insight into the mechanism of this transannulation reaction, the following deuterium labeling experiment was performed (eq. 3). A deuterated o-tolylacetylene was subjected to the standard transannulation reaction with 1a to produce 4a-d with complete preservation of the deuterium label. This result undoubtedly rules out possible involvement of the paths b and b′, which would result in partial or complete deuterium scrambling. Although, the crucial role of silver trifluoroacetate in path a is not clear, it possibly acts as a Lewis acid, which via a coordination to the imine, activates the electrophilic Rh carbene moiety toward the nucleophilic attack by an alkyne. The higher reactivity of electron-rich alkynes in this transformation reasonably fits into the most plausible reaction path a.
 |
(eq. 3) |
In conclusion, we have developed the transannulation reaction of N-tosyltriazoles with terminal alkynes in the presence of Rh2(oct)4/AgOCOCF3 binary catalyst system. This new protocol is highly efficient for the synthesis of 1,2,4-trisubstituted pyrroles from a diversely C-4-substituted triazoles and eletron-rich terminal alkynes. Furthermore, it has been also shown that trisubstituted pyrroles 4, can also be synthesized in a three-component semi-one-pot fashion from two different terminal alkynes and tosylazide. A mechanistic rationale, involving a direct nucleophilic attack of electron-rich alkyne at the Rh-iminocarbene intermediate, followed by cyclization step was proposed for this new transannulation reaction.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (Grant GM-64444) for financial support of this work.
Footnotes
Supporting Information Available. Experimental procedure and compound characterization. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Chuprakov S, Hwang FW, Gevorgyan V. Angew Chem, Int Ed. 2007;46:4757. doi: 10.1002/anie.200700804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.For representative examples on the Rh-catalyzed reactions of diazocarbonyl compounds with alkynes, see: Davies HML, Romines KR. Tetrahedron. 1988;44:3343.Kinder FR, Padwa A. Tetrahedron Lett. 1990;31:6835.Padwa A, Kassir JM, Xu SL. J Org Chem. 1991;56:6971.Davies HML, Cantrell WR, Romines KR, Jr, Baum JS. Org Synth. 1992;70:93.Doyle MP, Winchester WP, Hoorn JAA, Lynch V, Simonsen SH, Ghosh R. J Am Chem Soc. 1993;115:9968.Padwa A, Kinder FR. J Org Chem. 1993;58:21.Doyle MP, Protopopova M, Müller P, Ene D, Shapiro EA. J Am Chem Soc. 1994;116:8492.
- 3.For Rh-catalyzed cycloaddition of diazocarbonyl compounds with nitriles, see: Connell R, Scavo F, Helquist P. Tetrahedron Lett. 1986;27:5559.
- 4.For cyclopropanation of alkenes with 2-pyridyl diazo compounds, see: Davies HML, Townsend RJ. J Org Chem. 2001;66:6595. doi: 10.1021/jo015617t.
- 5.Horneff T, Chuprakov S, Chernyak N, Gevorgyan V, Fokin VV. J Am Chem Soc. 2008;130:14972. doi: 10.1021/ja805079v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For Rh-catalyzed transannulation of triazoles with alkenes, see: Chuprakov S, Kwok SW, Zhang L, Lercher L, Fokin VV. J Am Chem Soc. 2009;131:18034. doi: 10.1021/ja908075u.Grimster N, Zhang L, Fokin VV. J Am Chem Soc. 2010;132:2510. doi: 10.1021/ja910187s.
- 7.Transannulation of triazoles with Pd-cat, see: Nakamura I, Nemoto T, Shiraiwa N, Terada M. Org Lett. 2009;11:1055. doi: 10.1021/ol900113f.Miura T, Nishida Y, Morimoto M, Yamauchi M, Murakami M. Org Lett. 2011;13:1429. doi: 10.1021/ol103143a.
- 8.Transannulation of triazoles with Ni-cat, see: Miura T, Yamauchi M, Kosaka A, Murakami M. Angew Chem, Int Ed. 2010;49:4955. doi: 10.1002/anie.201001918.Miura T, Yamauchi M, Murakami M. Org Lett. 2008;10:3085. doi: 10.1021/ol8010826.Miura T, Yamauchi M, Murakami M. Chem Commun. 2009:1470. doi: 10.1039/b819162j.
- 9.For involvement of analogous azametallocycle intermediate in the Pd-catalyzed transannulation of benzotriazoles with internal alkynes, see ref. 7a.
- 10.Simple aliphatic alkynes, as well as electron-deficient arylalkynes were not effective in this transformation.
- 11.For Cu-catalyzed synthesis of N-tosyl-1,2,3-triazoles from tosylazide and terminal alkynes, see: Yoo EJ, Ahlquist M, Kim SH, Bae I, Fokin VV, Sharpless KB, Chang S. Angew Chem, Int Ed. 2007;46:1730. doi: 10.1002/anie.200604241.
- 12.Doyle KJ, Moody CJ. Tetrahedron. 1994;50:3761. [Google Scholar]
- 13.For involvement of ylide intermediates in the Rh-catalyzed reactions of diazocarbonyl compounds with nitriles, see: Connell R, Scavo F, Helquist P, Akermark B. Tetrahedron Lett. 1986;27:5559.See also ref. 5.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.