Abstract
Context
The impact of Society of Thoracic Surgeons (STS) predicted mortality risk score on resource utilization after aortic valve replacement (AVR) has not been previously studied.
Objective
We hypothesize that increasing STS risk scores in patients having AVR are associated with greater hospital charges.
Design, Setting, and Patients
Clinical and financial data for patients undergoing AVR at a tertiary care, university hospital over a ten-year period (1/2000–12/2009) were retrospectively reviewed. The current STS formula (v2.61) for in-hospital mortality was used for all patients. After stratification into risk quartiles (Q), index admission hospital charges were compared across risk strata with Rank-Sum tests. Linear regression and Spearman’s coefficient assessed correlation and goodness of fit. Multivariable analysis assessed relative contributions of individual variables on overall charges.
Main Outcome Measures
Inflation-adjusted index hospitalization total charges
Results
553 patients had AVR during the study period. Average predicted mortality was 2.9% (±3.4) and actual mortality was 3.4% for AVR. Median charges were greater in the upper Q of AVR patients [Q1–3,$39,949 (IQR32,708–51,323) vs Q4,$62,301 (IQR45,952–97,103), p=<0.01]. On univariate linear regression, there was a positive correlation between STS risk score and log-transformed charges (coefficient: 0.06, 95%CI 0.05–0.07, p<0.01). Spearman’s correlation R-value was 0.51. This positive correlation persisted in risk-adjusted multivariable linear regression. Each 1% increase in STS risk score was associated with an added $3,000 in hospital charges.
Conclusions
This study showed increasing STS risk score predicts greater charges after AVR. As competing therapies such as percutaneous valve replacement emerge to treat high risk patients, these results serve as a benchmark to compare resource utilization.
Keywords: Aortic Valve Replacement, Resource Utilization, Society of Thoracic Surgeons Risk Score
Introduction
The grim natural course of untreated symptomatic severe aortic stenosis (AS) was first shown by Braunwald in 1968, and re-affirmed by the medically managed cohort of patients in the recent publication of the PARTNER trial.1,2 In the PARTNER study, patients receiving optimal medical treatment experienced 50% 1-year mortality.1 In light of these sobering statistics, the effectiveness of aortic valve replacement (AVR) for severe aortic stenosis cannot be overstated. Not only do patients across all age ranges derive a dramatic survival advantage, but they also enjoy greater quality of life, whether undergoing standard sternotomy or a less invasive partial sternotomy approach.3–6
In 2002, the first successful percutaneous AVR was performed, avoiding the need for median sternotomy.7 Retrospective results with this transcatheter aortic-valve implantation (TAVI) approach have subsequently been widely reported.8–12 The first randomized, prospective trial in the United States was recently published, which revealed a significant survival advantage for TAVI over best medical management in inoperable patients with severe AS.1 As this new technology gains widespread application, it will be incumbent on the cardiovascular community to give consideration to cost effectiveness with regard to this new therapy. The Society of Thoracic Surgeons (STS) risk prediction models enable clinicians to compare groups of patients based on similar preoperative risk profiles.13 In the PARTNER study, STS risk scores were utilized to define the eligible patient population. To serve as a benchmark for cost effectiveness comparisons, we tested the hypothesis that increasing STS risk scores in patients undergoing AVR are associated with greater hospital charges and resource consumption.
Methods
Patient Data
This was a retrospective review of the cardiac surgery database, which is managed by a dedicated data center team within the Division of Cardiac Surgery at the Johns Hopkins Hospital. All patients undergoing AVR with either a tissue or mechanical prosthesis at our institution from 1/2000–12/2009 were included. Patients with prior sternotomy were included.. Those patients who underwent other concomitant cardiac surgical procedures (coronary artery bypass, ascending aortic aneurysm repair, atrial septal defect repair, radiofrequency ablation) as well as pediatric patients (<18 years) were excluded. There were 553 patients with isolated AVR who comprised the cohort for this analysis.
All charts of these patients were available for review, and following Institutional Review Board approval all relevant clinical information was extracted from the institutional cardiac surgery database and the electronic medical record as necessary. Demographic and clinical variables included age, gender, race, cardiovascular co-morbidities, smoking history, and ejection fraction. STS risk score for operative mortality was calculated according to version 6.21, which was introduced in 2008. For patients prior to 2008, all necessary data were used to extrapolate a risk score according to version 6.21.
Outcomes
Postoperative data included: operative mortality, length of stay (LOS), in-hospital drug-treated infections, post-operative cerebrovascular accidents (CVA), renal replacement therapy (RRT), and deep sternal infections. These complications were reviewed from both data submitted to the STS database as well as independent review of the electronic medical record. Survival status was supplemented using the Social Security Death Index.
Charges Data
Hospital charges are obtained through the hospital billing department as reported to the Maryland State authorities, and represent total hospital charges for the index admission only. These charges include those incurred during the operation, as well as all aspects of postoperative care. The state of Maryland Health Services Cost Review Commission (HSCRC) is the only system of its kind in the United States, and this commission minimizes cost-shifting by establishing payment rates for all insurers within the state of Maryland.
Charges data are divided into the following categories: Routine charges, operating room facility use, operating room supply use, pharmacy charges, laboratory charges, radiology charges, physical therapy charges, and other charges. For any patient hospitalized prior to AVR, index admission charges begin with the date of the operation. All financial information was inflation-adjusted according to the United States Department of Labor Consumer Price Index in United States dollars for the year 2009.
Statistical Analysis
Patients were stratified into quartiles according to STS risk scores. Differences between STS Q1–3 and STS Q4 patients were compared using two-tailed Student’s t-test for normally distributed continuous variables. Chi-square analysis was used for categorical variables. For nonparametric continuous data, Wilcoxon rank-sum test was used. To confirm non-parametric distributions, the data were visually inspected in graphical form and checked for skewness. The Wilcoxon rank-sum test compared index admission charges data between Q1–3 and Q4.
To test for an STS risk score that would predict increased charges receiver operating characteristic (ROC) curves were used. The outcome measure for high charges was defined as the upper 5% of median index admission charges. An area under the curve (AUC) above 0.7 was deemed significant.
A histogram of absolute charges revealed a non-normal distribution. After logarithmic transformation of charges data, visual inspection revealed a more normal distribution. To further confirm that no assumptions of linear regression had been violated, residual values of the regression model were plotted against fitted values and demonstrated equal variance across the spectrum of STS risk scores. Univariate linear regression assessed the correlation between continuous STS risk score and log index hospitalization charges. A separate univariate linear regression was performed with STS risk score above or below 10% as a binary independent variable. Multivariable linear regression determined the relative contributions of individual variables toward index admission charges. In addition to variables associated with charges on exploratory univariate analysis (p<0.1), those with biological plausibility were incorporated in a forwards and backwards stepwise fashion into the multivariable linear regression model. The likelihood ratio test and Akaike’s information criterion in a nested model approach were used to identify which covariates increased the explanatory power of the model. The final model incorporated the following covariates: STS risk score, age, ejection fraction, pre-operative CVA, chronic kidney dysfunction, chronic obstructive pulmonary disease (COPD), diabetes mellitus (DM), post-op major complication (composite of CVA, RRT, or pneumonia), and operative mortality.
Continuous variables are presented with the mean ± standard deviation (SD). Categorical variables are shown in whole numbers and percentages. All actual charges data are presented in median and interquartile range (IQR). Regression coefficients are presented with 95% confidence intervals (CI). Actual P-values are reported except when <0.001, and values less than 0.05 represented statistical significance. Analysis was performed using Stata statistical software, version 9.2 (StataCorp, College Station, Texas).
Results
Cohort Statistics
From January 2000 to December 2009, 553 patients underwent isolated AVR at our institution and comprise the cohort for this analysis. The mean age was 67.0 ±14.1 years with 40% females(n=222). The race distribution of the cohort was: 81% Caucasian(n=447), 14% African American(n=76), and 0.5% Hispanic(n=2) and 4.5% other(n=26). Forty-three (8%) reported a history of smoking. Throughout the study period, the number of yearly adult isolated AVR procedures remained relatively constant, ranging from 42–86 procedures annually.
STS results
Mean STS risk score for the entire isolated AVR cohort was 2.95(±3.4). Isolated AVR patients were grouped into the following STS quartiles: Q1, 0.37–1.02, n=139; Q2, 1.03–1.90, n=138; Q3, 1.91–3.44, n=138; Q4, 3.49–30.29, n=138. Baseline demographic information was compared between patients in Q1–3 against patients in Q4. As expected, patients in Q4 were older with significantly greater cardiovascular co-morbidities. A history of smoking was more common in patients in Q1–3.
Outcomes and Mortality Rates
By quartile, operative mortality rates for isolated AVR were 1(0.72%) Q1; 0(0%) Q2; 2(1.5%) Q3; and 16(11.6%) Q4. Median hospital LOS was longer in Q4: 10 days (IQR:7–17) versus 7 days (IQR:5–9) for Q1–3. Median duration of mechanical ventilation was also greater in Q4: 17 hours (IQR:11–37) compared with 9 hours (IQR:5–13). Postoperative CVA, RRT, and pneumonia were also more common in Q4, though deep sternal infection rates were equivalent. Demographic information and postoperative complications for isolated AVR are shown in Table 1.
Table 1.
Baseline Demographics and Postoperative Complications
| Variables | Isolated AVR cohort | ||
|---|---|---|---|
| Q 1–3 (N=415) | Q 4 (N=138) | P-value | |
| Mean age, years (SD) | 63.8 ± 13.2 | 76.5 ± 12.3 | <0.001 |
| Male gender, # (%) | 274 (66%) | 57 (41%) | <0.001 |
| Caucasian race, # (%) | 343 (83%) | 104 (75%) | 0.3 |
| Smoking history, # (%) | 38 (9%) | 5 (4%) | 0.04 |
| Redo sternotomy, # (%) | 17 (4%) | 9 (7%) | 0.2 |
| Cerebrovascular disease, # (%) | 28 (7%) | 21 (15%) | 0.002 |
| Diabetes mellitus, #(%) | 83 (20%) | 48 (35%) | <0.001 |
| Hypertension, # (%) | 274 (66%) | 114 (83%) | <0.001 |
| Ejection Fraction, % (SD) | 54% ± 14 | 50% ± 15 | 0.004 |
| COPD, # (%) | 40 (10%) | 36 (26%) | <0.001 |
| Chronic kidney dysfunction, # (%) | 23 (6%) | 30 (22%) | <0.001 |
|
Outcomes
| |||
| Length of stay (LOS), days (IQR) | 7 (5–9) | 10 (7–17) | <0.001 |
| Duration of mechanical ventilation, hours (IQR) | 9 (6–13) | 17 (11–38) | <0.001 |
| Pneumonia, # (%) | 8 (2.2%) | 10 (7.2%) | 0.01 |
| CVA, # (%) | 5 (1.2%) | 8 (5.8%) | <0.001 |
| Renal replacement therapy, # (%) | 9 (2.2%) | 17 (12.3%) | <0.001 |
| Deep sternal infection, # (%) | 2 (0.5%) | 0 (0%) | 0.4 |
Hospital Charges
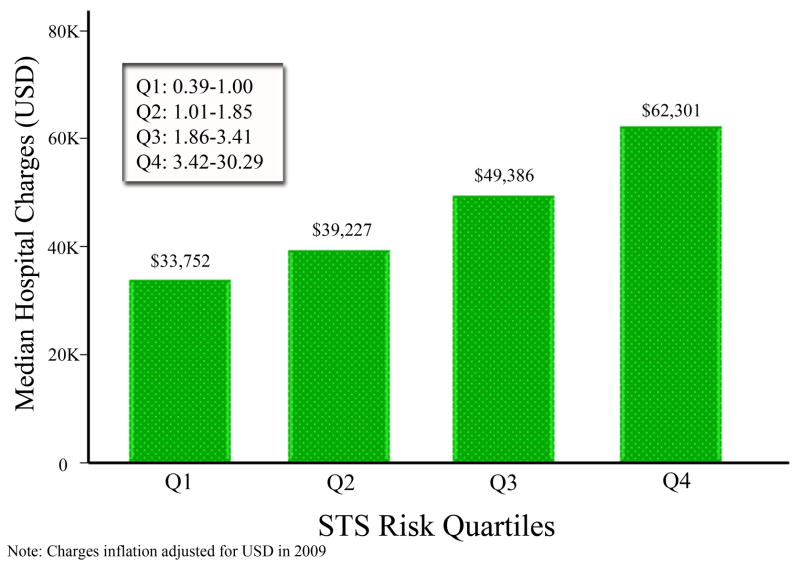
For isolated AVR, median index admission charges were higher in STS Q4 patients: $62,301 (IQR45,952–97,103) for Q4 compared with $39,949 (IQR32,708–51,323) for Q1–3, p<0.001. Median index admission charges for individual quartiles were: Q1, $33,820 (IQR:29,641–42,243); Q2, $39,534 (IQR: 33,156–45,929); Q3, $49,571 (IQR: 38,710–62,554); and Q4, $62,301 (IQR: 45,952–97,103). When examining each of the four STS quartiles by non-parametric ANOVA, there was an overall global difference in the group medians (P<0.001). Multiple pairwise comparisons with an adjusted significance level for the Bonferroni correction revealed that all of the quartiles were significantly different from each other for isolated AVR (Figure 2).
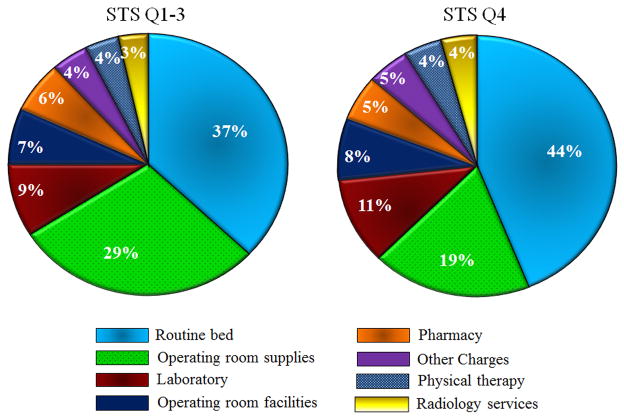
Figure 2.
Side-by-side pie charts showing breakdown of total hospital charges by individual category for isolated AVR patients only. Chart on left corresponds to STS risk score quartile 1–3 patients combined and chart on right depicts STS quartile 4 patients.
For STS Q4 and Q1–3, the relative contributions of each charge category to total index hospitalization charges for isolated AVR patients are depicted in Figure 3. Hospital charges according to category were compared between STS Q4 and Q1–3. When analyzing index admission charges, the following categories were higher for STS Q4: routine ward and ICU care, OR supplies charges, OR facilities charges, pharmacy charges, laboratory services, radiology, and physical therapy services.
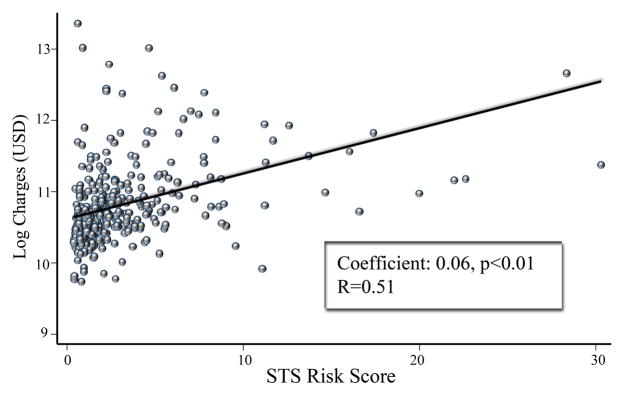
Figure 3.
Results of univariate linear regression analysis for isolated AVR with STS risk score as independent variable. Log charges used as dependent variable (outcome measure).
Isolated AVR patients who died prior to discharge had greater median charges than those who survived to discharge (survivors: $42,917 (IQR: 33,829–59,222) vs non-survivors: $136,769 (IQR:65,855–316,461, p<0.01). Additional sub-analysis was performed revealing isolated AVR patients with STS risk score >10% had significantly increased median hospital charges (<10%: $42,785 (IQR: 33,820–59,391) vs >10%: $88,241 (IQR: 58,518–129,693), p<0.01).
Area under the ROC curve was 0.72 (95% CI: 0.61–0.83). An STS risk score of 3.13% had the best discriminatory power for predicting the upper 5% of index admission charges, with sensitivity of 68% and specificity 73%.
Linear Regression Analysis
Linear regression of charges data after logarithmic transformation revealed a significant positive correlation between STS predicted risk score and index admission charges (correlation coefficient: 0.06, 95%CI 0.05–0.07, p<0.01), with a Spearman R-value of 0.51 (Figure 3). Using the binary independent variable of STS risk score >10%, there was a positive correlation (coefficient: 0.64, 95% CI 0.39–0.89, p<0.01) with increased hospital charges.
Following adjustment with multivariable linear regression, STS predicted risk score (coefficient: 0.04, 95% CI 0.03–0.05, p<0.01), diabetes mellitus (coefficient: 0.07, 95% CI 0.01–0.14, p=0.04), a major post-operative complication (coefficient: 0.45, 95% CI 0.35–0.55, p<0.01), and in-hospital mortality (coefficient: 0.54, 0.40–0.69, p<0.01) were all independently associated with increased hospital charges. The remaining variables did not reach significant associations and are shown in Table 2.
Table 2.
Multivariable Regression Analysis
| Variable | Coefficient | 95% CI | P-Value |
|---|---|---|---|
| Age, years | 0.01 | −.002–0.004 | 0.5 |
| Diabetes mellitus | 0.11 | 0.013–0.203 | 0.03 |
| COPD | 0.13 | 0.011–0.248 | 0.03 |
| Pre-op CVA | 0.19 | 0.049–0.331 | 0.01 |
| Chronic kidney dysfunction | 0.12 | −0.032–0.277 | 0.1 |
| Ejection fraction, % | −0.01 | −0.005–0.001 | 0.1 |
| Post-operative complication | 0.67 | 0.529–0.826 | <0.01 |
| In-hospital mortality | 0.46 | 0.222–0.696 | <0.01 |
| STS risk score | 0.02 | 0.010–0.034 | 0.01 |
Discussion
This is the first study to evaluate the impact of high STS predicted risk scores on hospital charges and resource utilization in a cohort of patients undergoing AVR. Patients were stratified into quartiles according to STS risk score in order to compare index admission hospital charges. When examined against other algorithms for mortality risk after isolated AVR, a recent study demonstrated that the STS score most closely correlates with outcomes.14 Consistent with our hypothesis, we observed a linear association between STS risk scores and hospital charges. Given the recent progress in percutaneous technology for AVR, the findings in this study establish a benchmark for future cost effectiveness comparisons.
Charges Analysis
As the focus of this study was to identify charges associated with delivering care during the operative procedure and all subsequent hospital care, daily charges were obtained in order to subtract all preoperative charges. Analysis in this fashion permits a clear examination of the effect of STS risk score on charges associated with operative and postoperative hospital care only; however, it should be noted that patients with high STS risk score may incur significant preoperative cost and utilize significant hospital resources. Patients in STS Q4 had higher median index admission charges than patients in STS Q1–3 combined, supporting the hypothesis. Additionally, when comparing individual quartiles by ANOVA, pairwise comparisons revealed each successive quartile increase was associated with greater charges. Median charges for patients in the highest STS risk quartile were $30,000 more than median charges for patients in the lowest quartile. This finding is further supported by the linear relationship observed in the regression analysis (figure 3). Univariate linear regression determined that STS risk score had a positive correlation with increasing index admission charges. Extrapolating the regression coefficient from log charges to absolute charges revealed that each 1% increase in STS risk score was associated with an additional $3,000 in hospital charges. Because of our rigorous regression methodologies, we believe the linear regression results are robust and valid
To explore which pre- and post-operative factors are associated with increased charges, an adjusted multivariable linear regression model was constructed. The formula to determine STS risk score is derived from many preoperative factors, however all variables with significant associations on univariate analysis or biological plausibility were incorporated in the multivariable linear regression model. Diabetes mellitus, chronic obstructive pulmonary disease, and pre-operative stroke were preoperative variables significantly associated with increased charges. This finding is expected, as patients with these co-morbidities are known to be at greater risk for postoperative mortality following AVR.15–17 When grouped together into a composite outcome, the occurrence of postoperative CVA, RRT, or pneumonia was associated with increased charges. As the complication rate following AVR is relatively low, we surmise that we would have low power to detect a significant association when examining any major complication independently. Therefore, we grouped the three major complications following AVR (CVA, RRT, or pneumonia) to be examined collectively. The high expenses associated with RRT in the setting of critical illness are already known, and we speculate that the cost associated with this therapy may be driving the findings in this study as well.18
In the context of recent emergence of TAVI, it is important to rigorously examine the cost effectiveness of these competing therapies. AVR is known to effectively improve survival and quality of life for patients with AS, including in octogenarians. According to the quality of life Short Form-36 instrument, patients greater than 75 years undergoing AVR had comparable quality of life to the age-matched general population.6 Furthermore, Wu et al. prospectively evaluated a cohort of over 4,500 patients during a four-decade span to assess cost effectiveness of AVR using economic modeling.19 They applied the value of life-years as determined by economists to calculate the economic value of additional life afforded to the cohort by AVR, concluding the net value of life-years gained by AVR to be 11.2 billion dollars. In contrast with our study, the study by Wu et al. preceded STS risk models and thus they were not able to incorporate these models in their cost savings analysis. Nevertheless their study illustrates the significant positive economic impact of traditional AVR.
The recent publication of the inoperable cohort of the PARTNER study utilized an STS risk score for predicted mortality of 50% (or permanent disability) as the threshold to define the eligible study population. TAVI demonstrated a significant survival advantage over optimal medical management, including balloon valvuloplasty, in this cohort.1 Data regarding the high risk cohort are yet unpublished, however an STS risk score for predicted mortality of 10% was used as a cut-off to define this patient subgroup. Thus, we compared patients with an STS risk score greater than 10% against those with a risk score <10%. Median index admission charges in the nineteen patients with an STS risk score >10% exceeded median charges for patients <10% by over $45,000. Furthermore, according to univariate regression analysis an STS score >10% was associated with a $36,000 increase in hospital charges. Given the current state of health care economics in the United States, it will be imperative that the cardiovascular community give full consideration to the cost-benefit ratio when implementing TAVI on a broad scale.
To examine resource utilization for patients who die prior to hospital discharge, we performed a charges comparison among patients who survived to hospital discharge versus non-survivors. Median hospital charges for patients who died prior to hospital discharge were nearly $100,000 more than survivors. Furthermore, in the risk-adjusted multivariable linear regression model, in-hospital mortality had an independent association with increased charges, with a regression coefficient of 0.54. Extrapolating this coefficient from log charges to absolute charges reveals that with all other variables being held constant, patients who died in the hospital had on average $30,000 in excess charges. The opposite finding was seen in a cohort of open thoraco-abdominal aneurysm repair patients. Not only was there no difference in median charges between open repair versus endovascular thoracic aortic aneurysm repair, but there was no difference in median hospital charges between survivors versus non-survivors.20 That analysis included patients undergoing repair for aortic rupture, however, and we suspect that because of different pathologies the higher rate of early deaths in that series blunted the charges associated with in-hospital death. It remains to be seen how charges will compare between the open versus endovascular approach for aortic valve disease in the future. Charges data are not yet available for TAVI at our institution; however we speculate that until this technology becomes widely available with broad competition in the marketplace, charges associated with the device will be high.
Limitations
This study is limited by the use of hospital charges as surrogate indices for cost. However, the unique medical reimbursement structure in the State of Maryland mitigates this issue. The Maryland Health Services Cost Review Commission (HSCRC) was established by the state legislature in 1971 to contain costs. The HSCRC determines hospital payment rates for insurers—both private and public—including Medicare and Medicaid within all Maryland hospitals. Therefore, the practice of “cost shifting” by overcharging privately insured patients is absent, making institutional charge data predictable and consistent. The authors’ institution HSCRC rate for charge payment has been cost + 1–3% during the study interval. Accordingly, we believe this study represents a novel and accurate assessment of charges associated with AVR. This analysis is further limited by single institution data. However, because of variable payment rates across states, a multi-institutional study involving centers in different states cannot be easily performed.
Re-admission rates and subsequent hospitalization charges were not captured by our database. Thus, additional study is needed to determine if patients with increased STS risk score consume greater resources following AVR in the long term also. A charges analysis of our institutional lung transplant recipients revealed that patients with increased preoperative risk score (according to lung allocation score), had increased index admission charges, but not 1-year charges.21
Conclusions
This is the first study to examine the impact of high STS risk scores on hospital charges and resource utilization after AVR. Patients in the highest STS risk quartile had increased index admission hospital charges, greater lengths of stay, and more complications when compared to patients in the lower 75% of STS risk scores. There is a linear relationship between hospital charges and STS risk score, as increasing risk scores predict higher hospital charges. As competing therapies such as percutaneous valve replacement gain broader application, these results serve as a benchmark to compare resource utilization across increasing strata of STS risk scores.
Figure 1.
Bar graph depicting median hospital charges by STS risk quartile for Isolated AVR. P<0.001 by Kruskal-Wallis non-parametric ANOVA and each individual quartile statistically different from each other by pairwise comparison using Bonferonni corrected P-values.
Acknowledgments
Dr. Arnaoutakis is the Irene Piccinini Investigator in Cardiac Surgery and Dr. George is the Hugh R. Sharp Cardiac Surgery Research Fellow.
The authors wish to thank Joe DiNatale and Barbara Fleischman for their dutiful assistance in managing the cardiac surgery database and for providing STS risk score information.
Footnotes
Conflicts: The authors have no conflicts of interest to disclose.
References
- 1.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 2.Ross J, Jr, Braunwald E. Aortic stenosis. Circulation. 1968;38:61–67. doi: 10.1161/01.cir.38.1s5.v-61. [DOI] [PubMed] [Google Scholar]
- 3.Elbardissi AW, Shekar P, Couper GS, Cohn LH. Minimally invasive aortic valve replacement in octogenarian, high-risk, transcatheter aortic valve implantation candidates. J Thorac Cardiovasc Surg. 2011;141:328–335. doi: 10.1016/j.jtcvs.2010.08.056. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz F, Baumann P, Manthey J, Hoffmann M, Schuler G, Mehmel HC, et al. The effect of aortic valve replacement on survival. Circulation. 1982;66:1105–1110. doi: 10.1161/01.cir.66.5.1105. [DOI] [PubMed] [Google Scholar]
- 5.Varadarajan P, Kapoor N, Bansal RC, Pai RG. Survival in elderly patients with severe aortic stenosis is dramatically improved by aortic valve replacement: Results from a cohort of 277 patients aged > or =80 years. Eur J Cardiothorac Surg. 2006;30:722–727. doi: 10.1016/j.ejcts.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 6.Sundt TM, Bailey MS, Moon MR, Mendeloff EN, Huddleston CB, Pasque MK, et al. Quality of life after aortic valve replacement at the age of >80 years. Circulation. 2000;102:III70–74. doi: 10.1161/01.cir.102.suppl_3.iii-70. [DOI] [PubMed] [Google Scholar]
- 7.Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106:3006–3008. doi: 10.1161/01.cir.0000047200.36165.b8. [DOI] [PubMed] [Google Scholar]
- 8.Modine T, Obadia JF, Choukroun E, Rioufoul G, Sudre A, Laborde JC, et al. Transcutaneous aortic valve implantation using the axillary/subclavian access: Feasibility and early clinical outcomes. J Thorac Cardiovasc Surg. 2011;141:487–491. e481. doi: 10.1016/j.jtcvs.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 9.Cribier A, Eltchaninoff H, Tron C, Bauer F, Agatiello C, Nercolini D, et al. Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J Am Coll Cardiol. 2006;47:1214–1223. doi: 10.1016/j.jacc.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 10.Grube E, Laborde JC, Gerckens U, Felderhoff T, Sauren B, Buellesfeld L, et al. Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study. Circulation. 2006;114:1616–1624. doi: 10.1161/CIRCULATIONAHA.106.639450. [DOI] [PubMed] [Google Scholar]
- 11.Thomas M, Schymik G, Walther T, Himbert D, Lefevre T, Treede H, et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2010;122:62–69. doi: 10.1161/CIRCULATIONAHA.109.907402. [DOI] [PubMed] [Google Scholar]
- 12.Webb JG, Chandavimol M, Thompson CR, Ricci DR, Carere RG, Munt BI, et al. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation. 2006;113:842–850. doi: 10.1161/CIRCULATIONAHA.105.582882. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2--isolated valve surgery. Ann Thorac Surg. 2009;88:S23–42. doi: 10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 14.Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg. 2008;135:180–187. doi: 10.1016/j.jtcvs.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Carnero-Alcazar M, Reguillo-Lacruz F, Alswies A, Villagran-Medinilla E, Maroto-Castellanos LC, Rodriguez-Hernandez J. Short- and mid-term results for aortic valve replacement in octogenarians. Interact Cardiovasc Thorac Surg. 2010;10:549–554. doi: 10.1510/icvts.2009.218040. [DOI] [PubMed] [Google Scholar]
- 16.Thourani VH, Myung R, Kilgo P, Thompson K, Puskas JD, Lattouf OM, et al. Long-term outcomes after isolated aortic valve replacement in octogenarians: a modern perspective. Ann Thorac Surg. 2008;86:1458–1464. doi: 10.1016/j.athoracsur.2008.06.036. discussion 1464–1455. [DOI] [PubMed] [Google Scholar]
- 17.Brown ML, Pellikka PA, Schaff HV, Scott CG, Mullany CJ, Sundt TM, et al. The benefits of early valve replacement in asymptomatic patients with severe aortic stenosis. J Thorac Cardiovasc Surg. 2008;135:308–315. doi: 10.1016/j.jtcvs.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 18.Srisawat N, Lawsin L, Uchino S, Bellomo R, Kellum JA. Cost of acute renal replacement therapy in the intensive care unit: results from The Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) study. Crit Care. 2010;14:R46. doi: 10.1186/cc8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y, Grunkemeier GL, Starr A. The value of aortic valve replacement in elderly patients: an economic analysis. J Thorac Cardiovasc Surg. 2007;133:603–607. doi: 10.1016/j.jtcvs.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 20.Arnaoutakis GJ, Hundt JA, Shah AS, Cameron DE, Black JH., 3rd Comparative Analysis of Hospital Costs of Open and Endovascular Thoracic Aortic Repair. Vasc Endovascular Surg. 2011;45:39–45. doi: 10.1177/1538574410380471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnaoutakis GJ, Allen JG, Merlo CA, Sullivan BE, Baumgartner WA, Conte JV, et al. Impact of the lung allocation score on resource utilization after lung transplantation in the United States. J Heart Lung Transplant. 2011;30:14–21. doi: 10.1016/j.healun.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]