Abstract
Objectives
The purpose of this study was to examine the effect of cigarette smoking on plasma uric acid concentration and to determine the correlation between this parameter and the biological tobacco markers, plasma thiocyanate and urinary cotinine.
Methods
The initial study was conducted on 300 subjects; 138 of them were nonsmokers (62 men and 76 women) aged 14–72 years and 162 were current smokers (145 men and 17 women) aged 16–85 years. Uric acid, creatinine, and urinary cotinine were determined by the enzymatic colorimetric method and plasma thiocyanate by selective electrode.
Results
Plasma uric acid concentration was significantly lower in smokers than in nonsmokers. A statistically significant negative correlation was noted between the smoking status parameters, including both the number of cigarettes smoked/day (F3–161 = 12.063; r = −0.9968; p = 0.0001) and the duration of smoking (F3–161 = 1.305; r = −0.9406; p = 0.0274), and the plasma uric acid. Among smokers, we noted a negative correlation between uric acid and both plasma thiocyanates (r = −0.437; p < 0.05) and urinary cotinine (r = −0.580; p < 0.05).
Conclusion
After excluding the other factors affecting the uric acid levels, the significant low plasma uric acid in smokers was attributed to a reduction of the endogenous production as a result of the chronic exposure to cigarette smoke that is a significant source of oxidative stress. Therefore, it is recommended to stop or reduce smoking and to introduce plasma uric acid estimation as a routine test, since it is cheap and simple to reflect the antioxidant level.
Keywords: Cigarette smoking, Urinary cotinine, Plasma thiocyanate, Uric acid, Oxidative stress
Introduction
Cigarette smoking is known to contribute to many diseases, including cancer, chronic obstructive pulmonary disease, stroke, cardiovascular diseases, and peptic ulcers [1, 2]. Investigators have attempted to elucidate the mechanisms of the pathogenesis associated with cigarette smoking, but the conclusions were not consistent. A basic hypothesis is that free radicals cause oxidative damage to macromolecules such as lipids, proteins, and DNA. Therefore, these radicals play an important role in the pathogenesis of diseases [3]. In accordance with this theory, antioxidants are believed to play important roles in resisting damage from oxidative stress resulting from cigarette smoking. Several studies were conducted concerning the relationship between smoking and antioxidants, focusing on the differences of the effect of antioxidants between smokers and nonsmokers. However, the results were controversial.
As cigarette smoke contains superoxide and reactive nitrogen species that readily react with various biomolecules, it has been hypothesized that some of the adverse effects of smoking may result from the oxidative damage to the endothelial cells, which results in nitric oxide deficiency. Nitric oxide regulates the vascular tone and its deficiency accelerates the insufficiency of the coronary artery and vasoconstriction in different tissues. Therefore, the imbalance between oxidants and antioxidants may play an important role in the smoker [4]. In addition, cigarette smokers have increased inflammatory responses that further enhance their oxidative stress; in humans, uric acid is the most abundant aqueous antioxidant, accounting for up to 60% of serum free radical scavenging capacity, and it is an important intracellular free radical scavenger during metabolic stress, including smoking [5, 6]. The measurement of its serum level, therefore, reflects the antioxidant capacity [7]. Nicotine is the main substance that results in addiction to smoking. Because nicotine is a substance specific to cigarette smoking, it can be used as a marker for the number of cigarettes consumed [8]. Therefore, it allows to quantify the amount that a subject smokes. Nicotine metabolites, including cotinine, nicotine-N-oxide, and a number of derivatives, offer a priority consideration. These are more accurate than other biomarkers such as carboxyhemoglobin or carbon monoxide, which are not predictors of the number of cigarettes smoked. In addition, such biomarkers may be present as a result of exposure to other environmental factors [8].
The aim of this study was to demonstrate the possible effect of smoking on plasma uric acid concentration and to determine the correlation between this parameter and the biological tobacco markers, plasma thiocyanate and urinary cotinine.
Materials and methods
Study design
Population
The initial study was conducted on 300 voluntary subjects; 138 of them were nonsmokers (62 men and 76 women) aged 35.6 ± 16.0 years and 162 were current smokers (145 men and 17 women) aged 38.0 ± 17.5 years. This study had been approved by the local ethical committee. Written informed consent was obtained from all voluntary adult participants and from the parents of minors.
Samples
For plasma uric acid, creatinine, and thiocyanates, venous blood was drawn in tubes containing lithium heparinate and it was immediately centrifuged. The plasma samples were stored at −20°C until analysis. Urine samples were obtained from the smokers and nonsmokers. These samples were either used on the same day or frozen at −20°C until they were required for analysis. All of the samples were analyzed for urinary cotinine.
Methods
Smoking questionnaire
Subject information and cigarette smoking outcome data were collected in a structured interview. The available data were limited to the classification of smoking to three categories: never, former, or current. The majority of current and former smokers were able to provide information on the number of cigarettes they smoked and the duration of smoking. All subjects were questioned concerning their medical history and sociodemographic characteristics, including age, gender, education, and employment.
Laboratory analysis
Uric acid, creatinine, and urinary cotinine levels were determined by the enzymatic colorimetric method on the Konelab 30™ analyzer (Thermo Electron Corporation). Cotinine was expressed as micrograms per micromol of creatinine in urine. SCN− levels were determined using selective electrodes (Ionometer SevenMulti S80™, Mettler Toledo, Schwerzenbach, Switzerland) and expressed as milligrams per liter in plasma.
Statistical analysis
The results were expressed as means ± standard deviation (SD). Standard descriptive statistics, correlation coefficients, and significance tests were calculated using the SPSS 17.0 program. Differences between mean values were evaluated by Student’s t-test and between frequency values by the χ2 test. Comparisons between smokers and nonsmokers in uric acid levels were applied by analysis of variance (ANOVA) after adjustment to potential confounders’ factors (factors of arthrosclerosis such as cholesterol, high-density lipoproteins [HDL] and low-density lipoproteins (LDL), alcohol drinking, creatinine, and body mass index [BMI]). A p-value of less than 0.05 was considered to represent a statistically significant difference between groups.
Results
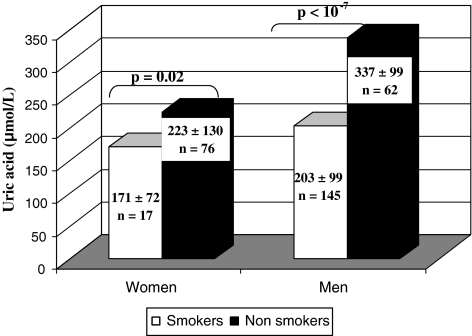
We noted that plasma uric acid was significantly lower in smokers than in nonsmokers (p = 0.0003, Table 1). It was also significantly lower in smoking men than nonsmoking men (203 ± 100 vs. 337 ± 100; p < 10−7). The same result was found for women (171 ± 72 vs. 223 ± 130; p = 0.02) (Fig. 1).
Table 1.
Variation of uric acid, creatinine, urea, SCN−, and urinary cotinine levels according smoking status
| Parameters | Smokers (n = 162) | Nonsmokers (n = 138) | p value |
|---|---|---|---|
| Age (years) | 38.0 ± 17.5 | 35.6 ± 16 | 0.172 |
| Sex ratio (men/women) | 8.52 (145/17) | 0.81 (62/76) | <10−3 |
| BMI (kg/m2) | 24.24 ± 3.17 | 25.63 ± 4.36 | 0.003 |
| Plasma creatinine (μmol/L) | 66.78 ± 20.7 | 72.3 ± 19 | 0.018 |
| Plasma uric acid (μmol/L) | 199 ± 97 | 250 ± 132 | 0.0003 |
| Plasma urea (mmol/L) | 4.0 ± 1.9 | 4.1 ± 3.3 | 0.05 |
| Urinary cotinine (μg/μmol Cr) | 231.4 ± 205.2 | 73.2 ± 73.7 | <10−7 |
| Plasma SCN− (μmol/L) | 100.25 ± 1.36 | 99.62 ± 0.91 | 0.0005 |
Fig. 1.
Variation of uric acid in men and women according to smoking status
After adjustment of plasma uric acid levels to confounders’ factors, we noted a significant difference between smokers and nonsmokers (p < 0.0001).
Table 1 clearly shows that smokers have lower plasma urea levels than nonsmokers. We found a significant dissimilarity between smokers and nonsmokers in the urinary cotinine (p < 10−7) and plasma SCN− levels (p < 0.0005).
In this study, we found a significant difference between subjects smoking more than 20 cigarettes/day and those smoking less than 20 cigarettes/day (χ2 = 22.4; p < 10−7). We noted a significant decrease of uric acid concentration when the smoking duration exceeds 5 years (χ2 = 3.89; p = 0.04) (Table 2).
Table 2.
Variation of uric acid according to the number of cigarettes smoked and consumption duration
| Cigarettes smoked/day | p value | Consumption duration (years) | p value | |||
|---|---|---|---|---|---|---|
| <20 | ≥20 | ≤5 | >5 | |||
| Uric acid <200 μmol/L | ||||||
| M (n = 64) | 141 ± 25 | 96 ± 27 | 0.004 | 140 ± 41 | 99 ± 65 | 0.01 |
| W (n = 8) | 185 ± 17 | 135 ± 27 | 0.03 | 175 ± 20 | 130 ± 28 | 0.04 |
| Uric acid >200 μmol/L | ||||||
| M (n = 81) | 269 ± 42 | 268 ± 52 | 0.96 | 271 ± 50 | 267 ± 45 | 0.69 |
| W (n = 9) | 276 ± 72 | 229 ± 25 | 0.38 | 304 ± 78 | 228 ± 22 | 0.39 |
| χ2, p | 22.4; p < 10−7 | 3.89; p = 0.04 | ||||
M men, W women
The number of cigarettes smoked/day was significantly higher in subjects having uric acid values below 200 μmol/L than in others (24 ± 9.58 vs. 19 ± 9.55 cigarettes/day; p = 0.01).
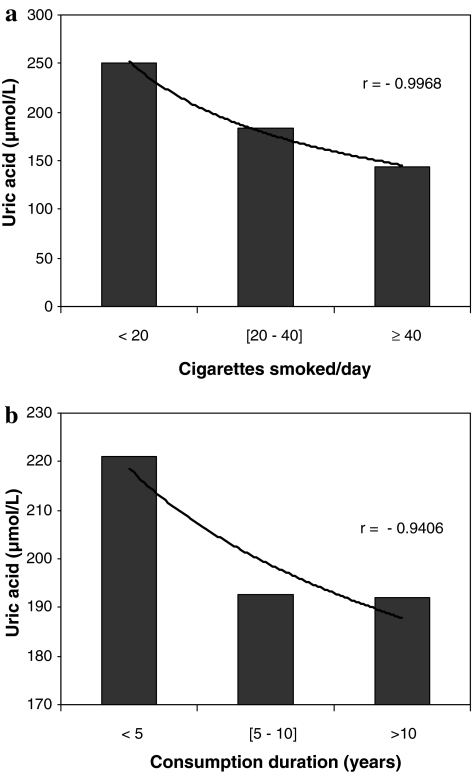
A statistically significant negative correlation was noted between the smoking status parameters, including both the number of cigarettes smoked/day (F3–161 = 12.063; r = −0.9968; p < 0.05; Fig. 2a) and the duration of smoking (F3–161 = 1.305; r = −0.9406; p = 0.0274, Fig. 2b), and plasma uric acid.
Fig. 2.
Correlation between uric acid concentration and smoking status (cigarettes smoked/day and consumption duration). a Uric acid levels and cigarettes smoked/day. b Uric acid levels and consumption duration
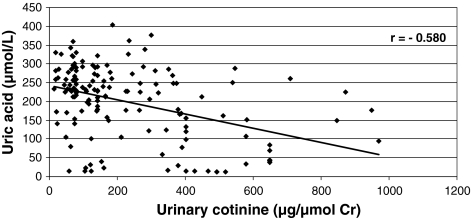
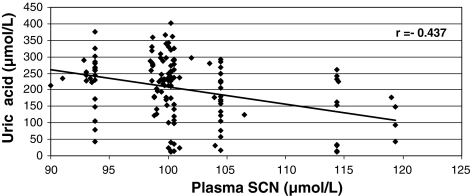
Figures 3 and 4 show a negative correlation between urinary cotinine and plasma uric acid levels (r = −0.580), and between plasma SCN− and uric acid concentrations (r = −0.437).
Fig. 3.
Correlation between uric acid concentration and urinary cotinine levels
Fig. 4.
Correlation between uric acid concentration and plasma SCN− levels
Discussion
Many, but not all, epidemiological studies have suggested that high plasma uric acid is a risk factor for cardiovascular diseases, and they aimed at evaluating its prognostic implications and potential utility in the therapy monitoring [9, 10]. This raised level of plasma uric acid, parallel to an increased risk of cardiovascular diseases, could be either primary or secondary to the underlying causes of the cardiovascular diseases [11]. However, the specific role of plasma uric acid in this constellation remains uncertain, although it may be involved in the platelet adhesiveness, aggregation, or inflammation, and it may be implicated in the genesis of hypertension [12]. In contrast, there is some evidence that the increase of plasma uric acid is protective against the cardiovascular diseases, since uric acid acts as an endogenous antioxidant [12, 13], and the higher plasma uric acid levels found in cardiovascular diseases patients suggest that any protective antioxidant effect of uric acid is hidden by other negative effects in these pathogeneses.
In this study, the plasma uric acid level in smokers was significantly lower than in nonsmokers (p = 0.0003), both in men (p < 10−7) and in women (p = 0.02). This could confirm the effect of cigarette smoking on uric acid levels independently of the gender. In addition, we noted a significant negative correlation with the smoking status, including the average number of cigarettes smoked/day and the smoking duration. Moreover, we noted that the uric acid levels decrease when the smoking duration exceeds 5 years. This finding is in agreement with other studies showing a low plasma uric acid in regular smokers [14] and a reduction of antioxidants, including uric acid, in smokers, indicating that oxidative stress increases each time a cigarette is smoked [15, 16]. Other studies proved that even nonsmokers exposed to cigarette smoke have a significantly lower plasma antioxidant status than unexposed nonsmokers, independently of the differences in the dietary antioxidant intake [16]. Others studies proved that the administration of uric acid increases the circulating antioxidant defenses and allows the restoration of endothelium-dependent vasodilatation [17]. A decrease of uric acid in smokers can be explained by the inactivation of xanthine oxidase by cyanide, which is eliminated as thiocyanate [18, 19]. Therefore, high plasma uric acid concentrations might be protective in situations characterized by an increase of cardiovascular risk and oxidative stress, such as smoking [14], and a reduction of its level, which increases susceptibility to oxidative damage and accounts for the excessive free radical production [16]. Therefore, the possibility that uric acid confers protection against the development of atherosclerosis, in view of its antioxidant properties, has been recognized [16].
In this study, we found a significant decrease of plasma creatinine levels in smokers compared to nonsmokers, although these values are not pathological. This can confirm that all of the subjects studied are without any renal failure, since the determination of creatinine has been reported to be useful in evaluating the renal handling of uric acid and as concentrations of this parameter are highly dependent on endogenous production as well as on renal excretion [19, 20]. Therefore, the low plasma uric acid level in smokers is attributed to a reduction of endogenous production.
This finding is in agreement with other studies that proved that the reduction of antioxidants, including uric acid in smokers, is due to both the chronic exposure to cigarette smoke, which is a significant source of oxidative stress, and to the low intake of dietary antioxidants [21].
Some of the relationships between tobacco and urea or uric acid are very significant; however, they are all very weak. If these relationships have the same origin, a hypothetical renal mechanism must first be considered. In fact, the blood urea is a product of the catabolism of proteins and their amino acids, whereas uric acid originates from the oxidation of purines. Moreover, the two molecules, while circulating in the blood, remain unlinked, either directly or by a common carrier. On the contrary, they are both excreted by the kidney, and in the disease processes, they generally vary in the same way: a rise in blood uric acid is well known as an early sign of renal failure. An increase in the renal excretion of urea and uric acid under the influence of tobacco is, therefore, a reasonable hypothesis, and it is supported by the known action of nicotine on the metabolism of catecholamines and the effect of these substances on renal function [22].
Urinary cotinine and plasma SCN− concentrations were both significantly higher in smokers than in nonsmokers, and they were well correlated with the number of cigarettes smoked per day and with the duration of consumption. Although cotinine and plasma SCN− are influenced by diet and industrial pollution, it remains a reliable indicator of the smoking status [23].
We found a negative correlation between the plasma uric acid level and both urinary cotinine concentration (r = −0.580) and plasma SCN− concentration (r = −0.437) in active smokers. The important correlation found between urinary cotinine and plasma uric acid in smokers was not surprising because the urinary cotinine and plasma SCN− levels were determined as a marker of tobacco smoke exposure [24].
In our study, plasma antioxidant levels were closely, but inversely, related to the levels of plasma nicotine metabolites. It can be explained that more regular cigarette smoking will markedly affect plasma nicotine metabolites, and, thus, decrease plasma antioxidant levels. Furthermore, our finding suggests that plasma nicotine metabolites are appropriate as biomarkers for smoking consumption. These biomarkers should be applied in future studies on cigarette smoking.
Limitations
Several methodological limitations should be considered when interpreting these findings. First, larger sample sizes of the groups would be beneficial. Second, our work is a cross-sectional study that does not permit to follow up biological parameters. Finally, the sample of smokers may not be representative of more heterogeneous populations.
Conclusion
After the exclusion of other factors affecting uric acid levels, the significant low plasma uric acid level in smokers was attributed to a reduction of endogenous production as a result of the chronic exposure to cigarette smoke that is a significant source of oxidative stress. Therefore, cigarette smoking may influence oxidative stress by affecting the levels of plasma antioxidants, which may be involved in the mechanisms underlying various diseases. As this reduction is proportionate with the smoking status and leads to cardiovascular diseases, it is recommended to stop or reduce smoking and to introduce plasma uric acid estimation as a routine test, since it is cheap and simple to reflect the antioxidant status.
Acknowledgments
The authors thank Mr. Samir Boukattaya for his assistance with the English language corrections.
Conflict of interest The authors stated that there are no conflicts of interest regarding the publication of this article.
References
- 1.Leistikow BN, Tsodikov A. Cancer death epidemics in United States Black males: evaluating courses, causation, and cures. Prev Med. 2005;41:380–385. doi: 10.1016/j.ypmed.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Mallampalli A, Guntupalli KK. Smoking and systemic disease. Clin Occup Environ Med. 2006;5:173–192. doi: 10.1016/j.coem.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Chiu YW, Chuang HY, Huang MC, Wu MT, Liu HW, Huang CT. Comparison of plasma antioxidant levels and related metabolic parameters between smokers and non-smokers. Kaohsiung J Med Sci. 2009;25:423–430. doi: 10.1016/S1607-551X(09)70537-6. [DOI] [PubMed] [Google Scholar]
- 4.Vaart H, Postma DS, Timens W, Hacken NHT. Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax. 2004;59:713–721. doi: 10.1136/thx.2003.012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathru M, Dries DJ, Barnes L, Tonino P, Sukhani R, Rooney MW. Tourniquet-induced exsanguination in patients requiring lower limb surgery. An ischemia–reperfusion model of oxidant and antioxidant metabolism. Anesthesiology. 1996;84:14–22. doi: 10.1097/00000542-199601000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Hellsten Y, Tullson PC, Richter EA, Bangsbo J. Oxidation of urate in human skeletal muscle during exercise. Free Radic Biol Med. 1997;22:169–174. doi: 10.1016/S0891-5849(96)00286-9. [DOI] [PubMed] [Google Scholar]
- 7.Maxwell SR, Thomason H, Sandler D, LeGuen C, Baxter MA, Thorpe GH, et al. Antioxidant status in patients with uncomplicated insulin-dependent and non-insulin-dependent diabetes mellitus. Eur J Clin Invest. 1997;27:484–490. doi: 10.1046/j.1365-2362.1997.1390687.x. [DOI] [PubMed] [Google Scholar]
- 8.Leone A. Biochemical markers of cardiovascular damage from tobacco smoke. Curr Pharm Des. 2005;11:2199–2208. doi: 10.2174/1381612054367391. [DOI] [PubMed] [Google Scholar]
- 9.Meisinger C, Koenig W, Baumert J, Döring A. Uric acid levels are associated with all-cause and cardiovascular disease mortality independent of systemic inflammation in men from the general population: the MONICA/KORA cohort study. Arterioscler Thromb Vasc Biol. 2008;28:1186–1192. doi: 10.1161/ATVBAHA.107.160184. [DOI] [PubMed] [Google Scholar]
- 10.Ioachimescu AG, Brennan DM, Hoar BM, Hazen SL, Hoogwerf BJ. Serum uric acid is an independent predictor of all-cause mortality in patients at high risk of cardiovascular disease: a preventive cardiology information system (PreCIS) database cohort study. Arthritis Rheum. 2008;58:623–630. doi: 10.1002/art.23121. [DOI] [PubMed] [Google Scholar]
- 11.Kawamoto R, Tomita H, Oka Y, Ohtsuka N. Relationship between serum uric acid concentration, metabolic syndrome and carotid atherosclerosis. Intern Med. 2006;45:605–614. doi: 10.2169/internalmedicine.45.1661. [DOI] [PubMed] [Google Scholar]
- 12.Waring WS, Webb DJ, Maxwell SR. Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J Cardiovasc Pharmacol. 2001;38:365–371. doi: 10.1097/00005344-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Schretlen DJ, Inscore AB, Jinnah HA, Rao V, Gordon B, Pearlson GD. Serum uric acid and cognitive function in community-dwelling older adults. Neuropsychology. 2007;21:136–140. doi: 10.1037/0894-4105.21.1.136. [DOI] [PubMed] [Google Scholar]
- 14.Waring WS, Convery A, Mishra V, Shenkin A, Webb DJ, Maxwell SR. Uric acid reduces exercise-induced oxidative stress in healthy adults. Clin Sci. 2003;105:425–430. doi: 10.1042/CS20030149. [DOI] [PubMed] [Google Scholar]
- 15.Tsuchiya M, Asada A, Kasahara E, Sato EF, Shindo M, Inoue M. Smoking a single cigarette rapidly reduces combined concentrations of nitrate and nitrite and concentrations of antioxidants in plasma. Circulation. 2002;105:1155–1157. doi: 10.1161/hc1002.105935. [DOI] [PubMed] [Google Scholar]
- 16.Dietrich M, Block G, Norkus EP, Hudes M, Traber MG, Cross CE, et al. Smoking and exposure to environmental tobacco smoke decrease some plasma antioxidants and increase gamma-tocopherol in vivo after adjustment for dietary antioxidant intakes. Am J Clin Nutr. 2003;77:160–166. doi: 10.1093/ajcn/77.1.160. [DOI] [PubMed] [Google Scholar]
- 17.Waring WS, McKnight JA, Webb DJ, Maxwell SRJ. Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabetes. 2006;55:3127–3132. doi: 10.2337/db06-0283. [DOI] [PubMed] [Google Scholar]
- 18.Massey V, Edmondson D. On the mechanism of inactivation of xanthine oxidase by cyanide. J Biol Chem. 1970;245:6595–6598. [PubMed] [Google Scholar]
- 19.Puig JG, Mateos FA, Jiménez ML, Ramos TH. Renal excretion of hypoxanthine and xanthine in primary gout. Am J Med. 1988;85:533–537. doi: 10.1016/S0002-9343(88)80091-3. [DOI] [PubMed] [Google Scholar]
- 20.Leyva F, Wingrove CS, Godsland IF, Stevenson JC. The glycolytic pathway to coronary heart disease: a hypothesis. Metabolism. 1998;47:657–662. doi: 10.1016/S0026-0495(98)90026-9. [DOI] [PubMed] [Google Scholar]
- 21.Bloomer RJ. Decreased blood antioxidant capacity and increased lipid peroxidation in young cigarette smokers compared to nonsmokers: impact of dietary intake. Nutr J. 2007;6:39–40. doi: 10.1186/1475-2891-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watts DT. The effect of nicotine and smoking on the secretion of epinephrine. Ann N Y Acad Sci. 1960;90:74–90. doi: 10.1111/j.1749-6632.1960.tb32619.x. [DOI] [PubMed] [Google Scholar]
- 23.Bruckert E, Jacob N, Lamaire L, Truffert J, Percheron F, Gennes JL. Relationship between smoking status and serum lipids in a hyperlipidemic population and analysis of possible confounding factors. Clin Chem. 1992;38:1698–1705. [PubMed] [Google Scholar]
- 24.Haj Mouhamed D, Ezzaher A, Neffati F, Douki W, Najjar MF. Comparison between plasma and urine thiocyanates and urinary cotinine determinations as indicators of cigarette smoking. J Health Sci. 2009;55:1–6. doi: 10.1248/jhs.55.1. [DOI] [Google Scholar]