Abstract
Chemokines and their receptors have been studied in several solid tumor models as mediators of inflammation. In turn, inflammation has been implicated in the promotion and progression of tumors, and as such, chemokines have been proposed as novel molecular targets for chemotherapy. While the expression of these molecules has been described in tumor cells, endothelial cells, macrophages, and neutrophils, less attention has been paid to the expression profile of these molecules by T lymphocytes in the periphery or infiltrating the tumor. Using the D1-DMBA-3 murine mammary adenocarcinoma model, we aimed to better characterize the differential expression of chemokines and/or their receptors in the host and in the tumor microenvironment, and specifically, in the T cells of tumor-bearing mice compared to normal control animals. We found that T lymphocytes from tumor-bearing mice express the pro-inflammatory chemokines, CCL2, CCL5, and CXCL2, as well as the chemokine receptors, CCR1, CCR2, CCR3 and CXCR2.
Keywords: chemokines, chemokine receptors, T lymphocytes, mammary tumor, angiogenesis
1. Introduction
All tumors, benign or malignant, are comprised of two main components: the neoplastic population and the reactive stroma surrounding that population. Mounting evidence continues to implicate the perivascular and vascular elements, as well as the inflammatory infiltrate of tumors, as contributors to tumor progression and metastasis [1; 2; 3; 4; 5; 6; 7; 8]. Chemokines expressed by the tumor cells recruit a variety of immune cells, including lymphocytes, which may be immunoregulatory or suppressive, or may enrich the chemokine milieu by secreting chemotactic molecules that contribute to the inflammatory process. It has been suggested that the inflammatory reaction at the breast tumor site enhances tumor growth and progression [9]. Since chemokines are known to have angiogenic and growth promoting characteristics, we sought to determine the role of tumor burden on T cell chemokine and chemokine receptor expression using the D1-DMBA-3 mammary adenocarcinoma model.
Chemokines are grouped into four families (C, CC, CXC and CX3C), based on the grouping of cysteine residues near the N-terminus of these proteins [10]. The CC or β chemokines are a large superfamily of small, inducible, secreted, pro-inflammatory cytokines that are expressed by various cell types including monocytes, endothelial cells, and fibroblasts [11]. CCL2/monocyte chemoattractant protein-1 (MCP-1) is upregulated in different tumor cell types, including breast, prostate and melanoma cells [12; 13; 14; 15]. CCL5/Regulated upon Activation Normal T cell Expressed and Secreted (RANTES) is expressed by T lymphocytes, as well as some tumor cells, and has been shown to have a controversial role in breast tumor progression. Soria et al. have established a positive correlation in breast cancer between aggressiveness of the tumor and tumor-derived CCL5 [16; 17], whereas Jayasinghe et al. have reported that tumor-derived CCL5 does not contribute to breast cancer progression [18]. While many of these studies correlated tumor-derived chemokines to disease progression, there are far fewer analyses of lymphocyte-derived chemokines in a tumor model. We have recently reported that CCL2 is elevated in the T lymphocytes of mammary tumor-bearing mice [19]. In this report, we further expand on CC chemokine expression in T lymphocytes of mammary tumor-bearing mice.
The CXC chemokines have been shown to regulate angiogenesis in both a positive and negative manner, depending on the presence or absence of an ELR motif (glutamic acid-leucine-arginine) in the amino terminus. The ELR positive chemokines that promote angiogenesis include CXCL1, -2, -3, -5, -6, -7 and -8 [20]. IL-8, the functional human analogue of murine CXCL2/macrophage inflammatory protein-2 (MIP-2), promotes the growth and invasiveness of breast cancer cells [21; 22; 23]. CXCL2 is produced by macrophages, endothelial cells, epithelial cells and tumor cells [24]. However, it is not known whether lymphocytes from tumor bearers are induced to produce this chemokine. In this study, we determined whether CXCL2 is expressed by T lymphocytes of tumor-bearing mice.
Leukocytes are recruited to sites of inflammation and specific microenvironments within secondary lymphoid tissues based on chemokine and chemokine receptor expression patterns [25; 26]. Previous studies have established similarities between the functions of chemokine receptors in the physiologic homing of leukocytes and in the roles of these receptors in cancer metastasis [27]. Since leukocyte infiltration into tumors is regulated by chemokine production in the tumor microenvironment [28], chemokine receptor expression by lymphocytes may play a role in tumor infiltration, and in shaping the tumor microenvironment via inflammation. For example, it has been well documented that CCR2 and its principal ligand, CCL2, induce monocyte infiltration and promote inflammation [29], while Huang et al. have found that breast, gastric and ovarian tumor growth is enhanced by CCR2+ myeloid suppressor cells attracted to the liver by hepatocyte-produced CCL2 [30]. Furthermore, Robinson et al. reported that CCL5 was produced by 410.4 murine breast cancer cells, and that its receptors, CCR1 and CCR5, are expressed by the leukocyte infiltrates which contribute towards tumor growth [28]. Because chemokine and chemokine receptor expression is crucial for lymphocyte migration, we evaluated the expression of chemokine receptors in T lymphocytes and how that expression relates to tumor infiltration.
2. Materials and Methods
2.1. Mice and cell lines
8–12 weeks of age BALB/c mice used in these studies were obtained from Charles River Laboratories (Charles River Laboratories International, Inc., Wilmington, MA). Animals were cared for and used according to the guidelines of the National Institutes of Health at the animal facility at Florida Atlantic University. The D1-DMBA-3 tumor, syngeneic to BALB/c mice, is a transplantable mammary adenocarcinoma derived from a non-viral, noncarcinogen-induced preneoplastic nodule after treatment with 7,12-dimethylbenzanthracene [31]. The D1-DMBA-3 tumor is immunogenic to the host of origin and nonmetastatic to the spleen, but metastases to the lung and bone marrow do occur. The DA-3 mammary tumor cell line was derived in our laboratory from the D1-DMBA-3 tumor and was maintained in DMEM/high glucose, 10% characterized heat inactivated FCS, 100 U/ml of penicillin, 100 μg/ml of streptomycin, and OPI media supplement (Sigma Chemical Co., St. Louis, MO). Tumors were implanted in BALB/c mice by subcutaneous injection of 1×106 tumor cells resulting in a measurable tumor 7–10 days post implantation.
2.2. Purification of splenic T cells
Spleens were compressed in Teflon tissue homogenizers and the resulting single cell suspensions were pelleted at 300g, subjected to hypotonic shock for red cell removal, washed and counted. Macrophages were removed from the cell suspensions by plastic adherence in pre-warmed RPMI-1640, 5% FCS at 37°C for 1 h in a CO2 incubator. The non-adherent T lymphocytes were purified on nylon wool columns according to the method of Julius et al. [32] and by positive selection using the MACS magnetic separation system (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions. Briefly, single-cell suspensions in cold PEB buffer (PBS supplemented with 2mM EDTA and 0.5% BSA) were incubated with supermagnetic microbeads conjugated to anti-mouse CD90 (Thy1.2), anti-mouse CD4, or anti-mouse CD8 mAb at 4° C for 15 min. Cells were washed twice and loaded onto the magnetic separation columns. The columns were washed three times with cold PEB buffer, and the positively selected Thy1.2+, CD4+, or CD8+ T cells were then eluted. After purification, the cells were routinely >95% viable, as assessed by trypan blue exclusion. FACS analysis using a Becton Dickinson LSR analyzer and anti-mouse FITC-CD90, anti-mouse FITC-CD4, and anti-mouse PE-CD8 antibodies (BD Biosciences Pharmingen, San Diego, CA) confirmed the populations to be ≥93% Thy1.2+, ≥94% CD4+, and ≥90% CD8+ T lymphocytes.
2.3. Cell culture
After purification, splenic T cells were cultured for 2h, 4h, or overnight at 2 × 106 cells/ml in complete media consisting of RPMI-1640, 10% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 mM 2-ME. Cell-free supernatants were removed and stored at −80°C until use. Some of the T lymphocyte cultures were stimulated with Concanavalin A (Con A, Sigma Chemical Co., St. Louis, MO) or with recombinant murine CCL2/MCP-1 (rmCCL2/MCP-1) (PeproTech Inc., Rocky Hill, NJ).
2.4. RNA analyses
Total RNA was isolated using TriReagent (Molecular Research Center, Inc., Cincinnati, Ohio). The detection and quantification of chemokine ligand and receptor mRNA species was analyzed by RNase protection assay using the RiboQuant Multiprobe RPA kit (BD Biosciences Pharmingen) according to the manufacturer’s instructions. Briefly, 20 μg RNA was hybridized overnight to the [α-32P]UTP-radiolabeled anti-sense RNA probe set, which had been synthesized from the following template sets: mCK-5 murine chemokine, mCR-5 murine chemokine receptors, and a custom designed probe set which included CXC receptors CXCR2, -4, and -5 (all from BD Biosciences Pharmingen), after which single-stranded RNA and free probe were digested by RNase A and T1. Subsequently, protected RNA was purified and resolved on a 5% denaturing polyacrylamide gel. The chemokine transcripts were identified by the length of the respective fragments. The quantity of each species was determined by the intensity of the appropriately-sized, protected probe fragment. Equivalence of loading was ascertained by the intensity of the fragments for the housekeeping genes, L32 and glyceraldehyde-3–phosphate dehydrogenase (GAPDH) for each lane. Blots were exposed to Kodak BioMax autoradiography film (PerkinElmer Life And Analytical Sciences, Inc., Waltham, MA) overnight.
2.5. Cytokine ELISA
Cell culture supernatants, tumor cystic fluid, and sera were analyzed for murine chemokine protein levels by OptEIA™ ELISA (BD Biosciences Pharmingen) for CCL2, and analyzed for CXCL-2 and CCL5 protein levels by DuoSet® ELISA development systems (R&D Systems, Minneapolis, MN) according to manufacturers’ instructions. Absorbance at 450 nm with wavelength correction at 570 nm was read on a Tecan SLT Rainbow Reader (Lab Instruments, Research Triangle Park, NC) and OD values of samples were converted to picograms against a standard curve of known quantities of recombinant murine chemokines.
2.6. Western Blot analyses
T lymphocytes were cultured for 3 h in media alone or media with 10 μg/ml Con A. Total protein from T cells of normal and tumor-bearing mice was isolated as previously described [19]. Protein concentration was normalized by comparison with BSA standards (Sigma Chemical Co.). The proteins were resolved on 10% SDS-polyacrylamide gels under reducing conditions and transferred to Protran nitrocellulose-1 membrane (0.45 μm pore size; Schleicher & Schuell, Keene, NH) using a Trans-Blot electrophoretic cell (Bio-Rad, Hercules, CA). The membranes were blocked for 1 h at room temperature in PBS containing 5% nonfat dry milk and 0.05% Tween 20, and subsequently incubated at room temperature for 1 h with rabbit anti-mouse CCR1, -2, and -3 (Santa Cruz Biotechnology Inc., Santa Cruz, CA) and CXCR2 (AbCam Inc., Cambridge, MA). Blots were washed for 30 min with five changes of 1× TBS-0.1% Tween 20 solution followed by 1 h incubation at room temperature with HRP-conjugated anti-rabbit IgG (Santa Cruz Biotechnology Inc.). Blots were washed again for 30 min and incubated for 3 min with Supersignal West Pico chemiluminescent substrate (Pierce, Rockford, IL). The results were visualized by exposing blots to BioMax autoradiographic film. The membranes were then stripped and reprobed with anti-mouse-β-Actin Abs (Sigma Chemical Co.) to confirm equivalent protein loading.
2.7. Immunohistochemistry
To obtain frozen sections, tumors were quickly dissected, embedded in Tissue-Tek® O.C.T. compound (Sakura Finetek U.S.A., Inc., Torrance, CA), frozen in chilled isopentane (−50°C) and stored at −80°C. Sections through the tumors were cut in a cryostat (−20°C) at 16 μm, collected onto Superfrost®/Plus charged slides (Fisher Scientific, Pittsburgh, PA), post-fixed in 4% paraformaldehyde for 10 min and then air dried. Cut tissue sections were stored at −20°C. Sections were blocked in 4% BSA in 0.1 M phosphate buffer (PB) [33], pH 7.4, for 30 min, then labeled with anti-CD3 using goat polyclonal antibody to CD3 (Santa Cruz Biotechnology, Inc.) diluted 1:50 in PB containing 3% BSA and rabbit anti-CCR1 polyclonal antibody specific for CCR1 (Abcam, Inc.) diluted 1:100 and incubated overnight at 4°C. For lymphocyte subset study, sections were labeled with rat anti-CD8 (Santa Cruz Biotechnology, Inc.) diluted 1:50 and goat anti-CCL5 (15 μg/ml) (R&D Scientific) in PB containing 3% BSA. Labeling was localized using rabbit-, rat- or goat-specific donkey IgGs conjugated to AlexaFluor 488 or 568 diluted 1:1000 (Molecular Probes, Eugene, OR). Sections were cover-slipped with Vectashield (Vector Laboratories, Burlingame, CA), and examined using an Olympus AX-70 fluorescence microscope equipped with appropriate barrier filters (ChromaTech) for viewing the individual emission wavelengths, as well as for simultaneous localization of both fluorescent labels. Photomicrographs were taken using a Magnafire digital camera system. Double labeled sections were examined using a Bio-Rad Radiance 2100 Laser Scanning Confocal Microscope (Bio-Rad Laboratories, Inc., Hercules, CA). Digital images were imported in Adobe Photoshop, with minor adjustments made for brightness. Controls for staining included the omission of primary antisera.
2.8. In situ hybridization: Co-localization of CD3 and CCL5 or CCR3
Tissue was first processed for detection of CD3 immunoreactivity (IR) to identify T cells, then the expression of cytokine and receptor mRNA was localized by in situ hybridization of [35S]-labeled cRNAs. All buffers used for immunohistochemistry were removed for RNAses by DEPC treatment and autoclaving. Cryosections (20 μm) were fixed in 4% paraformaldehyde in 0.1 M PB (pH 7.4) for 15 min and blocked in 5 % BSA in PB for 60 min. Sections were then incubated overnight at 4°C in goat antibody to murine CD3 (1:50 dilution in 5% BSA in PB) followed by treatment with biotinylated rabbit anti-goat IgG (both Santa Cruz Biotech, Inc.) for 2 h at room temperature. Antibody binding was localized using avidin-biotin-HRP with diaminobenzidine (DAB) as chromagen, using kit reagents according to the manufacturer’s instructions (Santa Cruz Biotech, Inc.). Following immunostaining, slides were processed for in situ hybridization and emulsion autoradiography (Kodak NTB) as described in detail by Guthrie et al. [34], with the RNA probes added to the hybridization incubation at a concentration of 1 × 107 cpm/ml. The 360 base CCR3 riboprobe was complementary to positions of 241–601 of the GenBank sequence NM 009914. The CCL5 cRNA comprised 214 bases complementary to positions 61–254 of GenBank sequence BC 033508. Autoradiographic exposure intervals ranged from 4 to 8 weeks. Localization of silver grains, indicative of [35S]-cRNA hybridization, and brown DAB reaction product, indicative of CD3-IR, was assessed by microscopic examination using an Olympus AX-70 microscope equipped with a Magnafire digital camera system. Images were imported into Adobe Photoshop for adjustments in brightness and contrast and assembly of composite images.
2.9. Statistical analyses
Data are expressed as the mean ± SD. Statistical significance was determined using two-tailed Student’s t tests. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Expression of CCL2, CCL5, and CXCL2 in tumor-bearing mice
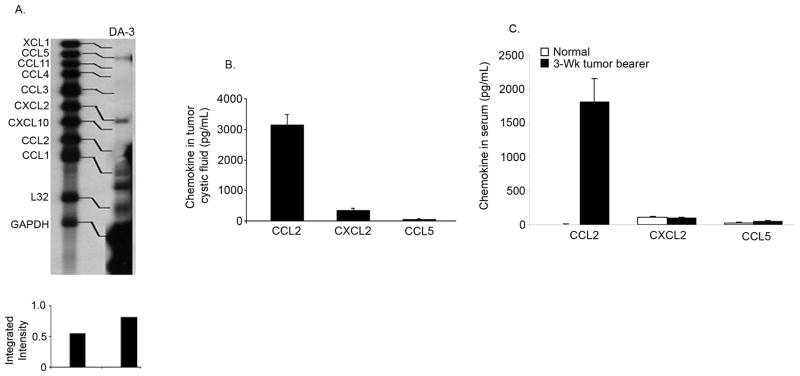
Prior to assessing the contribution of T cells to the inflammatory cytokine mileu within the host and the tumor microenvironment, we wanted to identify any chemokine secretion by the tumor cells themselves. As various breast cancer cell lines are reported to express CCL2, CCL5, and CXCL12 [35; 36], we screened for the expression of various CC and CXC chemokines using multi-probe RNase protection assays (RPA). Analyses of the DA-3 mammary tumor cell line, derived from the parental D1-DMBA-3 tumor, revealed that the tumor cells constitutively expressed low levels of CCL5 and CXCL2 mRNA, but not CCL2 (Fig. 1A). To validate protein secretion of the mRNA expressed by the tumor cells, we evaluated the production of CXCL2 and CCL5 proteins in the tumor cystic fluid and in the sera from control BALB/c and D1-DMBA-3 tumor-bearing mice, using ELISAs. CXCL2 was secreted in moderate quantities of 334 ± 80 pg/mL in the tumor cystic fluid of 3-week tumor bearers; however, there was no significant production of CCL5 within the fluid (65 ± 4 pg/mL, Fig. 1B). We have previously shown that CCL2 is expressed by tumor-infiltrating T lymphocytes of the D1-DMBA-3 tumor [19]. Thus, we also looked at CCL2 production within the tumor cystic fluid and found high levels of CCL2 protein, 3133 ± 351 pg/mL, in that fluid (Fig. 1B). The expression of CXCL2 and CCL5 mRNA by the tumor cells, and the production of CXCL2 within the tumor cystic fluid, did not correspond to higher levels of protein in the sera from 3-week tumor bearers (Fig. 1C). While there were no significant differences in the levels of CXCL2 and CCL5 protein in the sera from control and tumor-bearing mice, the levels of CCL2 were significantly elevated in tumor-bearing animals, confirming earlier experiments [19] (Fig. 1C).
Fig. 1. Expression of proinflammatory chemokines in mammary tumor-bearing mice.
A) DA-3 mammary tumor cells express CCL5 and CXCL2, but not CCL2 mRNA. B) Production of CCL2 and CXCL2 protein in the tumor cystic fluid of 3-week tumor bearers. C) CCL2 protein levels are increased in the circulation of 3-week mammary tumor-bearing mice. These data are representative of at least three independent experiments with at least six mice per group. *Significant difference (p<0.005) between control and tumor bearers’ serum levels of CCL2.
3.2. Expression of CCL2, CCL5 and CXCL2 is increased in T cells from tumor-bearing mice
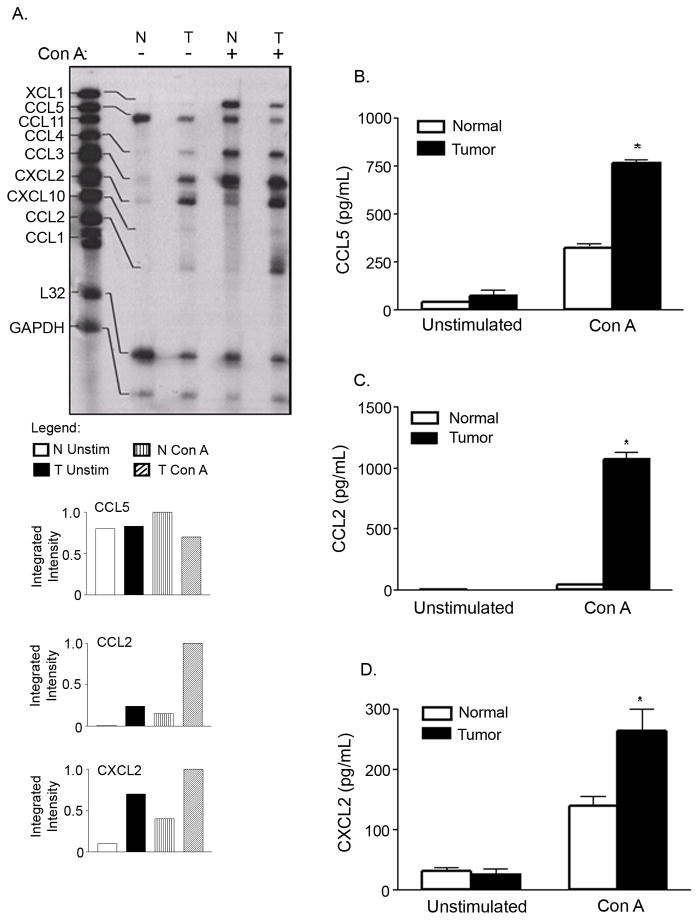
Our previous studies have shown that proinflammatory factors produced by the tumor directly affect the expression of CCL2 in T lymphocytes [19]. To gain insight into differential chemokine expression in T cells from mammary tumor-bearing mice, RPAs were performed on total RNA isolated from purified T lymphocytes from normal and tumor-bearing mice, as described in Materials and Methods. As shown in Figure 2A, the gene expression of CCL5 differed between the T cells of control and tumor-bearing mice. Since mRNA of CCL5 appeared to be down-regulated in tumor bearers’ T cells, both constitutively and upon activation with Con A, we investigated whether the protein levels of this chemokine were likewise altered. Contrary to expectation, the protein levels in unstimulated splenic T cells of normal mice were lower compared to the protein levels from non-activated splenic T cells of tumor bearers. Furthermore, the protein levels of CCL5 were significantly increased in Con A stimulated cells of tumor bearers as determined by ELISA (Fig. 2B). A possible explanation for these results is that the mRNA expressed in unstimulated T cells of normal mice may be silenced, preventing translation of the mRNA. Conversely, post-translational modification may be occurring in the T cells of tumor bearers, resulting in increased protein levels.
Fig. 2. Increased expression of chemokines by T lymphocytes of tumor-bearing mice.
A) Increased expression of chemokine mRNA by T lymphocytes from tumor-bearing mice. B) Increased protein levels of CCL5. C) Increased protein levels of CCL2. D) Increased CXCL2 protein secretion in tumor-bearing mice. For mRNA expression studies of chemokines, purified splenic T cells from normal (N) and 4-week tumor-bearing mice (T) were cultured for 4 h +/− 10 μg/ml Con A. Total RNA was isolated and 20 μg was subjected to a multi-probe RNase protection assay. These data are representative of at least three independent experiments with six mice per group. For protein secretion studies, purified splenic T cells from normal and 4-week tumor-bearing mice were cultured overnight +/− 10 μg/ml Con A. Cell-free supernatants were collected and assayed by specific ELISAs. These data are representative of at least four independent experiments with at least five mice per group. *Significant difference (p<0.005) between control and tumor bearers’ T lymphocytes treated with Con A. No significant differences were noted for unstimulated T cells between control and tumor-bearing mice for CCL2, CCL5, and CXCL2.
We have previously reported an increase in the expression of CCL2 in tumor-bearing mice compared to normal BALB/c mice [19]. In this study, we confirmed that Thy1.2+ cells from normal mice did not express CCL2 constitutively, while Thy 1.2+ cells from mice bearing mammary tumors did. Furthermore, a significant increase in CCL2 mRNA expression was observed in tumor bearers’ T cells cultured with 10 μg/ml Con A (Fig 2A). While negligible amounts of CCL2 protein were produced by normal T cells, T cells of mammary tumor bearers were capable of producing high levels of CCL2 protein (Fig. 2C). Treatment with 10 μg/ml Con A was required to induce this CCL2 protein secretion. These results were consistent with other reports that mitogenic stimulation dramatically increased CCL2 secretion by freshly isolated human PBMCs [37] and myelomonocytic cell lines [38]. These results also confirmed that secretion of CCL2 parallels increased mRNA expression upon activation with Con A. Increased levels of CCL2 have correlated with angiogenesis and tumor progression in breast cancer models [39].
We next analyzed the expression of CXCL2, an angiogenic chemokine which has potent chemotactic activity for neutrophils [24]. A substantial increase in mRNA expression of this chemokine was demonstrated by T cells of tumor-bearing mice, both constitutively and with stimulation (Fig. 2A). To determine whether the increased mRNA expression of CXCL2 translated into enhanced protein levels, ELISAs were performed. Consistent with the mRNA levels, there was a significant difference in the secretion of this chemokine between normal and tumor bearers’ T cells, with higher levels of CXCL2 protein secretion observed in tumor bearers’ T cells cultured with Con A (Fig. 2D). However, the higher constitutive levels of CXCL2 mRNA seen in the RPAs did not translate into corresponding increases in constitutive protein levels in tumor bearers’ T cells. Possible explanations for the decreased protein secretion by the T cells of tumor-bearing mice may include decreased mRNA stability, inefficient mRNA translation, or increased proteosomal activity leading to protein degradation in the tumor bearers’ T cells.
Although there were differences at the mRNA level of XCL1, CCL3 and CCL4 between the control and 3-week tumor-bearing mice, there were no significant differences at the protein level (data not shown). We therefore focused our attention on CCL2, CCL5 and CXCL2 for the remainder of the studies.
3.3. Chemokine receptor expression is altered in tumor bearers’ T lymphocytes
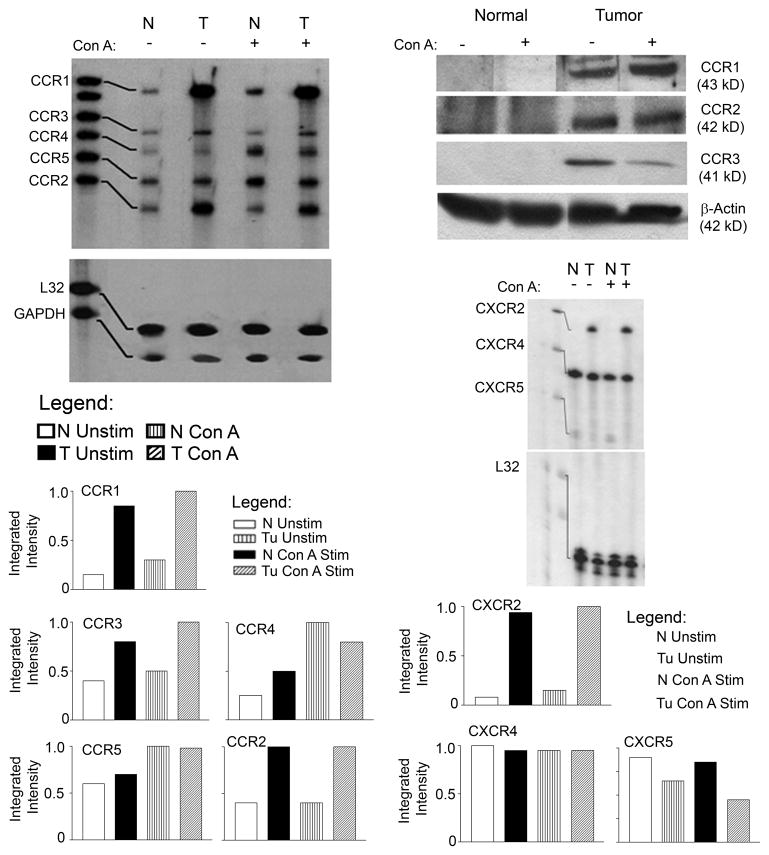
We then set out to characterize how mammary tumor burden alters chemokine receptor expression. In order to assess the expression of these receptors by splenic T lymphocytes, an RPA consisting of probes for the mRNA of CCR1, -1b, -2, -3, -4, and -5, along with two housekeeping genes, was utilized. Figure 3A shows that the CCL2 receptors, CCR1, -2 and -3, were up-regulated in the tumor bearers’ T cells compared to normal T cells, both constitutively and with stimulation. The expression of CCR5 and CCR4 was not significantly different between normal and tumor bearers’ T cells.
Fig. 3.
The expression of CC and CXC chemokine receptors is altered in splenic T cells of tumor-bearing mice. A) RNase protection assay of CC chemokine receptors. B) Western Blot of CC receptors. C) RNase protection assay of CXC receptors. For RNase protection studies, splenic T cells from normal and tumor-bearing mice were cultured for 2 h +/− 10 μg/ml Con A. Total RNA was isolated and 20 μg was subjected to a multi-probe CC or CXC chemokine protection assay. For Western Blotting studies, splenic T cells from normal and tumor-bearing animals were cultured for 3 h +/− 10 μg/ml Con A. Total protein was extracted and 50 μg from each sample was run. These data are representative of at least three independent experiments with six mice per group.
The T lymphocytes from both normal and tumor bearers were then analyzed for chemokine receptors at the protein level. Because very few murine antibodies are available for the flow cytometric analysis of chemokine receptor expression, Western blots were performed to analyze the protein levels of chemokine receptors using whole cell lysates of splenic T lymphocytes from normal and mammary tumor-bearing mice. Consistent with the gene expression analyses, the protein levels of CCR1, -2 and -3 were elevated in the T cells of tumor bearers compared to those of normal mice (Fig. 3B).
Additionally, we evaluated the expression of CXCR chemokine receptors using a multi-probe RPA consisting of CXCR2, -4 and -5, along with two housekeeping genes. Constitutive and induced CXCR4 receptor expression levels appeared to be equivalent in the T cells of both normal and tumor-bearing mice, indicating that expression of this receptor is not influenced by the presence of the tumor (Fig. 3C). CXCR5, a receptor normally expressed by memory and follicular T helper cells [40], was expressed at low levels by normal, but not by tumor-bearers’ T lymphocytes. More importantly, the CXCR2 receptor, although not expressed by T cells from normal mice, was expressed by the T cells from tumor-bearing mice, both constitutively and upon mitogenic stimulation (Fig. 3C).
3.4. Expression of chemokines and their receptors in T cell subsets
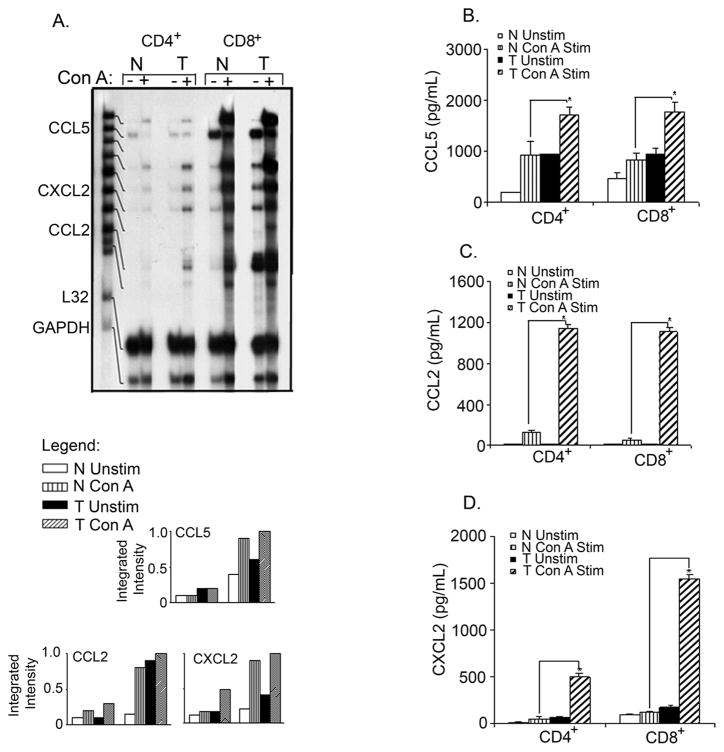
Selective recruitment of immune effector cells to the site of inflammation is known to be regulated by the expression of chemokines and the presence of specific chemokine receptors on different leukocyte subsets [41; 42]. Therefore, we analyzed splenic CD4+ and CD8+ T cell subsets for chemokine and chemokine receptor expression. A higher steady-state level of mRNA expression of chemokines was seen in cultured CD8+ cells compared to CD4+ cells of both normal and tumor bearers’ T lymphocytes (Fig. 4A). The constitutive expression of CCL2 and CXCL2 was higher in CD8+ T cells of tumor bearers compared to those of normal mice, while the constitutive expression of CCL5 appeared to be equivalent in both normal and tumor bearers’ CD8+ T lymphocytes. Stimulation of T lymphocytes with Con A resulted in the enhanced expression of CCL2 and CXCL2 in CD8+ T cells of tumor-bearing mice compared to those of normal mice, while the expression of CCL5 was minimally increased in CD8+ T cells of tumor-bearing mice (Fig. 4A).
Fig. 4.
The expression of CC and CXC chemokines is altered in splenic CD4+ and CD8+ T cell subsets of tumor-bearing mice. A) RNase protection of CC and CXC chemokines, and protein expression of: B) CCL5, C) CCL2, and D) CXCL2. Splenic CD4+ and C8+ T cells from normal and tumor-bearing mice were isolated by Miltneyi magnetic bead technique and then cultured for 2 h +/− 10 μg/ml Con A for RNA and overnight for protein. Total RNA was isolated and 20 μg was subjected to a multi-probe protection assay. For protein expression studies, cell-free supernatants were collected and assayed by specific ELISAs. These data are representative of at least three independent experiments with at least six mice per group. *Significant difference (p<0.005) between control and tumor bearers’ T lymphocytes treated with Con A.
Having observed that CCL2 and CXCL2 gene expression was elevated in tumor bearers’ CD8+ T cells, we analyzed the protein levels of CCL2, CCL5 and CXCL2 secreted by CD4+ and CD8+ T cells cultured with Con A. Unlike what was observed at the message level, both CD4+ and CD8+ T cells of tumor-bearing mice expressed high levels of CCL5 (Fig 4B), CCL2 (Fig 4C) and CXCL2 (Fig 4D). It is possible that post-transcriptional modification may be occurring in CD4+ T cells, resulting in higher levels of protein production in tumor bearers.
In contrast to the expression of the chemokine ligands, the expression of the receptors CCR2, -3 and -4 was higher in the CD4+ T cells compared to the CD8+ T cell subset in tumor-bearing animals (Fig. 5). However, the levels of CCR1 and CCR5 seemed to be higher in CD8+ cells. Although previous studies have found differential expression of chemokine receptors in TH1/TH2 lymphocyte subsets [43], to our knowledge, there are no reports of chemokine receptor studies in CD4+/CD8+ T lymphocytes in a mammary tumor model.
Fig. 5.
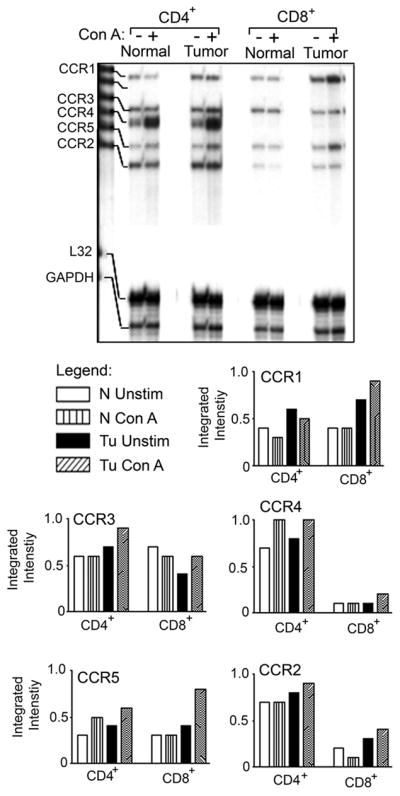
The expression of CC and CXC chemokine receptors is altered in splenic CD4+ and CD8+ T cell subsets of tumor-bearing mice. Total RNA was isolated and 20 μg was subjected to a multi-probe protection assay. These data are representative of at least three independent experiments with at least six mice per group.
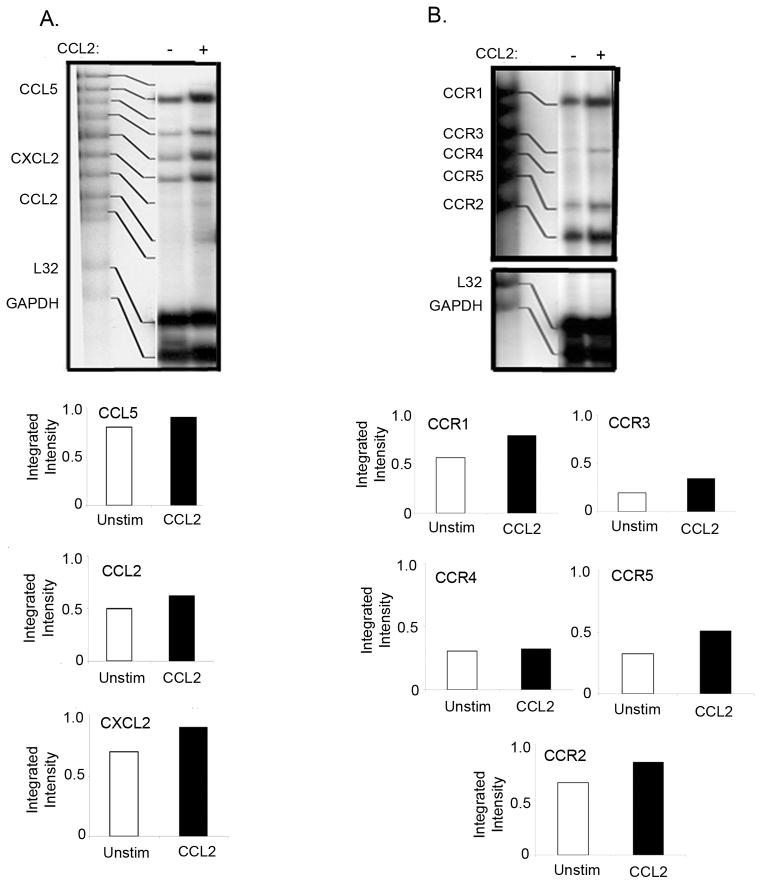
3.5. The effect of CCL2 treatment on chemokine and chemokine receptor expression in T cells from mammary tumor-bearing mice
Chemokines are one of the key components of the tumor microenvironment which shape leukocyte recruitment and induce the production of factors to promote tumor growth and angiogenesis [44]. Since CCL2 is overexpressed in splenic T cells and in TILs of the tumor-bearing mice, we hypothesized that CCL2 may modulate the expression of other chemokines and chemokine receptors in a paracrine manner. Although we did not notice appreciable differences in chemokines or chemokine receptor mRNA expression in T cells from normal mice treated with recombinant murine CCL2 (data not shown), we did see changes in the expression of chemokine and chemokine receptor mRNA in the T cells from tumor-bearing mice. Treatment of T lymphocytes from mammary tumor-bearing mice resulted in significant increases in CCL5 and CXCL2 mRNA (Fig. 6A), suggesting a role for CCL2 in the increased expression of CCL5 and CXCL2. Furthermore, CCL2 treatment of T cells resulted in the increased expression of CCR1, -2, -3 and -5 receptors, but had no effect on the expression of CCR4 (Fig. 6B). This study indicates that CCL2 affects the expression of chemokine receptors in tumor-bearing animals.
Fig. 6.
The effect of CCL2 on chemokine (A) and chemokine receptor (B) expression in T cells from mammary tumor-bearing mice. Splenic T cells from mammary tumor-bearing mice were cultured in the presence of 10 ng/ml of recombinant murine CCL2 (rmCCL2) for 4 h. Total RNA was isolated and 20 μg was subjected to a multi-probe protection assay for chemokines and chemokine receptors. These data are representative of at least three independent experiments with six mice per group.
3.6. Chemokine receptors and chemokines are expressed by tumor-infiltrating T lymphocytes
Our previous studies established the infiltration of the D1-DMBA-3 mammary tumors by T lymphocytes three weeks post-implantation [19]. To further elucidate the effect of tumor-host interactions on T cell chemokine and chemokine receptor expression, we analyzed the expression of these molecules in the mammary tumors using immunohistochemistry and in situ hybridization. Immunohistochemisty analyses revealed that although CCR1 appeared to be expressed by T lymphocytes in the tumor, as indicated by co-localization of CD3 with CCR1, this receptor was also expressed in CD3 negative cells (Fig. 7, A–C). CCR3 is a receptor for both CCL2 and CCL5, and we have observed increased levels of CCR3, CCL2 and CCL5 in splenic T cells of mammary tumor-bearing mice. Combined immunolabeling in frozen tissue sections with in situ hybridization was performed to determine whether splenic cells also express CCR3. Immunolabeling of T cells with CD3 antibody (brown reaction product) combined with in situ hybridization (silver grains) using 35S-labeled cRNAs for CCR3 was performed. These studies revealed that CCR3 mRNA is expressed by T cells clustered within periarteriole lymphoid sheaths in the spleens of normal mice (Fig. 7, D), and that this expression increased in the spleens of tumor-bearing mice (Fig. 7, E). However, grain density overlying individual T cells within tumors did not exceed background levels, compared to that associated with splenic T cells (data not shown), indicating that similar to other studies [45], receptor desensitization may be occurring here.
Fig. 7.
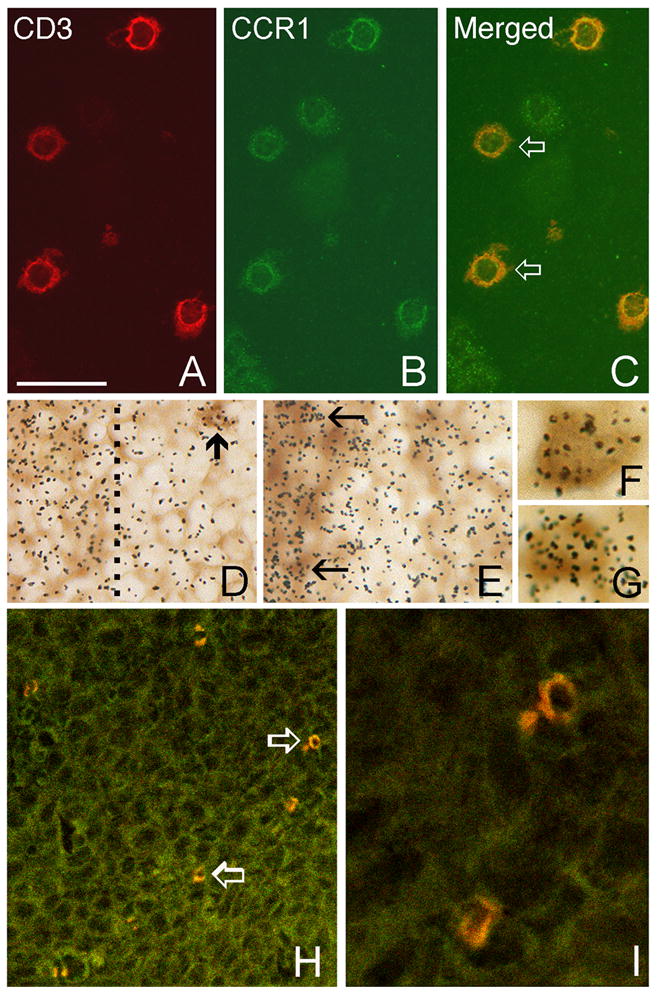
Expression of chemokines and chemokine receptors by T cells. A–C. Immunostaining of tumor sections at 3-weeks of growth showing co-localization of CD3-immunoreactivity (red in A) with CCR1-immunoreactivity (green in B) in tumor-infiltrating T cells. Double-labeled cells in the merged image are indicated by open arrows (C). D–I. Immunolabeling of T-cells with CD3 antibody (brown reaction product) combined with in situ hybridization (silver grains) using 35S-labeled cRNAs for detection of CCR3 (D–E) and CCL5 mRNAs (F–G). Grain density indicative of CCR3 expression is concentrated over T cells located within periarteriole lymphoid sheaths in the spleens of normal mice (D), and is increased in the spleens of tumor-bearing mice (E). T cells within the sheaths are located to the left and the dashed line in D indicates the sheath margin. The arrow in D indicates a cluster of labeled T cells outside the sheath. The arrows in E indicate labeled cells within the sheath. F–G. Double labeling of tumor-infiltrating T cells at 3-weeks post tumor implantation for CCL5 and CD3. H-I. Merged confocal microscope images of CD8 (red) and CCL5 (green) immunostained tumor section showing CD8+ T cells that express CCL5 (open arrows in H). These cells are shown in higher maginification in I. Bar in A = 35 μm in A–C and I, 17 μm in D–E, 7 μm in F–G and 93 μm in H.
Our previous studies have shown that although the DA-3 mammary tumor cells do not express CCL2, expression of this chemokine is induced in the T lymphocytes of mammary tumor-bearing mice and that it is expressed by the tumor-infiltrating T cells [19]. In this study, we have shown that CCL5 is expressed by the DA-3 mammary tumor cells and that it is also produced by splenic T cells of mammary tumor-bearing mice. To determine the effect of tumor microenvironment on CCL5 expression, we localized CCL5 cRNA using in situ hybridization. Figure 7 illustrates grain density indicative of CCL5 mRNA expression by tumor-infiltrating T cells (Fig. 7, F–G). To further delineate the subset of lymphocytes expressing CCL5 in the tumor, immunohistochemical analyses were performed using frozen tumor sections. This study revealed that CCL5 is expressed by CD8+ T cells, as indicated by co-localization of CCL5 and CD8 (Fig. 7, H–I). These results also indicate that CCL5 is expressed in vivo within the tumor microenvironment, confirming the expression of CCL5 in DA-3 tumor cells shown by RPA.
4. Discussion
Chemokines have been reported to be elevated in the plasma of breast cancer patients, compared to healthy individuals [46; 47], and may serve as prognostic indicators for disease spread and relapse [48; 49]. The inflammatory microenvironment rich in chemokines is thought to be ideal for tumor cell development and growth. Although breast cancer cells and cell lines are known to secrete various pro-inflammatory chemokines including CCL2, CCL5 and CXCL2 (34, 46–48), relatively little is known about the effect of mammary tumors on the expression of these chemokines and their receptors by T lymphocytes. In this study, we report that CCL2 and CCL5 were upregulated in both splenic and tumor-infiltrating T cells and that CXCL2 is overexpressed by splenic T lymphocytes of tumor-bearing mice but not normal controls. We also demonstrate that the chemokine receptors, CCR1, -2 and -3 are upregulated in tumor bearers’ T lymphocytes at both mRNA and protein levels, while CXCR2 is expressed at the mRNA level. Furthermore, treatment of T lymphocytes with rmCCL2 increased the expression of CCL5, CXCL2, CCR1, -2 and -5.
The pro-inflammatory and pro-angiogenic chemokine, CCL2, has been shown to be highly expressed by breast cancer cells at primary tumor sites [35] and is known to contribute towards tumor growth. We have previously demonstrated that this chemokine is also secreted by splenic and tumor-infiltrating T lymphocytes of mammary tumor-bearing mice and that this induced secretion is mediated by tumor-derived phosphatidyl serine and GM-CSF. In this study, we further explored these findings and found that CCL2 is expressed primarily by the CD8+ subset of T lymphocytes in our tumor model. Since CCL2 is known to affect tumor growth directly via its pro-angiogenic activity and indirectly by attracting monocytes that secrete tumor-promoting factors [50], secretion of CCL2 by T cells may affect tumor growth in a similar manner. Our previous studies have shown that increased production of CCL2 may also play a role in compromised T cell effector function, as exposure of the T cells to recombinant CCL2 resulted in the decreased secretion of IFN-γ [19]. Thus, the increased levels of CCL2 may contribute towards tumor progression by inhibiting anti-tumor effects of T lymphocytes, as well as by promoting angiogenesis.
The expression of CCL5 by tumor cells has been correlated with tumor progression in several types of cancer, including breast cancer [12; 47; 51]. The DA-3 mammary tumor cells used in this study expressed low levels of CCL5, which does not account for the levels observed in circulation. We therefore investigated for other sources of this chemokine. In this report, we have shown that splenic T lymphocytes, as well as tumor-infiltrating CD8+ T cells, express CCL5 and that the receptors for CCL5, CCR1 and CCR3 are expressed at high levels in TILs and T cells of tumor bearers, compared to normal mice. Two possible explanations may be made for the role of differential expression of CCL5 and CCL5 receptors in tumor bearers’ T cells in our study. It is possible that tumor derived CCL5 is responsible for lymphocyte infiltration into tumors to help establish anti-tumor immunity or conversely, CCL5 may recruit T cells which promote tumor growth. Fischer et al. [52] showed that CCL5 produced by Reed Sternberg cells is one mechanism by which CCR3+ mast cells can be attracted into the tumor tissue in Hodgkin’s lymphoma. Furthermore, Robinson et al. showed that CCR1+ monocytes are attracted to a mammary tumor expressing CCL5, and that treatment with a CCR1 receptor antagonist resulted in decreased tumor growth [28]. Likewise, the interaction of mammary tumor cells and lymphocytes may play an important role in tumor growth. In contrast, Mellado et al. have shown that the binding of CCL5 to CCR5 promotes cell death of tumor infiltrating cells via activation of a cytochrome c-dependent pathway [53]. Okita et al. have found that gastric tumor cells acquire an invasive potential through interaction with peripheral blood mononuclear cells and that CCL5 plays a role in this interaction [54]. Contrary to this, studies by Jayasinghe et al., investigating the role of CCL5 in tumor progression, found that tumor-derived CCL5 does not contribute to breast cancer progression and pointed towards a role for host-derived chemokines in the progress of the disease [18]. Future studies in our laboratory will investigate the role of T lymphocyte-derived CCL5 in mammary tumor progression.
The CXC chemokines are important regulators of tumor growth and metastasis, as they play an extensive role in angiogenesis. Production of CXCL2 is associated with macrophages, endothelial cells, epithelial cells and tumor cells [55; 56]. Tumor growth has been linked to increased angiogenesis due to the interaction of CXCL2 with endothelial cell expressed CXCR2 [57]; in addition, IL-8, which is the human analogue of mouse CXCL2, was found to act as a direct autocrine growth factor for malignant melanoma [58], liver and pancreatic tumors [59], and for colon carcinoma cells [60]. In this study, we show that in addition to being produced by the DA-3 mammary tumor cells, splenic T lymphocytes of mammary tumor-bearing mice also secrete CXCL2.
We have demonstrated elevated levels of CCL2 in the circulation of mammary tumor-bearing mice and have shown that T cells and more importantly, tumor-infiltrating T cells, express this chemokine [19]. In this study we analyzed whether CCL2 plays a role in the induction of other inflammatory chemokines or their receptors. We show for the first time that T cells treated with CCL2 upregulate the gene expression of CCL5 and CXCL2 and the receptors, CCR1, -2, and -3. It is important to note that CCL2 is known to recruit monocytes that differentiate into TAMs within the tumor microenvironment and facilitate tumor progression [61; 62]. Thus, CCL2 may prove to be a target for immunotherapy in cancer, and is currently being investigated in Phase I clinical trials [63].
Enhanced leukocyte infiltration of tumors has been associated with increased tumor vascularity [64]. The chemokines expressed in our tumor system can exert angiogenic effects either by directly acting on the cancer cells or indirectly through the effector cells they recruit. Soria et al. have shown that co-expression of CCL5 and CCL2 in the same tumor was associated with more advanced stages of breast cancer and have suggested that breast tumors “benefit” from interactions between the two chemokines [12]. Our studies show that splenic and tumor-infiltrating T lymphocytes of mammary tumor-bearing mice secrete CCL2, CCL5 and CXCL2 and express CCR1, -2, -3 and CXCR2. Other studies suggest that chemokine receptor expression, e.g., the CCL5 receptors CCR 1 and CCR5, contribute to breast tumor development as treatment with CCR1/CCR5 receptor antagonists reduced the volume and weight of the tumors [28]. Based on these findings, we postulate that T cells of tumor-bearing mice may be promoting tumor growth instead of inhibition due to increased expression of angiogenic chemokines and their receptors. In order to validate a role for these T lymphocyte-derived molecules in mammary tumor progression, we plan to explore the effects of gene silencing on D1-DMBA-3 tumor growth and angiogenesis.
Highlights
T lymphocytes from mammary tumor-bearing mice express CCL2, CCL5 and CXCL2
Chemokine receptors CCR-1, -2, -3 and CXCR2 are upregulated in T lymphocytes of tumor-bearing mice.
Both splenic T cellsand tumor-infiltrating T lymphocytes express CCL2 andCCL5 as well as CCR1.
CCL2 induces the expression of CCL2 in an autocrine manner
CCL2 induces expression of CCL5 and CXCL2 in a paracrine manner
Acknowledgments
The excellent technical assistance of Mantley Dorsey Jr. is gratefully acknowledged. We are grateful for Drs. Diana M. Lopez and Mahyar Nouri-Shirazi for reviewing this manuscript.
This work was supported by grant F02S-FAU-1 from the Florida division of the American Cancer Society and NIH R15 CA135513-01 and R15 CA135513-01-OS1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Solinas G, Marchesi F, Garlanda C, Mantovani A, Allavena P. Inflammation-mediated promotion of invasion and metastasis. Cancer Metastasis Rev. 2010;29:243–8. doi: 10.1007/s10555-010-9227-2. [DOI] [PubMed] [Google Scholar]
- 2.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 3.Coussens LM, Werb Z. Inflammatory cells and cancer: think different! J Exp Med. 2001;193:F23–6. doi: 10.1084/jem.193.6.f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–6. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 6.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 7.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 8.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 9.Azenshtein E, Luboshits G, Shina S, Neumark E, Shahbazian D, Weil M, Wigler N, Keydar I, Ben-Baruch A. The CC chemokine RANTES in breast carcinoma progression: regulation of expression and potential mechanisms of promalignant activity. Cancer Res. 2002;62:1093–102. [PubMed] [Google Scholar]
- 10.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 11.Van Coillie E, Van Damme J, Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 1999;10:61–86. doi: 10.1016/s1359-6101(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 12.Soria G, Yaal-Hahoshen N, Azenshtein E, Shina S, Leider-Trejo L, Ryvo L, Cohen-Hillel E, Shtabsky A, Ehrlich M, Meshel T, Keydar I, Ben-Baruch A. Concomitant expression of the chemokines RANTES and MCP-1 in human breast cancer: a basis for tumor-promoting interactions. Cytokine. 2008;44:191–200. doi: 10.1016/j.cyto.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Loberg R, Liao J, Ying C, Snyder LA, Pienta KJ, McCauley LK. A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Cancer Res. 2009;69:1685–92. doi: 10.1158/0008-5472.CAN-08-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molloy AP, Martin FT, Dwyer RM, Griffin TP, Murphy M, Barry FP, O’Brien T, Kerin MJ. Mesenchymal stem cell secretion of chemokines during differentiation into osteoblasts, and their potential role in mediating interactions with breast cancer cells. Int J Cancer. 2009;124:326–32. doi: 10.1002/ijc.23939. [DOI] [PubMed] [Google Scholar]
- 15.Graves DT, Barnhill R, Galanopoulos T, Antoniades HN. Expression of monocyte chemotactic protein-1 in human melanoma in vivo. Am J Pathol. 1992;140:9–14. [PMC free article] [PubMed] [Google Scholar]
- 16.Luboshits G, Shina S, Kaplan O, Engelberg S, Nass D, Lifshitz-Mercer B, Chaitchik S, Keydar I, Ben-Baruch A. Elevated expression of the CC chemokine regulated on activation, normal T cell expressed and secreted (RANTES) in advanced breast carcinoma. Cancer Res. 1999;59:4681–7. [PubMed] [Google Scholar]
- 17.Saji H, Koike M, Yamori T, Saji S, Seiki M, Matsushima K, Toi M. Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer. 2001;92:1085–91. doi: 10.1002/1097-0142(20010901)92:5<1085::aid-cncr1424>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 18.Jayasinghe MM, Golden JM, Nair P, O’Donnell CM, Werner MT, Kurt RA. Tumor-derived CCL5 does not contribute to breast cancer progression. Breast Cancer Res Treat. 2008;111:511–21. doi: 10.1007/s10549-007-9802-6. [DOI] [PubMed] [Google Scholar]
- 19.Owen JL, Lopez DM, Grosso JF, Guthrie KM, Herbert LM, Torroella-Kouri M, Iragavarapu-Charyulu V. The expression of CCL2 by T lymphocytes of mammary tumor bearers: role of tumor-derived factors. Cell Immunol. 2005;235:122–35. doi: 10.1016/j.cellimm.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 20.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–57. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 21.Yao C, Lin Y, Chua MS, Ye CS, Bi J, Li W, Zhu YF, Wang SM. Interleukin-8 modulates growth and invasiveness of estrogen receptor-negative breast cancer cells. Int J Cancer. 2007;121:1949–57. doi: 10.1002/ijc.22930. [DOI] [PubMed] [Google Scholar]
- 22.Freund A, Jolivel V, Durand S, Kersual N, Chalbos D, Chavey C, Vignon F, Lazennec G. Mechanisms underlying differential expression of interleukin-8 in breast cancer cells. Oncogene. 2004;23:6105–14. doi: 10.1038/sj.onc.1207815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Y, Huang R, Chen L, Li S, Shi Q, Jordan C, Huang RP. Identification of interleukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays. Int J Cancer. 2004;109:507–15. doi: 10.1002/ijc.11724. [DOI] [PubMed] [Google Scholar]
- 24.Baggiolini M, Loetscher P, Moser B. Interleukin-8 and the chemokine family. Int J Immunopharmacol. 1995;17:103–8. doi: 10.1016/0192-0561(94)00088-6. [DOI] [PubMed] [Google Scholar]
- 25.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 26.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 27.Kakinuma T, Hwang ST. Chemokines, chemokine receptors, and cancer metastasis. J Leukoc Biol. 2006;79:639–51. doi: 10.1189/jlb.1105633. [DOI] [PubMed] [Google Scholar]
- 28.Robinson SC, Scott KA, Wilson JL, Thompson RG, Proudfoot AE, Balkwill FR. A chemokine receptor antagonist inhibits experimental breast tumor growth. Cancer Res. 2003;63:8360–5. [PubMed] [Google Scholar]
- 29.Tylaska LA, Boring L, Weng W, Aiello R, Charo IF, Rollins BJ, Gladue RP. Ccr2 regulates the level of MCP-1/CCL2 in vitro and at inflammatory sites and controls T cell activation in response to alloantigen. Cytokine. 2002;18:184–90. doi: 10.1006/cyto.2002.1031. [DOI] [PubMed] [Google Scholar]
- 30.Huang B, Lei Z, Zhao J, Gong W, Liu J, Chen Z, Liu Y, Li D, Yuan Y, Zhang GM, Feng ZH. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007;252:86–92. doi: 10.1016/j.canlet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Medina D, DeOme KB. Response of hyperplastic alveolar nodule outgrowth-line D1 to mammary tumor virus, nodule-inducing virus, and prolonged hormonal stimulation acting singly and in combination. J Natl Cancer Inst. 1969;42:303–10. [PubMed] [Google Scholar]
- 32.Julius MH, Simpson E, Herzenberg LA. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645–9. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 33.Campbell MJ, Scott J, Maecker HT, Park JW, Esserman LJ. Immune dysfunction and micrometastases in women with breast cancer. Breast Cancer Res Treat. 2005;91:163–71. doi: 10.1007/s10549-004-7048-0. [DOI] [PubMed] [Google Scholar]
- 34.Guthrie KM, Woods AG, Nguyen T, Gall CM. Astroglial ciliary neurotrophic factor mRNA expression is increased in fields of axonal sprouting in deafferented hippocampus. J Comp Neurol. 1997;386:137–48. [PubMed] [Google Scholar]
- 35.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008 doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 36.Lee BC, Lee TH, Avraham S, Avraham HK. Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor. 1alpha in breast cancer cell migration through human brain microvascular endothelial cells. Mol Cancer Res. 2004;2:327–38. [PubMed] [Google Scholar]
- 37.Van Damme J, Proost P, Put W, Arens S, Lenaerts JP, Conings R, Opdenakker G, Heremans H, Billiau A. Induction of monocyte chemotactic proteins MCP-1 and MCP-2 in human fibroblasts and leukocytes by cytokines and cytokine inducers. Chemical synthesis of MCP-2 and development of a specific RIA. J Immunol. 1994;152:5495–502. [PubMed] [Google Scholar]
- 38.Steube KG, Meyer C, Drexler HG. Constitutive protein expression of monocyte chemotactic protein-1 (MCP-1) by myelomonocytic cell lines and regulation of the secretion by anti- and proinflammatory stimuli. Leuk Res. 1999;23:843–9. doi: 10.1016/s0145-2126(99)00107-1. [DOI] [PubMed] [Google Scholar]
- 39.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–85. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 40.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor. 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–62. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–8. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 42.Nelson PJ, Krensky AM. Chemokines, lymphocytes and viruses: what goes around, comes around. Curr Opin Immunol. 1998;10:265–70. doi: 10.1016/s0952-7915(98)80164-7. [DOI] [PubMed] [Google Scholar]
- 43.Francis JN, Lloyd CM, Sabroe I, Durham SR, Till SJ. T lymphocytes expressing CCR3 are increased in allergic rhinitis compared with non-allergic controls and following allergen immunotherapy. Allergy. 2007;62:59–65. doi: 10.1111/j.1398-9995.2006.01253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 45.Kurt RA, Baher A, Wisner KP, Tackitt S, Urba WJ. Chemokine receptor desensitization in tumor-bearing mice. Cell Immunol. 2001;207:81–8. doi: 10.1006/cimm.2000.1754. [DOI] [PubMed] [Google Scholar]
- 46.Potter SM, Dwyer RM, Curran CE, Hennessy E, Harrington KA, Griffin DG, Kerin MJ. Systemic chemokine levels in breast cancer patients and their relationship with circulating menstrual hormones. Breast Cancer Res Treat. 2008 doi: 10.1007/s10549-008-0078-2. [DOI] [PubMed] [Google Scholar]
- 47.Niwa Y, Akamatsu H, Niwa H, Sumi H, Ozaki Y, Abe A. Correlation of tissue and plasma RANTES levels with disease course in patients with breast or cervical cancer. Clin Cancer Res. 2001;7:285–9. [PubMed] [Google Scholar]
- 48.Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpe S, Vermeulen PB, Dirix LY. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res. 2004;10:7157–62. doi: 10.1158/1078-0432.CCR-04-0812. [DOI] [PubMed] [Google Scholar]
- 49.Lebrecht A, Grimm C, Lantzsch T, Ludwig E, Hefler L, Ulbrich E, Koelbl H. Monocyte chemoattractant protein-1 serum levels in patients with breast cancer. Tumour Biol. 2004;25:14–7. doi: 10.1159/000077718. [DOI] [PubMed] [Google Scholar]
- 50.Ben-Baruch A. The multifaceted roles of chemokines in malignancy. Cancer Metastasis Rev. 2006;25:357–71. doi: 10.1007/s10555-006-9003-5. [DOI] [PubMed] [Google Scholar]
- 51.Sugasawa H, Ichikura T, Kinoshita M, Ono S, Majima T, Tsujimoto H, Chochi K, Hiroi S, Takayama E, Saitoh D, Seki S, Mochizuki H. Gastric cancer cells exploit CD4+ cell-derived CCL5 for their growth and prevention of CD8+ cell-involved tumor elimination. Int J Cancer. 2008;122:2535–41. doi: 10.1002/ijc.23401. [DOI] [PubMed] [Google Scholar]
- 52.Fischer M, Juremalm M, Olsson N, Backlin C, Sundstrom C, Nilsson K, Enblad G, Nilsson G. Expression of CCL5/RANTES by Hodgkin and Reed-Sternberg cells and its possible role in the recruitment of mast cells into lymphomatous tissue. Int J Cancer. 2003;107:197–201. doi: 10.1002/ijc.11370. [DOI] [PubMed] [Google Scholar]
- 53.Mellado M, de Ana AM, Moreno MC, Martinez C, Rodriguez-Frade JM. A potential immune escape mechanism by melanoma cells through the activation of chemokine-induced T cell death. Curr Biol. 2001;11:691–6. doi: 10.1016/s0960-9822(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 54.Okita K, Furuhata T, Kimura Y, Kawakami M, Yamaguchi K, Tsuruma T, Zembutsu H, Hirata K. The interplay between gastric cancer cell lines and PBMCs mediated by the CC chemokine RANTES plays an important role in tumor progression. J Exp Clin Cancer Res. 2005;24:439–46. [PubMed] [Google Scholar]
- 55.Matzer SP, Rodel F, Strieter RM, Rollinghoff M, Beuscher HU. Constitutive expression of CXCL2/MIP-2 is restricted to a Gr-1high, CD11b+, CD62Lhigh subset of bone marrow derived granulocytes. Int Immunol. 2004;16:1675–83. doi: 10.1093/intimm/dxh169. [DOI] [PubMed] [Google Scholar]
- 56.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 57.Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP, Belperio JA. Cancer CXC chemokine networks and tumour angiogenesis. Eur J Cancer. 2006;42:768–78. doi: 10.1016/j.ejca.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol. 1993;151:2667–75. [PubMed] [Google Scholar]
- 59.Miyamoto M, Shimizu Y, Okada K, Kashii Y, Higuchi K, Watanabe A. Effect of interleukin-8 on production of tumor-associated substances and autocrine growth of human liver and pancreatic cancer cells. Cancer Immunol Immunother. 1998;47:47–57. doi: 10.1007/s002620050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brew R, Erikson JS, West DC, Kinsella AR, Slavin JSE. Christmas, Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine. 2000;12:78–85. doi: 10.1006/cyto.1999.0518. [DOI] [PubMed] [Google Scholar]
- 61.Loberg RD, Ying C, Craig M, Yan L, Snyder LA, Pienta KJ. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia. 2007;9:556–62. doi: 10.1593/neo.07307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ben-Baruch A. Host microenvironment in breast cancer development: inflammatory cells, cytokines and chemokines in breast cancer progression: reciprocal tumor-microenvironment interactions. Breast Cancer Res. 2003;5:31–6. doi: 10.1186/bcr554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garber K. First results for agents targeting cancer-related inflammation. J Natl Cancer Inst. 2009;101:1110–2. doi: 10.1093/jnci/djp266. [DOI] [PubMed] [Google Scholar]
- 64.Yu JL, Rak JW. Host microenvironment in breast cancer development: inflammatory and immune cells in tumour angiogenesis and arteriogenesis. Breast Cancer Res. 2003;5:83–8. doi: 10.1186/bcr573. [DOI] [PMC free article] [PubMed] [Google Scholar]