Abstract
With the genome of the malaria parasite Plasmodium vivax sequenced, it is important to determine the proteomes of the parasite in order to assist efforts in antigen and drug target discovery. Since a method for continuous culture of P. vivax parasite is not available, we tried to study the proteome of the erythrocytic stages using fresh parasite isolates from patients. In schizont-enriched samples, 316 proteins were confidently identified by tandem mass spectrometry. Almost 50% of the identified proteins were hypothetical, while other major categories include proteins with binding function, protein fate, protein synthesis, metabolism and cellular transport. To identify proteins that are recognized by host humoral immunity, parasite proteins were separated by two-dimensional gel electrophoresis and screened by Western blot using an immune serum from a P. vivax patient. Mass spectrometry analysis of protein spots recognized by the serum identified four potential antigens including PV24. The recombinant protein PV24 was recognized by antibodies from vivax malaria patients even during the convalescent period, indicating that PV24 could elicit long-lasting antibody responses in P. vivax patients.
Keywords: Antigen, Erythrocytic stage, Mass spectrometry, Plasmodium vivax, Proteome
1. Introduction
Plasmodium vivax is the most widespread human malaria parasite, which causes significant morbidity and socio-economic problems in endemic countries [1]. It has several unique characteristics that distinguish it from other human malaria parasite species. Most notably, P. vivax forms hypnozoites in hepatocytes, causing relapses of the disease [2]. P. vivax strains from the tropical and temperate zones can vary dramatically in terms of the pattern and frequency of the relapse. Moreover, P. vivax requires Duffy receptor on the red cell for invasion, and is thus absent in West Africa where Duffy negativity predominates [3]. It selectively invades reticulocytes [4], thus limiting parasitemias to low levels. Unlike Plasmodium falciparum infection that increases the rigidity of the host cell, P. vivax increases the size and deformability of infected red cells [5]. P. vivax also actively remodels the host cell, producing caveola-vesicle complexes along the plasmalemma in the infected erythrocyte cell, which are visible in Giemsa-stained smears as multiple red spots called “Schüffner's dots”. Apart from these characteristics, the ability of P. vivax to survive at much lower temperature has allowed this parasite to establish transmission foci in temperate zones. Despite that many of these unique features of P. vivax have been known for a long time, the underlying mechanisms remain poorly understood. Therefore, a better understanding of the fundamental biology of P. vivax is needed to effectively control and eventually eradicate this parasite.
The task to eliminate malaria globally requires integrated control measures, one of which is development of vaccines against malaria parasites. Several leading candidate vaccines from P. falciparum have been tested in clinical trials but do not offer protection against other Plasmodium species [6]. The deployment of such vaccines against P. falciparum may cause an unexpected outcome in malaria epidemiology in areas of P. vivax and P. falciparum coexistence, and therefore, multi-subunit and multi-species vaccines are needed in such endemic areas. Whereas the P. falciparum vaccine candidate repertoire is well characterized [7], few P. vivax antigens are well defined. Therefore, antigen discovery is a prerequisite for the development of vaccines against P. vivax.
Completion of the genome [8], transcriptome [9] and proteome [10,11] of P. falciparum has played a significant role in advancing research on this parasite. In comparison, research on P. vivax malaria has lagged much behind. One major reason is the unavailability of a continuous in vitro culture system for P. vivax, although recent work showed promises in this direction [12]. With the recent deciphering of the P. vivax genome [13] and transcriptome [14], the parasite proteome remains to be determined. The advance in highly sensitive mass spectrometry (MS) offers an extraordinary opportunity to determine the proteome of the P. vivax parasite in the absence of large amount of experimental materials from a continuous in vitro culture. In this study, we attempted to study the proteome of the erythrocytic stages of P. vivax field isolates by highly accurate tandem mass spectrometry (MS/MS). As a way of antigen discovery, we further tried to identify parasite antigens that are recognized by host humoral immunity using an immune serum from a P. vivax malaria patient.
2. Materials and Methods
2. 1. Sample collection
Fresh P. vivax isolates were collected from 10 symptomatic malaria patients attending a malaria clinic in Mae Sot district, Tak Province, Thailand. Twenty milliliters of P. vivax-infected blood were collected from each patient. White blood cells were removed by passing infected blood through a Plasmodipur® filter. Parasites were cultured with McCoy's 5A medium supplemented with 25% human AB serum at 37°C under 5% CO2 until they reached the schizont stage. To reduce contamination of red blood cell proteins, schizont-infected red blood cells were purified on 60% Percoll® and parasites were released by 0.01% saponin treatment. Parasite pellet was washed with phosphate buffered saline (PBS) pH 7.4 until the supernatant was clear and stored at -80°C for proteome analysis.
A total of 118 and 33 plasma samples were collected in 2001, 2002, and 2007-2009 from P. vivax and P. falciparum patients, respectively, who were attending an outpatient malaria clinic in Mae Sot, or the Hospital of Tropical Diseases, Bangkok, Thailand. Among these patients, follow-up was conducted in four P. vivax cases, from whom plasma samples were collected at the time of acute infection, and in three and six months after treatment. During the follow-up, the participants did not experience malaria infections. Control plasma samples from malaria naïve donors were collected from a non-endemic area in Thailand.
The study protocol was approved by The Pennsylvania State University Institutional Review Board and the Ethical Review Committee of Mahidol University. Informed consent or assent was obtained from volunteers before the blood samples were collected.
2.2. Preparation of protein extracts
For proteome analysis, ∼109 P. vivax schizonts were resuspended in 100 μl of 100 mM Tris-HCl (pH 7.2) and sonicated on ice for four 10-sec pulses. Proteins were dissolved in SDS-PAGE loading buffer and separated on a linear gradient (4-20%, 1.5 mm thick) Tris-glycine mini gel (Invitrogen, USA). The gel was stained by Colloidal blue (Invitrogen) and protein bands were horizontally sliced into 16 sections (Fig. 1.) Each gel section was cut into ∼1 mm cubes, destained in 50% acetonitrile (ACN) containing 25 mM NH4HCO3 (pH 8.0) until gel pieces became transparent, dehydrated in 100% ACN, and dried completely in a SpeedVac. Afterward, the samples were reduced in 45 mM dithiothreitol (DTT) and 25 mM NH4HCO3 (pH 8.0) for 45 min at 55°C and then alkylated in 100 mM iodoacetamide, 25 mM NH4HCO3 (pH 8.0) for 45 min at room temperature in the dark. All samples were washed twice in 50% ACN, 25 mM NH4CO3 (pH 8.0) and dried in a SpeedVac. For in-gel digestion, samples were digested overnight at 37°C with 0.01 μg/μl MS grade trypsin (Promega, Madison, Wisconsin, USA) in 50% ACN, 25 mM NH4HCO3 (pH 8.0). The resultant peptides were extracted twice with 100 μl of 5% trifluoroacetic acid (TFA) in 50% ACN for 15 min. Samples were dried down completely by SpeedVac, and then resuspended in 200 μl of deionized water. This procedure was repeated twice, with a third drying down halted when the remaining volume was approximately 10 μl. Then 1% TFA was added to make the final concentration of TFA at 0.1%. The peptide samples were cleaned with SCX ZipTips (Millipore, Billerica, Massachusetts, USA) according to the manufacturer's instructions. The eluant was dried completely in a SpeedVac and resuspended in 15 μl of 2% ACN and 0.1% TFA. Separation of the peptides were achieved by reverse-phase nanoflow liquid chromatography (LC) using a 150 × 0.1 mm chromolith caprod column injector loop (Merk, USA) on a Tempo LC matrix assisted laser desorption/ionization (MALDI) spotting system (ABI-MDS/Sciex) and eluted with a gradient of 2% ACN/0.1% TFA and 98% ACN/0.1% TFA, respectively.
Fig 1.

SDS-PAGE analysis of P vivax lysate Lysate from ∼109 parasites were separated on 12% polyacrylamide gel and stained with colloidal blue. Molecular standards are shown in lane 1. Protein bands were horizontally sliced into 16 sections (lane 2, S1-S16) before subjected to in-gel trypsin digestion and MS analysis.
2.3. Two-dimensional gel electrophoresis (2-DE) and Western blot
To identify parasite antigens, parasite proteins were subjected to 2-DE, immunoblotting, and LC/MS/MS analysis. Briefly, parasite crude extract (40 μg/gel) was solubilized in 2-D rehydration buffer (8 M urea, 0.5% CHAPS, 60 mM DTT), and 0.5% ampholyte (pH 3–10), thoroughly mixed and centrifuged at 15000 × g for 10 min. The resultant supernatant was subjected to 2-DE. Isoelectric focusing was performed with pre-cast 7 cm Immobiline® dry strips (pH 3-10) using the Ettan IPGphor 3 apparatus (Amersham Bioscience AB, Uppsala, Sweden). The running protocol was as follows: step 1, 300 v, 200 Vh; step 2, 1000 v, 300 Vh; step 3, 5000 v, 1,400 Vh; and step 4, 5000 v, 2000 Vh. The focused strips were equilibrated in 10 ml of equilibration solution (50 mM Tris-HCl, pH 8.8, 6 M urea, 30% glycerol, 2% SDS, 1% DTT) for 15 min, followed by incubation in 10 ml equilibration solution containing 2.5% iodoacetamide for another 15 min. The equilibrated strips were loaded on 12% SDS-PAGE gels for the second dimension separation. One gel was silver-stained using a MS compatible PlusOne Silver staining kit (Amersham Bioscience), whereas the other was used for immunoblotting.
For Western blot, proteins were transferred to a 0.45 μm nitrocellulose membrane (Pharmacia Biotech, California, USA) under cooling conditions and constant voltage (100 V) for 4 h. The membrane was blocked with 5% skimmed milk in Tris-buffered saline (TBS, pH 7.2) overnight at 4°C. After washing with TBS containing 0.05% Tween 20, the membrane was incubated with an immune serum from a P. vivax patient at 1:10 dilution for 2 h, and then with HRP-conjugated goat anti-human IgG antibody at 1:500 dilution for 1 h. The blot was visualized by incubating with the HRP substrate (4-chloro-1-napthol, methanol and H2O2 in TBS). Positive spots were picked from the silver-stained gel. Each gel spot was destained in 1% H2O2, 25 mM NH4HCO3 (pH 8.0) prior to in-gel trypsin digestion as described above.
2.4. MS analysis and database search
For MS analysis, the final eluant of the peptides separated by reverse phase nanoflow LC was mixed with the MALDI matrix [7 mg/ml recrystallized α-cyanohydroxycinnamic acid (Sigma), 2 mg/ml ammonium phosphate, 0.1% TFA in 50% ACN], and automatically spotted onto a stainless steel MALDI target plate and analyzed on an ABI 4800 MALDI-TOF/TOF mass spectrometer (Applied Biosystems, USA). The MS spectra were acquired from each sample spot using 500 laser shots from 40 random per spot using Reflectron Positive Ion mode with laser setting of 3200. The highest top ten peaks of each observed m/z value of each MS spectrum (excluding trypsin auto-digestion peaks) were chosen for subsequent MS/MS analysis with collision-induced dissociation fragmentation. Up to 2500 laser shots at the laser power 4200 were accumulated for each MS/MS spectrum.
For identifying parasite antigens, peptides from the immune-positive spots of the 2-DE were analyzed by LC/MS/MS using a SYNAPT™ HDMS mass spectrometer (Waters, Manchester, UK). Nanoscale LC separation of tryptic peptides was performed with a NanoAcquity system (Waters) equipped with a 5 μm, 180 μm × 200 μm symmetry C18 trap column and a 1.7 μm, 75 μm × 200 μm BEH130 C18 analytical reversed phase column (Waters). The samples were initially transferred with an aqueous 0.1% formic acid solution to the trap column with a flow rate of 3 μl/min for 3 min. Mobile phase A was water with 0.1% formic acid, and mobile phase B was 0.1% formic acid in ACN. The peptides were separated with a gradient of 2–40% mobile phase B over 30 min at a flow rate of 350 nl/min followed by a 10 min rinse with 80% of mobile phase B. The lock mass was delivered from the auxiliary pump of the NanoAcquity pump with a constant flow rate of 200 nl/min to the reference sprayer of the NanoLockSpray source of the mass spectrometer. After nanoelectrospray ionization, accurate MS/MS data were acquired.
The MS and MS/MS data were searched against the annotated P. vivax protein database from PlasmoDB (http://www.plasmodb.org) and non-redundant human protein database from National Center for Biotechnology Information. The MS and MS/MS data from MALDI-TOF/TOF and LC/MS/MS were analyzed by Protein Pilot software version 2.01 (Applied Biosystems/MDS Sciex) and ProteinLynx Global Sever 2.2.5 (Waters), respectively. Search criteria were trypsin-cleaved peptides; 200 parts per million mass error tolerance in MS mode; 0.4 Da mass error tolerance for MS/MS fragments; fixed modification of carbamidomethylation of cysteines, and allowed (variable) modification of oxidation of methionine. Protein identification acceptance was ProteinPilot Unused Score of >1.3 (>95% confidence interval) plus <5% false discovery rate.
The presence of a signal peptide (SP) and transmembrane domain (TM) in the protein sequence is an important indicator of secretory or cell surface protein. We used SignalP 3.0 and TMHMM 2.0 (http://www.cbs.dtu.dk) to determine the SP and TM in the proteins. To predict the antigenicity of the proteins, linear B-cell epitope and antigenicity were predicted using the BepiPred 1.0 and Kolarskar & Tongaonkar Antigenicity programs (www.immuneepitope.org).
2.5. Expression of a recombinant protein
To express a putative antigen, PV24 (PVX_002950), identified from the 2-DE/MS analysis, cDNA was synthesized from 1 μg of P. vivax total RNA using Superscript®III reverse transcriptase (Invitrogen) [15]. Primers (5′ ATGCGGATCCTACAATGCAAGCGAAAGACAAAATGG3′ and 5′ AGTCGTCGACGAACAAGTAGCCATAATATTTGG 3′, BamHI and SalI sites underlined) were designed to amplify a 540 bp fragment (79-618 bp of the open reading frame). The PCR product was cloned in the expression vector pET-28 (a+) (Novagen, Darmstadt, Germany) after digestion with BamHI and SalI. The recombinant PV24 protein (rPV24) was expressed in Escherichia coli BL21 after induction by 1 mM isopropyl β-D-1-thiogalactopyranoside for 2 h. The His-tagged recombinant protein was purified by a denaturing method using the Ni-NTA agarose resin (Qiagen, Hilden, Germany). Eluted protein fractions were analyzed by 12% SDS-PAGE and Western blot analysis with mouse anti-His antibody at 1:1000 dilution. Purified recombinant protein was extensively dialyzed against PBS (pH 7.4) at 4°C.
2.6. ELISA
Griener flat-bottomed, 96-well microtiter plates were coated with 50 μl per well of 1 μg/ml of purified rPV24 or crude P. vivax parasite protein (10 μg/ml) overnight at 4°C. The plates were blocked for 2 h with 100 μl of PBS with 0.05% Tween 20 (PBST) containing 0.5% bovine serum albumin. After washing twice with PBST, 50 μl of human plasma diluted to 1:50 with the blocking buffer were added and incubated for 2 h at room temperature. Plates were washed thrice with PBST and incubated with 50 μl of HRP-conjugated goat anti-human IgG antibody (Caltag, USA) at 1:2,000 for 1 h. Afterwards, the plates were washed thrice with PBST, and developed with 2, 2′, azino-diethylbenzothiazolinesulfonic acid (Kirkepaard & Perry Laboratories, USA) for 30 min. The optical density (OD) was read at 405 nm on an ELISA plate reader. Each sample was tested in duplicate. Mean OD given by naïve control sera plus three standard deviations (STD) was used as the cutoff OD value.
2.7. Statistical analysis
Statistical analysis was performed using the SPSS program (Version 11.5, Chicago, USA). The level of anti-rPV24 antibodies among plasma samples from P. vivax and P. falciparum patients, and naïve control were evaluated by the analysis of variance (ANOVA). The correlation between anti-rPV24 antibody level and parasitemia was determined by Spearman's rank test. The results were considered significant at P < 0.05.
3. Results
3.1. The proteome of P. vivax schizons
Due to the lack of a suitable continuous in vitro culture method for P. vivax, we used field isolates obtained from infected patients for proteomic analysis. Our preliminary studies using parasite samples immediately after saponin lysis suggested that host protein contamination was too overwhelming to obtain an adequate parasite protein coverage. In order to circumvent this problem, parasites were cultured until they reached the schizont stage and purified by Percoll. To determine the schizont stage proteome, protein extracts were obtained from ∼109 schizonts, separated by SDS-PAGE and subjected to MS analysis by MALDI-TOF/TOF. Search of the protein databases with the MS/MS spectra identified 316 P. vivax proteins with at least 95% confidence (supporting information 1). Almost half of the identified proteins (47%) were hypothetical proteins, among which 29% contained a putative SP and 37% contained at least one TM. Further, 15% of these hypothetical proteins contained both a SP and a TM. This result suggested that the identified proteins were either secretory or/and membrane associated, which might play important roles in parasite-host interactions (supporting information 2).
Eleven percent (36 in 316 proteins) of the identified proteins was unique as defined by the absence of orthologs in other human malaria parasites, P. falciparum, P. ovale and P. malariae (supporting information 3). The majority of the unique proteins (18 in 36 proteins) were hypothetical. In addition, 16 of 36 proteins did not have orthologs in other human malaria parasites and P. knowlesi, which provide potential targets for P. vivax-specific diagnosis.
The identified proteins were classified into functional classes based on the Munich Information Centre for Protein Sequences (MIPS) catalogue [16] (supporting information 4). Proteins were plotted as a function of their broad functional classification; only one class was assigned per protein in order to avoid redundancy (Fig. 2). The predominant class contained hypothetical proteins (47%). Other important functional classes of proteins were proteins with binding function (10%), and involved in protein fate (5%), protein synthesis (4%), metabolism (4%) and cellular transport (4%). These protein classes were mainly housekeeping proteins.
Fig 2.
Pie chart showing the distribution of functional classes of identified P vivax proteins as defined by the MIPS catalogue.
Enzymes of major metabolic pathways of the parasite including glycolysis, nucleic acid metabolism, and hemoglobin digestion were identified (Table 1). During parasite development, it modifies or exports proteins into red cell membrane which causes dramatic changes in host cell membrane composition, structure and function. Different transportation mechanisms of parasite have been reported, and several proteins shown to play significant roles in the transport machinery including Rab GTPases (Rab2, Rab5c, Rab6 and Rab7) [17, 18], translocation protein complex (sec61, sec62 and SEC63), heat shock protein (Hsp70 and HSP101) and EXP2 [19-22] were identified in the schizont stage proteome. Several exported proteins potentially involved in immune evasion and host cell invasion including vir and erythrocyte membrane protein 3 were identified [23, 24]. The P. vivax tryptophan rich antigen (PvtrAg) or Pv-fam-a is one of the most abundant parasite protein families with 34 predicted members (www.plasmodb.org), among which 11 members were detected.
Table 1.
The detected P. vivax proteins involved in parasite's metabolic pathways
| Gene ID | Enzyme | EC no.a | Reaction catalyzed |
|---|---|---|---|
| Glycolysis | |||
|
| |||
| PVX_095015 | Enolase | 4.2.1.11 | Glycerate-2P ↔ Phosphoenol-pyruvate |
| PVX_114445 | Pyruvate kinase | 2.7.1.40 | Phosphoenol-pyruvate + ADP ↔ Pyruvate + ATP |
| PVX_118495 | Triosephosphate isomerase | 5.3.1.1 | Glyceraldehyde-3P ↔ Glycerone-P |
| PVX_118255 | Fructose 1,6-bisphosphate aldolase | 4.1.2.13 | Aldolase ↔ Glyceraldehyde-3P |
| PVX_116630 | Lactate dehydrogenase | 1.1.1.27 | Pyruvate + NADH+H+↔Lactate + NAD+ |
|
| |||
|
Nucleic acid biosynthesis Purine salvage pathway | |||
|
| |||
| PVX_111245 | Adenosine deaminase | 3.5.4.4 | Adenosine + H2O ↔ Inosine + NH3 |
| PVX_092535 | Adenylate and Guanylate cyclase catalytic domain containing protein | 4.6.1.2 | GTP ↔ 3′,5′-cyclic GMP + PPi |
| PVX_094840 | Hypoxanthine phosphoribosyltransferase | 2.4.2.8 | Hypoxanthine + PRPP ↔ IMP + PPi |
|
| |||
| Pyrimidine metabolism | |||
|
| |||
| PVX_083135 | Aspartate carbamoyltransferase | 2.1.3.2 | L-Aspatate + Carmoyl-P ↔ N-carbamoyl-L-Aspartate + Pi |
|
| |||
| TCA cycle | |||
|
| |||
| PVX_084960 | ATP-specific succinyl-CoA synthetase beta subunit | 6.2.1.5 | ATP + Succinate + CoA ↔ ADP + Orthophosphate + Succinyl-CoA |
|
| |||
| Hemoglobin digestion | |||
|
| |||
| PVX_115000 | Falcilysin | 3.4.24.- | Acting on peptide bonds |
| PVX_097935 | Subtilisin-like protease precursor | 3.4.21.62 | Hydrolyzes peptide amides |
| PVX_086040 | Aspartic protease PM4 | 3.4.23.B14 | Cleavage of hemoglobin |
| PVX_122425 | M1-family aminopeptidase | 3.4.11.2 | Release of an N-terminal amino acid from a peptide |
Plasmodium metabolic pathways can be found at http://siteshujiacil/malaria.
Enzyme Commission (EC) numbers of P falciparum orthologs.
Since our parasite samples were enriched in schizonts, schizont-specific proteins were readily detected. These include proteins associated with merozoite surface and invasive organelles such as merozoite surface proteins (MSP1 [25], MSP5 [26], MSP7 [27], and MSP8 [28]), apical membrane antigen 1 (AMA1) [29], reticulocyte binding protein [30], rhoptry proteins (RAP1 [31], RAP 2 [32], RhopH2, EXP2, and CLAG), and actinomyosin motors (actin, myosin) [33-35]. The parasite samples also contained gametocytes and several sexual stage proteins were detected, including the P. falciparum orthologs of male fertility protein Pf47 (PVX_083240), Pfs16 (PVX_000930) and transmission-blocking vaccine candidate Pfs230 (PVX_000995) [36-38].
Parasite adhesins are of particular importance due to their roles in parasite invasion, sequestration or parasite-host interactions [24]. The P. vivax genome was predicted to encode 137 adhesins [39], of which 11 were detected in our schizont stage proteome and six were hypothetical proteins (Table 2). Some of the adhesion proteins such as MSP1 and AMA1 are known to involve in host cell invasion [40]. Glycosylphosphatidylinositol (GPI) anchor is the major carbohydrate modification of proteins mostly in schizont-stage parasites. Nine putative GPI-anchored proteins were detected in the schizont stage proteome (Table 3), which represent 10% of total GPI-anchored proteins predicted in the P. vivax genome. Four of the nine proteins were known GPI-anchored proteins. Some of these GPI-anchored proteins were known to be involved in parasite-host interaction and invasion, and are promising vaccine candidates [41-43]. As a potential novel antigen, PVX_088910 encodes a hypothetical protein with maximum expression in late-stage parasites. Its P. falciparum ortholog (PF08_0008) is a GPI-anchored micronemal antigen shown to mediate binding to red blood cell surface, indicating involvement in the host cell invasion process.
Table 2.
Plasmodium vivax adhesins predicted by MAAP (Malaria adhesins and adhesion like proteins predictor) [39].
| Accession Number | Annotation | scorea | SP | TM |
|---|---|---|---|---|
| PVX_097950 | Hypothetical protein, conserved | 0.742 | 0 | 0 |
| PVX_096995 | Tryptophan-rich antigen (Pv-fam-a) | 0.740 | 0 | 1 |
| PVX_084720 | Hypothetical protein, conserved | 0.935 | 1 | 0 |
| PVX_092275 | Apical merozoite antigen 1 | 1.001 | 0 | 1 |
| PVX_099980 | Major blood-stage surface antigen Pv200 | 1.001 | 1 | 1 |
| PVX_096245 | Hypothetical protein, conserved | 1.008 | 0 | 0 |
| PVX_117060 | Hypothetical protein, conserved | 1.027 | 0 | 0 |
| PVX_083560 | Hypothetical protein, conserved | 1.242 | 0 | 0 |
| PVX_112670 | Tryptophan-rich antigen (Pv-fam-a) | 1.615 | 1 | 0 |
| PVX_096070 | Early transcribed membrane protein (ETRAMP) | 2.113 | 1 | 1 |
| PVX_003555 | Hypothetical protein, conserved | 2.213 | 0 | 0 |
P. vivax proteins with Pmaap score of ≥ 0.7 were predicted as adhesins.
Table 3.
GPI-anchored proteins identified in the schizont-stage proteome.
| Accession number | Annotation |
|---|---|
| PVX_083135 | Aspartate carbamoyltransferase, putative |
| PVX_088910 | Hypothetical protein, conserved |
| PVX_097625 | Merozoite surface protein 8, putative |
| PVX_099320 | Acid phosphatase, putative |
| PVX_099980 | Merozoite surface protein 1 |
| PVX_100835 | Hypothetical protein, conserved |
| PVX_110895 | ADP/ATP transporter on adenylate translocase, putative |
| PVX_113775 | Membrane protein pf12 precursor, putative |
| PVX_122545 | COP-coated vesicle membrane protein p24 precursor, putative |
3.2. Identification of novel P. vivax antigens
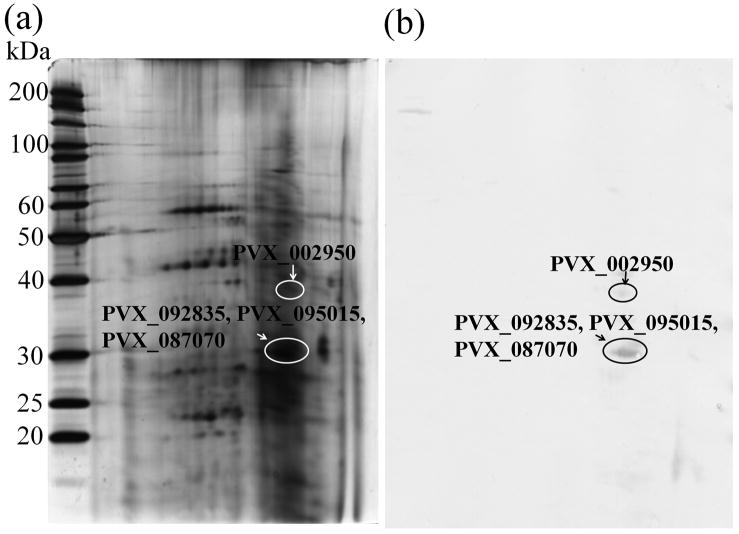
In order to identify parasite proteins that are recognized by host humoral immunity, 2-DE immunoblot of protein extract from schizont stage parasites was performed. Immune serum from an acute P. vivax infected patient showing a high antibody level to P. vivax lysate as determined by ELISA (data not shown) was used in Western blot analysis. Three immune-reactive protein spots were recognized by the antiserum. The positive protein spots were excised from the silver stained 2-DE gel for MS/MS analysis (Fig. 4). Four proteins were detected, corresponding to the protein products of PVX_002950, PVX_087070, PVX_095015 and PVX_092835 genes (Table 4). Antigenicity prediction suggested that all four proteins contained linear B-cell epitopes and had antiginicity score above the threshold. Of the four proteins, only PVX_002950 contained predicted SP and TM.
Fig 4.
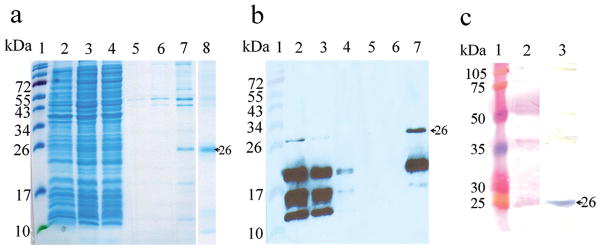
Purification of rPV24 using Ni-NTA chromatography (a) SDS-PAGE analysis and (b) chemiluminescence analysis of immunoblot using anti-His antibody. Lane 1 = molecular weight standards, lane 2 = whole lysate, lane 3 = soluble fraction, lane 4 = flow-through fraction, lane 5 = last washing fraction, and lane 6 and 7 = eluted fractions (c) Immunoblot analysis of rPV24 with the immune serum from a P vivax patient. Lane 1 = molecular weight standards, lane 2 = Ponceu S staining of rPV24 protein, and lane 3 = immunoblot of rPV24 Arrows indicate purified rPV24.
Table 4.
Plasmodium vivax proteins recognized by immune serum from P vivax patient identified by LC/MS/MS
| Gene ID | Description | Sequence | MW (kDa) |
pI | SP | TM |
|---|---|---|---|---|---|---|
| PVX_002950 | hypothetical protein | LIKDSNISFHFFYANNDPLSR | 24.1 | 9.81 | 1 | 3 |
| PVX_087070 | hypothetical protein | APPTQGEMLLLLVR | 110.1 | 6.53 | 0 | No |
| PVX_095015 | enolase | AAVPSGASTGIYEALELR | 48.8 | 9.70 | 0 | No |
| PVX_092835 | hypothetical protein | LGKSNKR, QKAKQVK, ENAERSK, AKISMFK, TNVKKNR, IFEREGK, HYKTNVK, DNKLGKSNK, TSENVNQSK, NILDEIAVK, GSTVNTYILK, QSKVSLKPIK, VKINLNNPVK, MHVFDLDKAK, KNRFTIIETR, KMHVFDLDKAK | 41.3 | 10.13 | 0 | No |
3.3. Expression and purification of rPV24
PVX_002950 is predicted to encode a 24 kDa hypothetical protein with 206 amino acids, referred to as PV24, which shared 41% similarities with the P. falciparum ortholog (PFB0515w). This gene encodes a putative dolichol-linked oligosaccharide biosynthesis enzyme. Microarray analysis indicates that it is expressed throughout the P. vivax erythrocytic cycle [14]. Since the predicted cleavage site of the SP is between amino acids 26 and 27, we decided to express the 180-residue recombinant protein without the first 26-residue SP sequence. The rPv24 expressed in E. coli had a molecular size of ∼26 kDa on SDS-PAGE gel and reacted with antiserum against 6X His-tag, which was consistent with the predicted molecular size (26.4 kDa) of rPV24 (Fig. 4a and 4b). Moreover, rPV24 reacted with the immune serum previously used in the 2-DE immunoblot (Fig. 4c).
3.4. Antigenicity of rPV24
To determine the antigenicity of rPV24, ELISA was performed by testing rPV24 protein using plasma samples from P. vivax and P. falciparum patients, and malaria naïve donors. The reactivity of plasma from P. vivax patients was significantly higher than that of the naïve group (P<0.001), and from that of plasma from P. falciparum patients (P<0.001) (Fig. 5a). Compared with the naïve group, the reactivity to rPV24 of plasma from P. falciparum infected patients was not significantly different (P>0.05). Plasma samples from P. vivax patients collected in different years (2001-2009) did not show significant differences in terms of reactivity with rPV24 (P > 0.05) (Fig. 5b). Ninety-nine percent of plasma samples from P. vivax patients showed reactivity to rPV24 above the cutoff value. Interestingly, the reactivity of plasma samples from all follow-up cases (3 and 6 months post treatment) was also higher than the cutoff level (Fig. 5c). In addition, there was a significant inverse correlation between the level of anti-rPV24 antibody and the P. vivax parasitemia (correlation coefficient = -0.372, P = 0.039). Altogether, these results indicated that the PV24 was strongly antigenic and elicited long-lasting immunity in P. vivax patients.
Fig 5.
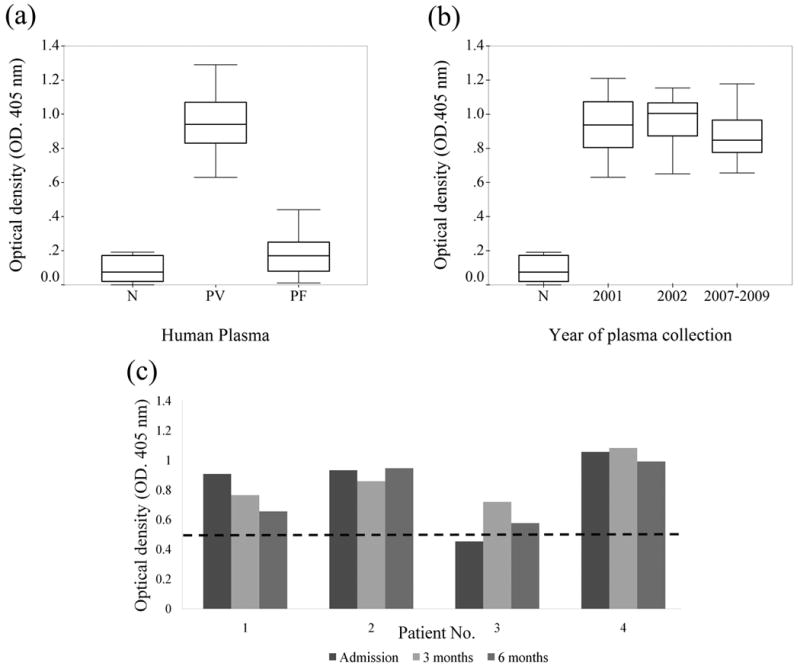
Determination of antibody levels in plasma samples to rPV24 by ELISA. (a) Different groups of human plasmas; sera of P vivax infected patients (PV), malaria naïve sera (N), and sera of P falciparum infected patients (PF). (b) plasma from PV collected in different years compared with N. (c) Antibody levels during acute infection and convalescence period collected at 3 and 6 months after treatment (dash line indicates cut-off level at mean + 3 standard deviation).
4. Discussion
With the completion of P. vivax genome sequencing and transcriptome projects, it is important to determine the P. vivax proteomes. Due to host protein contamination in other erythrocytic stages, we only attempted to purify schizont stage for proteomic analysis. Consequently, our proteome was enriched in schizont stage proteins. In schizonts, 1,212 transcripts were detected (www.plasmodb.org), among which 316 proteins were detected in our proteomic analysis. Of those proteins, 65% overlapped with P. falciparum proteins [11]. Over half of the proteins identified were abundant proteins with putative functions in metabolism, protein synthesis, cellular transport and binding, etc. The hypothetical proteins accounting for almost 50% of this proteome could be a key to unravel the unique biology of P. vivax. In addition, numerous hypothetical proteins containing predicted SP (45) and/or TM (52) were discovered, some of which may represent potential antigens. Thirty six (11%) out of 316 proteins were unique in P. vivax, as defined by the absence of orthologs in other human malaria parasites. Given that most of the rapid diagnostic tests are based on the pan-Plasmodium antigens (e.g., lactate dehydrogenase and aldolase) [44], the unique proteins in P. vivax could serve as potential candidates for developing P. vivax-specific tests.
Transcriptome analysis of P. vivax revealed that members of vir and pvtrag gene families have two distinct phases of transcription, immediately after invasion and during schizogony [14]. Protein members of these gene families (10 pvtrag and 4 vir) detected in the schizont stage proteome appeared to be derived from the first wave of gene expression since all of them showed maximum transcription at the ring stage. The cognate proteins of 52 vir and 13 pvtrag genes with maximum transcription in schizont stage could not be detected in our schizont stage proteome possibly due to a delay in protein translation.
Antimalarial vaccines are an important component for the integrated approaches to malaria control during the malaria elimination phase [45]. In particular, malaria vaccine development efforts need to target other malaria parasite species, especially P. vivax. Currently, there are only two P. vivax vaccine candidates being tested in clinical trials, circumsporozoite protein and transmission-blocking vaccine candidate pvs25 [46]. Compared with P. falciparum, the number of vaccine candidates in P. vivax is very limited, and antigen discovery is essential for P. vivax vaccine development. In the schizont stage proteome, we have identified several potential membrane proteins that could serve as vaccine candidates, including those that are predicted adhesions and GPI-anchored proteins. These groups include antigenic proteins such as MSP1 and AMA1 that are already in the vaccine development pipeline. In addition, we used a MS-based method to identify P. vivax antigens, which proved to be a viable approach to vivax antigen discovery. In our schizont-stage proteome, four antigens were identified by a P. vivax patient serum. We have further characterized one of these proteins, PV24, and showed that P. vivax infection induced long-lasting reactivity with this protein, and antibody titers against this protein could still be detected during the convalescence stage six months after successful treatment of the primary infection. During malaria infection, T cells play a central role in the regulation of immune responses and the formation of immunologic memory which helps control and eliminate the infection [47]. For antigen discovery, we need to understand how the potential antigens elicit humoral and cellular immune responses during natural infection [48]. In this study, the persistence of anti-PV24 antibodies in P. vivax patients implies the involvement of T cells in the production of antibodies against this protein [49]. Our study has demonstrated PV24 as a usefulness marker for malaria epidemiology. Whether PV24 could induce protective immunity against malaria infection and serve as a vaccine candidate awaits further investigations.
Supplementary Material
Fig 3.
2-DE analysis of blood stage proteins of P vivax (a) Silver stained gel, and (b) immunoblot with an immune serum of P vivax patient. Circles indicate proteins of P vivax reacted with the immune serum.
Acknowledgments
This work was supported by grant R21AI069126 from NIAID, D43-TW006571 from the Fogarty International Center, NIH and The Office of the Higher Education Commission and Mahidol University under the National Research Universities Initiative. WR was a fellow supported by the Fogarty International Center, NIH (D43-TW006571) and by the Royal Golden Jubilee Ph.D. Program (5TMU47H2). We would like to thank the staff at Biotech, Thailand Science Park, and laboratory members at the Department of Entomology and the core facilities of the Hershey Medical Center, Penn State University for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Price RN, Tjitra E, Guerra CA, Yeung S, et al. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87. [PMC free article] [PubMed] [Google Scholar]
- 2.Krotoski WA, Collins WE, Bray RS, Garnham PC, et al. Demonstration of hypnozoites in sporozoite-transmitted Plasmodium vivax infection. Am J Trop Med Hyg. 1982;31:1291–3. doi: 10.4269/ajtmh.1982.31.1291. [DOI] [PubMed] [Google Scholar]
- 3.Miller LH, Mason SJ, Clyde DF, McGinniss MH. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N Engl J Med. 1976;295:302–4. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- 4.Mons B. Preferential invasion of malarial merozoites into young red blood cells. Blood Cells. 1990;16:299–312. [PubMed] [Google Scholar]
- 5.Suwanarusk R, Cooke BM, Dondorp AM, Silamut K, et al. The deformability of red blood cells parasitized by Plasmodium falciparum and P. vivax. J Infect Dis. 2004;189:190–4. doi: 10.1086/380468. [DOI] [PubMed] [Google Scholar]
- 6.Ogutu BR, Apollo OJ, McKinney D, Okoth W, et al. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PLoS ONE. 2009;4:e4708. doi: 10.1371/journal.pone.0004708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu J, Chen Z, Gu J, Wan M, et al. Safety and Immunogenicity of a Malaria Vaccine, Plasmodium falciparum AMA-1/MSP-1 Chimeric Protein Formulated in Montanide ISA 720 in Healthy Adults. PLoS ONE. 2008;3:e1952. doi: 10.1371/journal.pone.0001952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner MJ, Hall N, Fung E, White O, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozdech Z, Llinás M, Pulliam BL, Wong ED, et al. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 2003;1:e5. doi: 10.1371/journal.pbio.0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Florens L, Washburn MP, Raine JD, Anthony RM, et al. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419:520–6. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- 11.Lasonder E, Ishihama Y, Andersen JS, Vermunt AMW, et al. Analysis of the Plasmodium falciparum proteome by high-accuracy mass spectrometry. Nature. 2002;419:537–42. doi: 10.1038/nature01111. [DOI] [PubMed] [Google Scholar]
- 12.Panichakul T, Sattabongkot J, Chotivanich K, Sirichaisinthop J, et al. Production of erythropoietic cells in vitro for continuous culture of Plasmodium vivax. Int J Parasitol. 2007;37:1551–7. doi: 10.1016/j.ijpara.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Carlton JM, Adams JH, Silva JC, Bidwell SL, et al. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature. 2008;455:757–63. doi: 10.1038/nature07327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozdech Z, Mok S, Hu G, Imwong M, et al. The transcriptome of Plasmodium vivax reveals divergence and diversity of transcriptional regulation in malaria parasites. Proc Natl Acad Sci USA. 2008;105:16290–5. doi: 10.1073/pnas.0807404105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui L, Fan Q, Hu Y, Karamycheva SA, et al. Gene discovery in Plasmodium vivax through sequencing of ESTs from mixed blood stages. Mol Biochem Parasitol. 2005;144:1–9. doi: 10.1016/j.molbiopara.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Mewes HW, Frishman D, Güldener U, Mannhaupt G, et al. MIPS: a database for genomes and protein sequences. Nucleic Acids Res. 2002;30:31–34. doi: 10.1093/nar/30.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–17. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 18.Vanlandingham PA, Ceresa BP. Rab7 Regulates Late Endocytic Trafficking Downstream of Multivesicular Body Biogenesis and Cargo Sequestration. J Biol Chem. 2009;284:12110–24. doi: 10.1074/jbc.M809277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ungermann C, Neupert W, Cyr DM. The role of Hsp70 in conferring unidirectionality on protein translocation into mitochondria. Science. 1994;266:1250–3. doi: 10.1126/science.7973708. [DOI] [PubMed] [Google Scholar]
- 20.Lyman SK, Schekman R. Interaction between BiP and Sec63p is required for the completion of protein translocation into the ER of Saccharomyces cerevisiae. J Cell Biol. 1995;131:1163–71. doi: 10.1083/jcb.131.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer H, Grau H, Kraft R, Kostka S, et al. Mammalian Sec61 Is Associated with Sec62 and Sec63. J Biol Chem. 2000;275:14550–7. doi: 10.1074/jbc.275.19.14550. [DOI] [PubMed] [Google Scholar]
- 22.de Koning-Ward TF, Gilson PR, Boddey JA, Rug M, et al. A newly discovered protein export machine in malaria parasites. Nature. 2009;459:945–9. doi: 10.1038/nature08104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.del Portillo HA, Lanzer M, Rodriguez-Malaga S, Zavala F, Fernandez-Becerra C. Variant genes and the spleen in Plasmodium vivax malaria. Int J Parasitol. 2004;34:1547–54. doi: 10.1016/j.ijpara.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Carvalho BO, Lopes SCP, Nogueira PA, Orlandi PP, et al. On the cytoadhesion of Plasmodium vivax-infected erythrocytes. J Infect Dis. 2010;202:638–47. doi: 10.1086/654815. [DOI] [PubMed] [Google Scholar]
- 25.del Portillo HA, Longacre S, Khouri E, David PH. Primary structure of the merozoite surface antigen 1 of Plasmodium vivax reveals sequences conserved between different Plasmodium species. Proc Natl Acad Sci U S A. 1991;88:4030–4. doi: 10.1073/pnas.88.9.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black CG, Barnwell JW, Huber CS, Galinski MR, Coppel RL. The Plasmodium vivax homologues of merozoite surface proteins 4 and 5 from Plasmodium falciparum are expressed at different locations in the merozoite. Mol Biochem Parasitol. 2002;120:215–24. doi: 10.1016/s0166-6851(01)00458-3. [DOI] [PubMed] [Google Scholar]
- 27.Mongui A, Perez-Leal O, Soto SC, Cortes J, Patarroyo MA. Cloning, expression, and characterisation of a Plasmodium vivax MSP7 family merozoite surface protein. Biochem Biophys Res Commun. 2006;351:639–44. doi: 10.1016/j.bbrc.2006.10.082. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Leal O, Sierra AY, Barrero CA, Moncada C, Martinez P, Cortes J, et al. Plasmodium vivax merozoite surface protein 8 cloning, expression, and characterisation. Biochem Biophys Res Commun. 2004;324:1393–9. doi: 10.1016/j.bbrc.2004.09.202. [DOI] [PubMed] [Google Scholar]
- 29.Cheng Q, Saul A. Sequence analysis of the apical membrane antigen I (AMA-1) of Plasmodium vivax. Mol Biochem Parasitol. 1994;65:183–7. doi: 10.1016/0166-6851(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 30.Rayner JC, Galinski MR, Ingravallo P, Barnwell JW. Two Plasmodium falciparum genes express merozoite proteins that are related to Plasmodium vivax and Plasmodium yoelii adhesive proteins involved in host cell selection and invasion. Proc Natl Acad Sci USA. 2000;97:9648–53. doi: 10.1073/pnas.160469097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez-Leal O, Mongui A, Cortes J, Yepes G, Leiton J, Patarroyo MA. The Plasmodium vivax rhoptry-associated protein 1. Biochem Biophys Res Commun. 2006;341:1053–8. doi: 10.1016/j.bbrc.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 32.Patarroyo MA, Perez-Leal O, Lopez Y, Cortes J, Rojas-Caraballo J, Gomez A, et al. Identification and characterisation of the Plasmodium vivax rhoptry-associated protein 2. Biochem Biophys Res Commun. 2005;337:853–9. doi: 10.1016/j.bbrc.2005.09.120. [DOI] [PubMed] [Google Scholar]
- 33.Bannister LH, Hopkins JM, Dluzewski AR, Margos G, et al. Plasmodium falciparum apical membrane antigen 1 (PfAMA-1) is translocated within micronemes along subpellicular microtubules during merozoite development. J Cell Sci. 2003;116:3825–34. doi: 10.1242/jcs.00665. [DOI] [PubMed] [Google Scholar]
- 34.Meyer EVS, Semenya AA, Okenu DMN, Dluzewski AR, et al. The reticulocyte binding-like proteins of P. knowlesi locate to the micronemes of merozoites and define two new members of this invasion ligand family. Mol Biochem Parasitol. 2009;165:111–21. doi: 10.1016/j.molbiopara.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh M, Mukherjee P, Narayanasamy K, Arora R, et al. Proteome analysis of Plasmodium falciparum extracellular secretory antigens at asexual blood stages reveals a cohort of proteins with possible roles in immune modulation and signaling. Mol Cell Proteomics. 2009;8:2102–18. doi: 10.1074/mcp.M900029-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quakyi IA, Carter R, Rener J, Kumar N, et al. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol. 1987;139:4213–7. [PubMed] [Google Scholar]
- 37.Lobo CA, Konings RN, Kumar N. Expression of early gametocyte-stage antigens Pfg27 and Pfs16 in synchronized gametocytes and non-gametocyte producing clones of Plasmodium falciparum. Mol Biochem Parasitol. 1994;68:151–4. doi: 10.1016/0166-6851(94)00155-3. [DOI] [PubMed] [Google Scholar]
- 38.van Schaijk BCL, van Dijk MR, van de Vegte-Bolmer M, van Gemert G, et al. Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum. Mol Biochem Parasitol. 2006;149:216–22. doi: 10.1016/j.molbiopara.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Ansari FA, Kumar N, Bala Subramanyam M, Gnanamani M, Ramachandran S. MAAP: malarial adhesins and adhesin-like proteins predictor. Proteins. 2008;70:659–66. doi: 10.1002/prot.21568. [DOI] [PubMed] [Google Scholar]
- 40.Richard D, MacRaild CA, Riglar DT, Chan J, et al. Interaction between Plasmodium falciparum apical membrane antigen 1 and the rhoptry neck protein complex defines a key step in the erythrocyte invasion process of malaria parasites. J Biol Chem. 2010;285:14815–22. doi: 10.1074/jbc.M109.080770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoessli DC, Poincelet M, Gupta R, Ilangumaran S, Nasir-ud-Din Plasmodium falciparum merozoite surface protein 1. Eur J Biochem. 2003;270:366–75. doi: 10.1046/j.1432-1033.2003.03397.x. [DOI] [PubMed] [Google Scholar]
- 42.Sanders PR, Gilson PR, Cantin GT, Greenbaum DC, et al. Distinct protein classes including novel merozoite surface antigens in Raft-like membranes of Plasmodium falciparum. J Biol Chem. 2005;280:40169–76. doi: 10.1074/jbc.M509631200. [DOI] [PubMed] [Google Scholar]
- 43.Gilson PR, Nebl T, Vukcevic D, Moritz RL, et al. Identification and stoichiometry of glycosylphosphatidylinositol-anchored membrane proteins of the human malaria parasite Plasmodium falciparum. Molecular & Cellular Proteomics. 2006;5:1286–99. doi: 10.1074/mcp.M600035-MCP200. [DOI] [PubMed] [Google Scholar]
- 44.Lee N, Baker J, Bell D, McCarthy J, Cheng Q. Assessing the genetic diversity of the aldolase genes of Plasmodium falciparum and Plasmodium vivax and its potential effect on performance of aldolase-detecting rapid diagnostic tests. J Clin Microbiol. 2006;44:4547–49. doi: 10.1128/JCM.01611-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The malERA Consultative Group on Vaccines. A research agenda for malaria eradication: vaccines. PLoS Med. 2011;8:e1000398. doi: 10.1371/journal.pmed.1000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arévalo-Herrera M, Chitnis C, Herrera S. Current status of Plasmodium vivax vaccine. Hum Vaccin. 2010;6:124–32. doi: 10.4161/hv.6.1.9931. [DOI] [PubMed] [Google Scholar]
- 47.Nardin EH, Nussenzweig RS. T cell responses to pre-erythrocytic stages of malaria: Role in protection and vaccine development against pre-erythrocytic stages. Annu Rev Immunol. 1993;11:687–727. doi: 10.1146/annurev.iy.11.040193.003351. [DOI] [PubMed] [Google Scholar]
- 48.Joshi SK, Bharadwaj A, Chatterjee S, Chauhan VS. Analysis of immune responses against T- and B-cell epitopes from Plasmodium falciparum liver-stage antigen 1 in rodent malaria models and malaria-exposed human subjects in India. Infect Immun. 2000;68:141–50. doi: 10.1128/iai.68.1.141-150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jangpatarapongsa K, Sirichaisinthop J, Sattabongkot J, Cui L, Montgomery SM, Looareesuwan S, Troye-Blomberg M, Udomsangpetch U. Memory T cells protect against Plasmodium vivax infection. Microbes Infect. 2006;8:680–6. doi: 10.1016/j.micinf.2005.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.