Abstract
To identify Toxoplasma gondii genes important in the establishment of a persistent infection, we previously used signature-tagged mutagenesis to identify mutants with reduced cyst numbers in the brains of mice. One of the mutants, 95C5, has an insertion within a predicted six transmembrane domain protein, which localizes to the parasite pellicle, thus we named it transmembrane pellicle protein 1 (TgTPP1). Although the 95C5 mutant was found be reduced in its ability to form brain cysts, it is defective during acute infection. Addition of TgTPP1 expressed from its endogenous promoter restored the acute lethality of the 95C5 mutant to parental levels. The 95C5 mutant does not have a growth defect in standard tissue culture conditions; however, we found a significant defect in host cell penetration after extracellular stress. Overall, TgTPP1 may function during acute infection by enhancing the parasites ability to invade after extracellular stress.
Introduction, Results and Discussion
Toxoplasma gondii is an obligate intracellular parasite that has the ability to invade any nucleated cell in its host including immune cells. Host cell invasion by T. gondii is rapid (<1 minute) and relies on a sequential discharge of a set of specialized secretory organelles, the micronemes, the rhoptries, and the dense granules. Invasion is an active process that is facilitated by an actin-dependent myosin motor found within the parasite pellicle, a unique triple bilayer structure composed of the plasma membrane and the inner membrane complex [1]. The myosin motor is also associated with short actin microfilaments that partner with the glycolytic enzyme aldolase to link the complex with the parasite’s plasma membrane through interactions with transmembrane microneme protein (MIC) complexes [2]. These transmembrane MIC complexes are believed to provide the forward motion of motility by binding host cell receptors, facilitating the mechanical force generated by the myosin motor [3].
Several T. gondii surface proteins have been shown to be vital for the pathogenesis of the parasite. Disruption of surface antigen 3 (SAG3) causes a two-fold reduction in the ability of the parasite to invade host cells [4]. Surface adhesins such as microneme proteins 1, 2, 3 and 8 (MIC1 [5], MIC2 [6], MIC3 [5] and MIC8 [7], respectively), MIC2-associated protein (M2AP [6]), and apical membrane antigen 1 (AMA1 [8]) are also important for productive infection by T. gondii. In previous work, we created of a library of signature-tagged mutants (STM) to discover additional genes that may play a role in the establishment of a persistent infection by T. gondii [9]. Here we report the characterization of one of the mutants from this screen, called 95C5. The protein disrupted in 95C5 contains multiple transmembrane domains and is localized to the parasite pellicle. Disruption of this transmembrane domain protein in 95C5 attenuates acute virulence in mice and decreases invasion efficiency after extracellular stress.
To characterize the gene disrupted in the 95C5 mutant, we first mapped the insertion site of the mutagenesis plasmid to the fifth predicted intron of the annotated gene TGME49_051410 (www.toxodb.org) on chromosome XII. The open reading frame (ORF) of TGME49_051410 was determined by sequencing cDNA from wild-type (WT) parasites. We determined the full transcript of TGME49_051410 using rapid amplification of cDNA ends (RACE). The 5’ untranslated region (UTR) is 567 nucleotides and contains a 1,090 nucleotide intron, while the 3’UTR is 578 nucleotides. In plants, introns in the 5’UTR have been shown to enhance the level of protein expression through a phenomenon called Intron-Mediated Enhancement (IME) [10–12]. IME has not been investigated in T. gondii and we have not determined if IME plays any role in the expression of protein from TGME49_051410. The full length mRNA is 7,352 nucleotides (Fig. 1A) and produces a predicted protein of 2,069 amino acids.
Fig. 1.
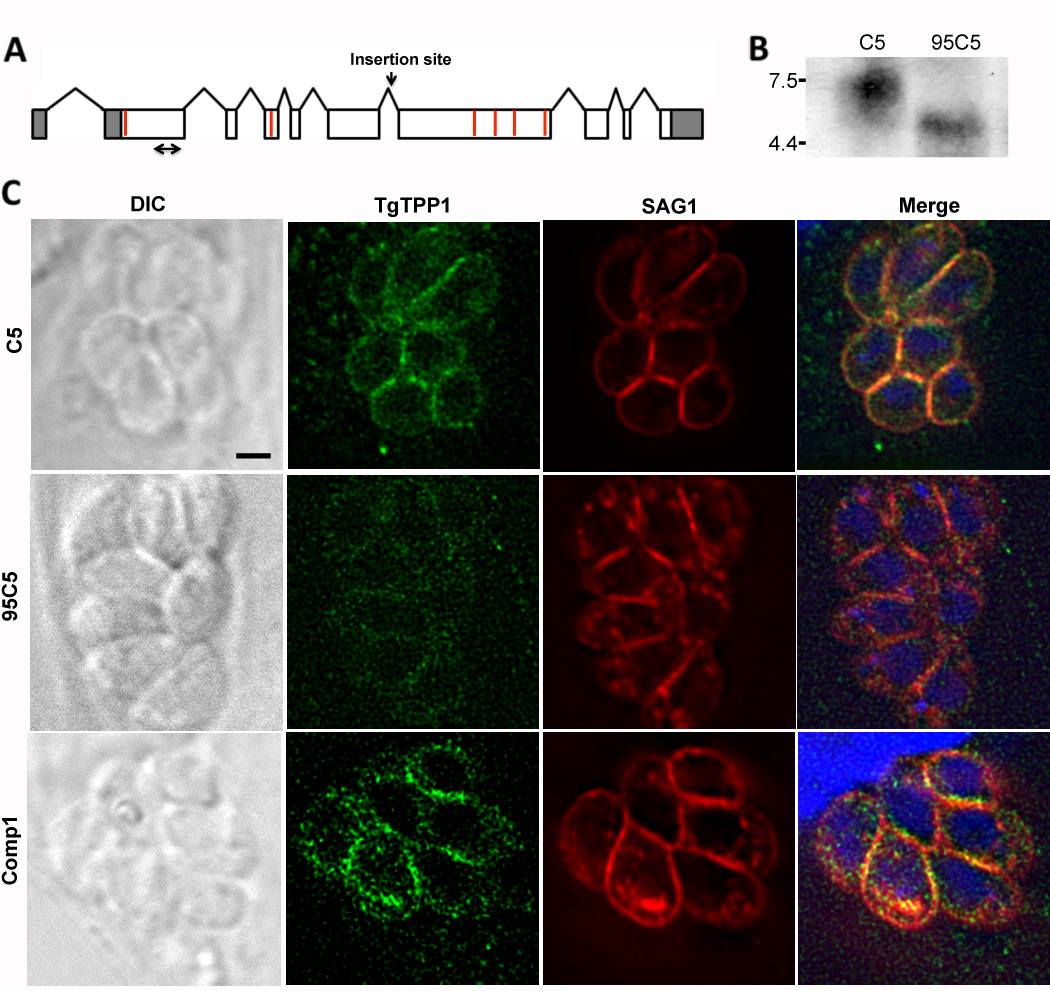
TgTPP1 is disrupted in the 95C5 mutant and is localized to the parasite surface. (A) A scale drawing of the mapped TGME49_051410 transcript with the UTRs shaded in gray, the exons in white, and the introns indicated by lines above the cartoon. The insertion site in 95C5 is indicated by a downward arrow. The location of the predicted transmembrane domains are indicated by red bars. The probe used in the northern hybridization in B is marked by a double arrow. (B) RNA from C5 and 95C5 parasites was examined by northern hybridization. The numbers to the left indicate the size of the markers of an RNA ladder in kilobases. (C) C5, 95C5 and complemented parasites (Comp1) parasites were seeded onto glass coverslips of human foreskin fibroblasts (HFFs) and allowed to grow for 48 hours. The cellular localization of TgTPP1 (green) was visualized using an scFv monoclonal antibody made against a TgTPP1 peptide using the Tomlinson I + J human single fold scFv libraries according to the manufacturer’s protocol. The cells were costained for SAG1 (red) and nucleic acid was visualized using 4’6’-diamidino-2-phenylindole (DAPI, blue). The black scale bar equals 2 µm.
To verify that TGME49_051410 is disrupted in 95C5 parasites, we compared the expression of TGME49_051410 in C5, the parental signature-tag strain used to produce 95C5, and 95C5 parasites by northern hybridization. The probe used to detect TGME49_051410 transcript corresponds to the second half of the first exon of TGME49_051410 (double arrow in Fig. 1A). C5 parasites showed a band at the predicted size of 7.3 kilobases while 95C5 parasites showed a significantly smaller message approximately 4.5 kilobases (Fig. 1B). Despite the presence of TGME49_051410 transcript in 95C5, the protein that would be produced from this transcript would be truncated, producing only the first 891 amino acids, or 43% of the predicted protein size.
The TGME49_051410 predicted protein was scanned at the Swiss Institute of Bioinformatics using the BLAST network service (http://www.expasy.org/cgi-bin/blast.pl) but no significant homology was found with any proteins in the database. Neospora caninum was the only other Apicomplexan found to have an ortholog. The predicted protein was also analyzed using a variety of other bioinformatics programs to identify potential functional domains. The presence of a signal peptide was predicted using SignalP-HMM ([13, 14] http://www.cbs.dtu.dk/services/SignalP/) with a predicted cleavage site between residues 24 and 25. The protein is also predicted to have six transmembrane domains according to TMpred (Fig. 1A, red lines).
To determine the cellular localization of the TGME49_051410 predicted protein, we created an antibody against amino acids 218–329 of the predicted protein using a library of over 100 million different single-chain variable fragments (scFv) from the Tomlinson I and J human single fold scFv libraries (Source BioScience, Nottingham, UK; [15]). The cellular localization of the predicted protein was examined by immunofluorescence assay (IFA). In C5 parasites, the predicted protein appears to be in the pellicle of the parasite and colocalized with the surface antigen SAG1 (Fig. 1C, top row). In 95C5 parasites, the staining with the scFv antibody was dramatically less intense compared to C5 parasites (Fig. 1C, middle row). Because of the high level of background staining with the scFv antibody, we verified the localization of the TGME49_051410 predicted protein by genetic complementation of 95C5 with an exogenous copy of TGME49_051410. The expression construct used for complementation includes 2 kilobases upstream of the transcription start site in order to place expression of TGME49_051410 under the control of its own promoter. The construct also includes the intron found in the 5’UTR as well as the first two introns in the coding sequence. The remaining coding sequence was amplified from cDNA due to the large size of the genomic sequence of TGME49_051410 (Table 1). Similar to C5 parasites, complemented 95C5 (Comp1) showed the predicted protein in the pellicle of the parasite, colocalizing with SAG1 (Fig. 1C, bottom row). The peptide used to create the scFv antibody corresponds to a part of the 95C5 protein that is upstream of the insertion site in 95C5 and thus it would detect a shortened form of the protein if it was translated in 95C5 parasites. We attempted to confirm this protein reduction in 95C5 by western immunoblot, however, no signal was detected in either C5 or 95C5 parasites (data not shown). Due to the localization of the TGME49_051410 predicted protein to the pellicle of T. gondii as well as the presence of multiple predicted transmembrane domains, we have designated this gene transmembrane pellicle protein 1 (TgTPP1).
Table 1.
The 95C5 complementation construct was created by ligating five different PCR products into the pCR2.1-TOPO vector (Invitrogen). The name of the primer indicates which piece it amplifies, the direction of the primer as it relates to the gene, and the restriction endonuclease used for cloning. For instance, the primer P1-F-KpnI is the forward primer used to create the first PCR fragment and was cloned using KpnI. Pieces 1, 2, and 5 were amplified using genomic DNA and pieces 3 and 4 were amplified from cDNA.
| Primer name | Primer sequence |
|---|---|
| P1-F-KpnI | 5’-GAAGGTACCTTCGTCTGTCCACAAT-3’ |
| P1-R-ClaI | 5’-GCAGAGGCGCCGAGACAAAGACG-3’ |
| P2-F-ClaI | 5’-GTAGTCAACCGCCCCACATCAG-3’ |
| P2-R-NotI | 5’-CCGGTATGCTGGGCTGTATGTA-3’ |
| P3-F-NotI | 5’-GCAGACGAGCAAGGACCAGGAT-3’ |
| P3-R-HindIII | 5’-CGGCACGCGTCGATACACCT-3’ |
| P4-F-HindIII | 5’-GCAGAGGCGAGCGACAACT-3’ |
| P4-R-PacI | 5’-TTAATTAAGCCATCAGCCCCGTCAC-3’ |
| P5-F-PacI | 5’-GGCTTAATTAATGGAGAAAGGGTGTC-3’ |
| P5-R-XbaI | 5’-GGGTCGACGCCGGAACATAAG-3’ |
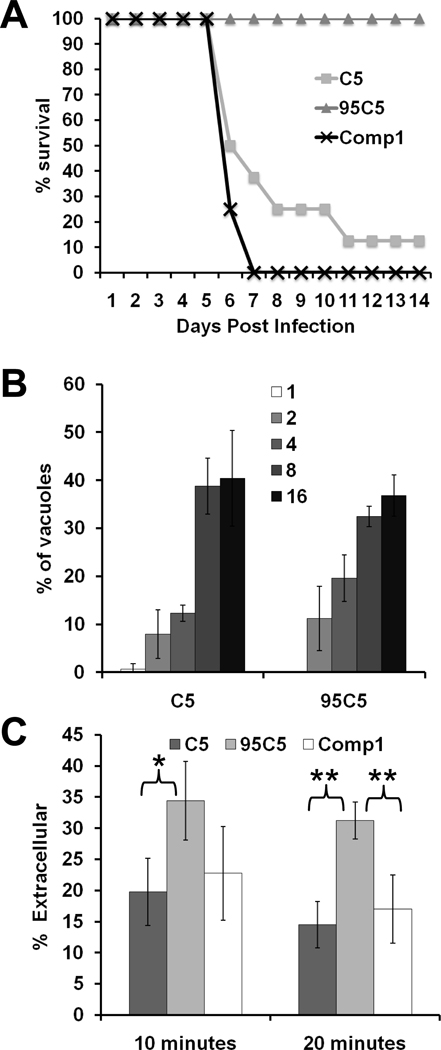
The mouse model used in the STM screen only indicates that a given mutant produced fewer cysts in mouse brains compared to WT parasites. This decrease in cyst counts could be caused by a defect in the ability of the parasite to switch to the cyst-forming bradyzoite form at the onset of chronic infection or by a defect during the acute stage of infection. We examined the ability of C5, 95C5 and Comp1 parasites to develop into bradyzoite cysts in tissue culture using minus bicarbonate, high pH media. In vitro, 95C5 parasites do not appear to have a development defect as they differentiate into bradyzoites similar to both parental C5 and Comp1 parasites (Fig. S1). To determine if the 95C5 decrease in vivo cyst counts is due to an acute stage defect, we tested C5, 95C5 and Comp1 parasites in an acute infection mouse model. In an acute infection mouse model, a lethal dose of parasites is used to challenge mice and time of death is used for comparison between strains. Typically, a challenge of 2×106 C5 parasites will be lethal to the mouse by 7 to 10 days post infection. In the case of 95C5 parasites, mice can be challenged with up to 5×107 parasites and not show any of the typical symptoms of T. gondii infection such as ruffled fur or hunching (data not shown). Comp1 parasites were restored in their lethality as all of the mice infected with this strain succumbed to infection by day 7 post infection (Fig. 2A). The viability and number of parasites used for the infections were verified by plaque assay in order to ensure that each mouse is infected with similar numbers of viable parasites. The complete lack of symptoms even at challenges well over 10 times the normal lethal dose and the restoration of acute virulence by addition of TgTPP1 to 95C5 parasites shows that TgTPP1 is required for survival of T. gondii during a mouse infection.
Fig. 2.
TgTPP1 is involved in virulence and invasion after stress. (A) 2×107 freshly lysed C5, 95C5, or complemented 95C5 (Comp1) parasites were used to infect four mice per strain in two independent experiments. Morbidity and mortality were monitored throughout the experiment. The percentage of mice surviving infection is noted. (B) C5 and 95C5 parasites were seeded onto coverslips containing HFFs and allowed to grow for 28 hours. The cells were fixed and stained to visualize parasites. The number of parasites per vacuole (indicated by the numbers in the legend) was noted for 100 vacuoles, and the average of three coverslips is shown +/− SD. (C) To test the invasion efficiency of 95C5, freshly lysed C5 and 95C5 parasites were used to infect fibroblasts on glass coverslips. Invasion was allowed to progress for 10 or 20 minutes at which point the cells were fixed and processed for IFA. To differentiate between extracellular parasites (parasites that have attached to the host cell plasma membrane but have not yet penetrated the cell) and intracellular parasites, the cells were first stained with a mouse-derived antibody against SAG1 followed by permeabilization of the cells and a second round of staining with a rabbit-derived antibody against SAG1. By using antibodies derived from two different species, the extracellular and intracellular parasites can be differentiated using secondary antibodies conjugated to fluorophores that emit at different wavelengths. Freshly lysed C5, 95C5, or Comp1 parasites were incubated in the absence of host cells at 37°C for three hours to induce a stress response. The “stressed” parasites were then used to infect glass coverslips of HFFs for 10 or 20 minutes. After each time point, the cells were fixed and red/green invasion assays were performed as previously described [17]. One hundred total parasites were counted for each coverslip and the average of four coverslips are shown as an average of the percentage of parasites that remained extracellular. Statistical significance was calculated using a student’s t-test (* p<0.05; ** p<0.005).
To determine if insertion into TgTPP1 is responsible for the inability of 95C5 parasites to survive during a mouse infection, we examined the virulence of 95C5 genetically complemented with TgTPP1. C5, 95C5, and Comp1 parasites were tested in the acute infection mouse model by infecting mice with 2×107 parasites. As before, none of the 95C5 infected mice showed any clinical signs of T. gondii infection, and most of the C5 infected mice succumbed to infection by day 11 post infection (Fig. 2A). Comp1 parasites were restored in their lethality as all of the mice infected with this strain succumbed to infection by day 7 post infection (Fig. 2A). The restorationv of acute virulence by addition of TgTPP1 to 95C5 parasites shows that TgTPP1 is required for survival of T. gondii during a mouse infection.
The attenuated virulence of the 95C5 mutant in the acute mouse model could be caused by a general growth defect. To test whether 95C5 has a growth defect, we measured the growth rate of 95C5 parasites in tissue culture. While this method does not exclude the possibility of an in vivo growth defect, any general deficiencies in parasite growth would be detected. Freshly lysed C5 and 95C5 parasites were used to infect fibroblast monolayers and allowed to grow for 40 hours. The growth of each strain was examined by counting the number of parasites per vacuole in the infected fibroblasts. This method is possible because parasite division is tightly controlled with all the parasites within a vacuole dividing synchronously so that the number of parasites doubles with each round of replication. There was no significant difference in growth between C5 and the 95C5 mutant (Fig. 2B). Therefore, it is not likely that the attenuated acute virulence of 95C5 in mice is due to a general growth deficiency.
Due to the apparent association of TgTPP1 with the parasite pellicle, we hypothesized that disruption of TgTPP1 could have an effect on invasion. To measure invasion efficiency, we used the red/green invasion assay where external, attached parasites stain red and internal, penetrated parasites stain green [16]. Fibroblasts on glass coverslips were infected with freshly lysed C5 and 95C5 parasites for either 10 or 20 minutes, then the cells were fixed and processed for IFA. 95C5 showed a slight decrease in invasion efficiency compared to C5 in this assay although the difference was not statistically significant (data not shown).
The stress response of T. gondii has been shown to promote extracellular survival [17]. Thus we examined the invasion efficiency of 95C5 parasites following extracellular stress. To induce a stress response in the parasites, freshly lysed C5 and 95C5 parasites were incubated for 3 hours at 37°C in the absence of host cells. The “stressed” parasites were then used in the red/green invasion assay. After invasion times of 10 and 20 minutes, 20% and 15% of C5 parasites remained extracellular, respectively (Fig. 2C). Meanwhile, 95C5 showed a significant penetration defect with 34% of 95C5 parasites remaining extracellular at the 10 minute time point and 31% remaining extracellular at the 20 minute time point (p<0.05 and p<0.005, respectively; Fig. 2C). The Comp1 strain was also tested in this assay to determine if the 95C5 penetration defect can be complemented by addition of TgTPP1. Comp1 parasites were restored in their invasion efficiency as 23% remained extracellular at the 10 minute time point and 17% remained extracellular at the 20 minute time point (Fig. 2C). The difference between the invasion efficiency of 95C5 and Comp1 was only statistically significant at the 20 minute time point (p<0.005). These results indicate that TgTPP1 enhances T. gondii penetration after extracellular stress.
Here we show that insertion into TgTPP1 causes a severe decrease in the ability of T. gondii to survive during a mouse infection. TgTPP1 is a large 226 kDa protein of unknown function with a predicted signal sequence and six transmembrane domains. Using an antibody raised against a TgTPP1 polypeptide, TgTPP1 localized to the pellicle of T. gondii. We also show that 95C5 parasites are defective in their ability to penetrate host cells following extracellular stress. This decreased invasion efficiency following extracellular stress may explain the inability of 95C5 parasites to survive during a mouse infection yet still maintain a normal growth rate in tissue culture. While the parasite is unlikely to remain extracellular for extended periods of time in an animal, the immune response may heighten the extracellular stresses applied to T. gondii due to the presence of antimicrobial compounds. Alternatively, the in vivo phenotype might be due to other parameters, such as inability to invade specific cell types, which has not been examined. Future studies will determine the precise role of TgTPP1 during an animal infection.
Supplementary Material
C5, 95C5, and Comp1 convert to bradyzoite cystsin vitro. Glass coverslips of confluent HFFs were infected with 1 × 105 tachyzoites of C5, 95C5, or Comp1 parasites. The parasites were allowed to grow for 3 hours, then differentiated to bradyzoites by replacing the medium with RPMI1640 supplemented with 1% FBS and 42mM N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES), 100 units/ml penicillin and 100 µg/ml streptomycin, at pH 8.1, and incubating at 37°C, ambient CO2 for 3 days. Cysts were visualized using Dolichos biflorus agglutinin (DBA) conjugated to rhodamine(red). The cysts were costained for the bradyzoite heat shock protein BAG1 (green) and nucleic acid was visualized using DAPI (blue).
ACKNOWLEDGEMENTS
We sincerely thank Jay Bangs for the use of his microscope and the Knoll lab for technical and editorial assistance. This research was supported by American Heart Association 0840059N (L.J.K.), and NIH National Research Service Award T32 AI007414 (T.M.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disruption of TgTPP1 in Toxoplasma gondii attenuates acute virulence in mice potentially due to a decrease in invasion efficiency following extracellular stress.
The GenBank accession number for the TgTPP1 nucleotide sequence is: BankIt1450213 TgTPP1 JF900403.
REFERENCES
- 1.Carruthers V, Boothroyd JC. Pulling together: an integrated model of Toxoplasma cell invasion. Curr Opin Microbiol. 2007;10(1):83–89. doi: 10.1016/j.mib.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Zheng B, et al. MIC6 associates with aldolase in host cell invasion by Toxoplasma gondii. Parasitol Res. 2009;105(2):441–445. doi: 10.1007/s00436-009-1401-5. [DOI] [PubMed] [Google Scholar]
- 3.Soldati D, Dubremetz JF, Lebrun M. Microneme proteins: structural and functional requirements to promote adhesion and invasion by the apicomplexan parasite Toxoplasma gondii. Int J Parasitol. 2001;31(12):1293–1302. doi: 10.1016/s0020-7519(01)00257-0. [DOI] [PubMed] [Google Scholar]
- 4.Dzierszinski F, et al. Targeted disruption of the glycosylphosphatidylinositol-anchored surface antigen SAG3 gene in Toxoplasma gondii decreases host cell adhesion and drastically reduces virulence in mice. Mol Microbiol. 2000;37(3):574–582. doi: 10.1046/j.1365-2958.2000.02014.x. [DOI] [PubMed] [Google Scholar]
- 5.Cerede O, et al. Synergistic role of micronemal proteins in Toxoplasma gondii virulence. J Exp Med. 2005;201(3):453–463. doi: 10.1084/jem.20041672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jewett TJ, Sibley LD. The toxoplasma proteins MIC2 and M2AP form a hexameric complex necessary for intracellular survival. J Biol Chem. 2004;279(10):9362–9369. doi: 10.1074/jbc.M312590200. [DOI] [PubMed] [Google Scholar]
- 7.Kessler H, et al. Microneme protein 8-a new essential invasion factor in Toxoplasma gondii. J Cell Sci. 2008;121(7):947–956. doi: 10.1242/jcs.022350. [DOI] [PubMed] [Google Scholar]
- 8.Mital J, et al. Conditional expression of Toxoplasma gondii apical membrane antigen-1 (TgAMA1) demonstrates that TgAMA1 plays a critical role in host cell invasion. Mol Biol Cell. 2005;16(9):4341–4349. doi: 10.1091/mbc.E05-04-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frankel MB, Mordue DG, Knoll LJ. Discovery of parasite virulence genes reveals a unique regulator of chromosome condensation 1 ortholog critical for efficient nuclear trafficking. Proc Natl Acad Sci U S A. 2007;104(24):10181–10186. doi: 10.1073/pnas.0701893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callis J, Fromm M, Walbot V. Introns increase gene expression in cultured maize cells. Genes Dev. 1987;1(10):1183–1200. doi: 10.1101/gad.1.10.1183. [DOI] [PubMed] [Google Scholar]
- 11.Rethmeier N, et al. Intron-mediated enhancement of transgene expression in maize is a nuclear, gene-dependent process. Plant J. 1997;12(4):895–899. doi: 10.1046/j.1365-313x.1997.12040895.x. [DOI] [PubMed] [Google Scholar]
- 12.Chaubet-Gigot N, et al. Tissue-dependent enhancement of transgene expression by introns of replacement histone H3 genes of Arabidopsis. Plant Mol Biol. 2001;45(1):17–30. doi: 10.1023/a:1006487023926. [DOI] [PubMed] [Google Scholar]
- 13.Bendtsen JD, et al. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340(4):783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen H, Krogh A. Prediction of signal peptides and signal anchors by a hidden Markov model. Proc Int Conf Intell Syst Mol Biol. 1998;6:122–130. [PubMed] [Google Scholar]
- 15.de Wildt RM, et al. Antibody arrays for high-throughput screening of antibody-antigen interactions. Nat Biotechnol. 2000;18(9):989–994. doi: 10.1038/79494. [DOI] [PubMed] [Google Scholar]
- 16.Joyce BR, et al. Phosphorylation of eukaryotic initiation factor-2{alpha} promotes the extracellular survival of obligate intracellular parasite Toxoplasma gondii. Proc Natl Acad Sci U S A. 2010;107(40):17200–17205. doi: 10.1073/pnas.1007610107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huynh MH, et al. Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2-M2AP adhesive protein complex. Embo J. 2003;22(9):2082–2090. doi: 10.1093/emboj/cdg217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
C5, 95C5, and Comp1 convert to bradyzoite cystsin vitro. Glass coverslips of confluent HFFs were infected with 1 × 105 tachyzoites of C5, 95C5, or Comp1 parasites. The parasites were allowed to grow for 3 hours, then differentiated to bradyzoites by replacing the medium with RPMI1640 supplemented with 1% FBS and 42mM N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES), 100 units/ml penicillin and 100 µg/ml streptomycin, at pH 8.1, and incubating at 37°C, ambient CO2 for 3 days. Cysts were visualized using Dolichos biflorus agglutinin (DBA) conjugated to rhodamine(red). The cysts were costained for the bradyzoite heat shock protein BAG1 (green) and nucleic acid was visualized using DAPI (blue).