Abstract
Apelin is a newly discovered peptide hormone which has recently been linked to insulin resistance and obesity. Collected data from both the clinical and basic research settings show that apelin (1) correlates with states of insulin resistance and obesity, (2) stimulates glucose utilization, (3) decreases insulin secretion, and (4) negatively regulates catecholamine-mediated lipolysis. These and other lines of evidence demonstrate that apelin may be a potentially viable candidate in the search for treatments for type 2 diabetes and the insulin resistance (metabolic syndrome). This review will summarize the literature to date on apelin’s regulation of glucose and lipid metabolism and the signaling pathways
Introduction
Insulin resistance, defined as a decreased responsiveness to insulin, is a cardinal feature of type 2 diabetes mellitus (T2DM) and the metabolic syndrome. Given the prevalence of these conditions, restoration of insulin sensitivity has long been a focus of drug development over time. Sadly, despite the advancement of several effective medical therapies for insulin resistance, the successful deployment of these medications has been greatly outpaced by the staggering burden of morbidity and mortality inherent in the metabolic syndrome and T2DM worldwide. It is thus clear that the introduction of novel therapeutic modalities (i.e. arrows for the quiver) would be greatly welcomed by patients and caregivers.
In recent years, a number of adipocyte-derived regulatory hormones, termed adipokines, have become a focus of scientific inquiry. One of these adipokines, apelin, was initially discovered in 1998. In the 13 years since its identification, apelin has been found to exert a wide variety of biologically diverse actions in various organs. Additionally, consistent with its identity as an adipokine, it has become increasingly appreciated that apelin regulates insulin sensitivity. Despite a growing body of literature, however, research into apelin’s function in insulin resistance and its underlying mechanisms remains relatively nascent. The goal of this review is thus to summarize the current literature and highlight gaps in our knowledge about apelin’s relationship with insulin resistance.
Insulin resistance
Insulin resistance is a pathological condition in which a biological unit, be it a cell, tissue, organ, or organism, becomes less responsive to insulin. Insulin resistance is the pathophysiologic hallmark of T2DM, which accounts for about 90% to 95% of the estimated 26 million adult diabetics in the United States. Although T2DM is not particularly consequential in the short term, it is independently and strongly associated with an increased risk of heart disease, stroke, kidney failure, amputations, blindness, and mortality1. In addition to those with T2DM, insulin resistance also affects the estimated 79 million US adults with the so-called metabolic syndrome (Syndrome X)2. While the metabolic syndrome is felt to be a precursor to T2DM, it is also not without sequelae; for example, it is independently associated with cardiovascular mortality3 and multiple types of cancer4–7. Insulin resistance is thus rapidly gaining in importance as a disease entity in the Western world. Unfortunately, while multiple treatment modalities have been developed, our current therapeutic armamentarium is inadequate to effectively manage the burden of the disease. A clear need for novel therapies therefore exists.
Apelin
Apelin is a recently discovered endogenous peptide hormone which has garnered significant investigative attention during the last decade. Apelin was first isolated from bovine stomach extracts8 in a drug screen searching for endogenous ligands for a previously orphaned G protein coupled receptor named angiotensin-like receptor 1 (Agtrl1), or APJ (which itself was discovered in a search for isotypes of the vasopression receptor)9. APJ bears significant sequence homology to the type 1 angiotensin receptor9, and is known to associate with the heterotrimeric G proteins Gi10 and Gq11. At this time APJ is the only known receptor for apelin.
The human gene APLN is located on chromosome Xq25-26.112. Its gene product is a 77-amino acid prepropeptide, that is subsequently cleaved post-translationally into several active forms, which are 36, 17, 13, and 12 residues in length8. Of these, the 36-amino acid isoform is the most widely expressed in different organs, including adipocytes, gastric mucosa, and endothelia of small arteries13. Nonetheless, the shorter isoforms (especially pyroglutamated apelin-13) are thought to induce a greater vasoactive response13, though it has recently been suggested that apelin-36 possesses equal potency to pyr-apelin-1314. Though little is known about its metabolism in vivo, apelin is cleaved by the exopeptidase angiotensin converting enzyme 2 (ACE2)15 under experimental conditions. While further efforts to characterize its pharmacodynamics have been hindered by lack of a robust, widely available diagnostic assay16, apelin is believed to be rapidly cleared from the circulation with a half-life of no longer than 5 minutes17.
Apelin has been demonstrated to have multiple unique effects in several organs and tissues, including the brain, heart, gut, and kidney (for review see 18). In contrast to APJ, which is expressed ubiquitously19, apelin is secreted predominantly from endothelial cells20. This tissue distribution has led to the hypothesis that apelin exerts its actions in a paracrine fashion. More recently, both apelin21, 22 and APJ23 have also been found to be present in adipocytes. As such, an endocrine role for apelin (as an adipokine) has been proposed24, though this contention awaits further clarification.
The association between apelin and insulin resistance: clinical evidence
Consistent with its putative role as an adipokine, apelin has been linked to states of insulin resistance in recent years. In clinical studies, compared to normal controls, plasma apelin concentration is increased in insulin resistant subjects25, as well as in morbidly obese individuals with T2DM26, 27. When considered as a continuous variable, apelin also has been found to correlate positively with hemoglobin A1c (HbA1c)28. Notably, some recent reports have shown that plasma apelin levels are paradoxically decreased in newly diagnosed patients with T2DM29, 30. While it is difficult to reconcile these divergent findings, these data do suggest the possibility of alternative regulatory pathways for apelin production in the setting of insulin resistance.
At present, few genomic studies assessing for variants in the APLN and AGTRL1 genes have been published. To date, most recognized single nucleotide polymorphisms (SNPs) have been found to correlate with stroke31, heart failure32 and systemic hypertension33–36. That said, a recent study reported that male diabetics carrying the C allele of a SNP, rs2235306, in the APLN gene had a significantly increased fasting glucose level relative to those carrying the T allele37. However, this finding was not observed in females, and other measures of insulin sensitivity (e.g. 2 hour oral glucose tolerance test, fasting insulin, homeostatic model assessment-IR) showed no correlation, raising the possibility of a spurious result. Nevertheless, interest in identifying and assessing the significance of genetic variants in apelin and APJ related to insulin resistance remains high.
The association between apelin and insulin resistance: basic evidence
The clinical evidence demonstrating apelin’s association with insulin resistance has also been borne out in the laboratory setting. Early research building on the observation that apelin and APJ were expressed in adipocytes reported that apelin secretion is regulated by insulin21, 22, suggesting that apelin’s correlation with insulin resistance was due to hyperinsulinemia. Lending further support to this possibility was the demonstration that apelin was downregulated in mice treated with the beta cell toxin streptozotocin22. Interestingly, glucose-stimulated pancreatic secretion of insulin is decreased in the presence of apelin, consistent with a negative feedback loop38, 39.
Subsequent investigation has elucidated apelin’s direct effect on glucose handling and insulin sensitivity. Direct injection of apelin in mice is sufficient to improve glucose handling as measured by a glucose tolerance test40, 41. Apelin has also been demonstrated to increase glucose uptake in isolated soleus muscle41, cultured skeletal myotubes42, and adipose tissue43. Mice with a generalized deficiency of apelin also have abnormal insulin tolerance and insulin suppression tests, are hyperinsulinemic, and have decreased adiponectin levels, suggesting a primary effect on insulin sensitivity42. Moreover, exogenous apelin administration results in reversal of these abnormalities. These data indicate that apelin directly increases insulin sensitivity and suggest that the elevations in circulating apelin observed in states of insulin resistance are compensatory.
Apelin and insulin signaling: mechanistic insights
The insights gained from exploring apelin’s effects on insulin-glucose homeostasis have led to further investigation regarding the underlying mechanisms responsible for these effects. Thus far, Gi-, Gq- and AMPK-mediated pathways have been implicated in apelin’s regulation of glucose uptake (Figure 1).
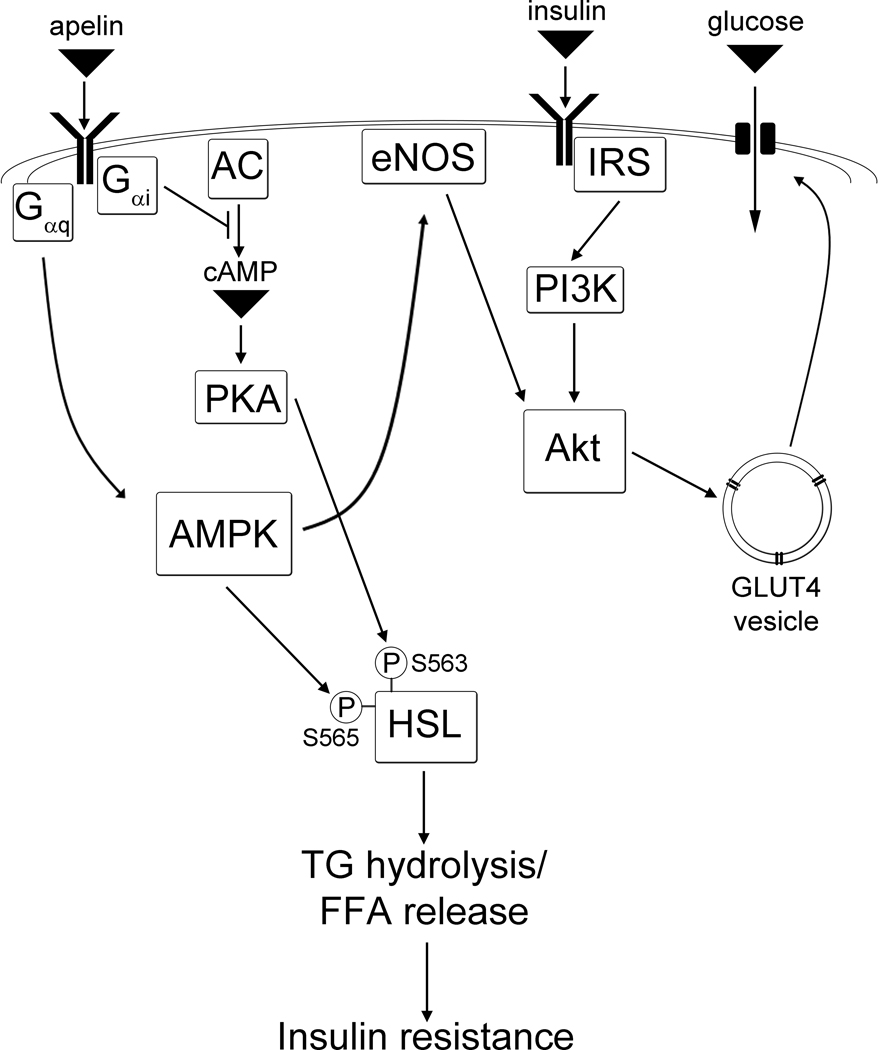
Figure 1.
Putative signaling mechanisms for apelin’s regulation of insulin sensitivity. Apelin directly enhances glucose uptake via a pathway involving AMPK and eNOS. Apelin also inhibits lipolysis via phosphorylative regulation of HSL, indirectly increasing insulin sensitivity by reducing FFA release to the circulation. Abbreviations: AC (adenylate cyclase), AMPK (AMP-mediated protein kinase), eNOS (endothelial nitric oxide synthase), FFA (free fatty acid), HSL (hormone sensitive lipase), IRS (insulin receptor substrate), PI3K (phosphoinositide 3-kinase).
As discussed above, apelin binds to APJ, a G protein coupled receptor. Shortly after its discovery APJ was found to couple with the heterotrimeric G protein Gi10. Accordingly, it has been shown that pertussis toxin (a potent Gi inhibitor) inhibits apelin-stimulated glucose uptake and Akt phosphorylation42, confirming that a Gi-mediated pathway participates in apelin’s control of glucose uptake. While further signaling events downstream of Gi are not well characterized, it has long been appreciated that Gi is involved in the regulation of glucose uptake. For example, hepatocyte- and adipocyte-specific deletion of Gi results in insulin resistance44, whereas hepatocyte-, adipocyte-, and myocyte-specific overexpression results in improved insulin sensitivity45.
In addition to Gi, it is also believed that APJ couples to Gq. Consistent with this, apelin has been demonstrated to increase accumulation of inositide triphosphate (IP3)46. Additionally, inhibition of Gq-dependent entities, such as phospholipase C, protein kinase C, and the sarcolemmal Na+/H+ and Na+/Ca2+ exchangers, has been demonstrated to block apelin-mediated contraction in isolated hearts11. Finally, three-dimensional modeling of APJ using a hidden Markov algorithm predicts a strong likelihood of coupling to Gq47. Taken together, these data strongly suggest that apelin activates Gq via its interaction with APJ. Whether apelin-mediated glucose uptake is dependent on Gq, however, remains undetermined.
Apelin’s effects on glucose uptake have also been shown to involve the energy sensing enzyme AMP-activated protein kinase (AMPK), which is well known to mediate the metabolic response to intracellular ATP depletion (for review see 48). Apelin is known to activate AMPK, and inhibition of AMPK activity, be it pharmacologic, genetic, or molecular, results in the abrogation of apelin-induced glucose uptake41, 42. The signaling events connecting APJ with AMPK have yet to be fully elucidated, though the likelihood of a Gi-mediated mechanism is lessened by the well established Gs/cAMP/PKA-mediated activation of AMPK49, 50. Interestingly, a signaling cascade involving phospholipase C, IP3, and Ca2+ release (a classic Gq response) was recently implicated in adiponectin-mediated AMPK activation51. Also, as with signaling proximal to AMPK, the distal pathways germane to apelin/APJ are not well understood. However, inactivation of AMPK has been shown to inhibit apelin-mediated Akt phosphorylation41, 42, suggesting the involvement of the latter.
An area of controversy concerns the relationship between apelin/APJ and canonical insulin signal transduction. It is clear that apelin stimulates several components of the insulin pathway, including phosphoinositol 3-kinase (PI3K)52 and Akt41, 42. However, it is less certain whether apelin potentiates insulin-mediated glucose uptake. It has been shown that apelin increases insulin-stimulated glucose uptake in soleus muscles and white adipose tissue41. The authors of this paper postulated that apelin’s effects were mediated by AMPK in a pathway also involving endothelial nitric oxide synthase (eNOS). Work in our laboratory, however, has not verified a similar effect in cultured skeletal myotubes42. While a definitive explanation for these discrepant results has not been advanced, the former study was conducted in the ex vivo setting, whereas our research was conducted in vitro. It is therefore possible that in ex vivo circumstances apelin may secondarily influence a process external to the target cell (e.g. eNOS activity) to produce the observed effects on insulin-mediated glucose uptake (and ultimately, insulin sensitivity).
While the APJ-AMPK-eNOS pathway represents the best-studied putative explanation for apelin’s effects on insulin sensitivity, other possibilities exist. For example, apelin has been shown to inhibit NF-κB expression by triggering an interaction between APJ and the type I angiotensin receptor53. NF-κB downregulation would, in turn, be expected to inhibit TNF-α-mediated insulin resistance. Further support for this mechanism comes from a recent report demonstrating an apelin-mediated improvement in insulin-mediated glucose uptake in cultured adipocytes54. Apelin has also been noted to reduce the production of inflammatory mediators other than NF-κB, including reactive oxygen species55, interleukin-6, and monocyte chemoattractant protein-1 (MCP1)56. Ultimately, it is likely that apelin’s salutary effects on insulin sensitivity occur via multiple pathways. Full elucidation of these pathways, as well as their significance relative to each other, awaits further research.
The effect of apelin on adiposity and fatty acid handling
Given its relationship with insulin resistance, it should come as no surprise that apelin is also associated with states of obesity. In clinical studies, compared to normal controls, plasma apelin concentration is increased in morbidly obese patients57, and a reduction of body weight results in a coincident decline in apelin expression58. Apelin also correlates positively with body mass index (BMI)57. In animal studies, apelin supplementation decreased white adipose tissue mass and body adiposity in obese mice fed with a high-fat diet40. Additionally, apelin null mice were found to have increased abdominal adiposity and epididymal fat weight; these changes were reversed with apelin infusion via osmotic pumps46.
Notably, obesity is associated with increased circulating free fatty acids (FFAs), and in turn, insulin resistance2. In fact, it is believed that increased FFAs, in part, underlie the pathogenesis of insulin resistance. For example, increases in circulating FFAs invariably result in diminished insulin sensitivity59–61. Furthermore, pharmacologically lowering FFAs is known to increase insulin sensitivity62. While the mechanism for this phenomenon has not been definitively established, it is believed that the link involves intracellular lipid accumulation, subsequent inactivation of glycolysis and glucose uptake, and buildup of toxic metabolites, including diacylglycerol and ceramide63.
Consistent with this framework, apelin null mice have an increased concentration of plasma FFAs46. Moreover, administration of apelin decreases circulating FFAs. In exploring possible etiologies for these findings, potential mechanisms can broadly be classified into two groups: (1) those that decrease FA entry into the circulation (i.e. “supply”), and (2) those that increase FA utilization (i.e. “demand”).
On the supply side, many research groups have looked into caloric intake. Unfortunately, apelin’s impact on this parameter is controversial, as investigators have separately reported an increase64, a decrease65, or no change28, 40 in food intake in various settings. The reasons these discrepant results may lie in the animal model chosen, the method of administration, and the chronicity of administration, all of which were different between the studies. Apelin’s effects on lipolysis (the catecholamine-mediated hydrolysis of triglycerides to FFAs in adipocytes) have also been investigated. In isolated murine adipocytes, as well as in cultured 3T3L1 cells, apelin has been shown to inhibit isoproterenol-induced FFA release in an AMPK, Gi, and Gq-dependent fashion46. In this study, apelin’s actions were found to be associated with a reduction in stimulatory phosphorylation of the triglyceride hydrolase hormone sensitive lipase (HSL) at the Ser-563 residue, as well as an increase in inhibitory phosphorylation of HSL at Ser-565. Notably, a more recent study has reported no apelin-mediated changes in lipolysis in human adipose explants43. These seemingly contradictory results may reflect differences in organism choice (human vs. mouse) and assay methodology.
On the demand side, apelin has been shown to increase body temperature and UCP3 expression in brown fat40. Apelin is also known to increase mitochondrial respiratory enzyme activity, expression of respiratory chain components, and protein content, suggesting an increase in mitochondrial biogenesis66. While the latter findings are of great interest given the importance of mitochondria to fatty acid and glucose metabolism, it remains to be seen whether apelin affects more significant assessments of mitochondrial function, such as oxygen consumption and membrane potential. Moreover, apelin’s effects on mitochondrial substrate utilization (i.e. fatty acid and glucose oxidation, glycolysis) have not been evaluated.
Conclusion
In recent years, there has been a growing appreciation of apelin’s involvement in the pathogenesis of insulin resistance. Apelin secretion is regulated by insulin, and clinical studies have demonstrated elevated plasma apelin concentrations in individuals with insulin resistance. Moreover, direct administration of apelin has been shown to increase insulin sensitivity, peripheral glucose uptake, and adiponectin levels, as well as decrease adiposity, hyperinsulinemia, and free fatty acid levels. While a human study evaluating apelin’s effects on insulin sensitivity has not been completed, the available evidence nevertheless suggests that apelin ameliorates insulin resistance, positioning apelin/APJ signaling as a possible pharmaceutical target for the treatment of T2DM and the metabolic syndrome. Despite this early promise, however, unresolved issues remain regarding apelin and its association with insulin sensitivity. The intracellular mechanisms governing apelin-induced glucose uptake, and its relationship with the classic insulin signaling cascade, have yet to be fully characterized. Moreover, apelin’s regulation of fatty acid homeostasis, as well as its significance relative to insulin sensitivity, needs to be further clarified. Nevertheless, targeting apelin/APJ signaling may represent a potentially novel avenue in designing therapies for insulin resistance.
Acknowledgements
Funding sources consist of a National Institutes of Health Mentored Clinical Scientist Research Career Development Award (1K08 DK080463; PY), an American Diabetes Association Basic Science Award (1-10-BS-204; PY), and an Established Investigator Award from the American Heart Association (0840172N; PT).
Footnotes
Disclosure
The authors disclose no conflicts of interest, financial or otherwise, related to the content of this review.
References
- 1.Prevention CfDCa. 2011 National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. [Accessed February 7, 2011];2011 www.cdc.gov/diabetes/pubs/factsheet11.htm.
- 2.Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988 Aug;37(8):1020–1024. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- 3.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004 Sep 7;110(10):1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 4.Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009 Dec 15;115(24):5651–5661. doi: 10.1002/cncr.24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update. Dig Dis. 28(4–5):645–656. doi: 10.1159/000320068. [DOI] [PubMed] [Google Scholar]
- 6.Porto LA, Lora KJ, Soares JC, Costa LO. Metabolic syndrome is an independent risk factor for breast cancer. Arch Gynecol Obstet. Jan 20; doi: 10.1007/s00404-011-1837-6. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqui AA. Metabolic syndrome and its association with colorectal cancer: a review. Am J Med Sci. Mar;341(3):227–231. doi: 10.1097/MAJ.0b013e3181df9055. [DOI] [PubMed] [Google Scholar]
- 8.Tatemoto K, Hosoya M, Habata Y, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998 Oct 20;251(2):471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 9.O'Dowd BF, Heiber M, Chan A, et al. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993 Dec 22;136(1–2):355–360. doi: 10.1016/0378-1119(93)90495-o. [DOI] [PubMed] [Google Scholar]
- 10.Masri B, Lahlou H, Mazarguil H, Knibiehler B, Audigier Y. Apelin (65–77) activates extracellular signal-regulated kinases via a PTX-sensitive G protein. Biochem Biophys Res Commun. 2002 Jan 11;290(1):539–545. doi: 10.1006/bbrc.2001.6230. [DOI] [PubMed] [Google Scholar]
- 11.Szokodi I, Tavi P, Foldes G, et al. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ Res. 2002 Sep 6;91(5):434–440. doi: 10.1161/01.res.0000033522.37861.69. [DOI] [PubMed] [Google Scholar]
- 12.Lee DK, Cheng R, Nguyen T, et al. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000 Jan;74(1):34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 13.Tatemoto K, Takayama K, Zou MX, et al. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept. 2001 Jun 15;99(2–3):87–92. doi: 10.1016/s0167-0115(01)00236-1. [DOI] [PubMed] [Google Scholar]
- 14.Japp AG, Cruden NL, Amer DA, et al. Vascular effects of apelin in vivo in man. J Am Coll Cardiol. 2008 Sep 9;52(11):908–913. doi: 10.1016/j.jacc.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Vickers C, Hales P, Kaushik V, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002 Apr 26;277(17):14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 16.Mesmin C, Dubois M, Becher F, Fenaille F, Ezan E. Liquid chromatography/tandem mass spectrometry assay for the absolute quantification of the expected circulating apelin peptides in human plasma. Rapid Commun Mass Spectrom. 2010 Oct 15;24(19):2875–2884. doi: 10.1002/rcm.4718. [DOI] [PubMed] [Google Scholar]
- 17.Japp AG, Cruden NL, Barnes G, et al. Acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failure. Circulation. 2010 Apr 27;121(16):1818–1827. doi: 10.1161/CIRCULATIONAHA.109.911339. [DOI] [PubMed] [Google Scholar]
- 18.Pitkin SL, Maguire JJ, Bonner TI, Davenport AP. International Union of Basic and Clinical Pharmacology. LXXIV. Apelin receptor nomenclature, distribution, pharmacology, and function. Pharmacol Rev. Sep;62(3):331–342. doi: 10.1124/pr.110.002949. [DOI] [PubMed] [Google Scholar]
- 19.Kleinz MJ, Skepper JN, Davenport AP. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul Pept. 2005 Mar 30;126(3):233–240. doi: 10.1016/j.regpep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Kleinz MJ, Davenport AP. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul Pept. 2004 May 15;118(3):119–125. doi: 10.1016/j.regpep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Glassford AJ, Yue P, Sheikh AY, et al. HIF-1 regulates hypoxia- and insulin-induced expression of apelin in adipocytes. Am J Physiol Endocrinol Metab. 2007 Dec;293(6):E1590–E1596. doi: 10.1152/ajpendo.00490.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boucher J, Masri B, Daviaud D, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005 Apr;146(4):1764–1771. doi: 10.1210/en.2004-1427. [DOI] [PubMed] [Google Scholar]
- 23.Wei L, Hou X, Tatemoto K. Regulation of apelin mRNA expression by insulin and glucocorticoids in mouse 3T3-L1 adipocytes. Regul Pept. 2005 Dec 15;132(1–3):27–32. doi: 10.1016/j.regpep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Castan-Laurell I, Boucher J, Dray C, Daviaud D, Guigne C, Valet P. Apelin, a novel adipokine over-produced in obesity: friend or foe? Mol Cell Endocrinol. 2005 Dec 21;245(1–2):7–9. doi: 10.1016/j.mce.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Yang G, Li Q, et al. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Exp Clin Endocrinol Diabetes. 2006 Nov;114(10):544–548. doi: 10.1055/s-2006-948309. [DOI] [PubMed] [Google Scholar]
- 26.Soriguer F, Garrido-Sanchez L, Garcia-Serrano S, et al. Apelin levels are increased in morbidly obese subjects with type 2 diabetes mellitus. Obes Surg. Nov 2009;19(11):1574–1580. doi: 10.1007/s11695-009-9955-y. [DOI] [PubMed] [Google Scholar]
- 27.Daviaud D, Boucher J, Gesta S, et al. TNFalpha up-regulates apelin expression in human and mouse adipose tissue. FASEB J. 2006 Jul;20(9):1528–1530. doi: 10.1096/fj.05-5243fje. [DOI] [PubMed] [Google Scholar]
- 28.Dray C, Debard C, Jager J, et al. Apelin and APJ regulation in adipose tissue and skeletal muscle of type 2 diabetic mice and humans. Am J Physiol Endocrinol Metab. 2010 Jun;298(6):E1161–E1169. doi: 10.1152/ajpendo.00598.2009. [DOI] [PubMed] [Google Scholar]
- 29.Erdem G, Dogru T, Tasci I, Sonmez A, Tapan S. Low plasma apelin levels in newly diagnosed type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2008 May;116(5):289–292. doi: 10.1055/s-2007-1004564. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Shen C, Li X, et al. Low plasma apelin in newly diagnosed type 2 diabetes in Chinese people. Diabetes Care. 2009 Dec;32(12):e150. doi: 10.2337/dc09-1146. [DOI] [PubMed] [Google Scholar]
- 31.Hata J, Matsuda K, Ninomiya T, et al. Functional SNP in an Sp1-binding site of AGTRL1 gene is associated with susceptibility to brain infarction. Hum Mol Genet. 2007 Mar 15;16(6):630–639. doi: 10.1093/hmg/ddm005. [DOI] [PubMed] [Google Scholar]
- 32.Sarzani R, Forleo C, Pietrucci F, et al. The 212A variant of the APJ receptor gene for the endogenous inotrope apelin is associated with slower heart failure progression in idiopathic dilated cardiomyopathy. J Card Fail. 2007 Sep;13(7):521–529. doi: 10.1016/j.cardfail.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Li WW, Niu WQ, Zhang Y, Wu S, Gao PJ, Zhu DL. Family-based analysis of apelin and AGTRL1 gene polymorphisms with hypertension in Han Chinese. J Hypertens. 2009 Jun;27(6):1194–1201. doi: 10.1097/HJH.0b013e32832a3eb1. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Q, Gu D, Kelly TN, et al. Association of genetic variants in the apelin-APJ system and ACE2 with blood pressure responses to potassium supplementation: the GenSalt study. Am J Hypertens. Jun;23(6):606–613. doi: 10.1038/ajh.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Q, Hixson JE, Rao DC, et al. Genetic variants in the apelin system and blood pressure responses to dietary sodium interventions: a family-based association study. J Hypertens. Apr;28(4):756–763. doi: 10.1097/HJH.0b013e3283370d32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niu W, Wu S, Zhang Y, et al. Validation of genetic association in apelin-AGTRL1 system with hypertension in a larger Han Chinese population. J Hypertens. Sep;28(9):1854–1861. doi: 10.1097/HJH.0b013e32833b1fad. [DOI] [PubMed] [Google Scholar]
- 37.Zhang R, Hu C, Wang CR, et al. Association of apelin genetic variants with type 2 diabetes and related clinical features in Chinese Hans. Chin Med J (Engl) 2009 Jun 5;122(11):1273–1276. [PubMed] [Google Scholar]
- 38.Guo L, Li Q, Wang W, et al. Apelin inhibits insulin secretion in pancreatic beta-cells by activation of PI3-kinase-phosphodiesterase 3B. Endocr Res. 2009;34(4):142–154. doi: 10.3109/07435800903287079. [DOI] [PubMed] [Google Scholar]
- 39.Sorhede Winzell M, Magnusson C, Ahren B. The apj receptor is expressed in pancreatic islets and its ligand, apelin, inhibits insulin secretion in mice. Regul Pept. 2005 Nov;131(1–3):12–17. doi: 10.1016/j.regpep.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Higuchi K, Masaki T, Gotoh K, et al. Apelin, an APJ receptor ligand, regulates body adiposity and favors the messenger ribonucleic acid expression of uncoupling proteins in mice. Endocrinology. 2007 Jun;148(6):2690–2697. doi: 10.1210/en.2006-1270. [DOI] [PubMed] [Google Scholar]
- 41.Dray C, Knauf C, Daviaud D, et al. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab. 2008 Nov;8(5):437–445. doi: 10.1016/j.cmet.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Yue P, Jin H, Aillaud M, et al. Apelin is necessary for the maintenance of insulin sensitivity. Am J Physiol Endocrinol Metab. 2010 Jan;298(1):E59–E67. doi: 10.1152/ajpendo.00385.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Attane C, Daviaud D, Dray C, et al. Apelin stimulates glucose uptake but not lipolysis in human adipose tissue ex vivo. J Mol Endocrinol. 2011;46(1):21–28. doi: 10.1677/JME-10-0105. [DOI] [PubMed] [Google Scholar]
- 44.Moxham CM, Malbon CC. Insulin action impaired by deficiency of the G-protein subunit G ialpha2. Nature. 1996 Feb 29;379(6568):840–844. doi: 10.1038/379840a0. [DOI] [PubMed] [Google Scholar]
- 45.Chen JF, Guo JH, Moxham CM, Wang HY, Malbon CC. Conditional, tissue-specific expression of Q205L G alpha i2 in vivo mimics insulin action. J Mol Med. 1997 Apr;75(4):283–289. doi: 10.1007/s001090050113. [DOI] [PubMed] [Google Scholar]
- 46.Yue P, Jin H, Xu S, et al. Apelin decreases lipolysis via G(q), G(i), and AMPK-Dependent Mechanisms. Endocrinology. Jan;152(1):59–68. doi: 10.1210/en.2010-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sgourakis NG, Bagos PG, Hamodrakas SJ. Prediction of the coupling specificity of GPCRs to four families of G-proteins using hidden Markov models and artificial neural networks. Bioinformatics. 2005 Nov 15;21(22):4101–4106. doi: 10.1093/bioinformatics/bti679. [DOI] [PubMed] [Google Scholar]
- 48.Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol Rev. 2009 Jul;89(3):1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 49.Moule SK, Denton RM. The activation of p38 MAPK by the beta-adrenergic agonist isoproterenol in rat epididymal fat cells. FEBS Lett. 1998 Nov 20;439(3):287–290. doi: 10.1016/s0014-5793(98)01392-1. [DOI] [PubMed] [Google Scholar]
- 50.Park H, Kaushik VK, Constant S, et al. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem. 2002 Sep 6;277(36):32571–32577. doi: 10.1074/jbc.M201692200. [DOI] [PubMed] [Google Scholar]
- 51.Zhou L, Deepa SS, Etzler JC, et al. Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. J Biol Chem. 2009 Aug 14;284(33):22426–22435. doi: 10.1074/jbc.M109.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masri B, Morin N, Cornu M, Knibiehler B, Audigier Y. Apelin (65–77) activates p70 S6 kinase and is mitogenic for umbilical endothelial cells. FASEB J. 2004 Dec;18(15):1909–1911. doi: 10.1096/fj.04-1930fje. [DOI] [PubMed] [Google Scholar]
- 53.Chun HJ, Ali ZA, Kojima Y, et al. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J Clin Invest. 2008 Oct;118(10):3343–3354. doi: 10.1172/JCI34871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu S, Sun F, Li W, et al. Apelin stimulates glucose uptake through the PI3K/Akt pathway and improves insulin resistance in 3T3-L1 adipocytes. Mol Cell Biochem. Apr 2; doi: 10.1007/s11010-011-0799-0. [DOI] [PubMed] [Google Scholar]
- 55.Jia YX, Pan CS, Zhang J, et al. Apelin protects myocardial injury induced by isoproterenol in rats. Regul Pept. 2006 Jan 15;133(1–3):147–154. doi: 10.1016/j.regpep.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 56.Leeper NJ, Tedesco MM, Kojima Y, et al. Apelin prevents aortic aneurysm formation by inhibiting macrophage inflammation. Am J Physiol Heart Circ Physiol. 2009 May;296(5):H1329–H1335. doi: 10.1152/ajpheart.01341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heinonen MV, Purhonen AK, Miettinen P, et al. Apelin, orexin-A and leptin plasma levels in morbid obesity and effect of gastric banding. Regul Pept. 2005 Aug 15;130(1–2):7–13. doi: 10.1016/j.regpep.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Castan-Laurell I, Vitkova M, Daviaud D, et al. Effect of hypocaloric diet-induced weight loss in obese women on plasma apelin and adipose tissue expression of apelin and APJ. Eur J Endocrinol. 2008 Jun;158(6):905–910. doi: 10.1530/EJE-08-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thiebaud D, DeFronzo RA, Jacot E, et al. Effect of long chain triglyceride infusion on glucose metabolism in man. Metabolism. 1982 Nov;31(11):1128–1136. doi: 10.1016/0026-0495(82)90163-9. [DOI] [PubMed] [Google Scholar]
- 60.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999 Jan;103(2):253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983 Nov;72(5):1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bajaj M, Suraamornkul S, Romanelli A, et al. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes. 2005 Nov;54(11):3148–3153. doi: 10.2337/diabetes.54.11.3148. [DOI] [PubMed] [Google Scholar]
- 63.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000 Jul;106(2):171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valle A, Hoggard N, Adams AC, Roca P, Speakman JR. Chronic central administration of apelin-13 over 10 days increases food intake, body weight, locomotor activity and body temperature in C57BL/6 mice. J Neuroendocrinol. 2008 Jan;20(1):79–84. doi: 10.1111/j.1365-2826.2007.01617.x. [DOI] [PubMed] [Google Scholar]
- 65.Clarke KJ, Whitaker KW, Reyes TM. Diminished metabolic responses to centrally-administered apelin-13 in diet-induced obese rats fed a high-fat diet. J Neuroendocrinol. 2009 Feb;21(2):83–89. doi: 10.1111/j.1365-2826.2008.01815.x. [DOI] [PubMed] [Google Scholar]
- 66.Frier BC, Williams DB, Wright DC. The effects of apelin treatment on skeletal muscle mitochondrial content. Am J Physiol Regul Integr Comp Physiol. 2009 Dec;297(6):R1761–R1768. doi: 10.1152/ajpregu.00422.2009. [DOI] [PubMed] [Google Scholar]