Abstract
Pro-inflammatory CD4+ T cell mediated autoimmune diseases, such as multiple sclerosis, are hypothesized to be initiated and maintained by self-reactive interferon-gamma (IFN-γ) and interleukin-17 (IL-17) producing CD4+ T cells. Previous studies have shown moderate to significant alterations in inflammatory T cell responses and potentially treatment of autoimmune disease by administration of anti-histamine or tricyclic antidepressants alone. The goal of the present study was to determine if treatment of PLP139–151-induced relapsing-remitting experimental autoimmune encephalomyelitis (R-EAE) in SJL/J mice with a combination of two FDA approved drugs for other indications could decrease R-EAE disease. The findings show that combination treatment with desloratadine and nortriptyline decreases the mean clinical score, disease relapse frequency, and number of CD4+ T cells infiltrating into the CNS. In addition, combination treatment of PLP139–151 primed mice decreases the level of IFN-γ and IL-17 secreted via a decrease in both the number of cells secreting and the amount of cytokine secreted per cell following PLP139–151 reactivation ex vivo. This is in contrast to an increase in the level of IL-4 produced and the number of IL-4 secreting cells. The data also show that combination treatment with desloratadine and nortriptyline inhibits the production of IFN-γ and IL-17 produced by naive CD4+ T cells activated in the presence of Th1 cell- and Th17-cell promoting conditions, while increasing the level of IL-4 produced by naive CD4+ T cells activated in the presence of Th2 cell-promoting conditions. The present findings suggest a novel method for the development of a putative autoimmune therapy.
Keywords: Autoimmunity, Experimental Autoimmune Encephalomyelitis, Multiple Sclerosis, Antihistamine, Antidepressant, Tolerance, Suppression, Anergy: T cell, Cytokine
1. Introduction
The present study was designed to elucidate the potential in vivo translation of a novel combinatorial treatment for autoimmune diseases, such as multiple sclerosis, using drugs that are already FDA approved for other indications. MS is a disease triggered by an initiating event in which myelin autoreactive CD4+ T cells are activated and subsequently induce damage of central nervous system (CNS) myelin [1; 2; 3], and disease is characterized by perivascular CD4+ T cell and mononuclear cell infiltration [4] with subsequent primary demyelination of axonal tracks leading to progressive paralysis [5]. As such, MS is generally considered to be an autoimmune disease characterized by IFN-γ and IL-17 producing CD4+ T cell responses to a variety of myelin proteins including myelin basic protein (MBP) [6; 7; 8; 9; 10], myelin proteolipid protein (PLP) [9], and/or myelin-oligodendrocyte glycoprotein (MOG) [11; 12; 13]. In order to study the potential disease mechanisms involved and the subsequent alterations due to therapies, experimental autoimmune encephalomyelitis (EAE), a myelin specific peptide/protein-induced disease in mice is a best-fit model. EAE is characterized by transient ascending hind limb paralysis, perivascular mononuclear-cell infiltration, and fibrin deposition in the brain and spinal cord with adjacent areas of acute and chronic demyelination [14]. In the PLP139–151-induced disease model of relapsing-remitting EAE (R-EAE) in SJL/J mice, peripheral PLP139–151-specific CD4+ T cell reactivity is maintained throughout the disease, but prior to the first relapse, PLP178–191-specific CD4+ T cell reactivity arises, i.e., intramolecular epitope spreading, and during the second relapse, T cells specific for a myelin basic protein epitope, MBP84–104, arise, i.e., intermolecular epitope spreading [15; 16]. The development of these spread epitope responses correlates with the extent of myelin destruction during the acute disease phase, and can be utilized experimentally to determine the efficacy of potential therapeutics.
The preliminary in vitro discovery phase of this study was designed to determine the ability of various FDA approved drugs to act in combination to inhibit inflammatory T cell responses in vitro. CRx-153 was identified by CombinatoRx via a proprietary screening assay for novel drug combinations demonstrating enhanced inhibition of TNFα, interferon gamma (IFN-γ) and interleukin 2 (IL-2) release; cytokines that are potentially involved in the pathogenesis of Th1/Th17 cell-mediated autoimmune disease. CRx-153 consists of the antihistamine, desloratadine, in combination with nortriptyline hydrochloride (HCl), a tricyclic antidepressant (TCA). Desloratadine (NeoClarityn®, Claramax®, Clarinex®, and Aerius®) is the histamine receptor H1 (H1R) antagonist. Histamine is a biogenic amine with both neurotransmitter and vasoactive properties [17]. Findings from other laboratories suggest that the blockade of histamine inhibits both the recruitment of immune cells to and the ability for these cells to traffic into sites of inflammation via H1R-induced secretion of RANTES from vascular epithelial cells via the activation of AP-1 and NF-κB [18]. Histamine also acts as an immunomodulatory molecule in both allergic and inflammatory reactions. For example, CD4+ T cells from H1R-deficient mice produce decreased levels of IFN-γ and increased levels of IL-4 in vitro as compared to wildtype mice [19]. Consequently, H1R-deficient mice present with a decreased level of EAE as compared to wildtype mice [19; 20]. Published data also show that H1R is a susceptibility gene in both EAE [21] and experimental autoimmune orchitis [22], which are two classical T cell-mediated models of organ-specific autoimmune disease. There are two potential mechanisms by which treatment with an antihistamine antagonist decreases the level of disease severity in EAE. First, H1R antagonists alter both the ability of immune cells to traffic into sites of inflammation via alteration of chemokine release, i.e., CCL5, CXCL8, and CXCL10, [23], and second blockade of H1R decreases the level of IFN-γ produced by Th1 cells [24; 25].
Besides the role of H1R in modulating immune cell function, further evidence show that blockade of histamine receptor H2 (H2R), also decreases the severity of disease symptoms in EAE via a decrease in pro-inflammatory cytokines, i.e., tumor necrosis factor-alpha (TNF-alpha) and interleukin-12 (IL-12) and reactive oxygen species are involved in ongoing EAE [26]. For example, since H2R activation suppresses production of reactive oxygen species, TNF-α, and IL-12 by inflammatory cells, H2R agonist reduced the severity of EAE via decreasing blood-brain barrier leakage and monocyte/macrophage activation. T cells isolated from H2R-deficient mice produced decreased levels of IFN-γ when activated in the presence of Th1 cell-promoting conditions, while producing equivalent levels of IL-4 as compared to wildtype mice [27]. This argues that the decrease in the level of EAE in both H1R- and H2R-deficient mice is due to an alteration in the level of IFN-γ produced, and not merely an increase in the level of IL-4 produced [27]. However, it should be noted that the level of IL-17 produced was not assayed in these studies. Taken together, while the immune modulatory effect of histamine is classically associated with release from mast cells and basophiles during an allergic response [28], histamine binding of both H1R and H2R also positively regulates the level of IFN-γ produced by Th1 cells.
The second drug investigated in the present study is nortriptyline, (Pamelor®), which is a second-generation tricyclic antidepressant indicated for the treatment of major depression. Besides its primary indication as an antidepressant, nortriptyline also has indications for the treatment of chronic illnesses such as chronic fatigue syndrome [29], chronic pain and migraines [30], and labile affect in some neurological conditions [31]. Consequently, nortriptyline is prescribed to MS patients for the treatment of both depression and parasthesias. Nortriptyline inhibits the activity of such diverse targets as histamine, 5-hydroxytryptamine, and acetylcholine. Published findings suggest that nortriptyline interferes with the transport, release, and storage of catecholamines [32], consequently altering the sympathetic output to secondary lymphoid organs which has been shown to decrease Th1 cell-mediated responses [33; 34]. Several pharmacological mechanisms may be involved following the treatment with a tricyclic antidepressant like nortriptyline. First, the sympathetic nervous system output is also altered following nortriptyline treatment, which would alter the level of norepinephrine released from sympathetic nerve terminals in secondary lymphoid tissue during the immune response. Sympathetic release of norepinephrine during an immune response in vivo has been shown to have positive and negative on Th1 cell responses via beta-2-adrenergic receptor (β2AR) binding dependent upon the time of release and the model system used [33; 35; 36]. Second, nortriptyline treatment may alter cytokine profile of CD4+ T cells via the inhibition of serotonin, the activity of serotonergic neurons have been shown to modulate immune cell function both positively and negatively [37; 38; 39; 40].
While nortriptyline is approved for the treatment of parasthesias and depression in patients with MS, no data exists to determine if nortriptyline has indications for decreasing the severity of MS disease severity. Initial in vitro studies showed that the present combination of desloratadine and nortriptyline inhibits the release of pro-inflammatory cytokines. Based upon these preliminary findings, the purpose of the present study was designed to investigate the ability of desloratadine and nortriptyline combination treatment to inhibit an inflammatory autoimmune disease using the PLP139–151-induced model of R-EAE in SJL/J mice. Our present data show that co-treatment of mice with desloratadine and nortriptyline decreases disease severity, while the mice are maintained on the therapy. There is a significant decrease in the number of infiltrating cells in to the CNS as well as a decrease in the epitope spreading to PLP178–191 and MBP84–104. We have also shown that co-treatment of mice with desloratadine and nortriptyline skews the CD4+ T cell cytokine profile away from IFN-γ/IL-17 pro-inflammatory profile toward an IL-4 anti-inflammatory profile. We go on to determine that the skewing of the CD4+ T cell population appears to be happening at the level of naïve CD4+ T cell activation and differentiation into effector CD4+ T cell populations.
2. Materials and Methods
2.1. Mice, cell isolation, peptides, and reagents
Female SJL/J mice were purchased from Harlan Labs (Indianapolis, IN) and 5B6 TCR transgenic (PLP139–151/I-As-specific) either on wildtype or Thy1.1+ background are currently bred in the Northwestern University Center for Comparative Medicine. Naïve CD4+ T cells were purified using mouse naïve CD4+ T cell AutoMacs Magnetic Bead isolation kit (Miltenyi Biotech; Auburn, CA) and found to be >98% CD4+, CD25−, CD62Lhi via flow cytometry. Peptides (PLP139–151, PLP178–191, and MBP84–104) were purchased from Peptides International (Louisville, KY) and purified by HPLC (purity of 96–99%).
2.2. CRx-153 development/screening and pharmacokinetics study
The ability of CRx-153, compared to its components, to attenuate proinflammatory cytokine release in vitro, was investigated in human primary blood mononuclear cells (PBMC) (20,000 cells/well) collected from healthy donors stimulated with phorbol 12-myristate 13 acetate (PMA) and ionomycin in 384-well microtiter plates for 18 hours. Cytokines released in the supernatant were measured using ELISA. To determine the pharmacokinetics of CRx-153 in vivo female SJL/J mice (n=3–5 per PK time point) were dosed with 3 mg/kg Desloratadine, 10 mg/kg Nortriptyline, or combination of the above doses of the two drugs that were diluted in 100 μl of vehicle [20% ethanol, 20% propylene glycol, 20% polyethylene glycol 300 (PEG300), and 40% water] administered via gavage. Blood sampling for pharmacokinetics analysis was done after a single dose and after 21 days of chronic dosing. Samples were analyzed by a reversed phase HPLC/MS/MS method.
2.3. R-EAE, 5B6 transgenic CD4+ T cell transfer, and co-treatment
Six- to 7-wk-old female SJL/J mice were immunized s.c. with 100 μl of an emulsion containing 200 μg of or 100 Mycobacterium tuberculosis H37Ra (BD Biosciences; San Jose, CA) and 50 μg of PLP139–151 or 100 μg of PLP178–191 distributed over three sites on the flank. In experiments where PLP139–151-specific 5B6 CD4+ Tg T cells were transferred to PLP139–151-primed mice, 3×106 naïve transgenic CD4+ T cells were transferred i.v. on day -2 before priming. For transfer EAE draining lymph nodes were collected on day 8 post priming, and total draining lymph node cells reactivated in the presence of 20μg/ml of PLP139–151, at a cell density of 8×106 cells/ml for 72 hours. After culture 2–3×106 blast cells were transferred to recipient SJL/J mice. At the onset of remission (approximately day +15–20 for most animals) mice received twenty-one consecutive daily doses of vehicle, desloratadine, nortriptyline, or combination of both drugs (1–10 mg/kg). Individual animals were observed at the indicated time points and clinical scores assessed in a blinded fashion on a 0–5 scale: 0, no abnormality; 1, limp tail; 2, limp tail and hind limb weakness; 3, hind limb paralysis; 4, hind limb paralysis and forelimb weakness; and 5, moribund. The data are reported as the mean daily clinical score; the cumulative mean disease score (summation of the daily mean clinical scores for each day over the duration of the experiment), and the relapse frequency (mean number of relapses per mouse per treatment group). A relapse is defined as worsening of at least one grade of clinical disease after stabilization for at least two days.
2.4. Delayed-Type Hypersensitivity Assay and Ex Vivo Recall
Active EAE was induced in 10 SJL/J mice as described above. Mice were treated according to the treatment groups described above and were followed for disease. On day 75 post disease induction mice were assayed for delayed type hypersensitivity (DTH). Mice were anaesthetized by inhalation of isoflurene and the thickness of both ears was measured. Mice received a subcutaneous injection in the dorsal surface of the ear of 10μg of PLP139–151 and PLP178–191 in 10μl of PBS in the left and right ear respectively. The increase in ear thickness was determined after 24 hours and mice were sacrificed. The spleens and draining lymph nodes were collected, made into a single cell suspension, and 1×106 cell were cultured in the presence of medium alone, OVA323–339, PLP139–151, PLP178–191, and MBP84–104 (20μg/ml). At 24 hours the cultures were pulsed with 1μCi of tritiated thymidine, and the cultures were harvested at 72 hours will the level of tritiated thymidine uptake detected using a Topcount Microplate Scintillation Counter and results are expressed as the mean counts per minute (CPM) of triplicate cultures.
2.5. Flow Cytometry and Immunohistochemistry
CNS leukocytes were isolated from the spinal cords of individual mice perfused with 20 mL of PBS. Single cell suspensions were prepared as previously described [41]. Flow cytometric analysis was performed on cells from individual animals (8 mice per group). Cells were stained with anti-CD4-APC (clone RM4–5), anti-CD90.1-PE-Cy7 (clone OX-7), anti-CD25-APC-Cy7 (clone 7D4), anti-CD44-Fitc (clone IM7), anti-FoxP3-PE (clone FJK-16e), and anti-CD69-PerCP-Cy5.5 (clone H1.2F3) (eBioscience; San Diego, CA). 5×105 viable cells were analyzed per individual sample using a BD Canto II cytometer (Becton Dickinson), and the data were analyzed using BD FACSDiva™ version 6.1 software (BD Bioscience). Immunohistochemistry was performed on 6-m–thick frozen lumbar spinal cord sections of PBS-perfused mice as previously described [42]. We analyzed staining using a Leica DM5000B fluorescent microscope and Advanced SPOT software. Confocal microscopy was performed using a Zeiss LSM 510 META laser scanning microscope.
2.6. CD4+ Th cell-promoting culture conditions
Naïve T cells were activated in the presence of anti-CD3/anti-CD28 coated beads (Dynal; Carlsbad, CA) at a ratio of 1 bead: 1 naïve CD4+ T cell in Th0 cell- (IL-2 200U/ml), Th1 cell- (IL-2 200U/ml; IL-12 10ng/ml; anti-IL-4 1μg/ml), Th2 cell- (IL-2 200U/ml; IL-4 10ng/ml; anti-IFN-γ 1μg/ml), or Th17 cell-(TGF-beta 10ng/ml; IL-6 50ng/ml; anti-IFN-γ 1μg/ml; anti-IL-4 1μg/ml; anti-IL-2 1μg/ml) promoting conditions in the presence of vehicle, desloratadine, nortriptyline (101–106 nM), either alone or in combination. After 3–7 days of culture, the T effector cells are isolated and culture supernatants collected and the level of cytokine determined via multiplex Luminex LiquiChip (Millipore; Billerica, MA).
2.7. Statistical analyses
Comparisons of the percentage of animals showing clinical disease were analyzed by X2 using Fisher’s exact probability and two-way ANOVA with a Bonferroni post-test was used to determine statistical differences between mean clinical disease scores. The statistical significance of cytokine and percentage of viable or apoptotic cells was analyzed using a one-tailed analysis of variance with group means compared using the Scheffe’ multiple comparison test. Single comparisons of two means were analyzed by Student’s t-test.
3. Results
3.1. Identification of desloratadine and nortriptyline combination treatment in vitro and in vivo
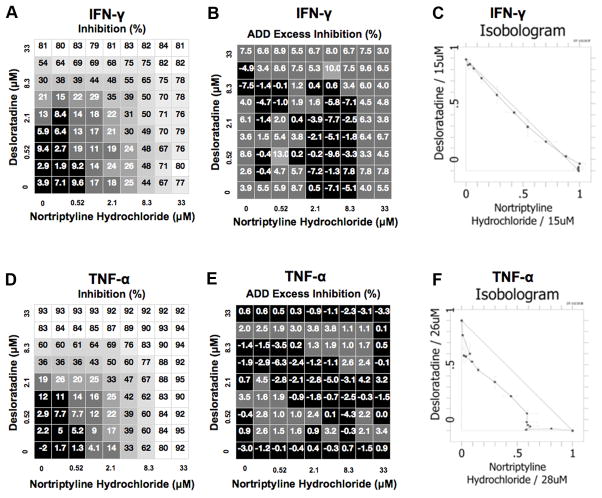
The goal of the present work was to determine the efficacy of R-EAE treatment using a combinatorial drug candidate that combines two agents presently not approved for the treatment of MS. While Nortriptyline is approved for the treatment of paresthesia and depression in patients with MS, no data exists to determine if nortriptyline has indications for decreasing the severity of MS disease severity. Initial in vitro studies showed that the combination of desloratadine and nortriptyline inhibits the release of pro-inflammatory cytokines. The release of interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) was stimulated from human peripheral blood mononuclear cells (PBMCs) by treatment with a mixture of phorbol-12-myristate 13- acetate and ionomycin; release of TNF-α was also induced by treatment with lipopolysaccharide (LPS). Cells were treated with nortriptyline and desloratadine, vehicle (DMSO) or recombinant TNF-α or IFN-γ as positive controls, and cytokine release was examined via ELISA. Combination activity was assessed by comparison to the additivity (ADD) reference model. Treatment with nortriptyline and desloratadine resulted in additive inhibition of IFN-γ (Fig. 1A, B, and C) and TNF-α (Fig. 1D, E, and F) from ionomycin- or LPS-stimulated PBMCs.
Fig 1. Identification of CRx-153, i.e., the combination of desloratadine and nortriptyline activity.
The ability of CRx-153, compared to its individual components was tested for the ability to inhibit inflammatory cytokine production by PBMCs. Total PBMCs were activated in vitro in the presence of PMA and ionomycin plus an increasing concentration of desloratadine, nortriptyline, or a combination of both. The level of IFN-γ (A-C) and TNF-α (D-F) were assayed via ELISA. The data is presented as the percent inhibition in the absence (A and D) or presence (B and E) of exogenous IFN-γ and TNF-α, respectively. The data is also presented in an isobologram illustrating several different dose combinations that attain the specified effect of decreasing the level of IFN-γ (C) and TNF-α (F).
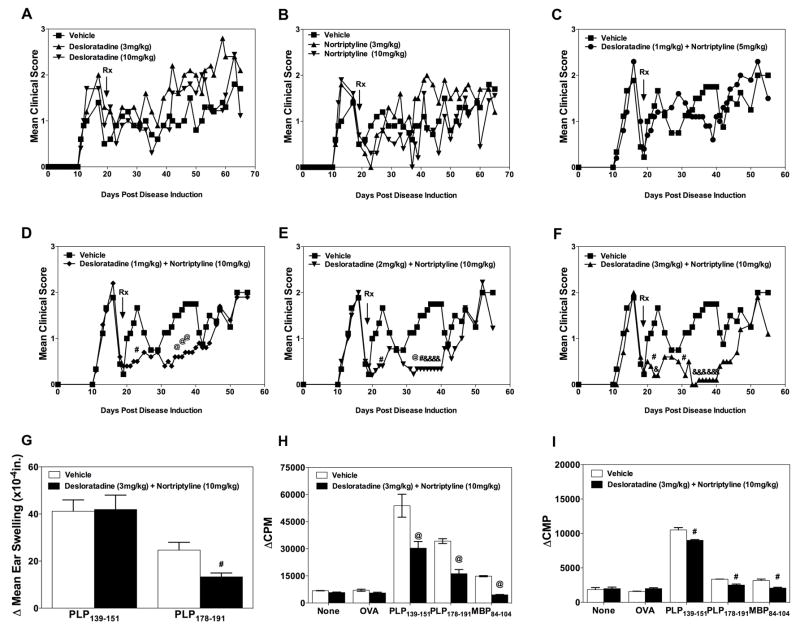
We next sought to determine if the combination treatment of mice with desloratadine and nortriptyline is able to decrease disease severity in PLP139–151-induced R-EAE in SJL/J mice. In the first study, groups of ten mice were treated via oral gavage with a vehicle control, desloratadine or nortriptyline alone at either a high dose (10 mg/kg) or a low dose (3 mg/kg) at the onset of disease remission. As shown in the Fig. 2A and B respectively, none of the single agent doses produced a significant reduction in clinical disease score. However, it should be noted that treatment with the high dose of each agent did show a moderate trend toward a decrease in disease severity. Therefore, we next tested a combination range of doses similar to those currently used in clinical settings, and sought to determine the optimal combination therapy dosage. Mice were treated with vehicle, desloratadine (1 mg/kg) + nortriptyline (5 mg/kg) (Fig. 2C), desloratadine (1 mg/kg) + nortriptyline (10 mg/kg) (Fig. 2D), desloratadine (2 mg/kg) + nortriptyline (10 mg/kg) (Fig. 2E), or desloratadine (3 mg/kg) + nortriptyline (10 mg/kg) (Fig. 2F) beginning at the onset of clinical remission via daily gavage for a period of 21 days. After administration of the test compounds, the mice were followed until Day 60 to assess clinical disease severity. While there was not a significant difference in disease severity between mice that received the various dosages of desloratadine (1, 2, and 3 mg/kg) + desloratadine (10mg/kg), mice that received desloratadine (3 mg/kg) + desloratadine (10mg/kg) or desloratadine (2 mg/kg) + desloratadine (10 mg/kg) showed the greatest decrease in disease severity as compared to vehicle treated mice. We next assessed the level of epitope spreading via DTH and ex vivo recall responses by total splenocytes and draining lymph node cells in the presence of PLP139–151, PLP178–191, and MBP84–104. SJL/J mice were primed with PLP139–151 in CFA, followed for disease severity, and treated with either vehicle or desloratadine (3 mg/kg) + desloratadine (10mg/kg) beginning at disease remission on 21 consecutive days via gavage. On Day 75 post disease induction the level of epitope spreading was assayed via DTH responses to PLP139–151 and PLP178–191. The present data show that combination treatment with desloratadine (3 mg/kg) + desloratadine (10mg/kg) decreases the level of in vivo esponses to the spread epitope PLP178–191. We next collected spleens and lymph nodes and determined the level of encephalitogenic peptide-specific proliferation in response to the disease inducing peptide, PLP139–151, and the spread epitopes, PLP178–191 and MBP84–104. The present data show that combination treatment with desloratadine (3 mg/kg) + desloratadine (10mg/kg) decreases epitope spreading as compared to vehicle treated mice, which is supported by the disease course data presented above. Therefore, combination treatment of SJL/J mice with PLP139–151-induced R-EAE during remission with desloratadine (2 or 3 mg/kg) + desloratadine (10mg/kg) induces a significant decrease in disease severity, and were the chosen as the treatment dose used in the experiments presented below.
Fig 2. Combination treatment with desloratadine and nortriptyline decreases disease severity in R-EAE.
Active R-EAE was induced in groups of 10 SJL/J mice with PLP139–151 in CFA on day 0. Following recovery from the acute disease episode (day +20), groups of 10 SJL/J mice were treated with Vehicle (■), desloratadine or nortriptyline (3mg/kg) (▲), or desloratadine or nortriptyline (10mg/kg) (▼) on twenty-one consecutive days via gavage, and monitored for clinical disease (A, B). Groups of 10 SJL/J mice were also treated with either a Vehicle (■), desloratadine (1mg/kg) plus nortriptyline (5mg/kg) (●) (C), desloratadine (1mg/kg) plus nortriptyline (10mg/kg) (◆) (D), desloratadine (2mg/kg) plus nortriptyline (10mg/kg) (▼) (E), and desloratadine (3mg/kg) plus nortriptyline (10mg/kg) (▲) (F) on twenty-one consecutive days via gavage, and monitored for clinical disease. Clinical results are expressed as the mean clinical score. To determine the effect of combination treatment on epitope spreading, 10 SJL/J mice were primed with PLP139–151 in CFA, and treated with desloratadine (3mg/kg) plus nortriptyline (10mg/kg) on twenty-one consecutive days via gavage beginning during disease remission. On day 75 post disease induction, the level of DTH response to PLP139–151 and PLP178–191 (G), as well as the ex vivo proliferative response of total splenocytes (H) and lymph node cells (I) to medium alone, OVA323–339, PLP139–151, PLP178–191, and MBP84–104 was assessed. A pound symbol (#) indicates a p value < 0.05, a commercial at symbol (@) indicates a p value < 0.01, and an ampersand symbol (&) indicates a p value < 0.001 in comparison to Vehicle treated mice. One representative experiment of two is shown.
3.2. Combination treatment with desloratadine and nortriptyline has a combinatorial effect in treatment of R-EAE
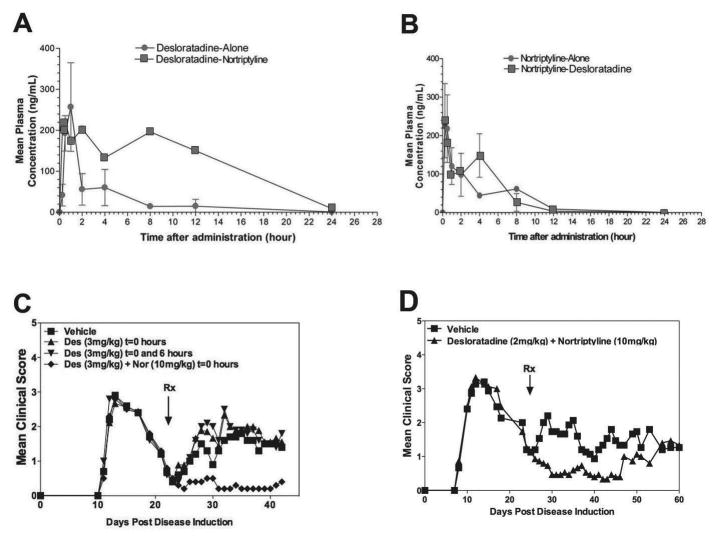
Based on the results presented in Fig. 2, we determined the pharmacokinetics (PK) of desloratadine and nortriptyline following treatment of each drug separately and in combination with one another. The study showed that desloratadine had no effect on the pharmacokinetics of nortriptyline, but there was some prolongation of desloratadine exposure when co-administered with nortriptyline. Therefore, the following experiments were designed to determine if a unique therapeutic effect of combination treatment existed versus an increase in the half-life of desloratadine and/or nortriptyline in serum following combination treatment, as suggested by the above PK studies (Fig. 3A and B). SJL/J mice were primed with PLP139–151 in CFA, and mice were treated with desloratadine (3 mg/kg) or desloratadine (3 mg/kg) + nortriptyline (10 mg/kg) once a day, or desloratadine (3 mg/kg) twice daily separated by 6 hours. After administration of the test compounds, the mice were followed until Day 42 to assess clinical disease. The data indicate that treatment of the mice twice daily with desloratadine, separated by 6 hours, does not decrease the mean disease score in PLP139–151-induced R-EAE in SJL/J, hence the therapeutic effect is not due to prolongation of desloratadine exposure. The study confirms that desloratadine and nortriptyline must be administered together for effective suppression of disease relapse (Fig. 3C). In previous studies, mice that received the combination treatment rapidly relapse following the removal of treatment. Since in active disease a bolus of PLP139–151 in CFA remains on the dorsal aspect of the mice, the possibility exists that disease relapse is do to the continual release of PLP139–151 and the reactivation of new PLP139–151-specific CD4+ T cells. To test this possibility, the efficacy of desloratadine and nortriptyline was determined in PLP139–151 transfer model of disease, thereby removing the continual release of PLP139–151. While treatment of mice with either combination of desloratadine and nortriptyline did decrease disease severity, the treated mice relapse with similar kinetics as mice in active disease (see Fig. 3D). Therefore, the rapid relapse seen in active disease may not be due to the bolus of PLP139–151 in CFA present in these mice. This finding suggests that combination treatment of mice with desloratadine and nortriptyline does indeed have a combination effect on the mean disease score, but the decrease in the PLP139–151-induced disease severity is not due to an induction of PLP139–151–specific tolerance.
Fig 3. Increased efficacy of combination treatment with desloratadine and nortriptyline is not due to an increase in desloratadine half-life.
To determine the pharmacokinetics of CRx-153 in vivo female SJL/J mice (n=3–5 per PK time point) were dosed with 3 mg/kg desloratadine or 10 mg/kg nortriptyline alone (●), or in combination (■). Blood sampling for pharmacokinetics analysis was done after a single dose and after 21 days of chronic dosing. Samples were analyzed by a reversed phase HPLC/MS/MS method (A and B). Active R-EAE was induced in groups of 10 SJL/J mice with PLP139–151 in CFA on day 0. Following recovery from the acute disease episode (day +21), groups of 10 SJL/J mice were treated with either a Vehicle (■), desloratadine (3mg/kg) once daily (▲), desloratadine (3mg/kg) twice daily at 0 and 6 hours (▼), or a combination of desloratadine (3mg/kg) plus nortriptyline (10mg/kg) (◆) on twenty-one consecutive days via gavage, and monitored for clinical disease (C). PLP139–151 transfer disease was induced in SJL/J mice (10 mice per treatment group). At the onset of disease remission (day +23) mice were treated with Vehicle (■), desloratadine 2mg/kg plus nortriptyline (10mg/kg) (▲) on twenty-one consecutive days via gavage, and monitored for clinical disease (D). Clinical results are expressed as the mean clinical score. One representative experiment of two is shown.
3.3. Combination treatment with desloratadine and nortriptyline decreases the number of T cells infiltrating the CNS and alters peripheral T cell responses
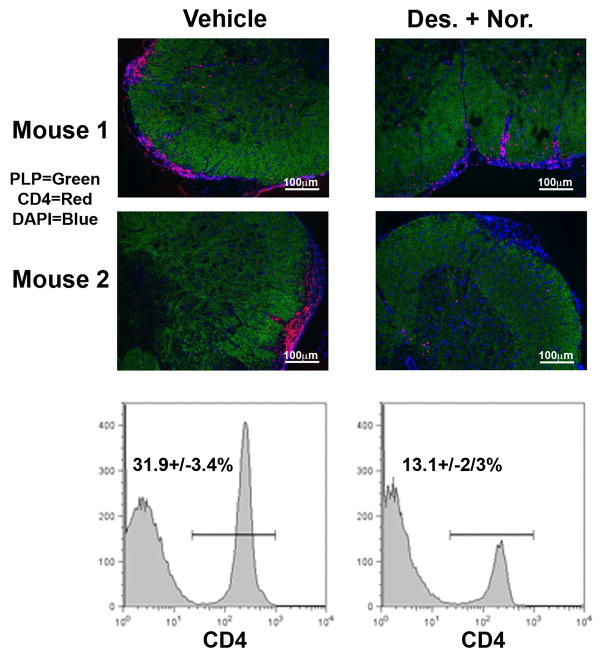
In the initial determination of the mechanism by which treatment of mice with a combination of desloratadine and nortriptyline reduced the severity of disease, mice were treated with the same combination of low dose desloratadine (3 mg/kg) and nortriptyline (10 mg/kg) via oral gavage for 21 days beginning during disease remission. Therefore, we next determined if treatment of mice altered the number of CD4+ T cells present within the lower lumbar spinal cord via histology and flow cytometric analysis. The data presented in Fig. 4 show that the percentage of CD4+ T cells within the lower lumbar spinal cord of desloratadine and nortriptyline treated mice is decreased from 31.9+/− 3.4% of the isolated infiltrating CD45hi cells in vehicle treated mice as compared to 13.1+/− 2.3% in desloratadine and nortriptyline treated mice. Therefore, combination treatment of SJL/J mice with desloratadine and nortriptyline during PLP139–151-induced EAE decreases the number of CD4+ T cell infiltrating into the low lumbar spinal cord during treatment.
Fig 4. Decrease in CD4+ T cell infiltration into the CNS.
Active R-EAE was induced in groups of 10 SJL/J mice with PLP139–151 in CFA on day 0. Following recovery from the acute disease episode (day +20), groups of 10 SJL/J mice were treated with either a Vehicle, or a combination of desloratadine (3mg/kg) plus nortriptyline (10mg/kg) on twenty-one consecutive days via gavage. After the final treatment mice were perfused PBS and lumbar spinal cords collected for histological analysis and flow cytometric analysis to determine the number of infiltrating CD4+ T cells. Representative data from individual mice is presented, and the mean percentage of 5 mice per group is listed on each histogram +/− standard deviation. One representative experiment of two is presented.
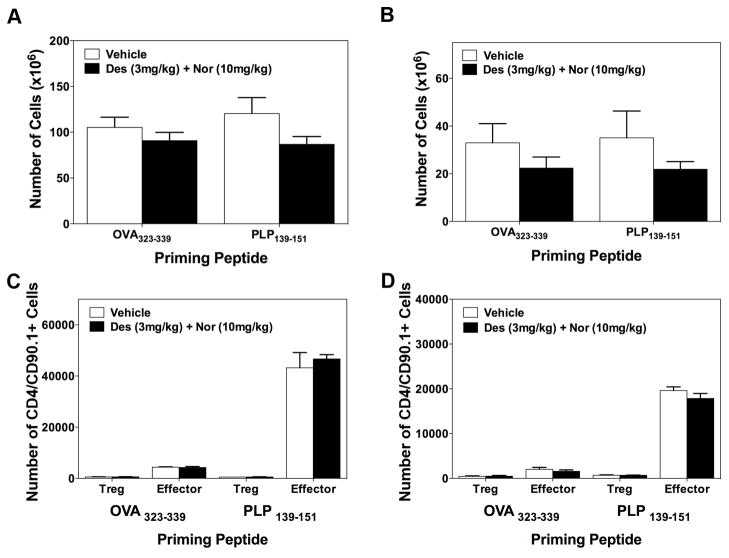
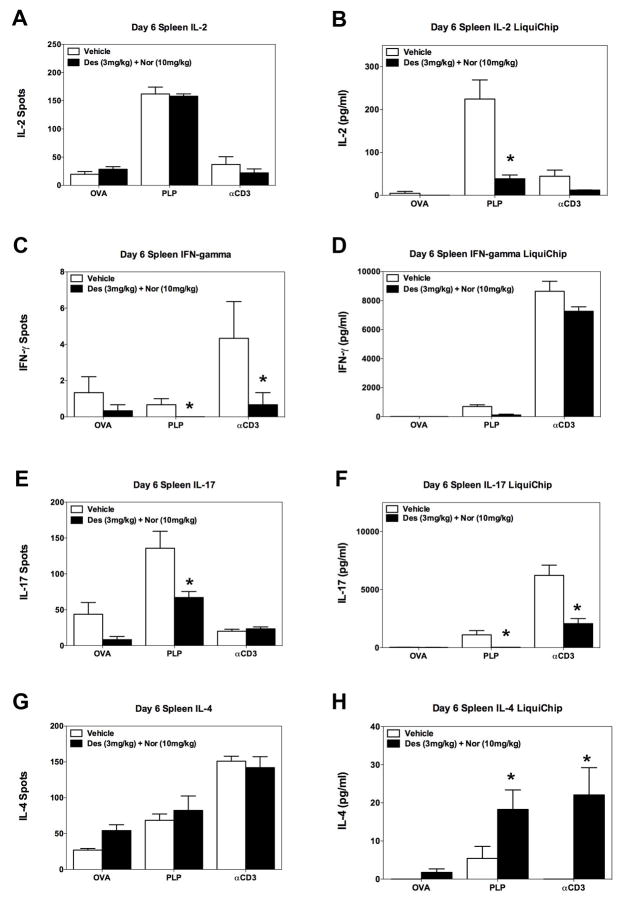
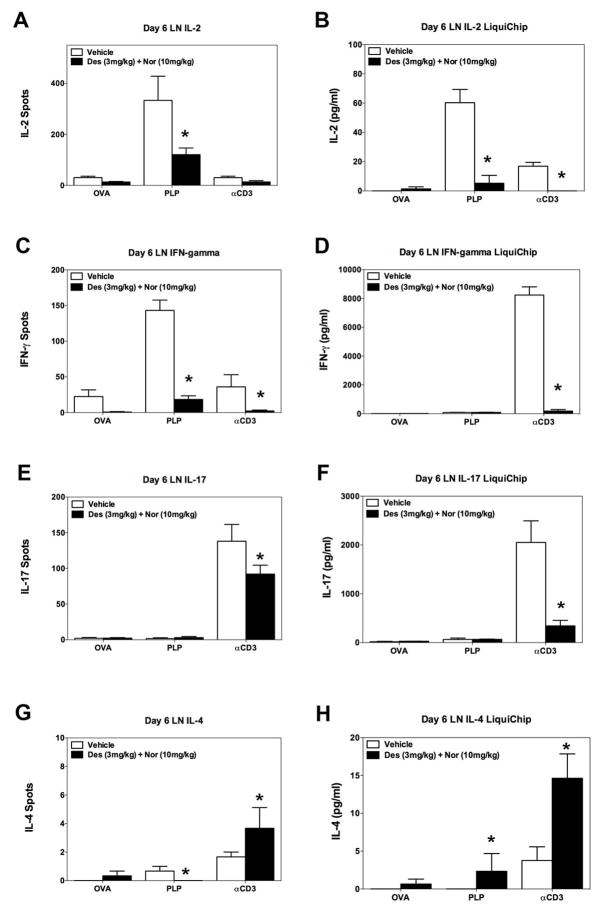
Based on the decrease in the number of CD4+ T cells present within the low lumbar spinal cord following treatment with desloratadine and nortriptyline, we determined if treatment with desloratadine and/or nortriptyline has a direct effect on CD4+ T cell phenotype or activation in vivo via utilizing the transfer of a traceable peptide-specific CD4+ T cell population, i.e., PLP139–151-specific TCR transgenic T cells (5B6 T cells), into PLP139–151 primed recipient SJL/J mice. SJL/J mice received 3×106 naive 5B6 T cells on day -2 prior to PLP139–151 in CFA priming, and groups of mice received either vehicle, or a combination of desloratadine and nortriptyline via gavage. On Day 6 cells were isolated from spleen and draining lymph nodes for to determine if combination treatment alters the number of PLP139–151-specific CD4+ T cells that express the effector CD4+ T cell phenotype, as compared to a regulatory T cell phenotype. In this same experiment we also determined the number of cytokine secreting cells, and amount of cytokine produced by cells present within the spleen and draining lymph nodes. Spleens and lymph nodes were collected on Day 6 post priming with either OVA323–339 in CFA or PLP139–151 in CFA respectively. Treatment of mice with desloratadine and nortriptyline did not alter the total number of cells present within the spleen or the lymph nodes of mice as compared to vehicle treated mice (Fig. 5A-B). We also assessed the ability of desloratadine and nortriptyline treatment to alter the number of PLP139–151-specific CD4+ T cells that were expressing either an effector T cell phenotype or Treg cell phenotype following priming recipient mice with either OVA323–339 in CFA or PLP139–151 in CFA. As shown in Fig. 5C-D, the flow cytometric data show that the overall number of 5B6 effector CD4+ T cells (as determined by CD4+/CD90.1+/CD69+/CD44+) and CD4+ regulatory T cells (as determined by CD4+/CD90.1+/CD25+/FoxP3+) is not altered by the combination treatment. Since there was not an alteration in the number of PLP139–151-specific CD4+ T cells that were expressing an effector T cell phenotype, then we also assayed the cytokine profile being produced by the bulk population of splenocytes or lymph node cells present within these same mice. As shown in Fig. 6, combination treatment of mice with desloratadine and nortriptyline decreases the number of IFN-γ (Fig. 6C) and IL-17 (Fig. 6E) producing cells, while not altering the number of IL-2 (Fig. 6A) and IL-4 (Fig. 6G) producing cells. When the level of cytokine produced was assayed, the level of IL-2 (Fig. 6B) and IL-17 (Fig. 6F) produced was significantly decreased. In contrast, there was a trend to a decreased level of IFN-γ (Fig. 6D) produced and there was a significant increase in the level of IL-4 produced (Fig. 6H). Likewise, when the cells from the draining lymph nodes were analyzed, combination treatment of mice with desloratadine and nortriptyline decreased the number of IL-2 (Fig. 7A), IFN-γ (Fig. 7C) and IL-17 (Fig. 7E) producing cells as well as decreasing the amount of the respective cytokines secreted as shown in Fig. 7B, D, and F respectively. In contrast, combination treatment with desloratadine and nortriptyline increased the number of IL-4 producing cells, and the amount of IL4 secreted (Fig. 7G-H). These findings suggest that combination treatment with desloratadine and nortriptyline skews the CD4+ T cell response while not inhibiting the overall level of CD4+ T cell activation.
Fig 5. Combination treatment with desloratadine and nortriptyline does not alter the number of peptide-specific T cells following priming.
SJL/J mice received 3×106 naive PLP139–151-specific TCR transgenic T cells (5B6 T cells) on day -2 prior to PLP139–151 in CFA priming. Groups of 5 mice received either Vehicle, or a combination of desloratadine (3mg/kg) plus nortriptyline (10mg/kg) via gavage on days 0–10 post PLP139–151 sensitization. On day 6 spleens and draining lymph nodes were collected and total cells enumerated for the spleen (A) and the lymph nodes (B). We also determined the number 5B6 effector CD4+ T cells (as determined by CD4+/CD90.1+/CD69+/CD44+) and CD4+ regulatory T cells (as determined by CD4+/CD90.1+/CD25+/FoxP3+) present via gating on singlet, live, CD90.1+ congenic CD4+ T cells within the spleen (C) and the lymph nodes (D). The data is presented as the mean number of cell present from 5 mice per treatment group that were analyzed individually. One representative experiment of two is presented.
Fig 6. Decrease in the number and level of inflammatory cytokine produced ex vivo by splenocytes.
SJL/J mice received 3×106 naive PLP139–151-specific TCR transgenic T cells (5B6 T cells) on day -2 prior to PLP139–151 in CFA priming. Groups of 5 mice received either Vehicle, or a combination of desloratadine (3mg/kg) plus nortriptyline (10mg/kg) via gavage on days 0–10 post PLP139–151 sensitization. On day 6 spleens were collected and total splenocytes (5×105 cells per well) activated ex vivo in the presence of OVA323–339 (20μM), PLP139–151 (20μM), or anti-CD3 (1μg/ml) in ELISPOT plates and culture plates to determine the number of IL-2 (A), IFN-γ (C), IL-17 (E), and IL-4 (G) secreting cells and level IL-2 (B), IFN-γ (D), IL-17 (F), and IL-4 (H) secreted, respectively. The level of cytokine secreted was determined via 10-plex LiquiChip. The data is presented as the mean number of cytokine secreting cells, and level of cytokine secreted in pg/ml from splenocytes collected from individual mice reactivated in triplicate wells. An asterisk symbol (*) indicates a p value < 0.05 in comparison to Vehicle treated mice. One representative experiment of two is presented.
Fig 7. Decrease in the number and level of inflammatory cytokine produced ex vivo by lymph node cells.
SJL/J mice received 3×106 naive PLP139–151-specific TCR transgenic T cells (5B6 T cells) on day -3 prior to PLP139–151 in CFA priming. Groups of 5 mice received either Vehicle, or a combination of desloratadine (3mg/kg) plus nortriptyline (10mg/kg) via gavage on days 0–10 post PLP139–151 sensitization. On day 6 lymph nodes were collected and total lymph node cells (5×105 cells per well) activated ex vivo in the presence of OVA323–339 (20μM), PLP139–151 (20μM), or anti-CD3 (1μg/ml) in ELISPOT plates and culture plates to determine the number of IL-2 (A), IFN-γ (C), IL-17 (E), and IL-4 (G) secreting cells and level IL-2 (B), IFN-γ (D), IL-17 (F), and IL-4 (H) secreted, respectively. The level of cytokine secreted was determined via 10-plex LiquiChip. The data is presented as the mean number of cytokine secreting cells, and level of cytokine secreted in pg/ml from lymph node cells collected from individual mice reactivated in triplicate wells. An asterisk symbol (*) indicates a p value < 0.05 in comparison to Vehicle treated mice. One representative experiment of two is presented.
3.4. Combination treatment with desloratadine and nortriptyline induces IL-4 production
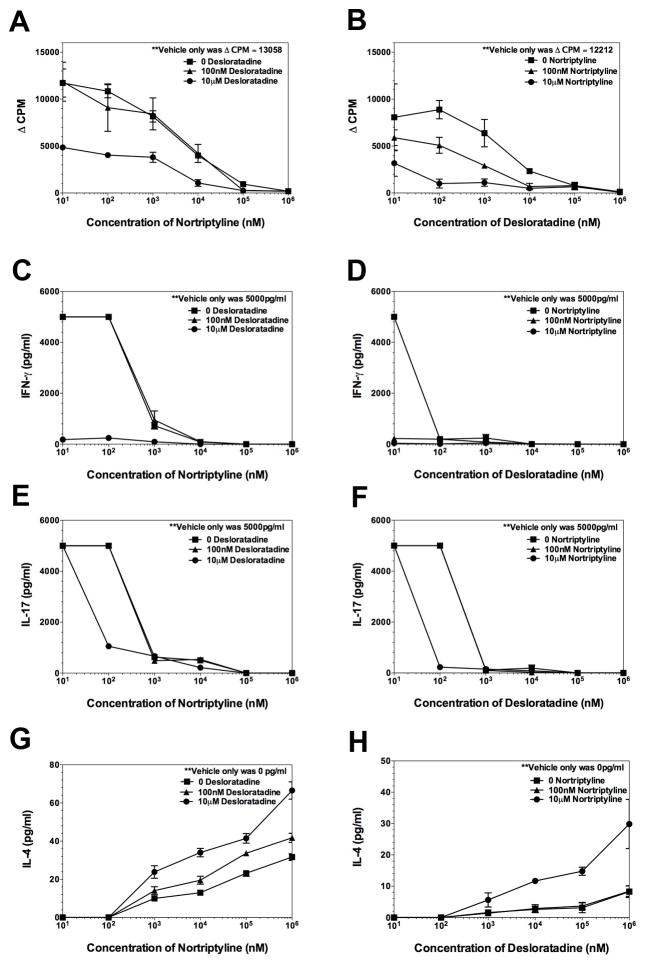
The above data has shown that combination treatment of mice with desloratadine and nortriptyline decreases the severity of PLP139–151-induced EAE in SJL/J mice by decreasing the infiltration of CD4+ T cells into the CNS. The findings also suggest that the combination treatment with desloratadine and nortriptyline decrease the amount of IFN-γ and IL-17 produced by decreasing the number of IFN-γ and IL-17 producing cells. Therefore, we next determined if desloratadine or nortriptyline alone, or in combination alters CD4+ T cell recall response ex vivo. Donor SJL/J mice were immunized with PLP139–151/CFA. On day 8 following immunization the draining lymph nodes were collected, and total lymph node cells were reactivated in the presence of 20 μM of either PLP139–151 or OVA323–339. Parallel cultures were set up, one for cellular proliferation and one for determining the level of cytokine produced in the presence of various concentration of desloratadine plus or minus nortriptyline. As shown in Fig. 8A and B, while desloratadine or nortriptyline treatment alone of PLP139–151 sensitized lymph node cells inhibits the proliferative response, there is a dose-dependent decrease when the level of proliferation when cells are reactivated in the presence of both desloratadine and nortriptyline. In agreement with the proliferation data, a treatment of PLP139–151 sensitized lymph node cells with desloratadine and nortriptyline decreases the level of IFN-γ (Fig. 8C and D) and IL-17 (Fig. E and F) produced in a dose-dependent manner while increasing the level of IL-4 (Fig. 8G-H) produced. Therefore, combination treatment with desloratadine and nortriptyline appears to inhibit Th1 cell and Th17 cell activation.
Fig 8. Dose-dependent decrease in inflammatory cytokine.
SJL/J mice were primed with PLP139–151 in CFA and draining lymph nodes from 5 individual were collected on Day 8 post priming. Total lymph node cells from individual mice were reactivated in the presence of PLP139–151 (20μM), and OVA323–339 (20μM) as a negative control ex vivo in the presence of various conditions and combinations of desloratadine and nortriptyline (0–1mM). Cultures were analyzed at 72 hours for the level of cellular proliferation via tritiated thymidine incorporation (A, B), and level of IFN-γ (C, D), IL-17 (E, F), and IL-4 (G, H) secreted via 10-plex LiquiChip. The data is presented as the mean ▵CPM and level of cytokine secreted as pg/ml. One representative experiment of two is presented.
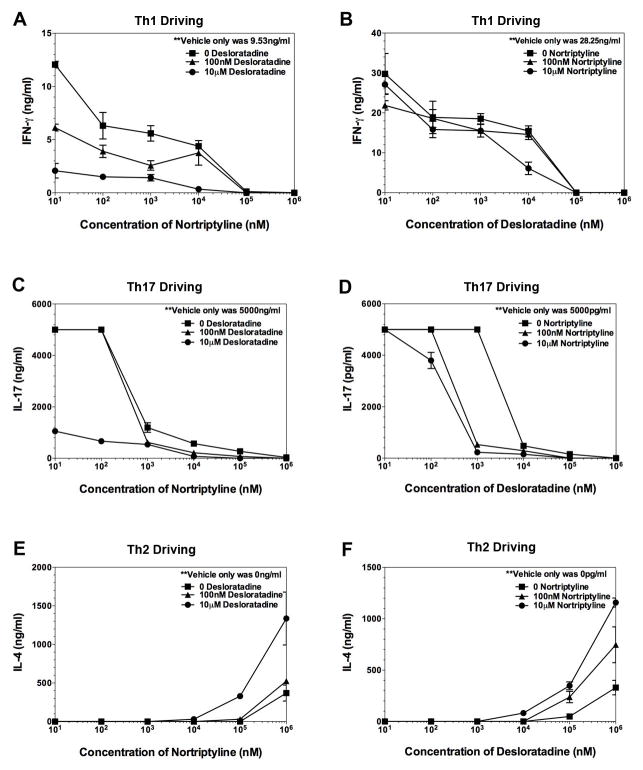
Since combination treatment of ex vivo activation cultures of lymph nodes from PLP139–151 primed mice decreased the level of inflammatory cytokines produced, while increasing the level of anti-inflammatory cytokines produced, we next determine if desloratadine or nortriptyline alone, or in combination alters naïve CD4+ T cell differentiation into Th1 or Th17 cells in vitro. Naïve CD4+ T cells (3–5×105 naïve CD4+ T cells per well) activated in the presence of 5×105 anti-CD3/anti-CD28 coated beads in neutral (Th0), Th1 cell-, Th2 cell-driving, or Th17 cell-promoting conditions. As shown in Fig. 9, both desloratadine and nortriptyline are able to decrease the level of IFN-γ (Fig. 9A) and IL-17 (Fig. 9C and D) produced by naive CD4+ T cells activated in the presence of Th1 cell- and Th17 cell-promoting conditions respectively. Subsequently, this decrease in the level of Th1 cell and Th17 cell differentiation is decrease further following combination treatment of the cultures with both desloratadine and nortriptyline in a dose-dependent manner. In contrast, both desloratadine and nortriptyline are able to increase the level of IL-4 (Fig. 9E and F) produced by naive CD4+ T cells activated in the presence of Th2 cell-promoting conditions, and the level of IL-4 produced is increase further following combination treatment of the cultures with both desloratadine and nortriptyline in a dose-dependent manner. The present data show that combination treatment of CD4+ T cells activated in culture inhibits Th1 cell and Th17 cell responses, while stimulating Th2 cell responses.
Fig 9. Dose-dependent alteration in naive CD4+ T cell differentiation.
Naive CD4+ T cells were isolated from unprimed wild-type SJL/J mice and cultured (1×106 cells) activated in the presence of anti-CD3/CD28 beads in the presence of Th1 cell-promoting conditions [IL-2 (100U/ml), IL-12 (4ng/ml), and anti-IL-4 (10μg/ml)] (A, B), Th17 cell-promoting conditions [TGF-β (10ng/ml), IL-6 (50ng/ml), IL-23 (10ng/ml), anti-IFN-γ (10μg/ml), and anti-IL-2 (10μg/ml)] (C, D), or Th2 cell-promoting conditions [IL-4 (10ng/ml) and anti-IFN-γ (10μg/ml)] (E, F). To triplicate wells the cells were activated in the presence of various conditions and combinations of desloratadine and nortriptyline (0–1mM). The level of secreted cytokine was assayed, and the data is presented as the mean as pg/ml. One representative experiment of two is presented.
4. Discussion
The goal of the present study was to determine the efficacy of combination treatment of mice with desloratadine and nortriptyline during PLP139–151-induced R-EAE in SJL/J mice. The present findings suggest that combination treatment with desloratadine and nortriptyline decreases disease severity and epitope spreading. A putative mechanism for the decrease in disease severity is a reduction in the number of immune cells (T cells and monocyte/APCs) infiltrating the CNS during treatment, also combination treatment with desloratadine and nortriptyline appears to skew the CD4+ T cells cytokine profile toward the production anti-inflammatory cytokines. i.e., IL-4, and away from proinflammatory cytokines, i.e., IFN-γ, IL-2, and IL-17. Studies were also designed to determine if a unique therapeutic effect of combination treatment existed versus an increase in the half-life of desloratadine and/or nortriptyline in serum following combination treatment, as suggested by the PK studies and published data [43]. Our present data show that following combination treatment with both desloratadine and nortriptyline, the half-life of desloratadine in serum is extended, however mice treated twice daily with desloratadine did not show a decrease in disease severity (see Fig. 2). Therefore, we conclude that the decrease in disease severity following combination treatment with desloratadine and nortriptyline is due to a unique therapeutic effect of the combination treatment, and is not merely due to an increase in the half-life of desloratadine in vivo.
Classically, histamine released from activated mast cells and basophils is an important mediator during allergy [44; 45] and is generally not associated with the Th1 cell/Th17 cell response during autoimmune diseases, such as EAE and MS. However, histamine is also produced by both CD4+ and CD8+ T cells activated in the presence of concanavalin A [46]. In support of the ability of an H1R antagonist to skew the immune response toward a Th2 cell-type response in vivo, CBA mice sensitized with bee venom allergen extracts produce increased levels of IgE when the H1R antagonist clemastine is co-administered at the time of sensitization. This is in contrast to vehicle treated mice that produce increased levels of IgG2a [47]. This Th2 cell response shift in the humoral response appears to be due to a reduced level of Th1 cell-associated IFN-γ produced and an enhanced level of IL-4 produced by the bee venom allergen extracts-specific T cells in the absence of H1R stimulation. This finding is further supported by data showing that activation of H1R-knockout CD4+ T cells in the presence of anti-CD3/CD28 beads produce decreased levels of IL-2 and IFN-γ [46]. While H1R expression on naive CD4+ T cells has been shown to be required for maximal IFN-γ production, data also show that H1R expression is dispensable with regard to cellular proliferation [27]. This is in contrast to our data that show that co-treatment of ex vivo reactivation cultures from PLP139–191 sensitize total splenocytes or lymph node cells with desloratadine and nortriptyline decreases the level of cellular proliferation (see Fig. 8). This may in part be explained by the differences in cell source and cellular activation status used in both studies, since the present study used a mix population of cells from PLP139–151 primed mice and not purified CD4+ T cells alone.
One potential mechanism by which H1R blockade alters Th1 cell/Th17 cell activation is that H1R signaling at the time of TCR ligation is required for activation of p38 MAPK, which is a known regulator of IFN-γ expression [19]. This potential mechanism is supported further by data showing selective re-expression of H1R in CD4+ T cells fully complemented both the IFN-γ production and the EAE susceptibility of H1R-deficient mice [21]. These findings suggest that the presence of H1R in CD4+ T cells and its interaction with histamine regulates early TCR signals that lead to Th1 cell differentiation and autoimmune disease. While we did not directly assess APC function in the present study, previous studies have shown that histamine is able to modulate the function of dendritic cells (DCs). Histamine appears to be required for the normal differentiation of DCs, and regulating the chemotaxis of immature DCs [48]. Histamine is also able to modulate the DC endocytosis of Ag, and peptide-presentation to CD4+ T cells. For example, the endocytosis of soluble HRP and FITC-OVA and presentation of OVA is significantly increased when DCs are cultured in the presence of histamine [49]. Therefore, histamine is able to alter CD4+ T cells function and the resultant level of IFN-γ produced via both direct stimulation of H1R expressed by the CD4+ T cell, and indirect alteration in DC endocytosis and presentation of Ag to CD4+ T cells.
As more MS patient data is cross-referenced with animal models of disease, a very complex picture of disease onset and etiology is beginning to emerge. Not only are there alterations in CD4+ T cell subset function (Th1, Th2, Th17, and Treg cells), but also there is a complex involvement of CD8+ T cells, antigen presenting cells (APC) (macrophages, dendritic cells, and B cells) and resident CNS cells (microglia, astrocytes, oligodendrocytes, and their precursors). A protective role for CD4+CD25+FoxP3+ regulatory T cells (Treg cells) has been reported in multiple autoimmune diseases. It is noteworthy that regulation of self-reactive T cells most likely involves multiple populations of immunoregulatory T cells, including nTreg cells, IL-10-producing induced (iTreg) cells and TGF-β-producing Treg cells (Th3) [50; 51; 52]. However, it is currently hypothesized that that in CD4+ T cell-mediated autoimmune disease, such as MS, is a consequence of an alteration in the balance between pro-inflammatory T cells, i.e., Th1 cells and Th17 cells, and Treg cells is a determinant between autoimmune disease and self-tolerance. In the current model system we did not see an alteration in the number of Treg cells following combination treatment with desloratadine and nortriptyline. However, it should be noted that published data show that the ability of Treg cells to suppress the in vitro proliferation of effector CD4+ T cells in response to anti-CD3/CD28-coated beads is reduced in the presence of syngeneic bone marrow-derived mast cells activated by Fc epsilonR cross-linking, and also in the presence of exogenous histamine. In the aforementioned study specificity of H1R activity was shown by co-administration of the H1R selective antagonist loratadine that restored Treg cell function. In contrast, co-administration of the H2R antagonist famotidine was not able to restore Treg cell function [53]. Taken together, the argument can be made that combination treatment with desloratadine and nortriptyline should decrease Treg cell function in vivo, which argues against Treg cells having a major role in the decrease in disease severity seen in the present model system following combination treatment with desloratadine and nortriptyline.
While the studies completed to date demonstrate the ability of combination treatment with desloratadine and nortriptyline to decrease disease severity in R-EAE via a decrease in the number of immune cells infiltrating the CNS and decreasing the level of proinflammatory cytokines (IFN- γ, IL-2, and IL-17) produced by CD4+ T cells, the pharmacological mechanism of drug action following combination treatment with desloratadine and nortriptyline in the present model system is not know. While desloratadine is a H1R antagonist, tricyclic antidepressants like nortriptyline have also been shown to bind H1R [54; 55]. Therefore, one potential mechanism is the combination effect by H1R antagonism. As an alternative mechanism, nortriptyline may antagonize a secondary pathway allowing for the synergistic decrease in the level of disease severity following combination treatment with desloratadine and nortriptyline. Nortriptyline is a tricyclic antidepressant that inhibits the re-uptake of norepinephrine and serotonin, which is approved for use in patients with MS [56]. As such, published findings in the field of neuroimmunology suggest several pharmacological mechanisms may also be involved. Nortriptyline treatment may alter cytokine profile of CD4+ T cells via the inhibition of serotonin; the activity of serotonergic neurons have been shown to modulate immune cell function both positively and negatively [37; 38; 39; 40]. Secondly, the sympathetic nervous system output is also altered following nortriptyline treatment, which would alter the level of norepinephrine released from sympathetic nerve terminals in secondary lymphoid tissue during the immune response. Sympathetic release of norepinephrine during an immune response in vivo has been shown to have both positive and negative effects on Th1 cell responses depending on the time of release and the model system used. Norepinephrine binds the beta-2-adrenergic receptor (β2AR) that is expressed by naive CD4+ T cells and Th1 cells [35]. Functionally, when Th1 and Th2 cell clones are activated with anti-CD3 at the time of β2AR agonist exposure, Th1 cell clones produced decreased levels of IL-2 and increased levels of IFN-γ, while Th2 cell clones showed no change in the level of IL-2 and IL-4 produced [57]. These data, in conjunction with radioligand binding and cAMP analysis [33; 36] to determine the level of functional β2AR protein expression, revealed that Th1 cells express a functional β2AR, while Th2 cells do not. Likewise, chemical sympathectomy of scid mice prior to transfer of Ag-specific Th1 cell clones and Ag-specific B cells decreased the level of IgG2a present in serum following Ag challenge as compared to control mice, and this decrease in the level of IgG2a could be reversed by the administration of the β2AR selective agonist [33]. These findings present a secondary mechanism by which the combination treatment with desloratadine and nortriptyline may decrease Th1 cell activity in vivo.
In conclusion, the present study was designed to determine the efficacy of the combination treatment using desloratadine and nortriptyline over a dose range similar to those in use in current clinical settings. Our data show that combination treatment with desloratadine and nortriptyline decrease disease severity and epitope spreading via a unique therapeutic effect of combination treatment versus an increase in the half-life of desloratadine and/or nortriptyline in serum following combination treatment. Subsequently, a putative mechanism by which combination treatment with desloratadine and nortriptyline treatment decreases disease severity is via an alteration CD4+ T cell cytokine production and trafficking into the CNS. The findings from this study suggest that combination with desloratadine and nortriptyline skews the cytokine response away from a proinflammatory cytokine profile (IFN-γ, IL-2, and IL-17), toward an anti-inflammatory profile (IL-4). While the present hypothesis that combination treatment with two FDA approved drugs from other indications may prove to be a viable alternative in drug discovery and shows promise for clinical therapy in the treatment of autoimmune disease, caution must also be taken. For example, the initiation of a high-dose treatment with an anti-histaminic might cause sleepiness and patient discomfort, and initiation of high-dose anti-depressant treatment would also have to be carefully monitored for patient mental health. Therefore, while the present doses in the combinational treatment show clinical efficacy in R-EAE, the proper safety trials would still have to be completed in a patient population.
Highlights.
Treatment with desloratadine and nortriptyline decreases EAE in SJL/J mice
Drug treatment inhibits clinical relapses and epitope spreading
Drug treatment inhibits inflammatory cell infiltration to the CNS
Drug therapy inhibits recall production of antigen-induced IFN-γ and IL-17
Drug therapy inhibits Th1 and Th17, while promoting Th2, differentiation
Acknowledgments
This work was supported in part by CombinatoRx, Incorporated, J.R.P. is supported by National Multiple Sclerosis Society Postdoctoral Fellowship Grant FG-1667A1/2.
Abbreviations
- CTLA-4
cytotoxic T lymphocyte associated antigen-4
- MBP
myelin basic protein
- MS
multiple sclerosis
- OVA
ovalbumin
- PLP
myelin proteolipid protein
- R-EAE
relapsing experimental autoimmune encephalomyelitis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hafler DA, Weiner HL. MS: a CNS and systemic autoimmune disease. Immunology Today. 1989;10:104–107. doi: 10.1016/0167-5699(89)90236-3. [DOI] [PubMed] [Google Scholar]
- 2.Olsson T, Zhi WW, Hîjeberg B, Kostulas V, Jiang YP, Anderson G, Ekre HP, Link H. Autoreactive T lymphocytes in multiple sclerosis determined by antigen -induced secretion of interferon-gamma. J Clin Invest. 1990;86:981–985. doi: 10.1172/JCI114800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pouly S, Antel JP. Multiple sclerosis and central nervous system demyelination. Journal of Autoimmunity. 1999;13:297–306. doi: 10.1006/jaut.1999.0321. [DOI] [PubMed] [Google Scholar]
- 4.Trotter JL, Hickey WF, van der Veen RC, Sulze L. Peripheral blood mononuclear cells from multiple sclerosis patients recognize myelin proteolipid protein and selected peptides. Journal of Neuroimmunology. 1991;33:55–62. doi: 10.1016/0165-5728(91)90034-5. [DOI] [PubMed] [Google Scholar]
- 5.Steinman L, Martin R, Bernard C, Conlon P, Oksenberg JR. Multiple sclerosis: deeper understanding of its pathogenesis reveals new targets for therapy. Annu Rev Neurosci. 2002;25:491–505. doi: 10.1146/annurev.neuro.25.112701.142913. [DOI] [PubMed] [Google Scholar]
- 6.Veillette A, Zu:niga-Pfl:ucker JC, Bolen JB, Kruisbeek AM. Engagement of CD4 and CD8 expressed on immature thymocytes induces activation of intracellular tyrosine phosphorylation pathways. Journal of Experimental Medicine. 1989;170:1671–1680. doi: 10.1084/jem.170.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Nun A, Liblau RS, Cohen L, Lehmann D, Tournier-Lasserve E, Rosenzweig A, Zhang JW, Raus JC, Bach MA. Restricted T-cell receptor V beta gene usage by myelin basic protein -specific T-cell clones in multiple sclerosis: predominant genes vary in individuals. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:2466–2470. doi: 10.1073/pnas.88.6.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns J, Littlefield K, Gill J, Trotter J. Autoantigen-induced self lysis of human myelin basic protein- specific T lymphocytes. Journal of Neuroimmunology. 1991;35:227–236. doi: 10.1016/0165-5728(91)90177-9. [DOI] [PubMed] [Google Scholar]
- 9.Theiler M, Gard S. Encephalomyelitis of mice. II. A method for the measurement of virus activity. Journal of Experimental Medicine. 1940;72:69–77. doi: 10.1084/jem.72.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soderstrom M, Link H, Sun JB, Fredrikson S, Kostulas V, Hojeberg B, Li BL, Olsson T. T cells recognizing multiple peptides of myelin basic protein are found in blood and enriched in cerebrospinal fluid in optic neuritis and multiple sclerosis. Scandinavian Journal of Immunology. 1993;37:355–368. doi: 10.1111/j.1365-3083.1993.tb02565.x. [DOI] [PubMed] [Google Scholar]
- 11.Sun J, Link H, Olsson T, Xiao BG, Andersson G, Ekre HP, Linington C, Diener P. T and B cell responses to myelin-oligodendrocyte glycoprotein in multiple sclerosis. Journal of Immunology. 1991;146:1490–1495. [PubMed] [Google Scholar]
- 12.Zhang Y, Burger D, Saruhan G, Jeannet M, Steck AJ. The T-lymphocyte response against myelin-associated glycoprotein and myelin basic protein in patients with multiple sclerosis. Neurology. 1993;43:403–407. doi: 10.1212/wnl.43.2.403. [DOI] [PubMed] [Google Scholar]
- 13.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 14.Paterson PY, Swanborg RH. Demyelinating diseases of the central and peripheral nervous systems. In: Sampter M, Talmage DW, Frank MM, Austen KF, Claman HN, editors. Immunological Diseases. Little, Brown and Co; Boston: 1988. pp. 1877–1916. [Google Scholar]
- 15.Vanderlugt CL, Eagar TN, Neville KL, Nikcevich KM, Bluestone JA, Miller SD. Pathologic role and temporal appearance of newly emerging autoepitopes in relapsing experimental autoimmune encephalomyelitis. Journal of Immunology. 2000;164:670–678. doi: 10.4049/jimmunol.164.2.670. [DOI] [PubMed] [Google Scholar]
- 16.Vanderlugt CL, Miller SD. Epitope spreading. Curr Opin Immunol. 1996;8:831–836. doi: 10.1016/S0952-7915(96)80012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boerth RC. Pharmacologic blockade of reflex vasodilation: peripheral actions of antihistamines. Eur J Pharmacol. 1972;20:312–20. doi: 10.1016/0014-2999(72)90192-6. [DOI] [PubMed] [Google Scholar]
- 18.Roumestan C, Henriquet C, Gougat C, Michel A, Bichon F, Portet K, Jaffuel D, Mathieu M. Histamine H1-receptor antagonists inhibit nuclear factor-kappaB and activator protein-1 activities via H1-receptor-dependent and -independent mechanisms. Clin Exp Allergy. 2008;38:947–56. doi: 10.1111/j.1365-2222.2008.02990.x. [DOI] [PubMed] [Google Scholar]
- 19.Noubade R, Milligan G, Zachary JF, Blankenhorn EP, del Rio R, Rincon M, Teuscher C. Histamine receptor H1 is required for TCR-mediated p38 MAPK activation and optimal IFN-gamma production in mice. J Clin Invest. 2007;117:3507–18. doi: 10.1172/JCI32792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedotti R, DeVoss JJ, Youssef S, Mitchell D, Wedemeyer J, Madanat R, Garren H, Fontoura P, Tsai M, Galli SJ, Sobel RA, Steinman L. Multiple elements of the allergic arm of the immune response modulate autoimmune demyelination. Proc Natl Acad Sci USA. 2003;100:1867–1872. doi: 10.1073/pnas.252777399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noubade R, Saligrama N, Spach K, Del Rio R, Blankenhorn EP, Kantidakis T, Milligan G, Rincon M, Teuscher C. Autoimmune disease-associated histamine receptor H1 alleles exhibit differential protein trafficking and cell surface expression. J Immunol. 2008;180:7471–9. doi: 10.4049/jimmunol.180.11.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teuscher C, Blankenhorn EP, Hickey WF. Differential susceptibility to actively induced experimental allergic encephalomyelitis and experimental allergic orchitis among BALB/c substrains. Cellular Immunology. 1987;110:294–304. doi: 10.1016/0008-8749(87)90124-9. [DOI] [PubMed] [Google Scholar]
- 23.Traidl-Hoffmann C, Munster I, Ring J, Behrendt H. Impact of desloratadine and loratadine on the crosstalk between human keratinocytes and leukocytes: Implications for anti-inflammatory activity of antihistamines. Int Arch Allergy Immunol. 2006;140:315–20. doi: 10.1159/000093709. [DOI] [PubMed] [Google Scholar]
- 24.Akdis CA, Blaser K. Bypassing IgE and targeting T cells for specific immunotherapy of allergy. Trends Immunol. 2001;22:175–8. doi: 10.1016/s1471-4906(01)01862-2. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto T, Iwata S, Ohnuma K, Dang NH, Morimoto C. Histamine H1-receptor antagonists with immunomodulating activities: potential use for modulating T helper type 1 (Th1)/Th2 cytokine imbalance and inflammatory responses in allergic diseases. Clin Exp Immunol. 2009;157:27–34. doi: 10.1111/j.1365-2249.2009.03958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emerson MR, Orentas DM, Lynch SG, LeVine SM. Activation of histamine H2 receptors ameliorates experimental allergic encephalomyelitis. Neuroreport. 2002;13:1407–10. doi: 10.1097/00001756-200208070-00012. [DOI] [PubMed] [Google Scholar]
- 27.Teuscher C, Poynter ME, Offner H, Zamora A, Watanabe T, Fillmore PD, Zachary JF, Blankenhorn EP. Attenuation of Th1 effector cell responses and susceptibility to experimental allergic encephalomyelitis in histamine H2 receptor knockout mice is due to dysregulation of cytokine production by antigen-presenting cells. Am J Pathol. 2004;164:883–92. doi: 10.1016/S0002-9440(10)63176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White MV, Slater JE, Kaliner MA. Histamine and asthma. Am Rev Respir Dis. 1987;135:1165–76. doi: 10.1164/arrd.1987.135.5.1165. [DOI] [PubMed] [Google Scholar]
- 29.Gracious B, Wisner KL. Nortriptyline in chronic fatigue syndrome: a double blind, placebo-controlled single case study. Biol Psychiatry. 1991;30:405–8. doi: 10.1016/0006-3223(91)90297-y. [DOI] [PubMed] [Google Scholar]
- 30.Swain RA, Kaplan B. Diagnosis, prophylaxis, and treatment of headaches in the athlete. South Med J. 1997;90:878–88. doi: 10.1097/00007611-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Jensen TS, Finnerup NB. Neuropathic pain treatment: a further step forward. Lancet. 2009;374:1218–9. doi: 10.1016/S0140-6736(09)61205-8. [DOI] [PubMed] [Google Scholar]
- 32.Bissada NK, Finkbeiner AE, Welch LT. Uropharmacology: XII. Miscellaneous drugs affecting lower urinary tract. Urology. 1979;14:309–16. doi: 10.1016/0090-4295(79)90512-0. [DOI] [PubMed] [Google Scholar]
- 33.Sanders VM, Baker RA, Ramer-Quinn DS, Kasprowicz DJ, Fuchs BA, Street NE. Differential expression of the beta2-adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. J Immunol. 1997;158:4200–10. [PubMed] [Google Scholar]
- 34.Swanson MA, Lee WT, Sanders VM. IFN-gamma production by Th1 cells generated from naive CD4+ T cells exposed to norepinephrine. J Immunol. 2001;166:232–40. doi: 10.4049/jimmunol.166.1.232. [DOI] [PubMed] [Google Scholar]
- 35.Kohm AP, Sanders VM. Norepinephrine: a messenger from the brain to the immune system. Immunology Today. 2000;21:539–542. doi: 10.1016/s0167-5699(00)01747-3. [DOI] [PubMed] [Google Scholar]
- 36.Bartik MM, Bauman GP, Brooks WH, Roszman TL. Costimulatory signals modulate the antiproliferative effects of agents that elevate cAMP in T cells. Cellular Immunology. 1994;158:116–130. doi: 10.1006/cimm.1994.1261. [DOI] [PubMed] [Google Scholar]
- 37.Young MR, Kut JL, Coogan MP, Wright MA, Young ME, Matthews J. Stimulation of splenic T-lymphocyte function by endogenous serotonin and by low-dose exogenous serotonin. Immunology. 1993;80:395–400. [PMC free article] [PubMed] [Google Scholar]
- 38.Aune TM, Golden HW, McGrath KM. Inhibitors of serotonin synthesis and antagonists of serotonin 1A receptors inhibit T lymphocyte function in vitro and cell-mediated immunity in vivo. J Immunol. 1994;153:489–98. [PubMed] [Google Scholar]
- 39.Diamond M, Kelly JP, Connor TJ. Antidepressants suppress production of the Th1 cytokine interferon-gamma, independent of monoamine transporter blockade. Eur Neuropsychopharmacol. 2006;16:481–90. doi: 10.1016/j.euroneuro.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Abramchik GV, Leonovich AL, Ulashchik VS, Starostenko LI, Tanina RM. Clinical aspects of serotonin therapy of autoimmune disturbances. Hum Physiol. 1984;10:105–9. [PubMed] [Google Scholar]
- 41.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 42.Tompkins SM, Padilla J, Dal Canto MC, Ting JP, Van Kaer L, Miller SD. De novo central nervous system processing of myelin antigen is required for the initiation of experimental autoimmune encephalomyelitis. Journal of Immunology. 2002;168:4173–4183. doi: 10.4049/jimmunol.168.8.4173. [DOI] [PubMed] [Google Scholar]
- 43.Gupta S, Banfield C, Kantesaria B, Flannery B, Herron J. Pharmacokinetics/pharmacodynamics of desloratadine and fluoxetine in healthy volunteers. J Clin Pharmacol. 2004;44:1252–9. doi: 10.1177/0091270004269518. [DOI] [PubMed] [Google Scholar]
- 44.Riley JF, West GB. Histamine in tissue mast cells. J Physiol. 1952;117:72P–73P. [PubMed] [Google Scholar]
- 45.Riley JF, West GB. Mast cells and histamine in normal and pathological tissues. J Physiol. 1953;119:44P. [PubMed] [Google Scholar]
- 46.Sonobe Y, Nakane H, Watanabe T, Nakano K. Regulation of Con A-dependent cytokine production from CD4+ and CD8+ T lymphocytes by autosecretion of histamine. Inflamm Res. 2004;53:87–92. doi: 10.1007/s00011-003-1227-z. [DOI] [PubMed] [Google Scholar]
- 47.Johansen P, Senti G, Maria Martinez Gomez J, Kundig TM. Medication with antihistamines impairs allergen-specific immunotherapy in mice. Clin Exp Allergy. 2008;38:512–9. doi: 10.1111/j.1365-2222.2007.02904.x. [DOI] [PubMed] [Google Scholar]
- 48.Renkl A, Berod L, Mockenhaupt M, Idzko M, Panther E, Termeer C, Elsner P, Huber M, Norgauer J. Distinct effects of sphingosine-1-phosphate, lysophosphatidic acid and histamine in human and mouse dendritic cells. Int J Mol Med. 2004;13:203–9. [PubMed] [Google Scholar]
- 49.Amaral MM, Davio C, Ceballos A, Salamone G, Canones C, Geffner J, Vermeulen M. Histamine improves antigen uptake and cross-presentation by dendritic cells. J Immunol. 2007;179:3425–33. doi: 10.4049/jimmunol.179.6.3425. [DOI] [PubMed] [Google Scholar]
- 50.Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, Morris PJ, Powrie F, Wood KJ. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166:3789–96. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- 51.Pop SM, Wong CP, Culton DA, Clarke SH, Tisch R. Single cell analysis shows decreasing FoxP3 and TGFbeta1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. J Exp Med. 2005;201:1333–46. doi: 10.1084/jem.20042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 53.Forward NA, Furlong SJ, Yang Y, Lin TJ, Hoskin DW. Mast cells down-regulate CD4+CD25+ T regulatory cell suppressor function via histamine H1 receptor interaction. J Immunol. 2009;183:3014–22. doi: 10.4049/jimmunol.0802509. [DOI] [PubMed] [Google Scholar]
- 54.Taylor JE, Richelson E. High affinity binding of tricyclic antidepressants to histamine H1- receptors: fact and artifact. Eur J Pharmacol. 1980;67:41–6. doi: 10.1016/0014-2999(80)90006-0. [DOI] [PubMed] [Google Scholar]
- 55.Sadava D, Wilmington K. Tricyclic antidepressant drugs affect histamine receptors in human leukocytes. Life Sci. 1984;35:2545–8. doi: 10.1016/0024-3205(84)90441-7. [DOI] [PubMed] [Google Scholar]
- 56.Chitsaz A, Janghorbani M, Shaygannejad V, Ashtari F, Heshmatipour M, Freeman J. Sensory complaints of the upper extremities in multiple sclerosis: relative efficacy of nortriptyline and transcutaneous electrical nerve stimulation. Clin J Pain. 2009;25:281–5. doi: 10.1097/AJP.0b013e318190862b. [DOI] [PubMed] [Google Scholar]
- 57.Ramer-Quinn DS, Baker RA, Sanders VM. Activated T helper 1 and T helper 2 cells differentially express the beta-2-adrenergic receptor: a mechanism for selective modulation of T helper 1 cell cytokine production. J Immunol. 1997;159:4857–67. [PubMed] [Google Scholar]