Abstract
Introduction
Z4032 is a randomized clinical trial conducted by the American College of Surgeons Oncology Group that compared sublobar resection alone (SR) to sublobar resection with brachytherapy (SRB) for high-risk operable patients with non-small cell lung cancer (NSCLC). This current report evaluate the early impact that adjuvant brachytherapy has on pulmonary function tests (PFT), dyspnea and perioperative (30-day) respiratory complications on this impaired patient population.
Methods
Eligible stage I NSCLC patients with tumors 3cm or less were randomized to SR or SRB. The outcomes measured included the % predicted forced expiratory volume (FEV1%), % predicted carbon monoxide diffusion capacity (DLCO%), dyspnea score using the UC San Diego Shortness of Breath Questionnaire. Pulmonary morbidity was assessed using the Common Terminology Criteria for Adverse Events (AE) Version 3.0 (CTCAE). Outcomes were measured at baseline, and at 3-months. A 10% change in PFT or a 10-point change in dyspnea score was deemed clinically meaningful.
Results
Z4032 permanently closed to patient accrual in January 2010 with a total of 224 patients. At 3-month follow-up, PFT data is currently available on 148 (74 SR/74 SRB) patients described in this report. There were no differences in baseline characteristics between the arms. In the SR arm, 9 (12%) patients reported grade-3 respiratory AE compared to 12 (16%) in the SRB arm (p=0.49). There was no significant change in the percent change in DLCO%, or dyspnea score from baseline to 3-month within either arm. In the case of FEV1%, the percent change from baseline to 3-month was significant within SR arm (p=0.03), with patients reporting an improvement in the FEV1% at month 3. Multivariable regression analysis (adjusted for baseline values) showed no significant impact of treatment arm, tumor location (upper versus other lobe), or surgical approach (VATS versus thoracotomy) on the 3-month values for FEV1%, DLCO% and dyspnea scores. There was no significant difference in the incidence of clinically meaningful (10% PFT change, or 10-point dyspnea score) change between the two arms. Twenty-two percent of patients with lower lobe tumors compared to 9% with upper lobe tumors demonstrated a 10% decline in FEV1% (odds ratio 2.79; 95 CI=1.07 – 7.25; p=0.04).
Conclusions
Adjuvant intraoperative brachytherapy performed in conjunction with sublobar resection does not significantly worsen pulmonary function, or dyspnea at 3-months in a high-risk population with NSCLC. SRB was not associated with increased perioperative pulmonary AE. Lower-lobe resection was the only factor that was significantly associated with a clinically meaningful decline in FEV1%.
Introduction
Z4032 is a prospective randomized clinical trial by the American College of Surgeons Oncology Group [ACOSOG] that compares sublobar resection with intraoperative brachytherapy [SRB] to sublobar resection alone [SR] for patients with stage I non-small cell lung cancer [NSCLC] who are considered at increased risk for lobectomy. This study has recently completed accrual. The primary aim of the study is local control, and this will be reported when sufficient follow-up becomes available. This initial report examines the impact of these procedures on pulmonary function, dyspnea symptoms and perioperative respiratory complications to determine in particular any adverse effect that brachytherapy has in a patient group that is already compromised from a pulmonary standpoint.
Methods
Eligible patients for this study, included patients with stage I lung cancers 3cm or less in maximum diameter [i.e. stage IA or the subset of stage IB with visceral pleural involvement] on pre-operative CT scan. Patients were defined as high risk for lobectomy if they met at least one major criterion or two minor criteria as described in Table 1. To facilitate enrollment, pre-registration and randomization occurred prior to surgery to allow a diagnostic wedge resection to be performed on the day of surgery. In order to confirm that patients did not have nodal involvement, all suspicious lymph nodes seen on PET or CT scan required biopsy by mediastinoscopy, endobronchial or esophageal ultrasound, or at the time of resection. Sublobar resection included wedge or segmental resection, and could be performed by video-assisted thoracic surgery (VATS) or thoracotomy. The type of sublobar resection and approach (VATS versus thoracotomy) was at the discretion of the treating surgeon. Lymph node dissection or sampling was strongly encouraged but was not mandatory in this high-risk population. Two methods of brachytherapy were allowed.1–2 In the first technique, polyglactin sutures containing 125I seeds [Oncura, Princeton, NJ] were placed parallel to and 5 mm away from the staple line on each side of the resection margin. The suture strands were fixed to the lung surface with several 3.0 silk or polyglactin sutures placed 1–2cm apart. With the second brachytherapy technique a polyglycolic mesh implant is created during the procedure. The same 125I suture strands were weaved into a piece of vicryl mesh. The strands were placed at 1cm intervals. The mesh is then sutured over the staple line using 2.0 or 3.0 sutures. The goal of the brachytherapy was to deliver 100 Gy at a 5–7mm along the central axis of the resection margin.
Table 1.
Major and Minor Eligibility Criteria for Z4032 trial*
| Major Criteria |
|
| Minor Criteria |
|
Eligible patients must have met either 1 Major or 2 Minor Criteria
The primary objective of this phase III trial was to ascertain whether patients treated by SRB have longer time to local recurrence as compared to patients treated by SR. A secondary aim of this study was to assess the impact of brachytherapy on pulmonary function, dyspnea symptoms and perioperative respiratory complications. In this manuscript, we focus on the pulmonary function results up to month 3 utilizing data from patients who had complete pulmonary function data at both baseline and 3 months following surgery.
All patients provided written informed consent before trial enrollment in accordance with applicable guidelines. At each participating site, Institutional Review Board approval was obtained in accord with an assurance filed with and approved by the United States Department of Health and Human Services.
Data
Pulmonary function tests (PFTs) included % predicted forced expiratory volume 1 (FEV1%) and % predicted carbon monoxide diffusion (DLCO%), both of which were obtained pre-operatively (baseline-day 0), and at 3 months and will continue to be collected at 12 and 24 months post intervention. Dyspnea score was measured using the UC San Diego (UCSD) Shortness of Breath Questionnaire at the same time intervals as the PFTs.3 The 24-item UCSD questionnaire uses a 6-point scale (0=not at all breathless, to 5=maximal breathless or too breathless to do the activity) to assess the severity of each patient’s self-reported shortness of breath. Dyspnea was also assessed using the Common Terminology Criteria for Adverse Events Version 3.0 [CTCAE] at day 0 (baseline), and at 30-days, and at 3, 6 and 12 months post-intervention. Pulmonary and respiratory complications were assessed using the CTCAE, and included pneumonia from the infection category. It should be noted that the CTCAE definition of prolonged intubation [>24 hours] is less than the definition used in the Society of Thoracic Surgeons general thoracic surgery database [>48 hours].
Statistical Analysis
Chi-square tests for categorical variables and Wilcoxon rank sum tests for continuous variables were used to compare the baseline patient characteristics between the SR and the SRB arms. The grade 3 or higher perioperative respiratory complications were compared between treatment arms using a Fisher’s exact test. The UCSD scale was reversed and scored by summing responses across all the 24 items to form a total score, ranging from 0 to 120. The total score was then transformed into a 0 to 100 scale, where 0 represented worst dyspnea (poor QOL) and 100 represented no dyspnea (best QOL). The median percent change in the dyspnea scores, DLCO% and the FEV1% values from baseline to month 3 was compared within the SR and the SRB arms using a Wilcoxon signed rank test. Additionally, a 10 point increase or decrease in dyspnea score, and a 10% increase or decrease in DLCO%, and FEV1% were deemed “clinically meaningful” and compared between the arms using the Fisher’s exact test. Exploratory logistic regression models were used to assess the impact of surgical approach (VATS versus thoracotomy) and tumor location (upper lobe versus other lobes) on clinically significant increases or decreases in DLCO%, FEV1% and UCSD dyspnea scores. In addition, general linear models adjusting for baseline values were used to assess the impact of treatment arm, surgical approach, and tumor location on the 3-month FEV1%, DLCO%, and dyspnea scores, all considered as continuous variables. P-values ≤ 0.05 were considered statistically significant.
Results
This trial met its target accrual and was permanently closed as of January 22, 2010. A total of 224 patients were registered. One patient on SR arm had the intervention at a non-IRB approved hospital and hence was deemed not evaluable. SR was performed in 114 and SRB in 109 patients. See Figure 1 for the patient cohort diagram.
Figure 1.
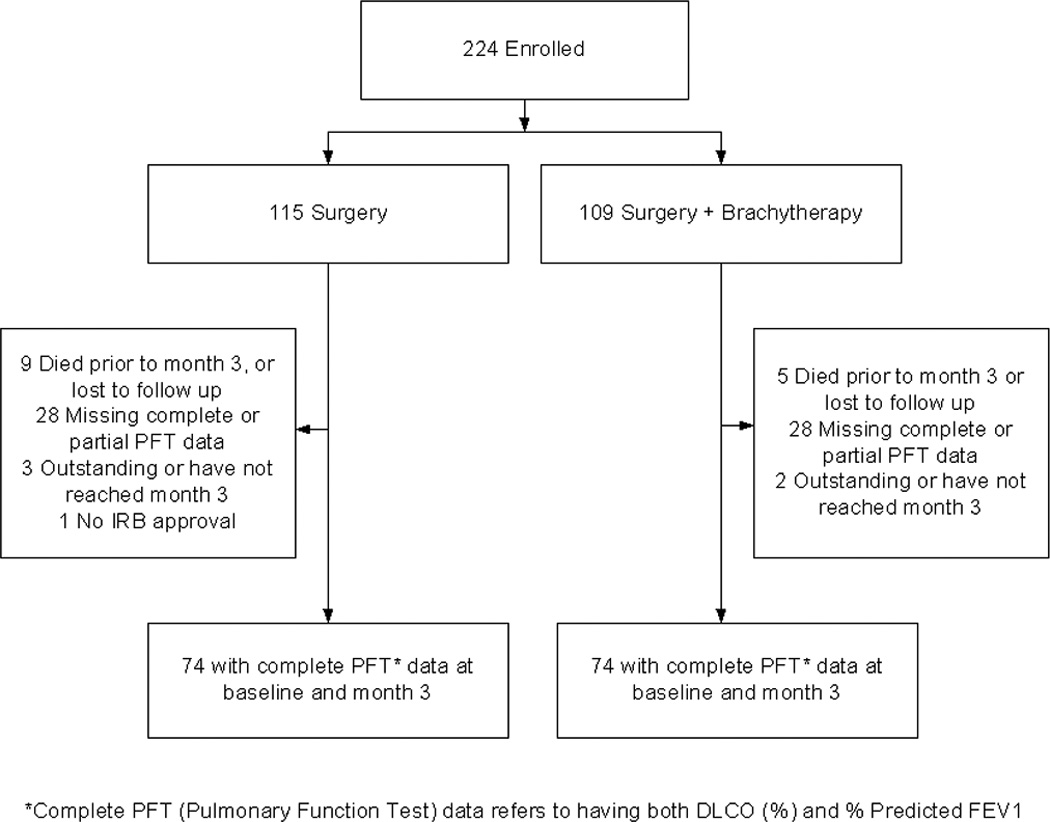
Patient Cohort Diagram
Data was frozen on March 9, 2010 for this analysis. At 3-month follow-up, complete PFT data was available on 148 [74 SR/74 SRB] patients. Of the 148 patients with baseline and 3-month follow-up, 12-month data is available in only 91 [40 SR /51 SRB] patients; therefore, the 3-month cohort is this review’s focus. Adjuvant chemotherapy was not allowed on this protocol. However if a patient was found after resection to have a stage more advanced than IA then adjuvant chemotherapy could be given at the treating physicians discretion. At 3-month follow-up only 3 patients (4%) in the SR arm received non-protocol cancer therapy. One patient had Lupron injections for prostate cancer and two patients had adjuvant cancer therapy for their primary NSCLC.
Baseline patient characteristics are summarized in Table 2. There were no differences between the arms. VATS was undertaken in 51 [69%] SR patients and 46 [62%] SRB patients [p=0.39]. There were a similar number of upper lobe location tumors in each group, specifically 46 [62%] of the patients who had SR and 42[57%] of the patients who had SRB [p=0.50]. The 3-month cohort was also similar to the remaining patients not included in this preliminary analysis for both the SR and the SRB arms.
Table 2.
Baseline Patient Characteristics
| Factors | Month 3 Cohort | Non-Month 3 Cohort SR patients (N=40) vs. Month 3 SR patients (N=74) |
Non-Month 3 Cohort SRB patients (N=35) vs. Month 3 SRB patients (N=74) |
||
|---|---|---|---|---|---|
| SR (N=74 |
SRB (N=74) |
P- value* |
P-value* | P-value* | |
| Age (Median, range) |
70 (49–85) | 71 (53–87) | 0.691 | 0.681 | 0.181 |
| Sex | 1.00 | 0.63 | 0.91 | ||
| Female | 41 (55%) | 41 (55%) | |||
| Male | 33 (45%) | 33 (45%) | |||
| Performance Status | 0.29 | 0.51 | 0.26 | ||
| 0 | 13 (18%) | 21 (28%) | |||
| 1 | 45 (61%) | 39 (53%) | |||
| 2 | 16 (21%) | 14 (19%) | |||
| T Stage | 0.15 | NA | 0.34 | ||
| T1 | 74 (100%) | 72 (97%) | |||
| T2 | 0 (0%) | 2 (3%) | |||
| M Stage | NA | NA | NA | ||
| M0 | 74 (100%) | 74 (100%) | |||
| N Stage | 0.32 | 0.17 | 0.49 | ||
| N0 | 74 (100%) | 73 (99%) | |||
| N1 | 0 (0%) | 1 (1%) | |||
| N2 | 0 (0%) | 0 (0%) | |||
| Surgery in Upper Lobe | 0.50 | 0.17 | 0.42 | ||
| Yes | 46 (62%) | 42 (57%) | |||
| No | 28 (38%) | 32 (43%) | |||
| Surgery Type | 0.39 | 0.90 | 0.54 | ||
| VATS | 51 (69%) | 46 (62%) | |||
| Thoracotomy | 23 (31%) | 28 (38%) | |||
| Surgery Extent | 0.07 | 0.58 | 0.07 | ||
| Segmentectomy | 26 (35%) | 16 (22%) | |||
| Wedge Resection | 48 (65%) | 58 (78%) | |||
SR= Sublobar Resection; SRB=Sublobar Resection with Intraoperative Brachytherapy
Wilcoxon rank sum test;
Chi-square test
Data on grade 3 or higher perioperative [30-day] respiratory complications by arm are presented in table 3. No grade 5 respiratory complications were reported in either arm. In the SR arm, nine patients (12%) reported 11 grade 3 respiratory adverse events. In the SRB arm, twelve patients (16%) reported 17 grade 3 respiratory adverse events, of which, one patient also reported grade 4 hypoxia. This was not statistically significant (Fisher’s exact p-value=0.49]. There were 3 perioperative deaths (1 SR; 2 SRB), two of whom had lower lobe resections. Two deaths were attributed to cardiovascular events and one to pulmonary embolus. One patient who died following a lower lobe sublobar resection had a baseline FEV1% of 25.
Table 3.
Grade 3 or higher Perioperative (30-day) Respiratory complications by arm
| Grade | All | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | |||||||
|
Body System |
Adverse Event |
Arm |
N | % | N | % | N | % | N |
| Infection/Febrile Neutropenia | PNEUMONIA* | SR | 1 | 1.4 | 0 | 0 | 0 | 0 | 1 |
| SRB | 3 | 4.1 | 0 | 0 | 0 | 0 | 3 | ||
| Pulmonary | ARDS | SR | 1 | 1.4 | 0 | 0 | 0 | 0 | 1 |
| SRB | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| DYSPNEA | SR | 5 | 6.9 | 0 | 0 | 0 | 0 | 5 | |
| SRB | 7 | 9.5 | 0 | 0 | 0 | 0 | 7 | ||
| HYPOXIA | SR | 2 | 2.8 | 0 | 0 | 0 | 0 | 2 | |
| SRB | 5 | 6.8 | 1 | 1.4 | 0 | 0 | 6 | ||
| PNEUMOTHORAX | SR | 1 | 1.4 | 0 | 0 | 0 | 0 | 1 | |
| SRB | 1 | 1.4 | 0 | 0 | 0 | 0 | 1 | ||
| Prolonged air-leak | SR | 1 | 1.4 | 0 | 0 | 0 | 0 | 1 | |
| SRB | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| PULMONARY - Other | SR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| SRB | 1 | 1.4 | 0 | 0 | 0 | 0 | 1 | ||
| Same patient could have multiple adverse events. | |||||||||
Pneumonia both with and without absolute neutrophil count specified
Twenty-seven patients [13 SR, 14 SRB] had baseline FEV1% or DLCO% values < 30%, with 6 patients (1 SR, 5SRB) having a value < 20%. Among these 27 patients, 2 reported grade 3 respiratory complications (dyspnea) on day 30, 7 patients had a 10% decline in FEV1% or DLCO% at month 3 and 6 patients had lower lobe resection. One patient had grade 3 hypoxia [required continuous home oxygen] at baseline. This patient developed grade 4 hypoxia at 30 days. 7 additional patients had grade 3 hypoxia at 30 days, thus becoming oxygen dependent immediately after treatment. However no grade 3 or higher hypoxia was reported at 3-months.
Baseline and 3-month PFTs and dyspnea scores for the SR and SRB arms are provided in table 4. There was no significant change in the percent change in DLCO%, or dyspnea score from baseline to 3-month within either arm. In the case of FEV1%, the percent change from baseline to 3-month was significant within SR arm (p=0.03), with patients reporting an improvement in the median FEV1% from 49% to 53% at month 3. Results from the general linear models (adjusted for baseline values) showed no significant impact of treatment arm, tumor location, or surgical approach on the 3-month values for FEV1%, DLCO% and dyspnea scores.
Table 4.
Baseline and 3-month PFT and dyspnea scores by arm
| SR | SRB | |||||||
|---|---|---|---|---|---|---|---|---|
| Factor | N | Baseline | Month 3 | Wilcoxon Signed Rank P-value* |
N | Baseline | Month 3 | Wilcoxon Signed Rank P-value* |
| FEV1% (median, range) |
74 | 49 (22–117) | 53 (24–101) | 0.03 | 74 | 51 (25–110) | 53 (24–104) | 0.18 |
| DLCO% (median, range) |
74 | 46 (18–97) | 48 (14–144) | 0.38 | 74 | 46 (10–137) | 43 (5–96) | 0.16 |
| UCSD Dyspnea Score (median, range) |
73 | 75 (18–100) | 79 (17–100) | 0.13 | 73 | 71 (6–98) | 73 (27–100) | 0.10 |
percent change from baseline to 3-month
Table 5 summarizes the clinically meaningful change (10% or 10-points) from baseline to 3-months in FEV1%, DLCO% and dyspnea scores. There were no statistically significant differences between the two groups with regard to the fractions of patients having meaningful changes in these parameters. At 3-months, clinically meaningful declines in FEV1%, DLCO % and dyspnea scores occurred in 14%, 19% and 21% of the SR patients, and in 15%, 30% and 27% of the SRB patients. Clinically meaningful improvements in FEV%, DLCO % and dyspnea score occurred in 19%, 19% and 27% of the SR patients, as compared to 20%, 19% and 34% of the SRB patients. The majority of the patients remained stable. Specifically, 68%, 62% and 51% on the SR arm, versus 65%, 51% and 38% on the SRB arm, had neither a clinically meaningful improvement nor a clinically significant decline in their FEV%, DLCO% and dyspnea scores.
Table 5.
Distribution of Clinically meaningful (10% change in DLCO%, FEV1% and 10-unit change in UCSD Dyspnea Score) changes from baseline to month 3.
| Factor | SR (N=74) |
SRB (N=74) |
Fisher’s Exact p value |
|---|---|---|---|
| DLCO % | 0.29 | ||
| 10 units increase | 14 (18.9%) | 14 (18.9%) | |
| 10 units decrease | 14 (18.9%) | 22 (29.7%) | |
| Neither 10 units increase/decrease | 46 (62.2%) | 38 (51.4%) | |
| FEV1 % | 0.94 | ||
| 10 units increase | 14 (18.9%) | 15 (20.3%) | |
| 10 units decrease | 10 (13.5%) | 11 (14.9%) | |
| Neither 10 units increase/decrease | 50 (67.6%) | 48 (64.9%) | |
| UCSD Shortness of Breath (0–100) | 0.30 | ||
| Missing | 4 (5.4%) | 1 (1.4%) | |
| 10 units increase | 19 (25.7%) | 25 (33.8%) | |
| 10 units decrease | 15 (20.3%) | 20 (27.0%) | |
| Neither 10 units increase/decrease | 36 (48.6%) | 28 (37.8%) |
The impact of surgical approach (VATS versus thoracotomy), tumor location and development of grade 3 or 4 perioperative respiratory complications on 3-month decline in DLCO%, FEV% and dyspnea score are summarized below. Of the thirty-six patients [14 SR, 22 SRB] who had 10% decrease in DLCO%, 21 (58%) had surgery in the upper lobe area; 14 (39%) had thoracotomy, and 5 (14%) patients had grade 3 or 4 perioperative respiratory complications. Of the 21 patients [10 SR, 11 SRB] who had a 10% decrease in FEV1%, 8 (38%) had surgery in the upper lobe area; and 7 (33%) had thoracotomy. No grade 3 or 4 respiratory complications were reported in these patients. Thirty-five patients [15 SR, 20 SRB] reported a 10-point decrease in dyspnea score, of whom 25 (71%) had surgery in upper lobe area; 12 (34%) had thoracotomy, and four patients (11%) had grade 3 or 4 respiratory complications. There was no statistically significant impact of the surgical approach or tumor location on clinically significant changes in DLCO% and UCSD dyspnea scores from baseline to month 3. However, there was a significant association between tumor location and a 10% decline in FEV1%. Twenty-two percent of patients with lower lobe tumors compared to 9% of patients with upper lobe tumors had a 10% decline in FEV1% (odds ratio=2.79; 95% CI=1.07, 7.25; p=0.04).
Conclusions
Since the publication of the Lung Cancer Study Group’s randomized study of lobectomy to sublobar resection, lobectomy has been the standard of care for patients with stage I NSCLC.4 Currently sublobar resection is generally reserved as a compromise approach for high-risk patients with impaired lung function. The impact of surgical resection on these patients with baseline emphysema is a significant concern. These considerations become even more significant for treatment planning as non-resectional therapies such as stereotactic body radiation therapy [SBRT] and radiofrequency ablation [RFA] become more widely available.5–7
Miyazima et al reported the effects of pulmonary resection on early [4–6 months] and late [42–48 months] cardiopulmonary function.8 In their small patient cohort [n=8], forced vital capacity [FVC], maximum voluntary ventilation [MVV] were found to be significantly decreased at late follow-up. Interestingly DLCO% decreased from 85.4% to 79.5% at early follow-up, but was significantly increased to 106.9% at late follow-up. In the much larger randomized Lung Cancer Study Group Study PFT’s were measured pre-operatively and at 6-month intervals, however PFT’s were only obtained in 60% of eligible patients who had at least 9-month follow-up.4 At 6-months the changes from baseline for FEV1, FVC and MVV were significantly better in patients having sublobar compared to lobar resection. At 12–18 months only the FEV1 was statistically different between patients undergoing lobar or sublobar resection. DLCO was not measured in that study.
Keenan et al compared PFT between 147 patients who had lobectomy to 54 patients treated with segmental resection.9 At 1-year, significant declines were seen in FVC, FEV1%, MMV and DLCO% in the lobectomy group. In contrast, in the segmentectomy group, significant decline was only seen in DLCO% which dropped from a mean of 67.5% to 55% [p=0.0001]. A recent study from Japan measured pulmonary function before and after segmentectomy in 56 patients, and compared these to predicted values after virtual lobectomy.10 The predicted values were calculated by SPECT/CT scan. FEV1% was significantly higher after segmentectomy [88%] compared to that by virtual lobectomy [77%]. Another interesting finding in this study was that preservation of FEV1% was superior if only one or two segments were removed, compared to removal of three or more segments. Left upper lobe, upper division, segmentectomy (lingula sparing lobectomy) also resulted in significantly lower FEV compared to patients undergoing lingular segmentectomy. These findings suggest that segmental resection will preserve PFT primarily if “small” sublobar resections are undertaken, and that with larger sublobar resections [such as an upper lobe division segmentectomy] the benefit is marginal, and perhaps should only be reserved for those patients with very compromised function.
The Cancer and Leukemia Group B previously reported on a phase II trial of VATS wedge resection and local external beam radiotherapy for NSCLC.11 Although 65 patients were accrued, only 31 patients were ultimately eligible for radiotherapy, with 28 receiving their protocol treatment. Severe dyspnea was reported in 3 [11%] and moderate pneumonitis in 4 [14%]. This study illustrates the difficulty in actually delivering planned external beam radiation post-operatively to a high-risk patient group, and the potential for harm from the radiation when it is delivered. The advantage of intraoperative brachytherapy is that this can be delivered at the same time as the lung resection and hopefully lower-radiation morbidity as well as improved local tumor control.12 SRB was initially reported in 1998 in a small series of fourteen patients, of whom 10 required supplemental oxygen prior to surgery2. No significant radiation pneumonitis was observed at a mean follow-up of 7 months. In the present series no increased pulmonary morbidity was seen in the patient group randomized to brachytherapy.
In another series, 23 patients who underwent sublobar resection with mesh brachytherapy had follow-up PFT measurements performed.13 No specific morbidity related to the mesh implant was noted. Pulmonary function was measured at baseline and 3-months, but included FEV and FVC only. There was no significant decline in either value at early follow-up. Our preliminary 3-month data is consistent with these findings. Compared to baseline values, there was no significant decrease in FEV1% and DLCO% in either group.
Therapies such as RFA and SBRT, that do not involve resection, are becoming increasingly popular for patients with NSCLC. There have been only a few reports documenting the impact that these therapies have on PFT. A multi-center prospective non-randomized trial of RFA included 22 patients with NSCLC who had PFT’s recorded to 12 months.14 There was no significant decline in FEV1% or FVC%. It should be noted however that baseline FEV1% was surprisingly high at 68.8% in this series, suggesting that this was not a group that most surgeons would regard as being high-risk. Another study reported on 20 patients who were treated with cyberknife and had serial measurement of PFT recorded.15 Baseline FEV1% and DLCO% were 52% and 57% respectively, which was consistent with this being high-risk group. Post-treatment FEV% did not change significantly, although there was a statistically significant decline of DLCO% of 9% and 11% seen at 6 and 12 months respectively. In another series of 70 patients treated with SBRT there was a 15.7% incidence of grade 3–5 toxicities, most of which were pulmonary associated toxicities.16 Additionally in that series 27.7% of patients became oxygen dependent. The need for supplemental oxygen was not specifically reported in our current series, however the CTCAE defines grade 2 hypoxia, as the need for intermittent oxygen, and grade 3 as the need for continuous oxygen. Seven patients became oxygen dependant after treatment (at 30 days), however by 3-months none of these patients were dependent on oxygen.
More recently the Radiation Therapy and Oncology group completed a phase study of SBRT in high-risk patients.17 Grade 3 and 4 protocol specific toxicity was reported in 7/55[12.7%] and 2/55[3.6%] patients respectively. All but one [8/55; 14.5%] of these toxicities were respiratory-associated. In our current series there were 21/148 [14.2%] patients had perioperative grade 3 or 4 respiratory complications, suggesting that even in a compromised patient population, resection can be undertaken with similar outcomes to SBRT. Currently the American College Surgeons and the Radiation Therapy and Oncology Group are developing a randomized study to compare SR and SBRT in high-risk lung cancer patients. Treatment related toxicity and impact on pulmonary function will be a key endpoint in this study
In conclusion, this randomized study demonstrates that in a patient cohort with stage I NSCLC at greater than average risk for lobectomy, that brachytherapy will have no significant impact on lung function at short-term follow-up. FEV1% and DLCO% were equally preserved in both groups. The incidence of grade 3 and 4 30-day respiratory complications in the SR and SRB arms were not significantly different‥ Follow-up including recording of pulmonary function is ongoing at the 12- and 24- month time points to ascertain the long term impact of brachytherapy on lung function.
ACKNOWLEDGMENTS
We thank the ACOSOG staff, in particular the leadership of Heidi Nelson and David Ota and Angelina Tan for assistance in the development of this manuscript. We also thank all of the investigators and their site research teams. Finally, we wish to thank the brave patients with non-small cell lung cancer and their caregivers who participated in this study.
Supported by NCI U10 grant # CA076001 and by a grant from Oncura, Inc
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee W, Daly BDT, DiPetrillo TA, et al. Limited resection for non-small cell lung cancer: Observed local control with implantation of I-125 brachytherapy seeds. Ann Thorac Surg. 2003;75:237–243. doi: 10.1016/s0003-4975(02)04098-5. [DOI] [PubMed] [Google Scholar]
- 2.d'Amato TA, Galloway M, Szydlowski G, et al. Intraoperative brachytherapy following thoracoscopic wedge resection of stage I lung cancer. Chest. 1998;114:1112–1115. doi: 10.1378/chest.114.4.1112. [DOI] [PubMed] [Google Scholar]
- 3.Eakin EG, Resnikoff PM, Prewitt LM, et al. Validation of a new dyspnea measure: the UCSD Shortness of breath Questionnaire; University of California, San Diego. Chest. 1998;113:619–624. doi: 10.1378/chest.113.3.619. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg RJ, Rubenstein LV. Randomized trial of lobectomy versus limited resection for T1NO Non-small cell lung cancer. Ann Thorac Surg. 1995;60:615–623. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 5.Timmerman RD, Park C, Kavanagh BD. The North American experience with sterotactic body radiation therapy in non-small cell lung cancer. J Thorac Oncol. 2007;2 Suppl 3:S101–S112. doi: 10.1097/JTO.0b013e318074e4fa. [DOI] [PubMed] [Google Scholar]
- 6.Fernando HC. Radiofrequency ablation to treat non-small cell lung cancer and pulmonary metastases. Ann Thorac Surg. 2008;85:S780–S784. doi: 10.1016/j.athoracsur.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 7.Dupuy DE, DiPetrillo T, Gandhi S, et al. Radiofrequency ablation followed by conventional radiotherapy for medically inoperable stage I non-small cell lung cancer. Chest. 2006;129:738–745. doi: 10.1378/chest.129.3.738. [DOI] [PubMed] [Google Scholar]
- 8.Miyazawa M, Haniuda M, Nishimura H, et al. Longterm effects of pulmonary resection on cardiopulmonary function. J Am Coll Surg. 1999;189:26–33. doi: 10.1016/s1072-7515(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 9.Keenan RJ, Landreneau RJ, Maley RH, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg. 2004;78:228–233. doi: 10.1016/j.athoracsur.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimoto K, Nomori H, Mori T, et al. Quantification of the impact of segmentectomy on pulmonary function by perfusion single-photon emission computed tomography and multidetector computed tomography. J Torca Cardiovasc Surg. 2009;137:1200–1205. doi: 10.1016/j.jtcvs.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 11.El-Sherrif A, Fernando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancerl. Ann Surg Onco. 2007;14:2400–2405. doi: 10.1245/s10434-007-9421-9. [DOI] [PubMed] [Google Scholar]
- 12.Shennib H, Bogart J, Herndon JE, et al. Video-assisted wedge resection and local radiotherapy for peripheral lung cancer in high-risk patients: the Cancer and Leukemia Group B (CALGB) 9335, a phase II, multi-institutional cooperative group study. J Thorac Cardiovasc Surg. 2005;129:813–818. doi: 10.1016/j.jtcvs.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Fernando HC, Santos RS, Benfield JR, Grannis FW, Keenan RJ, Luketich JD, Close JM, Landreneau RA. Lobar and sublobar resection with and without brachytherapy for small stage Ia non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;129:261–267. doi: 10.1016/j.jtcvs.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Chen A, Galloway M, Landreneau R, et al. Intraoperative 125I Brachytherapy for high-risk stage I non-small cell lung carcinoma. Int J Radiation Oncology Biol Phys. 1999;44:1057–1063. doi: 10.1016/s0360-3016(99)00133-9. [DOI] [PubMed] [Google Scholar]
- 15.Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumors: a prospective, intent-to treat, multicenter clinical trial (the RAPTURE study) Lancet Oncol. 2008;9:621–628. doi: 10.1016/S1470-2045(08)70155-4. [DOI] [PubMed] [Google Scholar]
- 16.Collins BT, Vahdat S, Erickson K, et al. Radical cyberknife radiosurgery with tumor tracking: an effective treatment for inoperable small peripheral stage I non-small cell lung cancer. J Hematol Oncol. 2009;2:1–9. doi: 10.1186/1756-8722-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Sterotactic radiation therapy for early-stage non-small cell lung carcinoma:four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–682. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 18.Timmerman R, Paulus R, Galvin J, et al. Sterotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;30(303):1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]


