Abstract
The recent RAF inhibitor trial with PLX4032/RG7204 in late-stage mutant B-RAF melanoma patients has been lauded as a success story for personalized cancer therapy since short-term clinical responses were observed in the majority patients. However, initial responses were followed by subsequent tumor re-growth and a subset of patients showed intrinsic resistance. Bi-directional translational efforts are now essential to determine the mechanisms underlying acquired/secondary and intrinsic resistance to RAF inhibitors.
Keywords: Cot1, IGF-1R, MEK, PLX4032, RAF, RAS
Introduction
Metastatic dissemination of melanoma occurs to multiple sites and is associated with 2–16% ten-year survival expectancy depending on the site of metastasis. Melanoma is a paradigm for chemo-resistance and treatment options have remained limited for decades. Standard chemotherapy such as the alkylating agent, dacarbazine, elicits a response rate of only 10% (Flaherty, 2010). Immunotherapy options, such as high dose interleukin 2 and anti-CTLA4 (ipilimumab), have shown improved median survival benefits but again response rates are low and the side effects of these treatments can be severe. Recently, targeted therapies have been designed to selectively kill melanoma cells harboring mutations in the serine-threonine kinase, B-RAF. Approximately 50–60% of melanomas harbor B-RAF mutations (Davies et al., 2002); the most frequent mutation is a valine to glutamic acid substitution at codon 600 (V600E). V600E, as well as V600K/D mutations, lead to constitutive B-RAF kinase activity and elevate downstream signaling through the MEK-ERK1/2 pathway. B-RAFV600E is a driver mutation that promotes melanoma growth and survival in a variety of pre-clinical models and inhibiting B-RAF expression/activity results in growth inhibition and cell death (Dhomen and Marais, 2007). However, B-RAFV600E mutations are also found in benign nevi and, hence, are not sufficient for malignancy.
PLX4032/RG7204 was recently developed as a potent ATP-competitive inhibitor of RAFs, with modest preference in vitro for mutant B-RAF and C-RAF compared to wild-type B-RAF (Bollag et al., 2010). However, in cells it acts as a selective inhibitor of mutant B-RAF signaling (Bollag et al., 2010) probably due to the higher ATP Km(app) for B-RAFV600E in mM cellular concentrations of ATP compared to wild-type forms of B-RAF and C-RAF (Hatzivassiliou et al., 2010). In a Phase 1 trial, 81% of melanoma patients harboring B-RAFV600E showed objective tumor regression by RECIST criteria following PLX4032 treatment (Flaherty et al., 2010). Additionally, a second ATP competitive RAF inhibitor, GSK2118436, is showing promising results in Phase 1 trials with a 63% response rate observed in mutant B-RAFV600E/K/D patients (Kefford et al., 2010). However in the PLX4032 trial, the clinical effects were temporary and the length of tumor-free survival averaged seven months (Smalley and Sondak, 2010). Furthermore, 19% of patients in the Phase 1 trial did not show tumor regression greater than 30% (Flaherty et al., 2010). Thus, acquired and intrinsic modes of resistance are hampering the clinical efficacy of PLX4032. It is critical to understand the mechanisms of resistance in order to optimize PLX4032 activity and improve the response rates, as well as the duration of clinical benefit. Emerging evidence from patient-matched pre-treatment and post-relapse samples (Table 1) highlights that multiple mechanisms underlie resistance to PLX4032 and likely other RAF inhibitors (Figure 1). These mechanisms can be divided into four non-mutually exclusive categories: re-activation of RAF-MEK signaling, alterations in ERK1/2-regulated cell cycle events, activation of alternative signaling pathways, and chromatin-regulating events.
Table 1.
Evidence from patient-matched pre-treatment and post-relapse samples
| Gene | Alteration(s) in patients samples | Reference |
|---|---|---|
| Cot1 | Enhanced Cot1 mRNA levels following PLX4032 treatment in 2 out of 3 patient samples analyzed | (Johannessen et al., 2010) |
| IGF-1R | Enhanced IGF-1R staining in relapse samples in 2 out of 5 patients compared to pre-treatment samples | (Villanueva et al., 2010) |
| N-RAS | Two out of 16 relapse samples harbored acquired N- RAS mutations. The 2 tumors were independent metastases from the same patient. | (Nazarian et al., 2010) |
| PDGFRβ | Four out of 11 PLX4032-resistant tumor samples displayed elevated PDGFRβ staining compared to patient-matched samples from the pre-treatment condition. | (Nazarian et al., 2010) |
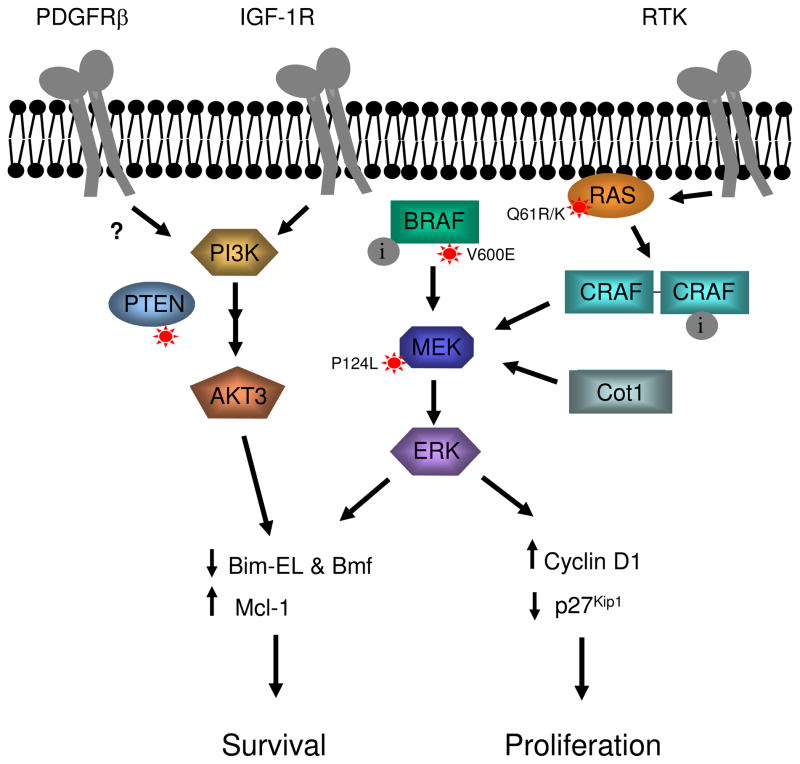
Figure 1. Multiple mechanisms of resistance to RAF inhibitors in mutant B-RAF cells.
Resistance to RAF inhibitor (i) blockade of signaling through the MEK-ERK1/2 pathway can occur via acquired mutation in N-RAS (Q61K or Q61R) or up-regulation of receptor tyrosine kinases (RTK). These mechanisms enhance RAS activity, which promotes C-RAF dimerization and activation. MEK-ERK1/2 pathway activation can also occur through mutations in the B-RAF target, MEK1 (P124L), and via up-regulation of the MAP3K, Cot1. Activation of the parallel PI-3 kinase-Akt pathway is promoted by loss of PTEN expression/activity often through mutation and up-regulation of RTKs including IGF-1R and possibly PDGFRβ. Re-activation of the ERK1/2 pathway and PI-3K-Akt signaling promote G1/S cell cycle events including cyclin D1 up-regulation and down-regulation of the cyclin-dependent inhibitor, p27Kip1. Additionally, these pathways promote survival events by promoting expression of the anti-apoptotic protein, Mcl-1, as well as down-modulating levels of the pro-apoptotic BH3-only proteins, Bim-EL and Bmf. Alterations in the expression of these cell cycle and survival proteins may also promote resistance to RAF inhibitors.
Re-activation of the RAF-MEK signaling pathway
The most direct route for a melanoma cell to by-pass RAF inhibitor action is by reactivation of the RAF-MEK-ERK1/2 pathway. Indeed, enhanced phosphoERK1/2 levels have been observed in mutant B-RAF melanoma cell lines that have acquired resistance to RAF inhibitors (PLX4032 and its non-clinical tool compound PLX4720, AZ628, and SB-590885) through continued culture in the presence of drug (Montagut et al., 2008; Paraiso et al., 2010, Tap, 2010 #10676; Villanueva et al., 2010). A notable difference (at least to-date) from resistance mechanisms to Abl kinase and EGFR inhibitors in chronic myelogenous leukemia and non-small cell lung cancer, respectively, is the lack of secondary gate-keeper mutations in the drug target. While mutation of the gatekeeper amino acid, threonine 529, renders B-RAFV600E resistant to PLX4720 in cell-based assays (Whittaker et al., 2010), deep sequencing analysis indicates that threonine 529 or other sites in B-RAF are not mutated in resistant tumor samples and cell lines (Nazarian et al., 2010; Tap et al., 2010). Rather up-regulation/amplification of other MAP3Ks appears to be a more prominent mechanism. C-RAF and A-RAF are paralogs of B-RAF. Settleman and colleagues showed that elevated C-RAF expression was associated with mutant B-RAF melanoma cell resistance to AZ628 (Montagut et al., 2008). Depletion of C-RAF from melanoma cells with acquired resistance to AZ628 enhanced susceptibility to RAF inhibition. Conversely, C-RAF over-expression in parental cells enhanced resistance to AZ628 (Montagut et al., 2008). AZ628-resistant cells displayed enhanced susceptibility to geldanamycin, an agent that reduces RAF protein levels by targeting its chaperone protein, HSP90 (Montagut et al., 2008). A modest increase in C-RAF expression was also observed in melanoma cells acquiring resistance to SB-590885 (Villanueva et al., 2010). In these studies, the authors propose that RAF isoform switching mediates the maintenance of elevated ERK1/2 phosphorylation. This notion is based on the requirement for molecular depletion of A-RAF and C-RAF, as well as SB-590885 (which is more potent against B-RAF compared to A- or C-RAF) to completely inhibit ERK1/2 phosphorylation in SB-590885-acquired resistant cells (Villanueva et al., 2010). However, it should be noted that maintenance of phosphoERK1/2 levels in these studies co-occurs with acquisition of alternative resistance pathways (see below).
Further support for a role for C-RAF in resistance to B-RAF inhibition was provided by Garraway and colleagues utilizing a cDNA screen for kinases that prevent PLX4720-mediated cell growth arrest (Johannessen et al., 2010). This screen also identified a non-RAF MAP3K, Cot1/Tpl2/MAP3K8. Cot1 activated MEK-ERK1/2 signaling in PLX4720-treated mutant B-RAF cell lines and cell lines endogenously expressing elevated levels of Cot1 were intrinsically resistance to PLX4720 (Johannessen et al., 2010). Importantly, Cot1 mRNA levels were increased in two out of three relapsed tumors samples analyzed from PLX4032-treated patients, indicating relevance to the in vivo situation (Johannessen et al., 2010). B-RAF inhibition in cell lines led to increased Cot1 levels, raising the possibility that Cot1 up-regulation is an adaptive response to PLX4032. However, RAF inhibitor-induced Cot1 up-regulation in cells was independent of mRNA alterations, indicating a distinct mechanism to the Cot1 up-regulation observed in patients.
The above studies highlight that compensatory up-regulation of MAP3Ks, such as Cot1, is likely to represent one mechanism of resistance to RAF inhibitors in patients. However, mechanisms upstream of MAP3Ks also appear likely in a subset of resistant tumors. Upstream of RAFs are the RAS GTPases that recruit RAFs to the membrane for activation. N-RAS is mutated in approximately 15% of melanomas in a manner that is mutually exclusive from B-RAF mutation. The potential role of RAS in altering the effects of RAF inhibitors was highlighted by a series of papers showing that RAF inhibitors lead to a paradoxical hyperactivation of MEK-ERK1/2 signaling in cells harboring mutant N-RAS/high RAS activity (reviewed in (Kaplan et al., 2010)). Indeed, ectopic expression of mutant N-RAS in a mutant B-RAF colorectal cancer cell line nullifies the inhibitory effects of PLX4720 (Poulikakos et al., 2010), a result that is reproducible in B-RAFV600E melanoma cells (Johannessen et al., 2010; Nazarian et al., 2010). Knockdown of mutant N-RAS reverses the insensitivity of PLX4032-resistant cell lines and, importantly, N-RAS mutations were detected in two independent progressing tumors from a PLX4032-treated patient (Nazarian et al., 2010). Elevated RAS activity in the absence of RAS mutations was detected in cell lines with acquired resistance to PLX4032 indicating that events that promote elevated RAS signaling will likely elicit similar effects. Thus, the co-occurrence of B-RAF mutations and RAS mutation/activation is likely to promote resistance to PLX4032 and other RAF inhibitors.
Another possible mechanism to by-pass the activity of RAF inhibitors is through alterations in B-RAF effectors. MEK1 and 2 are phosphorylated and activated by B-RAF and a P124L mutation in MEK1 was identified through a MEK1 random mutagenesis screen that confers cellular resistance to the MEK inhibitor, AZD6244 (Emery et al., 2009). MEK1P124L expression conferred resistance to PLX4720 in cell based assays and was identified in a metastasis from a patient with acquired resistance to AZD6244, indicating the clinical relevance of this mutation. Interestingly, the combination of PLX4720 plus AZD6244 overcame the resistance conferred by MEK1P124L expression. These and other data have prompted the use of MEK inhibitors in clinical trials enrolling mutant B-RAF melanoma patients who were previously treated with or without a BRAF inhibitor.
Alterations in ERK1/2-regulated cell cycle events
Mutant B-RAF signaling via ERK1/2 promotes G1/S cell cycle progression, at least in part, through control of cyclin D1 levels which enhance cyclin-dependent kinase (CDK) activity to promote hyper-phosphorylation of retinoblastoma (RB) (Bhatt et al., 2005). Studies using RB-null fibroblasts and tumor lines have demonstrated the requirement of an intact cyclin-CDK-RB axis for MEK inhibitor prevention of cell cycle entry (D’Abaco et al., 2002). Approximately 25% of mutant B-RAF melanoma cell lines and tumor samples harbor amplifications in cyclin D1 (Smalley et al., 2008). Furthermore, over-expression of CDK4 and cyclin D1 in a SB-590885-sensitive cell line promotes resistance to this RAF inhibitor (Smalley et al., 2008). An implication of this work is that mutant B-RAF patients with amplification of the cyclin D1 locus or other alteration on the cyclin-CDK-RB axis will likely be intrinsically resistant to PLX4032 and, thus, patients stratified on this basis may be predictive of the response.
Activation of alternative signaling pathways
Multiple laboratories have generated mutant B-RAF cell lines that have acquired resistance to RAF inhibitors but not all of these lines display re-activation of the ERK1/2 pathway (Montagut et al., 2008). These findings indicate that input from alternative, ERK1/2-independent pathways induce RAF inhibitor resistance mechanisms. In the Garraway screen, protein kinase C-ε, protein kinase C-η, and ErbB2 individually provided resistance to PLX4720 in the absence of ERK1/2 re-activation (Johannessen et al., 2010). Studies from Nazarian et al. implicate the PDGFRβ receptor tyrosine kinase in the resistance to PLX4032 (Nazarian et al., 2010). PDGFRβ is up-regulated in PLX4032 resistant patient biopsies. Furthermore, resistant cell lines with up-regulated PDGFRβ were dependent on PDGFRβ for proliferation and survival despite PLX4032 inhibition of the ERK pathway remained intact. Consistent with an ERK1/2-independent resistance mechanism, the growth of PDGFRβ-overexpressing resistant cells was insensitive to MEK inhibitors. However, the mechanism underlying PDGFRβ-mediated resistance to PLX4032 is currently unclear.
Other studies implicate the PI-3 kinase-Akt signaling pathway in resistance to RAF inhibitors. Elevated Akt activity was detected in two out of three cell lines with acquired resistance to PLX4032 (Tap et al., 2010). Additionally, enhanced Akt phosphorylation is acquired and associated with increased expression of IGF-1R or depletion of PTEN in relapsed tumor samples from PLX4032-treated patients (Villanueva et al., 2010). Consistent with activation of the Akt pathway providing resistance to RAF inhibitors, expression of constitutively active Akt3 inhibited PLX4720-induced apoptosis in 3D by preventing up-regulation of the pro-apoptotic BH3-only protein, Bim-EL and Bmf, following B-RAF inhibition (Shao and Aplin, 2010). Notably, MEK inhibitors in combination with either an insulin-like growth factor receptor (IGF-1R) inhibitor or a PI-3K inhibitor induces cell death in RAF inhibitor acquired resistant cell populations (Villanueva et al., 2010). Importantly, these data have provided new strategies to overcome resistance to RAF inhibitors and the combination of MEK and Akt inhibitors is being pursued in clinical trials.
Chromatin-regulating events
Acquired mutations are rare events and time is required for the resistant cells to outgrow from the tumor mass. To account for the ability of cells to withstand drug toxicity in the short term, it has been proposed that reversible “drug-tolerant” states exist before the acquisition of permanent resistance (Sharma et al., 2010). Tolerance is drug-induced suggesting an adaptive response to targeted therapies. Additionally, tolerance is reversible and a fraction of tolerant cells revert to a sensitive state during a “drug holiday”. Thus, a second round of drug treatment will promote tumor cell death and the cycle will repeat until secondary mutations lead to permanent resistance. When challenging tumor cell lines with various anticancer agents, Settleman and colleagues detected sub-populations of tumor cell lines that display higher (>100 fold) drug tolerance than the remainder of the cells (Sharma et al., 2010). This reversible drug-tolerant state is transiently acquired through elevated IGF-1R signaling and chromatin remodeling mediated by enhanced expression of JARID1A, a histone demethylase (Sharma et al., 2010). JARID1A associates with histone deacetylases and treatment of M14 melanoma cells with inhibitors of either IGF-1R or histone deacetylases in combination with the RAF inhibitor, AZ628, prevents the emergence of AZ628 tolerant cells. Melanoma cells are known for their plasticity and these studies indicate that an adaptive chromatin regulation response to targeted therapies that may contribute ultimately to the acquisition of a resistant state.
Concluding Remarks
There is a clear need to build on the initial success of the PLX4032 trial in melanoma. As an increasing number of patient-matched pre-treatment, during treatment and post-treatment samples become available, state-of-the art genomic and proteomic approaches need to be utilized to reveal the prominence of the mechanisms described above and reveal novel ways to promote resistance to RAF inhibitors. The findings are translational and will drive the specification of next generation RAF inhibitors and combinational strategies to provide lasting treatment to melanoma sufferers.
Acknowledgments
Work in the Aplin lab is supported by grants from the National Institutes of Health (GM067893, CA125103), American Cancer Society (RSG-08-03-01-CSM) and the Pennsylvania Department of Health (AF0301).
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest
References
- Bhatt KV, Spofford LS, Aram G, McMullen M, Pumiglia K, Aplin AE. Adhesion control of cyclin D1 and p27Kip1 levels is deregulated in melanoma cells through BRAF-MEK-ERK signaling. Oncogene. 2005;12:3459–71. doi: 10.1038/sj.onc.1208544. [DOI] [PubMed] [Google Scholar]
- Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Abaco GM, Hooper S, Paterson H, Marshall CJ. Loss of Rb overrides the requirement for ERK activity for cell proliferation. J Cell Sci. 2002;115:4607–16. doi: 10.1242/jcs.00161. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Dhomen N, Marais R. New insight into BRAF mutations in cancer. Curr Opin Genet Dev. 2007;17:31–9. doi: 10.1016/j.gde.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Emery CM, Vijayendran KG, Zipser MC, Sawyer AM, Niu L, Kim JJ, et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci. 2009;106:20411–6. doi: 10.1073/pnas.0905833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT. Narrative Review: BRAF Opens the Door for Therapeutic Advances in Melanoma. Annals of Internal Med. 2010;153:587–91. doi: 10.7326/0003-4819-153-9-201011020-00008. [DOI] [PubMed] [Google Scholar]
- Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. New Eng J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–72. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan FM, Mastrangelo MJ, Aplin AE. The wrath of RAFs: rogue behavior of B-RAF kinase inhibitors. J Invest Dermatol. 2010;130:2669–71. doi: 10.1038/jid.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefford R, Arkenau H, Brown M, Millward M, Infante J, Long G, et al. Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors. J Clin Oncol. 2010;28:15s. (abstract 8503) [Google Scholar]
- Montagut C, Sharma SV, Shioda T, McDermott U, Ulman M, Ulkus LE, et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res. 2008;68:4853–61. doi: 10.1158/0008-5472.CAN-07-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–7. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraiso KHT, Fedorenko IV, Cantini LP, Munko AC, Hall M, Sondak VK, et al. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer. 2010;102:1724–30. doi: 10.1038/sj.bjc.6605714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–30. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Aplin A. Akt3-mediated resistance to apoptosis in B-RAF targeted melanoma cells. Cancer Res. 2010;70:6670–81. doi: 10.1158/0008-5472.CAN-09-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Lee D, Li B, Quinlan M, Takahashi F, Maheswaran S, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley KS, Lioni M, Dalla Palma M, Xiao M, Desai B, Egyhazi S, et al. Increased cyclin D1 expression can mediate BRAF inhibitor resistance in BRAF V600E-mutated melanomas. Mol Cancer Ther. 2008;7:2876–83. doi: 10.1158/1535-7163.MCT-08-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley KSM, Sondak VK. Melanoma - an unlikely poster child for personalized cancer therapy. New Eng J Med. 2010;363:876–8. doi: 10.1056/NEJMe1005370. [DOI] [PubMed] [Google Scholar]
- Tap W, Gong K, Dering J, Tseng Y, Ginther C, Pauletti G, et al. Pharmacodynamic characterization of the efficacy signals due to selective BRAF inhibition with PLX4032 in malignant melanoma. Neoplasia. 2010;12:637–49. doi: 10.1593/neo.10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–95. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker S, Kirk R, Hayward R, Zambon A, Viros A, Cantarino N, et al. Gatekeeper mutations mediate resistance to BRAF-targeted therapies. Sci Trans Med. 2010;2:35ra41. doi: 10.1126/scitranslmed.3000758. [DOI] [PubMed] [Google Scholar]