Abstract
Acute respiratory distress syndrome (ARDS) is an inflammatory condition that can be associated with capillary leak of serum into alveoli causing inactivation of surfactant. Resistance to inactivation is affected by types and concentrations of surfactant proteins, lipids, and polymers. Our aim was to investigate the effects of different combinations of these three components. A simple lipid mixture (DPPC/POPG) or a more complex lipid mixture (DPPC/POPC/POPG/cholesterol) was used. Native surfactant proteins SP-B and SP-C obtained from pig lung lavage were added either singly or combined at two concentrations. Also, non-ionic polymers polyethylene glycol and dextran and the anionic polymer hyaluronan were added either singly or in pairs with hyaluronan included. Non-ionic polymers work by different mechanisms than anionic polymers, thus the purpose of placing them together in the same surfactant mixture was to evaluate if the combination would show enhanced beneficial effects. The resulting surfactant mixtures were studied in the presence or absence of serum. A modified bubble surfactometer was used to evaluate surface activities. Mixtures that included both SP-B and SP-C plus hyaluronan and either dextran or polyethylene glycol were found to be the most resistant to inhibition by serum. These mixtures, as well as some with either SP-B or SP-C with combined polymers were as or more resistant to inactivation than native surfactant. These results suggest that improved formulations of lung surfactants are possible and may be useful in reducing some types of surfactant inactivation in treating lung injuries.
Keywords: pulmonary surfactant lipids, surfactant proteins, dextran, polyethylene glycol, hyaluronan, polymer
Introduction
The normal functioning of pulmonary surfactant is critical for gas exchange. There are several important functions of lung surfactant. It lowers the work of breathing by reducing the effort needed to breathe in sufficient air for gas exchange to occur. It maintains patency of alveoli during inspiration and expiration, thereby allowing for continued gas exchange during low lung volumes. In addition, it provides an anti-infectious, anti-inflammatory surface exposed to the environment [1, 2]. Acute respiratory distress syndrome (ARDS) is a serious complication resulting from various acute lung injuries. Treatment of premature infants with neonatal RDS using animal-derived pulmonary surfactants is dramatically effective [3], but, (with the possible exception of children [4]) is ineffective in ARDS [5].
A growing body of work [6] shows that inactivation of surfactant activity may explain in part why surfactant treatment has been ineffective in ARDS. Surfactant inactivation is a broad term for the inability of surfactant under a variety of conditions to provide a normal low surface tension. The term covers degradation, alteration of subphase or surface structures, or interference with surfactant adsorption or surface compression. Inhibition may be defined as an interference of the normal functioning of surfactant, and may be seen as one specific example of inactivation. Inhibition may be caused in vitro and in vivo by serum proteins, secretory phospholipase A2, cholesterol and/or other inflammatory products [7–9]. This hypothesis has led to attempts to formulate surfactants more resistant to inhibition than those in current clinical use.
Surfactant proteins, as well as the composition and amount of various surfactant lipids, contribute to reducing susceptibility to inhibition. In general, surfactants rich in the two hydrophobic surfactant proteins are less susceptible than those with lower concentrations [10, 11]. Surfactants in clinical use are derived from animal lungs and contain variable amounts of two hydrophobic proteins (SP-B and SP-C) but lack the hydrophilic surfactant proteins, including SP-A, which is also important in reducing inhibition [12, 13].
We and others have found that various ionic and non-ionic polymers such as hyaluronan (HA), chitosan, polymyxin B, polyethylene glycol (PEG), or dextran can enhance the surface activity of a number of surfactants in the presence of inactivating substances such as serum, albumin, or meconium [6, 14–23].
It was the aim of the present study to determine the relative importance of the type and amount of the two hydrophobic surfactant proteins (SP-B and SP-C), polymers, and lipids for resistance to serum induced inhibition. Serum was used because of its relevance to the pathogenesis of ARDS in which serum leakage into alveoli occurs. Because SP-B and SP-C are important for the function of surfactant in situ [24, 25] [1, 2], we assess their effects, singly or combined, in either of two concentrations. In this study, we used two lipid mixtures, one simple and one more complex to assess the importance of surfactant lipid composition on surfactant function [26]. The anti-inhibitory effects of non-ionic polymers like PEG and dextran occur mainly by depletion and osmotic forces, while HA forms networks and can establish electrostatic interactions with lipids and proteins [27–30]. Since these polymers differ substantially from each other in structure, mechanism, and effect on surfactant, we have chosen to study them singly or paired with HA. For comparison, native surfactant, with its full complement of surfactant proteins, isolated from adult porcine lung lavage was used as a reference standard.
Methods
Materials
Lipids were purchased from Avanti Polar Lipids Inc. (Alabaster, Alabama). Hyaluronan (HA) 1240 kD (molecular weight range 850 kD to 1600 kD), polyethylene glycol (PEG) 10 kD (molecular weight range 8.5 kD to 11.5 kD) and dextran 148 kD (molecular weight range 90 kD to 210 kD) were purchased from Sigma (St. Louis, MO). The HA preparations were isolates from streptococcus fermentation and all polymers were used as supplied. Native surfactant and the hydrophobic surfactant proteins, SP-B and SP-C, were isolated from porcine lung lavage as previously described [31] [32].
Lipid and lipid/protein mixtures
Two lipid mixtures were compared. The simple lipid mixture (SLM) was dipalmitoyl phosphatidylcholine/palmitoyloleoyl phosphatidylglycerol (DPPC/POPG), 7:3 (w/w); lipids contained in clinical surfactants [13, 33]. A more complex lipid mixture (CLM), composed of DPPC, palmitoyloleoyl phosphatidylcholine (POPC), POPG, and cholesterol (52:26:16:5, w/w/w/w), was used to mimic more closely the balance of the sterol, saturated/unsaturated phospholipids, and zwitterionic/anionic phospholipids contained in native surfactant. Each lipid was dissolved in a chloroform/methanol 2:1 solution with the concentration of the phospholipids verified by phosphorous assay. The surfactant proteins B and C were added to the lipid mixtures in the following concentrations: SP-B 2%; SP-C 2%; SP-B 1% + SP-C 1%; SP-B 2% + SP-C 2% (all w/w with respect to phospholipid). These concentrations approximate those found in surfactants obtained from animal lungs [13, 34]. SLM or CLM with no added surfactant proteins was also used.
The lipid-protein mixtures were dried under nitrogen and the dried films were suspended by Vortex in 2.5 mM HEPES, 0.9% NaCl, and 2.5 mM CaCl2 and adjusted to a pH of 7.0. The final concentration was 1.25 mg phospholipid/mL. The samples were then placed in a 45°C water bath for one hour and mixed by Vortex every 10 minutes then stored at 4°C for use the next day.
Addition of polymers
Dry polymers were added singly to these mixtures in the following concentrations: HA 0.25%, PEG 5%, dextran 5% (w/v). The concentrations of polymer were chosen from previous studies [8, 20, 35]. We also added combinations of charged and uncharged polymers (HA+dextran or HA+PEG) using the above concentrations and also at half concentration for HA+dextran or HA+PEG. The mixtures were dispersed by Vortex in 5-sec bursts for 1–2 min until uniform in appearance at room temperature. These mixtures were studied within thirty minutes.
Serum inhibition
Serum, at a protein concentration of 580 mg/mL, was obtained from exchange transfusions done on newborn infants and pooled then frozen at −20°C until needed. It was then thawed and added to the surfactant mixtures (1.25 mg phospholipid/mL) in varying amounts to provide a range of serum concentrations relative to surfactant. The serum/surfactant combinations were mixed by Vortex for 20 seconds before testing (within 30 minutes). The lowest amount of serum protein per mL of surfactant mixture that caused minimum surface tension after five minutes of cycling (γmin 5 min) to exceed 7 mN/m was defined as the threshold for inhibition. Total serum protein was added in increments of 290 μg to determine the threshold for inhibition of each surfactant mixture. The final concentrations of serum protein used in surfactant ranged from 290 to 5220 μg/mL. Use of human serum was approved by the University of California Clinical Research Committee.
Surface activity measurements
Surface activities were measured in a modified pulsating bubble surfactometer (MPBS, Electronetics, Buffalo, NY) using a technique to prevent wetting of the capillary tube in the sample chamber [36]. The temperature of the 25 μL sample chamber was maintained at 37°C. The device was calibrated both electronically and with a water manometer. Measurements were also validated using pure fluids with known surface tensions. In this study we chose to use the MPBS because this technique allows relatively rapid testing of a large number of samples using reasonable amounts of material under physiological conditions of temperature and compression-expansion rates.
Five replicates from each surfactant mixture were measured and the results averaged. The following indices of surface activity were compared for the different mixtures:
Surface tension forty seconds after initial formation of a static bubble, defined as “adsorption”.
Minimum surface tension measured after cycling 30 seconds (10 cycles of inflation/deflation):γmin 10th.
Maximum surface tension measured after cycling 30 seconds (10 cycles of inflation/deflation): γmax 10th.
Minimum surface tension after five minutes of inflation/deflation: γmin 5 min.
Maximum surface tension after five minutes of inflation/deflation: γmax 5 min.
The percentage of surface area reduction of the bubble (from maximum) required for the surface tension to fall to 10 mN/m: A10. It is an estimate of surface film compressibility after five minutes of inflation/deflation. A low value indicates that the surface film is relatively non-compressible. If the surface tension did not fall to ≤10 mN/m after maximum surface compression (that is when the minimum bubble radius of 0.4 mm allowed by the apparatus was reached), a limit of >47% was assigned, corresponding to the difference in area between maximum and minimum bubble sizes.
Analyses
The data are presented as means ± SEM. Measurements were analyzed by one way analysis of variance (ANOVA) using SigmaPlot software (SPSS Science Chicago, IL). Comparisons between pairs of groups were done using the Student-Newman-Keuls Method or the Kruskal-Wallis test when necessary to correct for multiple comparisons. A p value ≤ 0.05 was considered statistically significant.
Results
Comparison of lipid mixtures, SLM and CLM without surfactant proteins
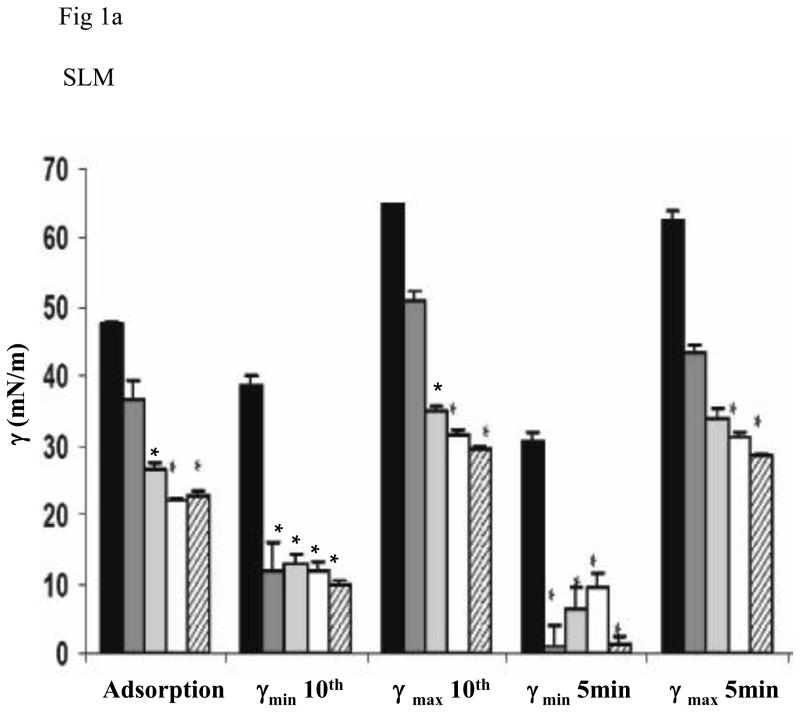
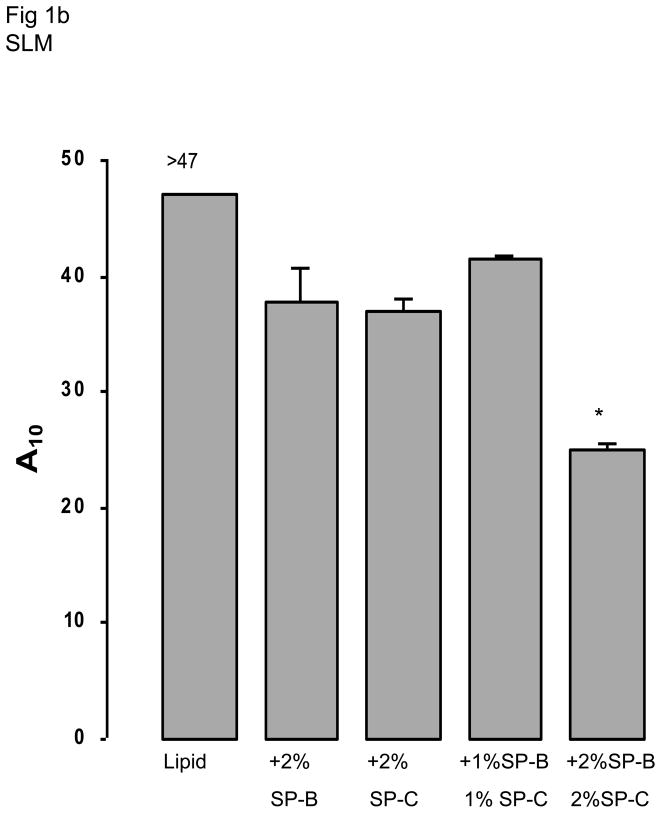
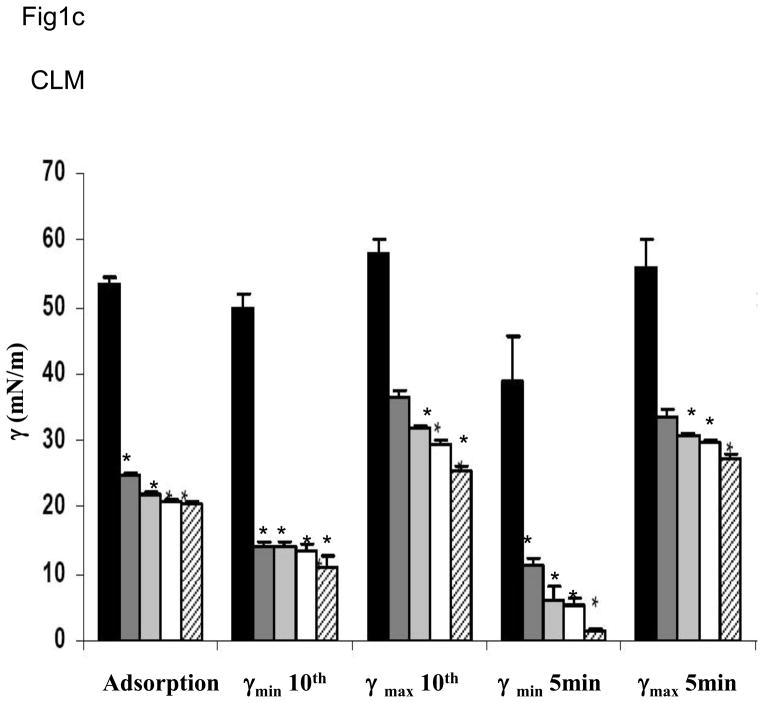
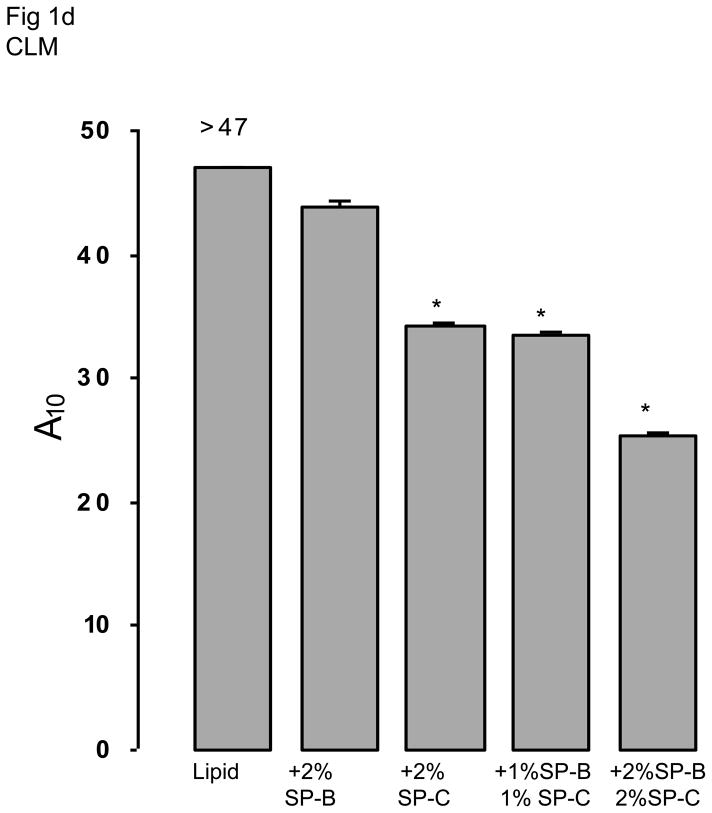
The two lipid mixtures (SLM or CLM) in the absence of surfactant proteins or polymers did not differ significantly on any of the measures of surface activity (Fig 1). All measures for both lipid mixtures were significantly less surface active than native surfactant. Adding polymers singly or in combination did not change the surface activities of the lipid mixtures (data not shown). Since these mixtures already showed poor surface activity, adding serum was moot.
Fig 1.
Fig 1a and 1c. Surface activities are shown for simple (SLM) (1a) and complex (CLM) (1c) lipid mixtures in the absence or presence of different concentrations of SP-B and/or SP-C. From left to right, the bars indicate means for samples containing lipid alone, lipid with 2% SP-B, with 2% SP-C, with 1% SP-B + 1% SP-C, and with 2% SP-B + 2% SP-C. The asterisk indicates statistically significant difference from the behavior of lipid alone as determined by single ANOVA, multiple comparison. Means are from five replicates ± SEM.
Fig 1b and 1d. A10, the percentage surface area reduction from the maximum for simple (SLM) (1b) and complex (CLM) (1d) lipid mixtures is shown in the absence or presence of different concentrations of SP-B and/or SP-C. The asterisk indicates statistically significant differences from the behavior of lipid alone as determined by single ANOVA, multiple comparison. Means are from five replicates ± SEM.
Comparison of lipid mixtures (SLM and CLM) with added surfactant proteins: SP-B and/or SP-C with no polymer no serum
Fig 1 shows results when surfactant proteins were added to each of the two lipid mixtures. Addition of either surfactant protein significantly improved surface activity but results did not differ significantly between SLM and CLM. Combinations of SP-B + SP-C tended to result in the best overall surface activity. For example, SP-B 2% + SP-C 2% in CLM was significantly better than SP-B 2% in CLM for minimum surface tension at five minutes and for A10 (p < 0.05, Fig 1).
Addition of polymers to lipid mixtures (CLM and SLM) with 1% SP-B plus 1% SP-C and no serum
With 1 % SP-B plus 1% SP-C, adding polymers singly or in combination improved surface activities. For example, with SLM, A10 was reduced three to seven fold for mixtures containing HA, HA+dextran, or HA+PEG compared to native surfactant mixtures, or ten to twenty fold compared with mixtures not containing polymers (Table 1). With CLM, A10 was reduced 1.5 to four fold for mixtures containing HA, HA+dextran, or HA+PEG compared with native surfactant, and two to five fold for mixtures not containing polymers (Table 1).
Table 1.
Effect of polymers on surface activity of simple lipid mixtures (SLM) and complex lipid mixtures (CLM) containing 1% SP-B and 1% SP-C. (Abbreviations defined in Methods).
| SLM | |||||||
|---|---|---|---|---|---|---|---|
| No Polymer | HA | PEG | Dextran | HA/PEG | HA + dextran | Native surfactant (No Polymer) | |
| Adsorption (mN/m) | 22±0.4 | 22±0.6 | 20±0.2* | 20±0.2* | 21±0.2* | 20.±0.2* | 20±0.2 |
| γmin (mN/m) 10th cycle | 12±1 | 0±0* | 8±1 | 2±0.5* | 1±0.3* | 0.4±0.2* | 10±0.7 |
| γmax (mN/m) 10th cycle | 32±0.8 | 54±2* | 27±0.6* | 28±0.2* | 38±1* | 46±3* | 30±0.7 |
| γmin (mN/m) 5 min | 9±2 | 0±0.1* | 0.2±0.2* | 0±0.1* | 0±0.1* | 0±0.1* | 2 ±0.6* |
| γmax (mN/m) 5 min | 31±0.5 | 48±2* | 27±0.4* | 27±0.2* | 34±0.5* | 43±2* | 30±0.5 |
| A10 | 41±3 | 2±0.5* | 22±0.7* | 19±0.6* | 7±1* | 3±0.7* | 23±2* |
| CLM | |||||||
|---|---|---|---|---|---|---|---|
| No Polymer | HA | PEG | Dextran | HA/PEG | HA/Dextran | Native surfactant (No Polymer) | |
| Adsorption (mN/m) | 21±0.3 | 22±0.5 | 20±0.2 | 21±0.2 | 20±0.2 | 21±0.3 | 20±0.2* |
| γmin (mN/m) 10th cycle | 14±0.9 | 7±1* | 12.2±1 | 12±1.2 | 6±1* | 5±1* | 10±0.7 |
| γmax (mN/m) 10th cycle | 29±0.5 | 34±1* | 28±0.1 | 29±0.8 | 33±1* | 40±1* | 30±0.7 |
| γmin (mN/m) 5 min | 5±1 | 0.8±0.8 | 5±1.3 | 3±0.8 | 1±0.5 | 1±0.8 | 2 ±0.6 |
| γmax (mN/m) 5 min | 30±0.6 | 35±0.6* | 29±0.7 | 31±1.3 | 31±1.5 | 40±1* | 30±0.5 |
| A10 | 33±2.3 | 17±3* | 33±2 | 29±1 | 10±0.2* | 6±0.6* | 23±2 |
Values are means ± SEM for five replicates. An asterisk indicates the value is significantly different from that for the mixture with no polymer (first column). P< 0.05 by ANOVA multiple comparison.
In general, the presence of HA, either alone or in combination with dextran or PEG, was most effective in producing low minimum surface tensions with minimal compression. At the same time, maximum surface tensions were generally increased when HA was added to surfactant mixtures. The increase in maximum surface tension associated with HA was greater with SLM than with CLM.
Addition of polymers to lipid mixtures with 2% SP-B, 2% SP-C and no serum
To establish whether the combined effect of surfactant proteins and polymers depended on protein concentration, we also tested samples containing 2% SP-B and/or 2% SP-C. When only SP-B or SP-C, was added to SLM or CLM, surface activities were affected most in the mixtures that contain HA+PEG or HA+dextran (Table 2). These results were similar to those found using mixtures containing 1% SP-B + 1% SP-C (Table 1).
Table 2.
Effect of polymers on surface activity of simple lipid mixtures (SLM) and complex lipid mixtures (CLM) containing 2% SP-B and 2% SP-C. (Abbreviations defined in Methods).
| SLM | |||||||
|---|---|---|---|---|---|---|---|
| No Polymer | HA | PEG | Dextran | HA/PEG | HA/Dextran | Native surfactant (No Polymer) | |
| Adsorption (mN/m) | 23±0.7 | 21±0.4* | 20±0.2* | 20±0.2* | 20±0.3* | 21±0.3* | 20±0.2* |
| γmin (mN/m) 10th cycle | 10±0.8 | 2.4±1* | 5±1.6 | 6±1 | 2±1* | 1±0.9* | 10±0.7 |
| γmax (mN/m) 10th cycle | 29±0.4 | 36±1* | 28±0.4 | 29±0.4 | 37±2* | 40.6±1* | 30±0.7 |
| γmin (mN/m) 5 min | 1.4±1 | 0±0 | 1.2±1 | 0.6±0.4 | 0±0 | 0±0 | 2 ±0.6 |
| γmax (mN/m) 5 min | 28.4±0.4 | 36±1* | 29±0.4 | 29±0.6 | 36±1.6* | 37±0.8* | 30±0.5 |
| A10 | 25±3 | 14±1* | 19±1.3 | 24±2 | 11±1.8* | 8±0.7* | 23±2 |
| CLM | |||||||
|---|---|---|---|---|---|---|---|
| No Polymer | HA | PEG | Dextran | HA/PEG | HA/Dextran | Native surfactant (No Polymer) | |
| Adsorption (mN/m) | 20±0.4 | 21±0.2 | 21±0.4 | 20±0.2* | 21±0.2 | 21±1 | 20±0.2 |
| γmin (mN/m) 10th cycle | 11±2 | 4±1* | 15±0.9 | 12±0.4 | 5±0.5* | 2±0.7* | 10±0.7 |
| γmax (mN/m) 10th cycle | 26±0.7 | 41±0.8* | 26±1 | 25±0.4 | 37±0.6* | 43±1* | 30±0.7 |
| γmin (mN/m) 5 min | 1.4±0.5 | 1.7±0.8 | 10±1* | 5±1.2 | 1.3±0.5 | 1.3±0.5 | 2 ±0.6 |
| γmax (mN/m) 5 min | 27±0.8 | 42±1* | 28±0.2 | 25±0.2* | 38±1* | 40±1* | 30±0.5 |
| A10 | 25±3 | 6±0.7* | 43±2.4* | 26±2.4 | 7±0.5* | 7±1* | 23±2 |
Values are means ± SEM for five replicates. An asterisk indicates the value is significantly different from that for the mixture with no polymer (first column). P< 0.05 by ANOVA multiple comparison.
When 2% SP-B + 2% SP-C were added to SLM with polymers singly or in combination, results were similar to results with 1% SP-B + 1% SP-C except that γ max 10th was lower for mixtures containing HA or HA+dextran, and A10 was higher for these mixtures (Table 1 and Table 2).
When 2 % SP-B + 2% SP-C was added to CLM, with HA alone or in combination with dextran or PEG, surface activities were improved compared to those observed in the absence of polymers. For example, A10 was reduced three to fourfold for mixtures containing HA, HA+dextran, or HA+PEG compared with mixtures of native surfactant or lipid mixtures with surfactant proteins not containing polymers (Table 2).
Serum inhibition of lipid/surfactant protein mixtures with and without polymers
A summary of serum inhibition for all the samples is shown in Table 3. The minimal serum concentration at which inhibition occurs is shown for each mixture as a multiple of the lowest serum protein concentration used (290 μg/mL).
Table 3.
Effect of protein and polymer composition on the susceptibility to serum inhibition of simple lipid mixtures (SLM) and complex lipid mixtures (CLM). The concentrations of serum protein (in multiples of 290 μg/mL) required to cause inhibition are shown.
| SLM | ||||
|---|---|---|---|---|
| polymer | protein | |||
| B 2% |
C 2% |
B + C 1% |
B + C 2% |
|
| None | 1 | 1 | 1 | 2 |
| HA | 4 | 1 | 6 | 6 |
| PEG | 2 | 1 | 6 | 6 |
| Dextran | 2 | 1 | 6 | 6 |
| HA+PEG | 8 | 4 | >18 | >18 |
| HA+Dextran | 10 | 4 | >18 | >18 |
| CLM | ||||
|---|---|---|---|---|
| polymer | proteins | |||
| B 2% |
C 2% |
B + C 1% |
B + C 2% |
|
| None | 1 | 1 | 2 | 3 |
| HA | 3 | 1 | 10 | 10 |
| PEG | 1 | 1 | 2 | 1 |
| dextran | 1 | 1 | 3 | 3 |
| HA+PEG | 6 | 12 | >18 | >18 |
| HA+Dextran | 14 | 12 | >18 | >18 |
With either SP-C or SP-B only
With only SP-C in the lipid mixture, the addition of any single polymer had no effect on inhibition. With only SP-B, the addition of any single polymer was slightly better with SLM compared to CLM. When HA plus PEG or dextran were added, the CLM mixtures were usually better than the SLM. It should be noted that results with anionic and non-ionic polymer combined gave substantial improvement to resistance to inhibition, especially the combination of HA and dextran in CLM, where the effect with either surfactant protein was equal to or superior to native surfactant (Table 3).
With both SP-B and SP-C
With both surfactant proteins present, and either PEG or dextran, the SLM mixtures were more resistant to inhibition than the CLM mixtures. However, in the presence of HA or HA combined with either nonionic polymer, the CLM mixtures were comparable to or better than the SLM mixtures. Mixtures containing combined polymers and combined surfactant proteins were the most resistant to inhibition by serum. For example, under these conditions, even in the presence of the highest serum concentrations used (5220 μg/mL serum protein—18 times the lowest inhibitory concentration) inhibition did not occur (Table 3). For comparison, the threshold for native surfactant inhibition, tested under identical conditions, was 3480 μg/mL serum protein or a factor of 12 above the minimum.
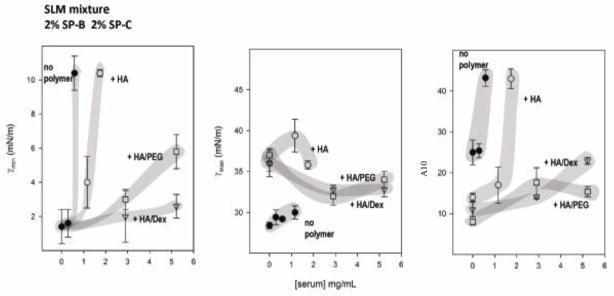
Since mixtures with HA alone or combined with PEG or dextran were the most resistant to inhibition with serum, more detailed results for these experiments are presented in Fig 2. This figure shows the effect of increasing concentrations of serum on some measures of surface activity for SLM or CLM containing 2% SP-B and 2% SP-C, with or without HA alone, or in combination with dextran or PEG. In the absence of polymer, lipid/surfactant protein mixtures reached low minimum surface tension only in the presence of low concentrations of serum protein (see left panels, Fig 2). If the samples contained a combination of HA and PEG, or HA and dextran, the concentration of serum required to produce inhibition was much higher, reflecting the improved ability of combinations of non-ionic and anionic polymers to impart resistance to inhibition.
Fig 2.
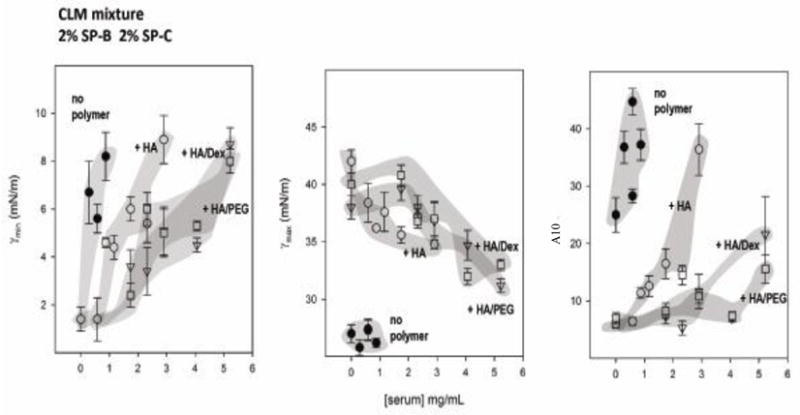
Three measures of surface activity are compared for simple (SLM) or complex (CLM) lipid mixtures containing 2% SP-B and 2% SP-C as a function of the concentration of serum in the subphase. HA ± PEG or dextran were added to some mixtures. Minimum (γmin) (left panels) and maximum (γmax) (central panels) surface tension after 5 min of cycling, and the percent of surface area reduction required to reach surface tensions below 10 mN/m (A10 right panels) for various mixtures have been plotted versus the concentration of added serum protein for samples without polymers (closed circles), with HA (open circles), with HA and PEG (squares), or with HA and dextran (triangles). Data points are means ± SEM after averaging five replicates. Shaded areas were drawn to guide the eye.
Susceptibility to inhibition was also analyzed with regard to film compressibility (right panels in Fig 2). Increasing concentrations of serum increased compressibility (increased A10). In the presence of HA alone or combined, a low compressibility was achieved even in the presence of substantial amounts of serum, for both SLM and CLM (with surfactant proteins). The low film compressibility with HA added to SLM or CLM (with surfactant proteins) was accompanied by higher maximum surface tensions (central panels in Figure 2).
Lipid/surfactant protein mixtures with lower concentrations of polymers with and without serum
To find whether lower concentrations of polymers would be as effective as those used in the above studies, we tested CLM or SLM with 2% SP-B and 2% SP-C with 0.125% HA + 2.5% dextran or 2.5% PEG. Without serum, minimum surface tensions were almost always higher than mixtures with double these polymer concentrations and A10 was always significantly higher (p<0.05). These mixtures were also inhibited by serum at concentrations a factor of 3 to 8 lower than the maximum used with full polymer concentrations which were still not inhibited (data not shown). The resistance to inhibition for the lower concentration polymers was essentially the same as those found with full strength single ionic polymers PEG or dextran.
Discussion
A major finding of this study is that SP-B and SP-C combined with HA and either PEG or dextran, when added to relatively simple lipid mixtures, exhibited less susceptibility to inhibition by serum than any other mixtures studied including native surfactant.
The mechanisms whereby surfactant proteins and combinations of polymers interact to prevent or reduce inhibition are complex. The non-ionic polymers PEG and dextran are believed to act mainly by depletion and osmotic forces in surfactant mixtures. In contrast, HA has been reported to establish interactions with itself forming a three dimensional network in addition to promoting depletion forces. Zasadzinski has suggested that depletion forces may promote association of large surfactant structures with the surface, overcoming the electrostatic barrier imposed by serum proteins that adsorb quickly to the interface [37], while the presence of surfactant proteins would be required for the transfer of surface active lipids to the actual interface and monolayer formation [1, 34, 38]. These differences in polymer interactions suggest that substantial additive effects should occur when both charged (HA) and uncharged (dextran or PEG) polymers are included with lipid/surfactant protein mixtures, as we have found.
Cooperative action between surfactant proteins and polymers may be established by their concerted action at different stages of interfacial lipid film formation and reformation. Polymer-promoted depletion forces also favor establishment of membrane-membrane contacts and formation of large surfactant aggregates. Polymers would then stabilize membrane-membrane and membrane-monolayer contacts and allow surfactant proteins to catalyze mobilization of phospholipids. Though the maximum protective effect of polymers was found with surfactant mixtures containing both surfactant proteins, mixtures with either SP-B or SP-C alone, with HA and dextran, could be as or more resistant to inhibition than native surfactant.
It is remarkable that HA exerts its counter-inhibitory action at much lower concentrations than the other polymers. HA forms networks in aqueous solutions [39], and we have shown that HA promotes formation of such networks in the presence of surfactant [28]. These charged networks formed by high molecular weight HA may impose spatial restrictions on the large surfactant aggregates, which might also contribute entropically to impel surfactant structures towards the interface [28]. Pasquali-Ronchetti et al have shown that HA can interact with phospholipids to form various complexes which depend on the molecular weight of HA. Phospholipids in the presence of HA tend to form aggregates [29]. On the other hand, binding of HA to albumin has been also reported by a number of investigators. Gramling et al. infer from electrophoretic mobility studies that HA forms stable complexes with albumin [40]. Gold et al. have found HA and other glycosaminoglycans binding to columns of albumin-agarose [41]. If HA binds with albumin, surfactant inhibition would be reduced since these larger structures would have more difficulty moving to the air-water interface. Therefore, inhibition of surfactant by serum components and counter-inhibition by HA may depend on the relative affinities of the three components in the complex surfactant/serum/HA scenario and the consequences on the reorganization of surfactant structures in the subphase. High molecular weight HA, along with other glycosaminoglycans, is a normal constituent in alveolar fluid [42, 43] and may play a role in vivo. Lung injury may also include a significant impairment of the structure and composition of this alveolar glycopolymeric matrix, which might be restored by supplementation with exogenous surfactant/HA combinations.
In the absence of serum, an important difference between the effects of HA vs. PEG or dextran is that HA reduces compressibility of SLM and CLM to values less than those for films formed by native surfactant (Table 1, 2). In the presence of HA, either alone or in combination with other polymers, little compression is required for the films to reach the lowest surface tensions (Fig 2). This effect would reinforce that of SP-B, which stabilizes mechanically compressed films, impeding their collapse and reducing the compressibility of the films, allowing them to reach low surface tensions with little compression [34]. We speculate that the HA network matrix modifies the rheological properties of the subphase, which in turn affects the surfactant adsorbed to the interface, making it less prone to fold and collapse, resulting in high lateral pressures (low surface tensions). In support of this concept, others have found that the viscoelastic properties of the bulk subphase can produce dramatic differences in the collapse properties of compressed surface layers [44]. HA-promoted exclusion of surfactant complexes from the bulk phase that leads to improved adsorption and effective displacement of serum components from the interface could then also prevent folding of compressed films into the subphase. Paradoxically, the effect of HA in preventing folding of interfacial films during compression may also impair re-spreading of compressed films during expansion. In the presence of HA, all surface films exhibit relatively high maximum surface tensions. Low maximum tensions have been usually interpreted as an expression of the efficiency of surfactant films to re-spread and refill the expanding interface, which depends on two simultaneous events: i) adsorption of new material from the subphase into the newly opened interface and ii) re-extension of compressed/collapsed structures to re-form a monolayer film. HA might promote adsorption but impair re-extension, both results as a consequence of its effect on the structure/rheology of the bulk subphase. The better performance of the CLM mixture, compared with SLM films, in the presence of HA, could be a consequence of the favorable dynamic behavior of the more fluid lipid mixture under the rheological restrictions imposed by the polymer.
Lipid mixtures were used that differed in complexity and represent in part the lipid composition of native surfactant [45]. These relatively simple mixtures were selected based on their potential use in developing new therapeutic formulations. The amount of SP-B and SP-C used approximate the concentrations found in native surfactant, and are more than double those found in therapeutic surfactants now available [45]. The concentrations of dextran, PEG and HA are within the range of those that have been found effective in other in vitro and in vivo experiments [15, 17] [14, 19]. The concentration of surfactant phospholipid used was 1.25 mg/mL, a limit imposed by the use of a pulsating bubble surfactometer. The serum protein concentration used was 290 to 5220 μg/mL giving a range of serum protein to phospholipid ratios from less than 1:4 to greater than 4:1. For native surfactant, the ratio of serum protein to phospholipids for inhibition was less than 3:1, while the optimal mixtures of combined polymers and surfactant proteins were not inhibited with ratios greater than 4:1 indicating less susceptibility to inhibition for these semisynthetic mixtures compared with native surfactant.
The concentration of phospholipid in commercial therapeutic surfactants is 25 mg to 80 mg/mL. Extrapolation from our results would suggest that inhibition of one mL of these full strength mixtures would occur with about 100 mg/mL of serum protein. Estimates of total serum protein in alveolar lung fluid in patients with ARDS range from 10 to 100 mg/mL [46]. At the low end of this range, the serum protein to phospholipid ratio is about 1:5, comparable to the low end of the serum range we studied. At the high end, the ratio would be about 2:1, a factor of two less than for our best mixtures. That is, the mixtures with combined polymer and both surfactant proteins would not be inhibited until at least 200 mg/mL serum protein are present. These estimates suggest the relevance of our in vitro results to possible pathologies existent in alveoli during lung injuries only one of which may involve influx of serum [47].
Given the extensive results from the comparisons of lipid, surfactant protein, and polymer combinations described above, more focused experiments with a few selected surfactant/polymer combinations using captive bubble surfactometry with much higher surfactant concentrations [34] will be useful. These studies are in progress with the aim of providing further information on the mechanisms by which polymer addition counteracts serum inhibition.
Conclusions
Inhibition of surfactant lipid mixtures was maximally reduced by the addition of HA and either dextran or PEG in the presence of SP-B and SP-C with results superior to those of whole native surfactant. The choice of lipid composition was found to depend on the polymer added, with single non-ionic polymers favoring the simple mixture and those with HA favoring the more complex, probably more dynamic, lipid formulation. The more complex mixture with surfactant proteins but no polymer also gave better results than the simple lipid mixture. Our experiments with different concentrations of polymers, surfactant proteins, and lipids suggest that new “recipes” for therapeutic surfactants may optimize adsorption and compressibility while not impairing readsorption during film expansion. The resulting lipid/protein/polymer preparations may be less prone to inhibition and therefore more efficacious for treatment of conditions such as ARDS. These findings also point to the importance and necessity of pursuing in vivo studies to see if these results translate into similar ones in animal models.
Research Highlights.
Surfactant proteins from lung lavage, two lipid mixtures, HA, PEG and dextran were combined and tested in a pulsating bubble surfactometer. Serum used as an inhibitor.
Addition of both SP-B and SP-C were better than either alone
Addition of any single polymer improved resistance to inhibition
Mixtures with SP-B and SP-C with combined polymers were more resistant to inactivation than native surfactant
A simple lipid mixture works well with non-ionic polymers, while a more complex (fluid) mixture works better with HA or HA combined with PEG or dextran
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pérez-Gil J. Structure of pulmonary surfactant membranes and films: the role of proteins and lipid-protein interactions. Biochim Biophys Acta. 2008;1778:1676–1695. doi: 10.1016/j.bbamem.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Gil J, Weaver T. Pulmonary surfactant pathophysiology: current models and open questions. Physiology (Bethesda) 2010;25:132–141. doi: 10.1152/physiol.00006.2010. [DOI] [PubMed] [Google Scholar]
- 3.Suresh G, Soll R. Overview of surfactant replacement trials. J Perinatology. 2005;25:S40–44. doi: 10.1038/sj.jp.7211320. [DOI] [PubMed] [Google Scholar]
- 4.Willson D, Thomas N, Markovitz B, Jefferson L, Conaway M, Egan E. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA. 2005;293:470–476. doi: 10.1001/jama.293.4.470. [DOI] [PubMed] [Google Scholar]
- 5.Adhikari N, Burns K, Meade M. Pharmacologic treatments for acute respiratory distress syndrome and acute lung injury: systematic review and meta-analysis. Treat Respir Med. 2004;3:307–328. doi: 10.2165/00151829-200403050-00005. [DOI] [PubMed] [Google Scholar]
- 6.Zuo Y, Veldhuizen R, Neumann A, Petersen N, Possmayer F. Current perspectives in pulmonary surfactant--inhibition, enhancement and evaluation. Biochim Biophys Acta. 2008;1778:1947–1977. doi: 10.1016/j.bbamem.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Hite R, Seeds M, Safta A, Jacinto R, Gyves J, Bass D, Waite B. Lysophospholipid generation and phosphatidylglycerol depletion in phospholipase A(2)-mediated surfactant dysfunction. Am J Physiol Lung Cell Mol Physiol. 2005;288:L618–624. doi: 10.1152/ajplung.00274.2004. [DOI] [PubMed] [Google Scholar]
- 8.Iwanicki J, Lu K, Taeusch H. Reductions of phospholipase A2 inhibition of pulmonary surfactant with hyaluronan. Exp Lung Res. 2010;36:167–174. doi: 10.3109/01902140903234186. [DOI] [PubMed] [Google Scholar]
- 9.Vockeroth D, Gunasekara L, Amrein M, Possmayer F, Lewis J, Veldhuizen R. Role of cholesterol in the biophysical dysfunction of surfactant in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;298:L117–L125. doi: 10.1152/ajplung.00218.2009. [DOI] [PubMed] [Google Scholar]
- 10.Rider E, Ikegami J, Whitsett J, Hull W, Absolom D, Jobe A. Treatment responses to surfactants containing natural surfactant proteins in preterm rabbits. American Review Respiratory Disease. 1993;147:669–676. doi: 10.1164/ajrccm/147.3.669. [DOI] [PubMed] [Google Scholar]
- 11.Amirkhanian JD, Bruni R, Waring AJ, Navar C, Taeusch HW. Full length synthetic surfactant proteins, SP-B and SP-C, reduce surfactant inactivation by serum. Biochim Biophys Acta. 1993;1168:315–320. doi: 10.1016/0005-2760(93)90188-f. [DOI] [PubMed] [Google Scholar]
- 12.Cockshutt A, Weitz J, Possmayer F. Pulmonary surfactant-associated protein A enhances the surface activity of lipid extract surfactant and reverses inhibition by blood proteins in vitro. Biochemistry. 1990;29:8424–8429. doi: 10.1021/bi00488a032. [DOI] [PubMed] [Google Scholar]
- 13.Blanco O, Perez-Gil J. Biochemical and pharmacological differences between preparations of exogenous natural surfactant used to treat Respiratory Distress Syndrome: Role of the different components in an efficient pulmonary surfactant. Eur J Pharmacol. 2007;568:1–15. doi: 10.1016/j.ejphar.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 14.Lu K, Goerke J, Clements J, Taeusch H. Hyaluronan decreases surfactant inactivation in vitro. Pediatr Res. 2005;57:237–241. doi: 10.1203/01.PDR.0000150726.75308.22. [DOI] [PubMed] [Google Scholar]
- 15.Taeusch H, Lu K, Goerke J, Clements J. Nonionic polymers reverse inactivation of surfactant by meconium and other substances. Am J Respir Crit Care Med. 1999;159:1391–1395. doi: 10.1164/ajrccm.159.5.9808047. [DOI] [PubMed] [Google Scholar]
- 16.Calkovska A, Mokra D, Drgova A, Zila I, Javorka K. Bronchoalveolar lavage with pulmonary surfactant/dextran mixture improves meconium clearance and lung functions in experimental meconium aspiration pneumonia. Eur J Pediatr. 2007;167:851–857. doi: 10.1007/s00431-007-0596-7. [DOI] [PubMed] [Google Scholar]
- 17.Lu K, Taeusch H, Robertson B, Goerke J, Clements J. Polymer-surfactant treatment of meconium-induced acute lung injury. Am J Respir Crit Care Med. 2000;162:623–628. doi: 10.1164/ajrccm.162.2.9909099. [DOI] [PubMed] [Google Scholar]
- 18.Lu K, Taeusch H, Robertson B, Goerke J, Clements J. Polyethylene glycol/surfactant mixtures improve lung function after HCl and endotoxin lung injuries. Am J Respir Crit Care Med. 2001;164:1531–1536. doi: 10.1164/ajrccm.164.8.2104016. [DOI] [PubMed] [Google Scholar]
- 19.Lu K, Goerke J, Clements J, Taeusch H. Hyaluronan reduces surfactant inhibition and improves rat lung function after meconium lung injury. Pediatr Res. 2005;58:206–210. doi: 10.1203/01.PDR.0000169981.06266.3E. [DOI] [PubMed] [Google Scholar]
- 20.Lu K, Robertson B, Taeusch H. Dextran or polyethylene glycol added to Curosurf for treatment of meconium lung injury in rats. Biol Neonate. 2005;88:46–53. doi: 10.1159/000084458. [DOI] [PubMed] [Google Scholar]
- 21.Lu J, Cheung W, Yu L, Policova Z, Li D, Hair M, Neumann A. The effect of dextran to restore the activity of pulmonary surfactant inhibited by albumin. Respir Physiol Neurobiol. 2002;130:169–179. doi: 10.1016/s0034-5687(02)00006-3. [DOI] [PubMed] [Google Scholar]
- 22.Stichtenoth G, Jung P, Walter G, Johansson J, Robertson B, Curstedt T, Herting E. Polymyxin B/pulmonary surfactant mixtures have increased resistance to inactivation by meconium and reduce growth of gram-negative bacteria in vitro. Pediatr Res. 2006;59:407–411. doi: 10.1203/01.pdr.0000200806.32822.e6. [DOI] [PubMed] [Google Scholar]
- 23.Zuo Y, Alolabi H, Shafiei A, Kang N, Policova Z, Cox P, Acosta E, Hair M, Neumann W. Chitosan enhances the in vitro surface activity of dilute lung surfactant preparations and resists albumin induced inactivation. Pediatr Res. 2006;60:125–130. doi: 10.1203/01.pdr.0000227558.14024.57. [DOI] [PubMed] [Google Scholar]
- 24.Almlén A, Stichtenoth G, Linderholm B, Haegerstrand-Björkman M, Robertson B, Johansson J, Curstedt T. Surfactant proteins B and C are both necessary for alveolar stability at end expiration in premature rabbits with respiratory distress syndrome. J Appl Physiol. 2008;104:1101–1108. doi: 10.1152/japplphysiol.00865.2007. [DOI] [PubMed] [Google Scholar]
- 25.Schürch D, Ospina O, Cruz A, Perez-Gil J. Combined and independent action of proteins SP-B and SP-C in the surface behavior and mechanical stability of pulmonary surfactant films. Biophysical Journal. 2010;99:3290–3299. doi: 10.1016/j.bpj.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walther F, Hernandez-Juviel J, Gordon L, Waring A, Stenger P, Zasadzinski A. Comparison of three lipid formulations for synthetic surfactant with a surfactant protein B analogue. Experimental Lung Research. 2005;31:563–579. doi: 10.1080/019021490951531. [DOI] [PubMed] [Google Scholar]
- 27.Braun A, Stenger P, Warriner H, Zasadzinski J, Lu K, Taeusch H. A freeze-fracture TEM and small angle X-ray diffraction study of the effects of albumin, serum, and polymers on clinical lung surfactant microstructure. Biophys J. 2007;93:1–17. doi: 10.1529/biophysj.106.095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu K, Perez-Gil J, Taeusch HW. Kinematic viscosities of surfactants with added polymers. Biochim Biophys Acta. 2009;1788:632–637. doi: 10.1016/j.bbamem.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasquali-Ronchetti I, Quaglino D, Mori G, Bacchelli B, Ghosh P. Hyaluronan-phospholipid interactions. J Struct Biol. 1997;120:1–10. doi: 10.1006/jsbi.1997.3908. [DOI] [PubMed] [Google Scholar]
- 30.Bray BA. The role of hyaluronan in the pulmonary alveolus. J Theor Biol. 2001;210:121–130. doi: 10.1006/jtbi.2001.2305. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Gil J, Cruz A, Casals C. Solubility of hydrophobic surfactant proteins in organic solvent/water mixtures. Structural studies on SP-B and SP-C in aqueous organic solvents and lipids. Biochim Biophys Acta. 1993;1168:261–270. doi: 10.1016/0005-2760(93)90181-8. [DOI] [PubMed] [Google Scholar]
- 32.Taeusch H, de la Sena J, Perez-Gil J, Alonso C, Zasadzinski J. Inactivation of pulmonary surfactant due to serum-inhibited adsorption and reversal by hydrophilic polymers: experimental. Biophys J. 2005;89:1769–1779. doi: 10.1529/biophysj.105.062620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mingarro I, Lukovic D, Vilar M, Pérez-Gil J. Synthetic pulmonary surfactant preparations: new developments and future trends. Curr Med Chem. 2008;15:393–403. doi: 10.2174/092986708783497364. [DOI] [PubMed] [Google Scholar]
- 34.Schürch D, Ospina O, Cruz A, Pérez-Gil J. Combined and independent action of proteins SP-B and SP-C in the surface behavior and mechanical stability of pulmonary surfactant films. Biophys J. 2010;99:3290–3299. doi: 10.1016/j.bpj.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu K, Taeusch H. Combined effects of polymers and KL(4) peptide on surface activity of pulmonary surfactant lipids. Biochim Biophys Acta. 2010;1798:1129–1134. doi: 10.1016/j.bbamem.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 36.Putz G, Goerke J, Taeusch W, Clements J. Comparison of captive and modified pulsating bubble surfactometers. J Appl Physiol. 1994;76:1425–1431. doi: 10.1152/jappl.1994.76.4.1425. [DOI] [PubMed] [Google Scholar]
- 37.Zasadzinski A, Taeusch H. Polymer depletion forces and surfactant adsorption. Polymer Preprints. 2005;46:134–138. [Google Scholar]
- 38.Hall S, Venkitaraman A, Whitsett J, Holm B, Notter R. Importance of hydrophobic apoproteins as constituents of clincal exogenoous surfactants. American Review of Respiratory Disease. 1992;145:24–30. doi: 10.1164/ajrccm/145.1.24. [DOI] [PubMed] [Google Scholar]
- 39.Scott J, Cummings C, Brass A, Chen Y. Secondary and tertiary structures of hyaluronan in aqueous solution, investigated by rotary shadowing-electron microscopy and computer simulation. Biochem J. 1991;274:699–705. doi: 10.1042/bj2740699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gramling E, Niedermeier W, Holley H, Pigman W. Some factors affecting the interaction of hyaluronic acid with bovine-plasma albumin. Biochimica Biophysica Acta. 1963;69:552–558. doi: 10.1016/0006-3002(63)91307-6. [DOI] [PubMed] [Google Scholar]
- 41.Gold E. An interaction of albumin with hyaluronic acid and chondroitin sulfate: a study of affinity chromatography and circular dichoism. Biopolymers. 1980;19:1407–1414. [Google Scholar]
- 42.Sahu S, Tanswell A, Lynn W. Isolation and characterization of glycosaminoglycans secreted by human foetal lung Type II pneumocytes in culture. Journal of Cell Science. 1980;42:183–188. doi: 10.1242/jcs.42.1.183. [DOI] [PubMed] [Google Scholar]
- 43.Skinner S, Post M, Torday J, Stiles A, Smith B. Characterization of proteoglycans synthesized by fetal rat lung type II pneumonocytes in vitro and the effects of cortisol. Exp Lung Res. 1987;12:253–264. doi: 10.3109/01902148709064304. [DOI] [PubMed] [Google Scholar]
- 44.Pocivavsek L, Dellsy R, Kern A, Johnson S, Lin B, Lee K, Cerda E. Stress and fold localization in thin elastic membranes. Science. 2008;320:912–916. doi: 10.1126/science.1154069. [DOI] [PubMed] [Google Scholar]
- 45.Bernhard W, Mottaghian J, Gebert A, Rau G, von der Hardt H, Poets C. Commercial versus native surfactants. Surface activity, molecular components, and the effect of calcium. Am J Respir Crit Care Med. 2000;162:1524–1533. doi: 10.1164/ajrccm.162.4.9908104. [DOI] [PubMed] [Google Scholar]
- 46.Ishizaka A, Matsuda T, Albertine K, Koh H, Tasaka S, Hasegawa N, Kohno N, Kotani T, Morisak H, Takeda J, Nakamura M, Fang X, Martin T, Matthay M, Hashimoto S. Elevation of KL-6, a lung epithelial cell marker, in plasma and epithelial lining fluid in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2003;286:L1088–1094. doi: 10.1152/ajplung.00420.2002. [DOI] [PubMed] [Google Scholar]
- 47.Papakonstantinou E, Karakiulakis G. The ‘sweet’ and ‘bitter’ involvement of glycosaminoglycans in lung diseases: pharmacotherapeutic relevance. Br J Pharmacol. 2009;157:1111–1127. doi: 10.1111/j.1476-5381.2009.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]