Abstract
Non-viral in vivo delivery of DNA, encoding for specific proteins, has traditionally relied on chemical or physical forces applied directly to tissues. Physical methods typically involve contact between an applicator/electrode and tissue and often results in transient subject discomfort. To overcome these limitations of contact-dependent delivery, a helium plasma source was utilized to deposit ionized gasses to treatment/vaccination sites without direct contact between the applicator and the tissues. The study reported here evaluated the efficacy of this strategy as an effective method to administer DNA vaccines. Balb/C mice were vaccinated with a DNA plasmid expressing an HIV gp120 envelope glycoprotein either with or without co-administration of helium plasma or electroporation. The results indicated, for the first time, the potential efficacy of helium plasma delivery for the induction and enhancement of antigen specific immune responses following DNA vaccination.
Keywords: Helium plasma delivery, electroporation, DNA vaccine, HIV gp120
1. Introduction
Advances in vaccine development have demonstrated the potential utility of plasmid-based genes (i.e. DNA vaccines), which code for biologically relevant proteins, to elicit cellular and humoral immunity, when delivered in vivo to cells and tissues [1-3]. When injected into tissues without applying a “forcing function”, these DNA constructs must often be administered at a “high” dose due to their limited ability to traverse cell membranes. Although, higher doses of plasmid do not always result in increased expression and increased therapeutic activity or immunogenicity, it is thought that an increase in dose is usually, at least initially, a prudent avenue to pursue in order to increase the efficiency of this method. Therefore, the delivery of these molecules with chemical or physical forces capable of causing transient membrane destabilization, often enables lower doses to be utilized. The most common physical delivery method that has been employed is electroporation (EP) [4]. This modality has been utilized for both the delivery of therapeutic drugs [5-7] as well as genes (i.e. DNA) for both therapeutics and vaccine applications [8-13]. While this delivery method has been demonstrated to have some effectiveness in eliciting both therapeutic effects as well as DNA vaccine mediated immune responses, they are often associated with adverse events such as temporary pain, involuntary muscle contraction, and thermal effects [14-15]. The avoidance of these effects, through the development of a more clinically desirable non-contact delivery method, served as the major impetus for performing this study.
One approach for potentially eliminating the discomfort caused by physical delivery mechanisms, is to decouple the applicator from the treatment/vaccination site. When considering alternative non-contact modalities it is obviously advantageous to maintain the mechanisms known to create transient pores in biological membranes which is thought to be necessary for the enhanced delivery and biological effectiveness of these methods. A viable means of achieving this is through ion deposition at a treatment site. Depositing charged species on an area for an extended period of time will lead to a charge accumulation, causing an electric potential to be established. Achieving a high potential would result in the formation of pores in a similar fashion to those generated by EP, without the deleterious effects caused by the contact between electrodes and tissues.
Delivery of DNA by plasma discharge exposure remains relatively novel, but has been proven efficacious with small molecules and genes encoding tracer molecules [16, 17]. One example supporting this molecular delivery technique entailed the exposure of cultured neuronal cells to a high frequency plasma discharge which achieved delivery of DNA encoding green fluorescent protein [18]. Another in vitro example of plasma delivery was performed by ionizing air molecules above a sample of melanoma cells with a high voltage direct current power source to delivery chemotherapeutic agents and tracer molecules [19]. Finally, a non-equilibrium direct current plasma source that ionizes a flowing stream of helium has been used in vitro to deliver tracer molecules, as well as deliver, to murine skin, a plasmid DNA encoding for luciferase [16, 17].
The focus of the study presented here was to utilize the aforementioned nonequilibrium direct current plasma source to delivery DNA encoding biologically relevant proteins for the purpose of generating an immune response. The specific plasmid selected for this study was pJRFLgp120, which contains a coding region for the 120 kilodalton (kDa) envelope glycoprotein, a protein present on the surface of a macrophage tropic HIV. Successful delivery by this technique would provide a novel method to augment the uptake and potential effectiveness of DNA vaccines [20].
2. Methods, results and discussion
2.1. Methods
The plasma generator utilized in this study was constructed from a hollowed Teflon dowel that was 15 cm in length with a 1 cm inner diameter. A steel hose barb, electrically connected to ground potential, was positioned on top of the Teflon tube to interface with the helium gas supply. A flat annular stainless steel electrode having a 3.5 mm internal diameter was positioned at the bottom of the tube. The annular electrode was electrically connected to a high voltage direct current power supply (Spellman Model CZE2000, New York, NY). Operating parameters, such as voltage, maximum current and treatment time for the power supply were controlled by a software interface platform (National Instruments LabVIEW, Austin, TX). Under operational conditions, the generator provided a reading of 10 μA at 8 kV to the annular electrode and produced a visible glow discharge measuring 3.0 cm in length. Figure 1 shows the plasma stream generated by the delivery conditions used in this study. The delivery time interval for both positive and negative plasma was 10min. The selection of this time interval was based on a previously published study that indicated that the 10min delivery time resulted in maximal expression of the luciferase reporter gene [16]. The fundamental difference between positive and negative plasma is the electric potential that is applied to the plasma generator to achieve breakdown, or ionization, of the helium. In the case of positive plasma a potential of +8kV was applied to the electrode in the generator to produce the breakdown. Whereas, in the case of negative plasma, the applied potential was -8kV. Each of these conditions has been previously used with luciferase encoding plasmids and was found to generate similar levels of expression [16]. It was decided to investigate both of these conditions with a plasmid encoding a biologically relevant molecule in order to determine if antibody titers would reveal any further information regarding a polarity dependence on the generation of protein expression and induction of immunogenicity. The EP conditions used in this study have been previously described and published [21] that optimizes luciferase expression using the 4 plate electrode. Specifically, the conditions used were 8 pulses at 100 V/cm, with duration of 150 ms per pulse. Prior to applying the electrical signals to the tissue the applicator surfaces were coated with a small amount of electrically conductive gel (Spectragel) to facilitate contact with the skin.
Figure 1.

Plasma generator operating at +8kV. Afterglow was captured with a 30 sec exposure.
Six to 10 week old female BALB/c mice (NIH, Bethesda, MD) were utilized for this study. At the conclusion of this investigation, all animals were humanely euthanatized in accordance with an approved Institutional Animal Care and Use Committee (IACUC) protocol.
Prior to vaccination/delivery exposure, animals were anesthetized in an induction chamber using 2% isoflurane mixed with oxygen. After anesthesia, the mice were maintained in an anesthetized state by delivering 2% isoflurane through a nose cone. To prevent excessive heat loss due to anesthesia exposure mice were placed on a 37°C heating pad during vaccination/treatment. The JRFLgp120 expressing DNA plasmid (see plasmid map in Figure 2) was constructed under the control of a CMV promoter and had been previously tested for appropriate expression (unpublished data). The rationale for the selection of the HIV JRFL envelope glycoprotein was based on the observation (personal communication) that this plasmid resulted in only moderate expression and immunogenicity under conditions of plasmid injection alone (i.e. without a concomitant delivery exposure). Therefore, it was reasoned that the potential expression and immune enhancing effects of a delivery method would be more evident and measurable using this plasmid.
Figure 2.
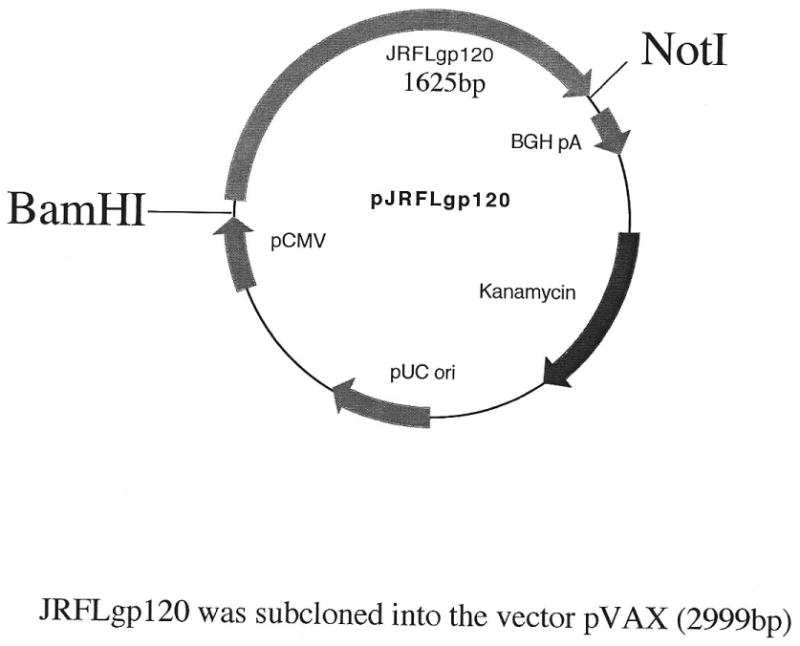
JRFL gp120 expressing DNA plasmid under the control of a CMV promoter.
Plasmid injections were performed with a 1 ml syringe and a 30-gauge needle (BD, Franklin Lakes, NJ). Plasmids were injected intradermally with 100μg pJRFLgp120 in 50μl if physiological saline. Discharge exposure times of 10 minutes were selected based on published in vivo data showing high levels of expression at this treatment time [16]. Each of the vaccination/delivery conditions were performed on groups of mice containing four animals each (n=4). The following vaccination groups were evaluated in this study: (a) no treatment, i.e. not injected with plasmid nor exposed to plasma ions (b) 100μg pVAX plasmid backbone only; (c) 100μg pVAX backbone plus 10 minutes of positive plasma exposure; (d) injection plus 100μg pVAX backbone delivered with 10 minutes of negative plasma exposure; (e) 100μg pVAX backbone followed by 8 electroporation pulses at 100 V/cm and 150 ms in duration; (f) 100μg pJRFLgp120 only; (g) 100μg pJRFLgp120 with 10 minutes positive plasma exposure; (h) 100μg pJRFLgp120 with 10 minutes negative plasma exposure and (i) 100μg pJRFLgp120 followed by 8 electroporation pulses at 100 V/cm and 150 ms in duration. In addition, identical groups (as indicated above) of animals were also injected with 25μg of plasmid. However, no significant antibody responses were noted. Data for these dose groups are not presented. This protocol involved an initial treatment on day 0, followed by three booster vaccinations on days 14, 28 and 130. Figure 3 indicates the timeline for treatment and sample collection for the study.
Figure 3.
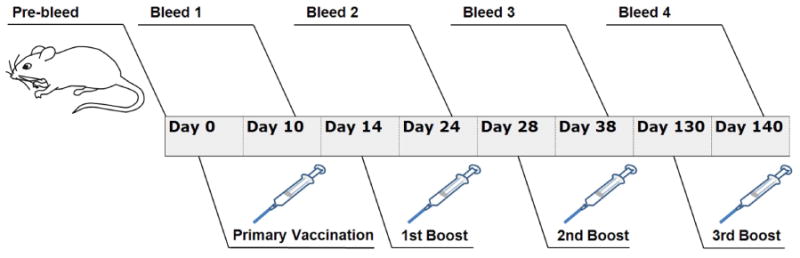
Time course of vaccination and sampling regimen.
At the sampling times listed in Figure 3, blood was collected from the mice and sera was separated for analysis in a indirect ELISA assay. Briefly, 50μl of a 2μg/ml solution of ADA gp120 (which shares 87.3% amino acid homology with the JRFL gp120 envelope) was added to Immulon 4 ELISA plates and incubated overnight at 4°C. This was followed by blocking with BSA, incubation with diluted sera samples from the different vaccination/delivery groups, incubation with a goat anti-mouse HRP conjugated IgG and development with an appropriate substrate. Optical density values of the different wells were determined at 450nm. Antigen specific geometric mean antibody titers were then determined. Details of the ELISA methodologies have been previously published [22,23]. Statistical differences between endpoint geometric mean antibody titers for the various groups was determined by a nonparametric ANOVA analysis (Kruskal-Wallis test).
2.2. Results
The major goal of this study was to determine whether helium plasma mediated delivery of a DNA vaccine could enhance the induction of antigen specific antibody responses compared to plasmid delivery alone or delivery through EP. To that end, we intradermally administered 100μg of a gp120 expressing DNA plasmid under different plasma delivery conditions or EP.
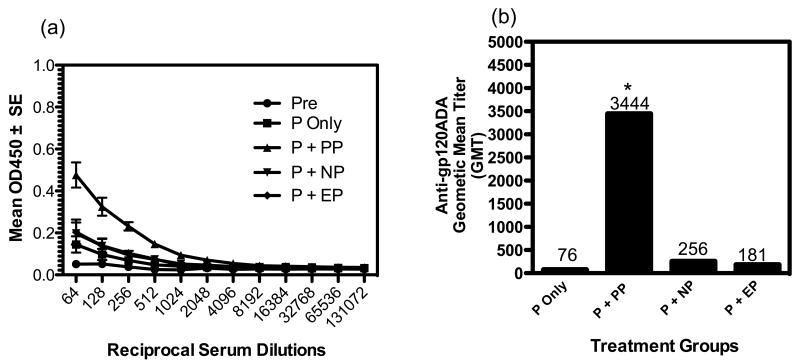
The data presented in Figure 4 was generated from sera samples from the different vaccination/delivery groups at day 140 of the study, which was 10 days after the final DNA vaccine boost (i.e. after a total of 4 vaccinations). Earlier bleeds from this study were also assessed for antibody levels. Antigen specific antibodies were also noted in the earlier bleeds; however, only data from the final terminal bleed are presented in this report. Figure 3a presents the anti-gp120 antibody levels, as indicated by OD values, among the different groups measured from reciprocal sera dilutions between 64 and 131,072. All vaccination delivery groups (through a reciprocal sera dilution of 4,096) had higher antibody levels than the pre-bleed samples. Importantly and interestingly, the plasmid plus positive plasma delivery groups demonstrated significantly higher antigen specific antibody responses than the other delivery groups.
Figure 4.
Effect of plasma delivery of a HIVgp120 expressing DNA vaccine on antigen specific antibody responses. Key: Pre=prevaccination sera samples; P=gp120 plasmid alone; P+PP=gp120 plasmid + positive plasma mediated delivery; P+NP=gp120 plasmid + negative plasma mediated delivery; P+EP=gp120 plasmid + electroporation mediated delivery. Panel (a) shows the anti-gp120 antibody responses/titers among the different vaccination/delivery groups. Panel (b) compares the antigen specific geometric mean antibody titers among the different vaccination/delivery groups. The asterisk (*) indicates statistically elevated GMT values compared to the other groups at the 5% level.
Figure 4b shows the anti-gp120 geometric mean anti-gp120 antibody titers (GMT) for the different vaccination/delivery groups. The GMT values for the plasmid alone, plasmid plus positive plasma, plasmid plus negative plasma and plasmid plus EP groups were 76, 3444, 256 and 181 respectively. Non-parametric ANOVA (Kruskal-Wallis) analysis (at the 5% level, p < 0.5) of the GMTs between the different groups indicated that the plasmid injection + positive plasma delivery group was significantly higher than the other groups. Also, further analysis indicated that the GMTs of the plasmid alone and plasmid + EP were not significantly different from each other. Whereas, plasmid + negative plasma was marginally significantly elevated compared to plasmid injection alone. These results indicate that positive plasma, based on GMT values, was 45 and 19 fold more effective in inducing antigen specific antibodies than the plasmid alone and plasmid plus EP groups, respectively. To our knowledge these data indicate, for the first time, the efficacy of a helium plasma based delivery system for enhancing the ability of a DNA vaccine to induce antigen specific humoral immune responses. Also, this initial study also indicates that positive plasma was more effective in enhancing induction of humoral immune responses than EP, at least under the electric pulsing conditions utilized in this study.
2.3. Discussion
Enhancement of delivery and subsequent expression of antigen encoded in DNA based plasmid vaccines is thought to be important for the utility and success of plasmid vaccine strategies. A number of methods to enhance delivery have been implemented. Recently, the use of in vivo EP has become a popular modality for enhancing the efficacy of DNA vaccines. It has met with some success in enhancing both humoral and cellular immune responses of antigen presenting DNA vaccines [9, 24]. However, effective non-contact delivery methods may be more useful in terms of clinical utility due to the elimination or attenuation of some of the side effects noted with a contact dependent approach which can include momentary shock and pain. To that end, this initial report demonstrates that positive but not negative polarity helium plasma was able to significantly enhance the ability of a gp120 expressing DNA plasmid to induce antigen specific humoral immune responses. This enhancing effect was relative to the administration of the gp120 expressing plasmid alone (i.e. without any concomitant delivery modality). Also, the data indicate that the positive plasma delivery method was more effective than EP, under the pulsing conditions utilized, in enhancing immunogenicity and presumably expression of antigen by the DNA vaccine.
The results of the study warrant further investigation of the mechanism of enhanced induction of immune responses by the non-contact helium ion delivery method. As well, it will be useful to determine the ability of this method to enhance antigen specific cellular immune responses. In particular, it will be instructive to determine the rationale and mechanism for the lack of enhancement of immune responses mediated by negative plasma delivery. This is particularly important in light of previously published results indicating that positive and negative plasma were equivalent in their ability to enhance the expression of a luciferase reporter gene [16]. Also, it will be important to determine whether shorter intervals of plasma exposure will be able to mediate the immune response enhancing effect of the 10min exposure. This will be relevant to establish since it is desirable, in terms of potential clinical applicability, to enhance the immune responses with the shortest plasma exposure time interval.
In summary, as indicated above, this is the first observation, to our knowledge, which document the ability of a helium ion plasma to enhance the delivery and immunogenicity of an antigen specific DNA plasmid vaccine. As such, further evaluation of this novel technology is warranted.
Acknowledgments
The project described was supported, in part, by grants AI090561 and AI079706 from the National Institute of Allergy and Infectious Diseases (NIAID) of the NIH. It’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID. In addition, the work was also supported, in part, by a pilot grant from the Florida Center of Excellence in Biomolecular Identification and Targeted Therapeutics (FCoE-BITT). Student support was provided by an NSF IGERT (DGE-0221681) grant, as well as by the University of South Florida Graduate and Multidisciplinary Scholars programs. We also acknowledge Drs. David Weiner and Jian Yan, from the University of Pennsylvania, for generously supplying the JRFLgp120 expressing DNA plasmid.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdulhaqq SA, Weiner DB. DNA vaccines: developing new strategies to enhance immune responses. Immunol Res. 2008;42:219–32. doi: 10.1007/s12026-008-8076-3. [DOI] [PubMed] [Google Scholar]
- 2.Liu MA. DNA vaccines: a review. J Intern Med. 2003;253:402–10. doi: 10.1046/j.1365-2796.2003.01140.x. [DOI] [PubMed] [Google Scholar]
- 3.Morrow MP, Weiner DB. DNA drugs come of age. Sci Am. 2010;303:48–53. doi: 10.1038/scientificamerican0710-48. [DOI] [PubMed] [Google Scholar]
- 4.Weaver JC. Electroporation theory. Concepts and mechanisms Methods Mol Biol. 1995;55:3–28. doi: 10.1385/0-89603-328-7:3. [DOI] [PubMed] [Google Scholar]
- 5.Heller R, Jaroszeski M, Leo-Messina J, et al. Treatment of B16 melanoma with the combination of electroporation and chemotherapy. Bioelectrochem Bioenerg. 1995;36:83–87. [Google Scholar]
- 6.Mir LM, Orlowski S, Belehradek J, Jr, Paoletti C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur J Cancer. 1991;27:68–72. doi: 10.1016/0277-5379(91)90064-k. [DOI] [PubMed] [Google Scholar]
- 7.Okino M, Mohri H. Effects of a high-voltage electrical impulse and an anticancer drug on in vivo growing tumors. Jpn J Cancer Res. 1987;78:1319–21. [PubMed] [Google Scholar]
- 8.Heller LC, Ugen K, Heller R. Electroporation for targeted gene transfer. Expert Opinion on Drug Delivery. 2005;2:255–68. doi: 10.1517/17425247.2.2.255. [DOI] [PubMed] [Google Scholar]
- 9.Hirao LA, Wu L, Satishchandran A, et al. Comparative Analysis of Immune Responses Induced by Vaccination With SIV Antigens by Recombinant Ad5 Vector or Plasmid DNA in Rhesus Macaques. Mol Ther. 2010;18:1568–76. doi: 10.1038/mt.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas ML, Heller L, Coppola D, Heller R. IL-12 plasmid delivery by in vivo electroporation for the successful treatment of established subcutaneous B16.F10 melanoma. Mol Ther. 2002;5:668–75. doi: 10.1006/mthe.2002.0601. [DOI] [PubMed] [Google Scholar]
- 11.Lucas ML, Heller R. Immunomodulation by electrically enhanced delivery of plasmid DNA encoding IL-12 to murine skeletal muscle. Mol Ther. 2001;3:47–53. doi: 10.1006/mthe.2000.0233. [DOI] [PubMed] [Google Scholar]
- 12.Titomirov AV, Sukharev S, Kistanova E. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim Biophys Acta. 1991;1088:131–34. doi: 10.1016/0167-4781(91)90162-f. [DOI] [PubMed] [Google Scholar]
- 13.Ugen KE, Kutzler MA, Marrero B, et al. Regression of subcutaneous B16 melanoma tumors after intratumoral delivery of an IL-15-expressing plasmid followed by in vivo electroporation. Cancer Gene Ther. 2006;13:969–74. doi: 10.1038/sj.cgt.7700973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denet AR, Vanbever R, Preat V. Skin electroporation for transdermal and topical delivery. Adv Drug Deliv Rev. 2004;56:659–74. doi: 10.1016/j.addr.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Prausnitz MR. The effects of electric current applied to skin: A review for transdermal drug delivery. Advanced Drug Delivery Reviews. 1996;18:395–425. [Google Scholar]
- 16.Connolly RJ, Lopez GA, Hoff AM, Jaroszeski MJ. Plasma facilitated delivery of DNA to skin. Biotechnology and Bioengineering. 2009;104:1034–40. doi: 10.1002/bit.22451. [DOI] [PubMed] [Google Scholar]
- 17.Connolly RJ, Lopez GA, Hoff AM, Jaroszeski MJ. Characterization of plasma mediated molecular delivery to cells in vitro. Int J Pharm. 2010;389:53–57. doi: 10.1016/j.ijpharm.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa Y, Morikawa N, Ohkubo-Suzuki A, et al. An epoch-making application of discharge plasma phenomenon to gene-transfer. Biotechnology and Bioengineering. 2005;92:865–70. doi: 10.1002/bit.20659. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran N, Jaroszeski M, Hoff AM. Molecular delivery to cells facilitated by corona ion deposition. IEEE Transactions on Nanobioscience. 2008;7:233–9. doi: 10.1109/TNB.2008.2002290. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Arthos J, Lawrence JM, et al. Enhanced immunogenicity of gp120 protein when combined with recombinant DNA priming to generate antibodies that neutralize the JR-FL primary isolate of human immunodeficiency virus type 1. J Virol. 2005;79:7933–37. doi: 10.1128/JVI.79.12.7933-7937.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heller LC, Jaroszeski MJ, Coppola D, McCray AN, Hickey J, Heller R. Optimization of cutaneous electrically mediated plasmid DNA delivery using novel electrode. Gene Therapy. 2007;14:275–80. doi: 10.1038/sj.gt.3302867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacGregor RR, Boyer JD, Ugen KE, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis. 1998;178:92–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- 23.Ugen KE, Goedert JJ, Boyer J, et al. Vertical transmission of human immunodeficiency virus (HIV) infection. Reactivity of maternal sera with glycoprotein 120 and 41 peptides from HIV type 1. J Clin Invest. 1992;89:1923–30. doi: 10.1172/JCI115798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirao LA, Wu L, Khan AS, et al. Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques. Vaccine. 2008;26:3112–20. doi: 10.1016/j.vaccine.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]