Abstract
Purpose of review
Hepatic ischemia reperfusion injury (IRI) linked to leukocyte recruitment and subsequent release of cytokines and free radicals remains a significant complication in organ transplantation. The aim of this review is to bring attention to advances made in our understanding of the mechanisms of leukocyte recruitment to sites of inflammatory stimulation in liver IRI.
Recent findings
Leukocyte transmigration across endothelial and extracellular matrix (ECM) barriers is dependent on adhesive events, as well as on focal matrix degradation mechanisms. While adhesion molecules are critical for the successful promotion of leukocyte transmigration by providing leukocyte attachment to the vascular endothelium, matrix metalloproteinases (MMPs) are important for facilitating leukocyte movement across vascular barriers. Among different MMPs, MMP-9, an inducible gelatinase expressed by leukocytes during hepatic IRI, is emerging as an important mediator of leukocyte traffic to inflamed liver.
Summary
It is generally accepted that the understanding of the molecular mechanisms involved in leukocyte recruitment will lead to the development of novel targeted therapeutic approaches for hepatic IRI and liver transplantation. Here, we review mechanisms of leukocyte traffic in liver IRI and the role of some of the proteins that are thought to be important for this process.
Keywords: Matrix metalloproteinases, adhesion molecules, fibronectin, liver ischemia and reperfusion injury
INTRODUCTION
Hepatic ischemia reperfusion injury (IRI) is a pathophysiological process in which the hypoxic insult is further accentuated by restoration of blood flow to the compromised organ. Hepatic IRI occurs in all transplanted livers, in trauma, shock, and in elective surgery where blood supply to liver is temporary interrupted. In human orthotopic liver transplantation (OLT), IRI is a major determinant of postoperative allograft dysfunction and morbidity as it causes up to 10% of early transplant failures, and increases the risk of acute and chronic rejections [1–3]. Furthermore, liver IRI limits the supply of organs available for transplantation [4;5*].
The mechanisms of ischemic damage in the liver associated with leukocyte adhesion/migration and with release of cytokines/free radicals play a central role in post-IR organ injury. They lead to a decline in liver function and, potentially, to an increase in organ immunogenicity, which may result in graft loss [2;6]. While the deleterious effects of cytokines and reactive oxygen species (ROS) released by leukocytes have been fairly documented, the mechanisms of leukocyte recruitment to sites of inflammation in the liver are far from being understood.
Overall, the process of leukocyte recruitment across endothelial and extracellular matrix (ECM) barriers involves complex cascades of adhesive and focal matrix degradation events leading to leukocyte tethering and rolling, firm adhesion and, finally, transmigration from the vasculature [7]. Liver is a venous driven vascular bed with slow flow rates and the recruitment of leukocytes to inflamed liver may require distinct adhesive and deadhesive mechanisms as compared with other organs with higher flow rates.
SELECTINS
The selectins (E-, L-, and P- selectin) are a family of glycoproteins that mediate low-affinity endothelial-leukocyte interactions, thus promoting the tethering and rolling of leukocytes through interactions with specific carbohydrate residues [8;9]. The three members of the selectin family share a similar structure containing an N-terminal lectin-like domain, an EGF-like domain, a variable number of consensus repeats, a single transmembrane domain, and a short cytoplasmic tail [10].
L-selectin (CD62L) is constitutively expressed on many leukocytes [11]. It regulates lymphocyte homing into the peripheral lymph nodes through interactions with peripheral lymph node addressins (PNAds), which are constitutively expressed on high endothelial venules (HEV) and induced in different chronically inflamed venules [12*]. P-and E- selectins, on the other hand, are mostly inducibly expressed in both acutely and chronically stimulated endothelium; P-selectin is also present on platelets [13;14]. The expression of these molecules on the vascular endothelium is induced upon exposure to a variety of proinflammatory stimuli; among the factors that induce selectin expression by endothelial cells are shear stress, several cytokines, and complement activation products [15;16]. Leukocytes are considered to first tether to and roll on P- and E-selectins expressed on activated endothelial cells [14]. Their roll on P- and E-selectins is mediated through interactions with P-selectin glycoprotein ligand-1 (PSGL-1), and other carbohydrate ligands, expressed on various leukocyte subsets [14;17–19*]. P- and E-selectins have been shown to play important roles in leukocyte recruitment in a variety of pathological conditions [20–22]; however, their role on leukocyte recruitment in liver IRI remains not fully understood. While a number of reports show that P-selectin blockade is beneficial in liver IRI [23–25], others minimize its role in the recruitment of leukocytes to sites of inflammation in liver [26–28]. In this regard, studies performed in mice deficient in P-selectin, or in both P- and E-selectins, demonstrated a minimal role for selectins in leukocyte recruitment into the inflamed liver microvasculature [27]. A selectin/rolling dependent leukocyte recruitment may not be necessary in the low shear conditions that prevail in the hepatic microvascular bed [29;30*]. Thus, leukocytes moving slowly through the narrow sinusoids may be able to interact directly with other adhesion molecules early expressed on the endothelium without the need of an initial selectin-mediated arrest. In view of the observations that steatosis further decreases sinusoidal blood flow by approximately 50% [31;32], this concept is perhaps even more relevant for marginal fatty livers, which are highly susceptible to hepatic IRI [33]. While there is an indication that selectin blockade have a beneficial role in liver IRI, future studies are still needed to further explain the apparent minimal contribution of selectins to leukocyte recruitment after inflammatory stimulation in liver.
CHEMOKINES
Chemokines are a large family of mostly 8- to 12-kDa proteins, which are essential in regulating directional leukocyte traffic [34]. They can be subdivided in four families according to the position of their cysteine residues (reviewed in Charo et al. [35]. The majority of chemokines belong to the CC (CCL1–28) and CXC (CXCL1-16) subfamilies. The C and CX3C subfamilies have only 2 members (XCL1 and XCL2) and 1 member (CX3CL1), respectively [36]. Exposure of leukocytes to chemokines released by inflamed tissues is particularly important for the activation of leukocyte integrins [37], which are key mediators of leukocyte firm adhesion to the vascular endothelium. There are a growing number of reports supporting a role for CC and CXC chemokines in the liver pathophysiology [38*]. Several CC chemokines have been shown to be major attractants for T cells, B cells and monocytes [35]. CCL2 [also known as monocyte chemotactic protein-1 (MCP-1)] is one of the best characterized chemokines in liver; CCL2 is a ligand for the receptor CCR2 and is secreted by several cells including hepatocytes, Kupffer cells and hepatic stellate cells (HSCs) [39]. CCL2 is highly expressed in livers after toxic or biliary injury and it has been shown that mice with a targeted deletion of the CCR2 gene develop reduced levels of hepatic fibrosis [40]. CCL2 expression has been detected in relatively high levels in hepatic IRI [41]. CXCL-1 [also known as keratinocyte-derived chemokine (KC)] and CXCL-2 [also known as macrophage inflammatory protein-2 (MIP-2)] are considered to be potent neutrophil chemoattractants in liver IRI [42;43]. The murine chemokines CXCL-1 and CXCL-2 bind to the chemokine receptor CXCR2 and are the functional murine homologues of the human IL-8 [44;45]. In our own studies in liver IRI, we found a modest correlation between neutrophil infiltration and the expression of the CXCL-2 chemokine [46], which is primarily induced by TNF-α [47]. The CXCL9 and CXCL10 chemokines are believed to mediate the infiltration of virus-specific T cells in liver [48] and their receptor CXCR3 has been detected on liver infiltrating leukocytes [49]. The CXCL16 chemokine is able to support lymphocyte adhesion by inducing conformational activation of β1 integrins, and its receptor CXCR6 is expressed on liver infiltrating lymphocytes [50]. Moreover, a recent study has provided evidence that CXCL16 may be involved in the recruitment of inflammatory cells in cholestatic liver disease [51*]. During the last several years, chemokines have emerged as important mediators in liver diseases; however, there is still much to be learned about the complexity of chemokine networks involved in leukocyte traffic in liver IRI.
INTEGRINS AND THEIR LIGANDS
It is well accepted that leukocytes have to acquire strong adhesion interactions to the vessel wall to migrate across the vascular endothelium [52]. The firm adhesion of leukocytes to the endothelium is mediated primarily by integrins. Integrins are αβ transmembrane adhesion receptors that mediate cell-cell and cell-ECM adhesion [53]. Each integrin contains one α and one β–subunit, and the 24 integrins identified in mammals are formed from combinations of 18 α-subunits and 8 β-subunits [54*]. The most characterized integrins expressed on leukocytes belong to the β1 and β2 integrin families. Of the β1 integrins, α4 (CD49d) has a central role; it interacts with the connecting segment-1 (CS-1), which is located within the V region of fibronectin (FN) [55], and with a recently described segment, PEDGIHELFP, located in the EIIIA fibronectin splicing domain [56]. In addition, it also binds to the endothelial Vascular Cell Adhesion Molecule-1 (VCAM-1: QIDSPL), recognizing yet a different sequence [57]. The α4β1 integrin, in the absence of the α5β1 integrin, is also able to interact with the RGD sequence, which is present in the cell adhesion domain of FN [55]. Cellular FN is a key ECM protein expressed by sinusoidal endothelial cells very early after liver injury [58], and its vascular expression precedes leukocyte recruitment in hepatic IRI [59]. In addition to its widely reported role on leukocyte adhesion and migration [60], FN is capable of mediating platelet adhesion [61], and may contribute to complement activation [62;63]. VCAM-1, the other ligand for the α4β1 integrin, is mostly detected on large-vessel endothelial cells after liver IRI. α4 integrin is particularly interesting because of its ability to support both leukocyte rolling and adhesion [64]. Clinical trials documented that a humanized α4-integrin antibody have been effective in inflammatory conditions such as multiple sclerosis (MS) [65], and inflammatory bowel disease [66]. Interestingly, it has been recently shown that the anti-α4 integrin antibody infused into MS patients reduced the ex-vivo adhesion of their leukocytes to activated human brain endothelial cells under flow conditions [67*]. Moreover, additional experiments blocking CS1 FN and VCAM-1 interactions, showed that the ligand of α4 integrin on the activated endothelial cells was FN and not VCAM-1 [67]. Our studies in rats also support an important role for the α4β1-CS1 FN interactions in leukocyte recruitment after hepatic IRI [59;68]. Others, using human HSEC and flow-based adhesion in vitro assays, have shown that lymphocyte adhesion to hepatic sinusoids can also be inhibited by blocking VCAM-1 [69]. The β2 integrin family is considered to play a role in neutrophil extravasation from the hepatic microcirculation into the parenchyma [70]. Intercellular adhesion molecule-1 (ICAM-1), a major endothelial-cell ligand for β2 integrins, is constitutively expressed on the liver vascular endothelium. However, hepatic IRI has been shown to be only moderately or not at all improved by anti-ICAM therapies [71]. Observations that leukocyte recruitment within the hepatic sinusoids is likely selectin-independent [30] have further attracted attention to integrins. However, there is still much to be unveiled about the role of integrins and their ligands in hepatic IRI.
MATRIX METALLOPROTEINASES
Leukocyte transmigration across endothelial and ECM barriers is dependent on the expression cell-activating chemokines, adhesive events, as well as on focal matrix degradation mechanisms. Interactions between ECM components and cell adhesion receptors regulate leukocyte functions; therefore, enzymatic degradation of ECM can alter leukocyte behaviors [72]. Leukocyte migration across ECM proteins is dependent on matrix degradation not only for facilitating “matrix permeability”, but also for generating ECM-derived fragments, which are biologically active and can be highly chemotactic for leukocytes [73]. The matrix metalloproteinases (MMPs) are a family of >24 specialized zinc-dependent proteases that play key roles in the responses of cells to their microenvironment [74–76*]. It is generally accepted that while MMP-facilitated degradation of ECM proteins is essential in physiological processes, such as remodeling and tissue repair, MMP inappropriate, prolonged, or overexpression has harmful consequences.
Among the different MMPs, a specific subset, the gelatinases, MMP-2 and MMP-9 (also known as gelatinase A and B or 72-kDa and 92-kDa type IV collagenases, respectively) are of particular interest. MMP-2 and MMP-9 are activated in damaged livers, and are thought to play a key role in liver injury [77]. MMP-9 is an inducible gelatinase expressed mostly by leukocytes, whereas MMP-2 is generally expressed constitutively. MMP-2 is thought to be derived largely from stromal cells, and not usually expressed by leukocytes [78]. These MMPs are characterized by the presence of fibronectin-like domain of three type II repeats, which facilitate enzyme binding to ECM substrates [79]. Gelatinases are responsible for the turnover and degradation of several ECM proteins, including FN and type IV collagen, the major component of basement membranes [79;80]. Indeed, MMP-9 expression has been linked to several pathological conditions that require disruption of the basement membrane, such as tumor invasion [81], inflammation [82], arthritis [83], multiple sclerosis [84], systemic lupus erythematosus [85], cerebral IRI [86], and traumatic brain injury [87].
In human orthotopic liver transplantation, MMP-9 has been detected in the serum of patients after surgery [88–90]; MMP-9 serum levels were found to be significantly increased in a few minutes after reperfusion [89] and remained elevated for several days after transplantation [88]. In rat livers, MMP-9 has been shown by our group to be upregulated after 6h following OLT [68], and by others after 3h of IRI [91]. Further studies from our laboratory have shown that MMP-9 is a critical mediator of leukocyte migration in liver IRI [92*]. MMP-9 deficiency and specific anti-MMP-9 antibody therapy clearly depressed the infiltration of CD4, Ly-6G, and Mac-1 leukocytes in periportal areas after IRI, significantly ameliorating hepatic IRI. A beneficial effect for MMP-9 inhibition has also been recently shown in a distinct model of small-for-size liver graft IRI [93*].
There is a growing body of evidence that, despite overlapping activities, MMP-2 and MMP-9 may have distinct biological functions [94]. For example, it has been demonstrated that MMP-2 and MMP-9 regulate platelet aggregation in opposite ways [95]. Moreover, while MMP-2−/− mice develop exacerbated experimentally-induced arthritis and acute colitis, MMP-9−/− mice show significantly reduced signs of these diseases [96;97]. The substrate specificities of MMP-2 and MMP-9 are similar but not identical [98], and this may account, in part, for the different roles that these gelatinases may have. In this regard, it has been shown that whereas MMP-9 is not able of cleaving monocyte chemoattractant protein-3 (MCP-3), MMP-2 is, and that the cleaved molecule acts as a general chemokine antagonist depressing inflammation [99]. Moreover, the same MMP can have opposing effects based upon the cell type in which is expressed [100].
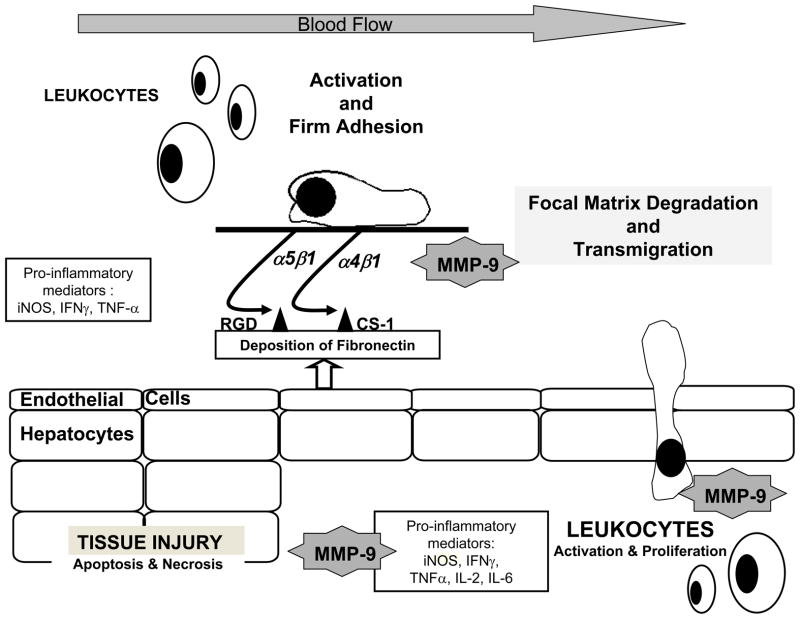
The regulation of MMP activity is complex and it takes place at transcriptional, post-transcriptional, and protein levels [72]. Tissue inhibitors of metalloproteinases (TIMPs) regulate the proteolytic activity of MMPs. There are at least four identified members (TIMP 1–4) in the TIMP family, which vary in tissue specific expression and in their ability to inhibit various MMPs [81]. TIMP-1, which inhibits MMP-9 with high affinity, has been detected in the serum of OLT patients [89]. Our unpublished studies (Duarte & Coito) suggest that TIMP-1 inhibition leads to increased levels of MMP-9 activity and leukocyte recruitment after hepatic IRI. There is a growing body of evidence supporting the view that cell attachment to ECM proteins and subsequent degradation are related events. Indeed, studies from our laboratory have shown that fibronectin interactions with its two α4β1 and α5β1 integrin receptors, expressed on leukocytes, are capable of regulating MMP-9 expression by leukocytes in hepatic IRI [68;101*]. Others have demonstrated that MMP-9 activation involves nitric oxide (NO)-mediated metalloproteinase S-nitrosylation S [86]. In liver IRI, the inability of nitric oxide synthase (iNOS) deficient mice to generate iNOS-derived NO profoundly inhibited MMP-9 activity as well as the recruitment of leukocytes [102*]. In addition, we also found that pro-inflammatory cytokines such as IFN-γ and IL-6 are capable of regulating MMP-9 activity by cultured neutrophils [102]. It is important to note that the ECM proteolysis mediated by metalloproteinases may not only facilitate leukocyte migration, but may also lead to detachment of liver cells resulting in apoptosis, a phenomenon called “anoikis” [103]. Indeed, MMP-9−/− deficient livers demonstrated significantly decreased numbers of hepatocytes undergoing apoptosis as compared with respective MMP-9 +/+ controls after IRI [102]. Thus, it is reasonable to postulate that MMP-9+ leukocytes infiltrating livers after IRI can cause parenchyma cell detachment from ECM and, consequently promote apoptosis/anoikis of these cells. Figure 1 illustrates the concept of a central role for MMP-9 in hepatic IRI [68;92;101;102].
Figure 1. Schematic representation of the role of MMP-9 in hepatic IRI.
Interactions between activated α4β1 and α5β1 integrins expressed on leukocytes and cellular fibronectin, newly synthesized by endothelial cells after injury, favor the induction of MMP-9 expression by leukocytes. Cytokines and other pro-inflammatory factors, such as iNOS-derived NO produced during the acute phase of IRI, can also mediate MMP-9 activation. MMP-9 assisted focal matrix degradation facilitates leukocyte transmigration into the liver. In addition, MMP-9+ leukocytes infiltrating livers after IRI can cause parenchyma cell detachment from ECM and, consequently promote apoptosis/anoikis of these cells leading to tissue injury.
Taken together, these studies emphasize the need for further exploring the individual functions of MMPs to support the development of potential therapeutic approaches to treat successfully liver IRI and other inflammatory diseases.
Conclusions
This review focuses primarily on the transmigration of leukocytes across the vascular endothelium and ECM barriers during liver IRI, and on the proteins that are thought to be important for this process. The understanding of leukocyte migration mechanisms remains a major challenge for the development of targeted therapies to treat pathological conditions that require modulation of immune responses [104–106]. While studies continue unraveling the complexity of mechanisms potentially involved in leukocyte traffic in hepatic IRI, recent developments support an important role for leukocyte-expressed MMP-9 as a key mediator of leukocyte transmigration and activation leading to liver injury.
Acknowledgments
Work in our laboratory is supported by National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID) grant R01AI057832.
Abbreviations
- CS-1
connecting segment-1
- ECM
extracellular matrix
- FN
fibronectin
- IRI
ischemia/reperfusion injury
- KO
knockout
- MMP
matrix metalloproteinase
- OLT
orthotopic liver transplantation
- ROS
reactive oxygen species
- TIMP
tissue inhibitor of metalloproteinases
Reference List
- 1.Howard TK, Klintmalm GB, Cofer JB, Husberg BS, Goldstein RM, Gonwa TA. The influence of preservation injury on rejection in the hepatic transplant recipient. Transplantation. 1990;49:103–107. doi: 10.1097/00007890-199001000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Strasberg SM, Howard TK, Molmenti EP, Hertl M. Selecting the donor liver. risk factors for poor function after orthotopic liver transplantation. Hepatology. 1994;20:829–838. doi: 10.1002/hep.1840200410. [DOI] [PubMed] [Google Scholar]
- 3.Henderson JM. Liver transplantation and rejection. an overview. Hepatogastroenterology. 1999;46 (Suppl 2):1482–1484. [PubMed] [Google Scholar]
- 4.Nocito A, El Badry AM, Clavien PA. When is steatosis too much for transplantation? J Hepatol. 2006;45:494–499. doi: 10.1016/j.jhep.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 5*.de Rougemont O, Dutkowski P, Clavien PA. Biological modulation of liver ischemia-reperfusion injury. Curr Opin Organ Transplant. 2010;15:183–189. doi: 10.1097/MOT.0b013e3283373ced. A valuable overview of important factors in hepatic IRI. [DOI] [PubMed] [Google Scholar]
- 6.Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169–173. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 7.Basbaum CB, Werb Z. Focalized proteolysis: spatial and temporal regulation of extracellular matrix degradation at the cell surface. Curr Opin Cell Biol. 1996;8:731–738. doi: 10.1016/s0955-0674(96)80116-5. [DOI] [PubMed] [Google Scholar]
- 8.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 9.Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 10.Huang K-S, Graves BJ, Wolitzky BA. The selectins: initiators of leukocyte endothelial adhesion. Harwood Academic Publishers; 1997. pp. 1–30. [Google Scholar]
- 11.Grailer JJ, Kodera M, Steeber DA. L-selectin: role in regulating homeostasis and cutaneous inflammation. J Dermatol Sci. 2009;56:141–147. doi: 10.1016/j.jdermsci.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Sperandio M, Gleissner CA, Ley K. Glycosylation in immune cell trafficking. Immunol Rev. 2009;230:97–113. doi: 10.1111/j.1600-065X.2009.00795.x. This article discusses the role of glycosylation in leukocyte traffic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tedder TF, Steeber DA, Chen A, Engel P. The selectins: vascular adhesion molecules. FASEB J. 1995;9:866–873. [PubMed] [Google Scholar]
- 14.McEver RP. Selectins: lectins that initiate cell adhesion under flow. Curr Opin Cell Biol. 2002;14:581–586. doi: 10.1016/s0955-0674(02)00367-8. [DOI] [PubMed] [Google Scholar]
- 15.Witz IP. The selectin-selectin ligand axis in tumor progression. Cancer Metastasis Rev. 2008;27:19–30. doi: 10.1007/s10555-007-9101-z. [DOI] [PubMed] [Google Scholar]
- 16.Atkinson C, Zhu H, Qiao F, Varela JC, Yu J, Song H, Kindy MS, Tomlinson S. Complement-dependent P-selectin expression and injury following ischemic stroke. J Immunol. 2006;177:7266–7274. doi: 10.4049/jimmunol.177.10.7266. [DOI] [PubMed] [Google Scholar]
- 17.Xia L, Sperandio M, Yago T, McDaniel JM, Cummings RD, Pearson-White S, Ley K, McEver RP. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J Clin Invest. 2002;109:939–950. doi: 10.1172/JCI14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hidalgo A, Peired AJ, Wild MK, Vestweber D, Frenette PS. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26:477–489. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yago T, Shao B, Miner JJ, Yao L, Klopocki AG, Maeda K, Coggeshall KM, McEver RP. E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin alphaLbeta2-mediated slow leukocyte rolling. Blood. 2010;116:485–494. doi: 10.1182/blood-2009-12-259556. This study supports a role for E-selectin on the induction of LFA-1-dependent slow leukocyte rolling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel KD, Cuvelier SL, Wiehler S. Selectins: critical mediators of leukocyte recruitment. Semin Immunol. 2002;14:73–81. doi: 10.1006/smim.2001.0344. [DOI] [PubMed] [Google Scholar]
- 21.Cai YH, Alvarez A, Alcaide P, Duramad P, Lim YC, Jarolim P, Lowe JB, Luscinskas FW, Lichtman AH. Abrogation of functional selectin-ligand expression reduces migration of pathogenic CD8+ T cells into heart. J Immunol. 2006;176:6568–6575. doi: 10.4049/jimmunol.176.11.6568. [DOI] [PubMed] [Google Scholar]
- 22.Lange-Sperandio B, Cachat F, Thornhill BA, Chevalier RL. Selectins mediate macrophage infiltration in obstructive nephropathy in newborn mice. Kidney Int. 2002;61:516–524. doi: 10.1046/j.1523-1755.2002.00162.x. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Criado FJ, Toledo-Pereyra LH, Lopez-Neblina F, Phillips ML, Paez-Rollys A, Misawa K. Role of P-selectin in total hepatic ischemia and reperfusion. J AmColl Surg. 1995;181:327–334. [PubMed] [Google Scholar]
- 24.Dulkanchainun TS, Goss JA, Imagawa DK, Shaw GD, Anselmo DM, Kaldas F, Wang T, Zhao D, Busuttil AA, Kato H, Murray NG, Kupiec-Weglinski JW, Busuttil RW. Reduction of hepatic ischemia/reperfusion injury by a soluble P-selectin glycoprotein ligand-1. Ann Surg. 1998;227:832–840. doi: 10.1097/00000658-199806000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuchihashi S, Fondevila C, Shaw GD, Lorenz M, Marquette K, Benard S, Shen XD, Ke B, Busuttil RW, Kupiec-Weglinski JW. Molecular characterization of rat leukocyte P-selectin glycoprotein ligand-1 and effect of its blockade: protection from ischemia-reperfusion injury in liver transplantation. J Immunol. 2006;176:616–624. doi: 10.4049/jimmunol.176.1.616. [DOI] [PubMed] [Google Scholar]
- 26.Young CS, Palma JM, Mosher BD, Harkema J, Naylor DF, Dean RE, Crockett E. Hepatic ischemia/reperfusion injury in P-selectin and intercellular adhesion molecule-1 double-mutant mice. Am Surg. 2001;67:737–744. [PubMed] [Google Scholar]
- 27.Wong J, Johnston B, Lee SS, Bullard DC, Smith CW, Beaudet AL, Kubes P. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J Clin Invest. 1997;99:2782–2790. doi: 10.1172/JCI119468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyllie S, Barshes NR, Gao FQ, Karpen SJ, Goss JA. Failure of P-selectin blockade alone to protect the liver from ischemia-reperfusion injury in the isolated blood-perfused rat liver. World J Gastroenterol. 2008;14:6808–6816. doi: 10.3748/wjg.14.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaboury JP, Kubes P. Reductions in physiologic shear rates lead to CD11/CD18-dependent, selectin-independent leukocyte rolling in vivo. Blood. 1994;83:345–350. [PubMed] [Google Scholar]
- 30*.Lee WY, Kubes P. Leukocyte adhesion in the liver: distinct adhesion paradigm from other organs. JHepatol. 2008;48:504–512. doi: 10.1016/j.jhep.2007.12.005. This article discusses data supporting the concept that “ selectins are not essential for recruitment” in liver. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi M, Tokunaga Y, Fujita T, Tanaka K, Yamaoka Y, Ozawa K. The effects of cold preservation on steatotic graft viability in rat liver transplantation. Transplantation. 1993;56:282–287. doi: 10.1097/00007890-199308000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Gao W, Connor HD, Lemasters JJ, Mason RP, Thurman RG. Primary nonfunction of fatty livers produced by alcohol is associated with a new, antioxidant-insensitive free radical species. Transplantation. 1995;59:674–679. doi: 10.1097/00007890-199503150-00005. [DOI] [PubMed] [Google Scholar]
- 33.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–113. doi: 10.1055/s-2001-12933. [DOI] [PubMed] [Google Scholar]
- 34.Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25:75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 36.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luissint AC, Lutz PG, Calderwood DA, Couraud PO, Bourdoulous S. JAM-L-mediated leukocyte adhesion to endothelial cells is regulated in cis by alpha4beta1 integrin activation. J Cell Biol. 2008;183:1159–1173. doi: 10.1083/jcb.200805061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Oo YH, Adams DH. The role of chemokines in the recruitment of lymphocytes to the liver. J Autoimmun. 2010;34:45–54. doi: 10.1016/j.jaut.2009.07.011. This article summarizes major developments on the roles of chemokines in the recruitment of lymphocytes to the liver. [DOI] [PubMed] [Google Scholar]
- 39.Wasmuth HE, Tacke F, Trautwein C. Chemokines in liver inflammation and fibrosis. Semin Liver Dis. 2010;30:215–225. doi: 10.1055/s-0030-1255351. [DOI] [PubMed] [Google Scholar]
- 40.Seki E, De Minicis S, Inokuchi S, Taura K, Miyai K, van Rooijen N, Schwabe RF, Brenner DA. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50:185–197. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bamboat ZM, Balachandran VP, Ocuin LM, Obaid H, Plitas G, DeMatteo RP. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology. 2010;51:621–632. doi: 10.1002/hep.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC. Hepatology. 1998;27:1172–1177. doi: 10.1002/hep.510270440. [DOI] [PubMed] [Google Scholar]
- 43.Mosher B, Dean R, Harkema J, Remick D, Palma J, Crockett E. Inhibition of Kupffer cells reduced CXC chemokine production and liver injury. J Surg Res. 2001;99:201–210. doi: 10.1006/jsre.2001.6217. [DOI] [PubMed] [Google Scholar]
- 44.Mukaida N, Hishinuma A, Zachariae CO, Oppenheim JJ, Matsushima K. Regulation of human interleukin 8 gene expression and binding of several other members of the intercrine family to receptors for interleukin-8. Adv Exp Med Biol. 1991;305:31–38. doi: 10.1007/978-1-4684-6009-4_4. [DOI] [PubMed] [Google Scholar]
- 45.Kockritz-Blickwede M, Rohde M, Oehmcke S, Miller LS, Cheung AL, Herwald H, Foster S, Medina E. Immunological mechanisms underlying the genetic predisposition to severe Staphylococcus aureus infection in the mouse model. Am J Pathol. 2008;173:1657–1668. doi: 10.2353/ajpath.2008.080337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamada T, Tsuchihashi S, Avanesyan A, Duarte S, Moore C, Busuttil RW, Coito AJ. Cyclooxygenase-2 deficiency enhances th2 immune responses and impairs neutrophil recruitment in hepatic ischemia/reperfusion injury. J Immunol. 2008;180:1843–1853. doi: 10.4049/jimmunol.180.3.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tessier PA, Naccache PH, Clark-Lewis I, Gladue RP, Neote KS, McColl SR. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J Immunol. 1997;159:3595–3602. [PubMed] [Google Scholar]
- 48.Hokeness KL, Deweerd ES, Munks MW, Lewis CA, Gladue RP, Salazar-Mather TP. CXCR3-dependent recruitment of antigen-specific T lymphocytes to the liver during murine cytomegalovirus infection. J Virol. 2007;81:1241–1250. doi: 10.1128/JVI.01937-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heydtmann M, Adams DH. Chemokines in the immunopathogenesis of hepatitis C infection. Hepatology. 2009;49:676–688. doi: 10.1002/hep.22763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heydtmann M, Lalor PF, Eksteen JA, Hubscher SG, Briskin M, Adams DH. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J Immunol. 2005;174:1055–1062. doi: 10.4049/jimmunol.174.2.1055. [DOI] [PubMed] [Google Scholar]
- 51*.Omenetti A, Syn WK, Jung Y, Francis H, Porrello A, Witek RP, Choi SS, Yang L, Mayo MJ, Gershwin ME, Alpini G, Diehl AM. Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production. Hepatology. 2009;50:518–527. doi: 10.1002/hep.23019. This study shows a role for the CXCL16 chemokine on leukocyte recruitment in cholestatic liver disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diamond MS, Springer TA. The dynamic regulation of integrin adhesiveness. Curr Biol. 1994;4:506–517. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 53.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 54*.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. This article provides new insides on integrin structure and activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moyano JV, Carnemolla B, Dominguez-Jimenez C, Garcia-Gila M, Albar JP, Sanchez-Aparicio P, Leprini A, Querze G, Zardi L, Garcia-Pardo A. Fibronectin type III5 repeat contains a novel cell adhesion sequence, KLDAPT, which binds activated alpha4beta1 and alpha4beta7 integrins. J Biol Chem. 1997;272:24832–24836. doi: 10.1074/jbc.272.40.24832. [DOI] [PubMed] [Google Scholar]
- 56.Liao YF, Gotwals PJ, Koteliansky VE, Sheppard D, Van De WL. The EIIIA segment of fibronectin is a ligand for integrins alpha 9beta 1 and alpha 4beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J Biol Chem. 2002;277:14467–14474. doi: 10.1074/jbc.M201100200. [DOI] [PubMed] [Google Scholar]
- 57.Osborn L, Vassallo C, Browning BG, Tizard R, Haskard DO, Benjamin CD, Dougas I, Kirchhausen T. Arrangement of domains, and amino acid residues required for binding of vascular cell adhesion molecule-1 to its counter-receptor VLA-4 (alpha 4 beta 1) J Cell Biol. 1994;124:601–608. doi: 10.1083/jcb.124.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127:2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amersi F, Shen XD, Moore C, Melinek J, Busuttil RW, Kupiec-Weglinski JW, Coito AJ. Fibronectin-alpha 4 beta 1 integrin-mediated blockade protects genetically fat Zucker rat livers from ischemia/reperfusion injury. Am J Pathol. 2003;162:1229–1239. doi: 10.1016/s0002-9440(10)63919-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coito AJ, de Sousa M, Kupiec-Weglinski JW. Fibronectin in immune responses in organ transplant recipients. Dev Immunol. 2000;7:239–248. doi: 10.1155/2000/98187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maurer LM, Tomasini-Johansson BR, Mosher DF. Emerging roles of fibronectin in thrombosis. Thromb Res. 2010;125:287–291. doi: 10.1016/j.thromres.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hindmarsh EJ, Marks RM. Complement activation occurs on subendothelial extracellular matrix in vitro and is initiated by retraction or removal of overlying endothelial cells. J Immunol. 1998;160:6128–6136. [PubMed] [Google Scholar]
- 63.Sjoberg AP, Trouw LA, Blom AM. Complement activation and inhibition: a delicate balance. Trends Immunol. 2009;30:83–90. doi: 10.1016/j.it.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 64.Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC. alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 65.O'Connor P. Natalizumab and the role of alpha 4-integrin antagonism in the treatment of multiple sclerosis. Expert Opin Biol Ther. 2007;7:123–136. doi: 10.1517/14712598.7.1.123. [DOI] [PubMed] [Google Scholar]
- 66.Fiorino G, Correale C, Fries W, Repici A, Malesci A, Danese S. Leukocyte traffic control: a novel therapeutic strategy for inflammatory bowel disease. Expert Rev Clin Immunol. 2010;6:567–572. doi: 10.1586/eci.10.40. [DOI] [PubMed] [Google Scholar]
- 67*.Man S, Tucky B, Bagheri N, Li X, Kochar R, Ransohoff RM. alpha4 Integrin/FN-CS1 mediated leukocyte adhesion to brain microvascular endothelial cells under flow conditions. J Neuroimmunol. 2009;210:92–99. doi: 10.1016/j.jneuroim.2009.03.008. This study shows that α4-FN, and not α4-VCAM-1, interactions mediate adhesion to brain endothelial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moore C, Shen XD, Gao F, Busuttil RW, Coito AJ. Fibronectin-{alpha}4{beta}1 Integrin Interactions Regulate Metalloproteinase-9 Expression in Steatotic Liver Ischemia and Reperfusion Injury. Am J Pathol. 2007;170:567–577. doi: 10.2353/ajpath.2007.060456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lalor PF, Edwards S, McNab G, Salmi M, Jalkanen S, Adams DH. Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. J Immunol. 2002;169:983–992. doi: 10.4049/jimmunol.169.2.983. [DOI] [PubMed] [Google Scholar]
- 70.Jaeschke H, Hasegawa T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006;26:912–919. doi: 10.1111/j.1478-3231.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- 71.Casillas-Ramirez A, Mosbah IB, Ramalho F, Rosello-Catafau J, Peralta C. Past and future approaches to ischemia-reperfusion lesion associated with liver transplantation. Life Sci. 2006;79:1881–1894. doi: 10.1016/j.lfs.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 72.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lohr KM, Kurth CA, Xie DL, Seyer JM, Homandberg GA. The amino-terminal 29- and 72-Kd fragments of fibronectin mediate selective monocyte recruitment. Blood. 1990;76:2117–2124. [PubMed] [Google Scholar]
- 74.Lee MH, Murphy G. Matrix metalloproteinases at a glance. J Cell Sci. 2004;117:4015–4016. doi: 10.1242/jcs.01223. [DOI] [PubMed] [Google Scholar]
- 75.Woessner JF., Jr MMPs and TIMPs--an historical perspective. Mol Biotechnol. 2002;22:33–49. doi: 10.1385/MB:22:1:033. [DOI] [PubMed] [Google Scholar]
- 76*.Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): Positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010 doi: 10.1016/j.semcancer.2010.05.002. This article summarizes major developments on the roles of MMPs and TIMPs in cancer cell adhesion and tumor progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245–G249. doi: 10.1152/ajpgi.2000.279.2.G245. [DOI] [PubMed] [Google Scholar]
- 78.Opdenakker G, Masure S, Grillet B, Van Damme J. Cytokine-mediated regulation of human leukocyte gelatinases and role in arthritis. Lymphokine Cytokine Res. 1991;10:317–324. [PubMed] [Google Scholar]
- 79.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 80.Yoshizaki T, Sato H, Furukawa M, Pagano JS. The expression of matrix metalloproteinase 9 is enhanced by Epstein-Barr virus latent membrane protein 1. Proc Natl Acad Sci USA. 1998;95:3621–3626. doi: 10.1073/pnas.95.7.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 82.Ram M, Sherer Y, Shoenfeld Y. Matrix metalloproteinase-9 and autoimmune diseases. J Clin Immunol. 2006;26:299–307. doi: 10.1007/s10875-006-9022-6. [DOI] [PubMed] [Google Scholar]
- 83.Tchetverikov I, Lard LR, DeGroot J, Verzijl N, TeKoppele JM, Breedveld FC, Huizinga TW, Hanemaaijer R. Matrix metalloproteinases-3, -8, -9 as markers of disease activity and joint damage progression in early rheumatoid arthritis. Ann Rheum Dis. 2003;62:1094–1099. doi: 10.1136/ard.62.11.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leppert D, Ford J, Stabler G, Grygar C, Lienert C, Huber S, Miller KM, Hauser SL, Kappos L. Matrix metalloproteinase-9 (gelatinase B) is selectively elevated in CSF during relapses and stable phases of multiple sclerosis. Brain. 1998;121 ( Pt 12):2327–2334. doi: 10.1093/brain/121.12.2327. [DOI] [PubMed] [Google Scholar]
- 85.Faber-Elmann A, Sthoeger Z, Tcherniack A, Dayan M, Mozes E. Activity of matrix metalloproteinase-9 is elevated in sera of patients with systemic lupus erythematosus. Clin Exp Immunol. 2002;127:393–398. doi: 10.1046/j.1365-2249.2002.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 87.Rylski M, Amborska R, Zybura K, Mioduszewska B, Michaluk P, Jaworski J, Kaczmarek L. Yin Yang 1 is a critical repressor of matrix metalloproteinase-9 expression in brain neurons. JBiol Chem. 2008;283:35140–35153. doi: 10.1074/jbc.M804540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuyvenhoven JP, Ringers J, Verspaget HW, Lamers CB, van Hoek B. Serum matrix metalloproteinase MMP-2 and MMP-9 in the late phase of ischemia and reperfusion injury in human orthotopic liver transplantation. Transplant Proc. 2003;35:2967–2969. doi: 10.1016/j.transproceed.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 89.Kuyvenhoven JP, Molenaar IQ, Verspaget HW, Veldman MG, Palareti G, Legnani C, Moolenburgh SE, Terpstra OT, Lamers CB, van Hoek B, Porte RJ. Plasma MMP-2 and MMP-9 and their inhibitors TIMP-1 and TIMP-2 during human orthotopic liver transplantation. The effect of aprotinin and the relation to ischemia/reperfusion injury. Thromb Haemost. 2004;91:506–513. doi: 10.1160/TH03-05-0272. [DOI] [PubMed] [Google Scholar]
- 90.Kuyvenhoven JP, Verspaget HW, Gao Q, Ringers J, Smit VT, Lamers CB, van Hoek B. Assessment of serum matrix metalloproteinases MMP-2 and MMP-9 after human liver transplantation: increased serum MMP-9 level in acute rejection. Transplantation. 2004;77:1646–1652. doi: 10.1097/01.tp.0000131170.67671.75. [DOI] [PubMed] [Google Scholar]
- 91.Cursio R, Mari B, Louis K, Rostagno P, Saint-Paul MC, Giudicelli J, Bottero V, Anglard P, Yiotakis A, Dive V, Gugenheim J, Auberger P. Rat liver injury after normothermic ischemia is prevented by a phosphinic matrix metalloproteinase inhibitor. FASEB J. 2002;16:93–95. doi: 10.1096/fj.01-0279fje. [DOI] [PubMed] [Google Scholar]
- 92*.Hamada T, Fondevila C, Busuttil RW, Coito AJ. Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology. 2008;47:186–198. doi: 10.1002/hep.21922. This study demonstrates that specific MMP-9 inhibition depresses leukocyte recruitment in hepatic IRI. [DOI] [PubMed] [Google Scholar]
- 93*.Ma ZY, Qian JM, Rui XH, Wang FR, Wang QW, Cui YY, Peng ZH. Inhibition of matrix metalloproteinase-9 attenuates acute small-for-size liver graft injury in rats. Am J Transplant. 2010;10:784–795. doi: 10.1111/j.1600-6143.2009.02993.x. This study supports a beneficial role for MMP-9 inhibition in small-for-size graft IRI. [DOI] [PubMed] [Google Scholar]
- 94.Le NT, Xue M, Castelnoble LA, Jackson CJ. The dual personalities of matrix metalloproteinases in inflammation. Front Biosci. 2007;12:1475–1487. doi: 10.2741/2161. [DOI] [PubMed] [Google Scholar]
- 95.Fernandez-Patron C, Martinez-Cuesta MA, Salas E, Sawicki G, Wozniak M, Radomski MW, Davidge ST. Differential regulation of platelet aggregation by matrix metalloproteinases-9 and -2. Thromb Haemost. 1999;82:1730–1735. [PubMed] [Google Scholar]
- 96.Itoh T, Matsuda H, Tanioka M, Kuwabara K, Itohara S, Suzuki R. The role of matrix metalloproteinase-2 and matrix metalloproteinase-9 in antibody-induced arthritis. J Immunol. 2002;169:2643–2647. doi: 10.4049/jimmunol.169.5.2643. [DOI] [PubMed] [Google Scholar]
- 97.Garg P, Rojas M, Ravi A, Bockbrader K, Epstein S, Vijay-Kumar M, Gewirtz AT, Merlin D, Sitaraman SV. Selective ablation of matrix metalloproteinase-2 exacerbates experimental colitis: contrasting role of gelatinases in the pathogenesis of colitis. J Immunol. 2006;177:4103–4112. doi: 10.4049/jimmunol.177.6.4103. [DOI] [PubMed] [Google Scholar]
- 98.Bjorklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755:37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 99.McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202–1206. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- 100.Martin MD, Matrisian LM. The other side of MMPs: protective roles in tumor progression. Cancer Metastasis Rev. 2007;26:717–724. doi: 10.1007/s10555-007-9089-4. [DOI] [PubMed] [Google Scholar]
- 101*.Fondevila C, Shen XD, Duarte S, Busuttil RW, Coito AJ. Cytoprotective effects of a cyclic RGD peptide in steatotic liver cold ischemia and reperfusion injury. Am J Transplant. 2009;9:2240–2250. doi: 10.1111/j.1600-6143.2009.02759.x. This article illustrates a role for FN-leukocyte interactions upon regulation of MMP-9 expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102*.Hamada T, Duarte S, Tsuchihashi S, Busuttil RW, Coito AJ. Inducible Nitric Oxide Synthase Deficiency Impairs Matrix Metalloproteinase-9 Activity and Disrupts Leukocyte Migration in Hepatic Ischemia/Reperfusion Injury. Am J Pathol. 2009 doi: 10.2353/ajpath.2009.080872. This study provides evidence for a novel mechanism by which MMP-9 can mediate iNOS-induced liver I/R injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 104.Mackay CR. Moving targets: cell migration inhibitors as new anti-inflammatory therapies. Nat Immunol. 2008;9:988–998. doi: 10.1038/ni.f.210. [DOI] [PubMed] [Google Scholar]
- 105.Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 106.Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]