Abstract
Polyprenoids, polymers containing varied numbers of isoprene subunits, serve numerous roles in biology. In Eukarya, dolichyl phosphate, a phosphorylated polyprenol bearing a saturated α-end isoprene subunit, serves as the glycan carrier during N-glycosylation, namely that post-translational modification whereby glycans are covalently linked to select asparagine residues of a target protein. As in Eukarya, N-glycosylation in Archaea also relies on phosphorylated dolichol. In this report, LC-ESI/MS/MS was employed to identify a novel dolichyl phosphate (DolP) in the thermoacidophilic archaeon, Sulfolobus acidocaldarius. The unusually short S. acidocaldarius DolP presents a degree of saturation not previously reported. S. acidocaldarius DolP contains not only the saturated α- and ω-end isoprene subunits observed in other archaeal DolPs, but also up to five saturated intra-chain isoprene subunits. The corresponding dolichol and hexose-charged DolP species were also detected. The results of the present study offer valuable information on the biogenesis and potential properties of this unique DolP. Furthermore, elucidation of the mechanism of the α-isoprene unit reduction in S. acidocaldarius dolichol may facilitate the identification of the alternative, as yet unknown polyprenol reductase in Eukarya.
Keywords: Archaea, dolichol, electrospray ionization mass spectrometry, polyprenol, polyprenol reductase, Sulfolobus acidocaldarius
1. INTRODUCTION
Polyprenoids correspond to a group of hydrophobic polymers that comprise up to 100 isoprene subunits linked head-to-tail, with the resulting chain presenting a hydroxy group at the α-end [for review, see 1,2]. Reduction of the double bond in the α-end isoprene subunit gives rise to the dolichols. While polyprenoids serve a variety of biological roles [1, 3–5], phosphorylated polyprenols and dolichols are central components in pathways of N-glycosylation, a post-translational modification involving the covalent attachment of glycans to select asparagine residues of target proteins. Across evolution, asparagine-linked glycans are initially assembled on phosphopolyprenol carriers, namely dolichyl phosphate (DolP)1 in Eukarya and Archaea and polyprenol phosphate (typically undecaprenol phosphate (UndP)) in Bacteria [1,2,6].
The biosynthesis of polyprenoids begins with formation of the building blocks of isoprene-based molecules, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). IPP and DMAPP are generated by either the classic mevalonate (MVA) pathway or by the mevalonate-independent deoxyxylulose phosphate (DOXP) pathway [7–10]. The subsequent union of DMAPP and several IPP subunits produces polyprenoids. These condensation reactions are mediated by prenyltransferases, which are also responsible for determining polyprenol cis/trans stereochemistry. In this manner, all-trans farnesyl (C15) or geranylgeranyl (C20) diphosphate are elongated into cis/trans polyprenol diphosphates.
Despite over 40 years of research and the resulting wealth of information available on polyprenol biosynthesis, many details remain unclear, particularly concerning those steps involved in the conversion of polyprenol diphosphate to DolP. It is generally thought that polyprenol diphosphate undergoes two rounds of dephosphorylation to produce polyprenol. Reduction of the polyprenol α-position isoprene subunit yields dolichol, which is in turn phosphorylated to produce DolP [11]. Most of the enzymes catalyzing these reactions have, however, yet to be identified. Recently, mammalian SRD5A3 and its yeast ortholog, DFG10, were shown to be polyprenol reductases responsible for converting polyprenol into dolichol [12]. Nonetheless, some dolichol is produced in cells lacking the encoding genes, indicating that additional polyprenol reductase(s) must exist. Moreover, those phosphatases responsible for transforming polyprenol diphosphate into polyprenol remain unknown.
The search for as yet unidentified enzymes involved in DolP biogenesis may benefit from the study of Archaea. Archaea are a group of microorganisms that comprise a distinct branch of the tree of life that also includes eukaryal and bacterial limbs [13]. Although their ubiquitous distribution is now clear [14,15], Archaea remain best known as extremophiles, namely organisms able to thrive in some of the most physically challenging environments on Earth, being found at extremes of temperature, pH and salinity and other conditions seemingly inhospitable to life [16]. Possibly in response to their harsh surroundings, Archaea have often adopted unique biochemical strategies. For example, in the archaeal version of the MVA pathway, mevalonate-5-phosphate is decarboxylated to produce isopentenyl phosphate, which is in turn phosphorylated to generate IPP [17–19]. This differs from the classic version of the pathway, where mevalonate-5-phosphate is phosphorylated to yield mevalonate-5-pyrophosphate, which is subsequently decarboxylated to form IPP. Likewise, examples of archaeal DolP structurally distinct from their eukaryal counterparts have been presented. In the halophilic archaeon, Haloferax volcanii, C55 and C60 DolP shown to participate in N-glycosylation both the α- and the ω-position isoprene subunits are saturated [20–22]; in the eukaryal lipid, only the α-position isoprene subunit is saturated [1,2].
In the following, another unusual DolP of archaeal origin is reported. Sulfolobus acidocaldarius, a thermoacidophilic archaeon that grows optimally at 80°C and pH 2 [23], contains a version of the molecule that is both shorter than eukaryal or other known archaeal DolP and which presents a degree of saturation never seen before.
2. MATERIALS AND METHODS
2.1. Strain and growth
S. acidocaldarius (DSM639) were grown in Brock’s medium at 76°C, pH adjusted to 3 using sulphuric acid and supplemented with 0.1% (w/v) tryptone [23].
2.2. Lipid extraction
A S. acidocaldarius lipid extract was prepared according to Bligh and Dyer [24]. Briefly, 1.6 ml of PBS, 2 ml chloroform (CHCl3) and 4 ml methanol (MeOH) were added to 0.5 g of lyophilized S. acidocaldarius cell powder in a 15 ml glass tube with a Teflon-lined cap to yield a single phase (CHCl3:MeOH:PBS = 1:2:0.8, v/v) solution. Following intermittent mixing by vortex for 5 min, the mixture was sonicated in a water bath for 15 min at room temperature (25°C). The sample was centrifuged (3,000 rpm) for 5 min at room temperature and the supernatant was transferred to a fresh 15 ml glass tub with a Teflon-lined cap. Two milliliters CHCl3 and 2 ml PBS were added to yield a two-phase Bligh-Dyer mixture (CHCl3:MeOH:PBS = 2:2:1.8, v/v). After mixing, the sample was centrifuged (3,000 rpm) for 5 min at room temperature to separate the phases. The upper phase was removed and the lower CHCl3 phase was dried under a stream of nitrogen. The dried lipid extract was stored at −20°C until further analyzed.
2.3. LC-ESI/MS
Normal phase LC-ESI/MS of the S. acidocaldarius lipid extract was performed using an Agilent 1200 Quaternary LC system coupled to a QSTAR XL quadrupole time-of-flight tandem mass spectrometer (Applied Biosystems, Foster City, CA). An Ascentis Si HPLC column (5 μm, 25 cm × 2.1 mm) was used. Mobile phase A consisted of chloroform/methanol/aqueous ammonium hydroxide (800:195:5, v/v/v). Mobile phase B consisted of chloroform/methanol/water/aqueous ammonium hydroxide (600:340:50:5, v/v/v/v). Mobile phase C consisted of chloroform/methanol/water/aqueous ammonium hydroxide (450:450:95:5, v/v/v/v). The elution program consisted of the following: 100% mobile phase A was held isocratically for 2 min and then linearly increased to 100% mobile phase B over 14 min and held at 100% B for 11 min. The LC gradient was then changed to 100% mobile phase C over 3 min and held at 100% C for 3 min, and finally returned to 100% A over 0.5 min and held at 100% A for 5 min. The total LC flow rate was 300 μl/min. The post-column splitter diverted ~10% of the LC flow to the ESI source of the Q-Star XL mass spectrometer, with MS settings as follows: Ion spray voltage (IS) = −4500 V, Curtain gas (CUR) = 20 psi, Ion source gas 1 (GS1) = 20 psi, De-clustering potential (DP) = −55 V, and FP = −150 V. Nitrogen was used as the collision gas for MS/MS experiments. Data acquisition and analysis were performed using the instrument’s Analyst QS software.
Reverse phase LC-ESI/MS of the S. acidocaldarius lipid extract was performed using a Shimadzu LC system (comprising a solvent degasser, two LC-10A pumps and a SCL-10A system controller) coupled to a QSTAR XL quadrupole time-of-flight tandem mass spectrometer (as above). LC was operated at a flow rate of 200 μl/min with a linear gradient as follows: 100% of mobile phase A was held isocratically for 2 min and then linearly increased to 100% mobile phase B over 14 min and held at 100% B for 4 min. Mobile phase A consisted of methanol/acetonitrile/aqueous 1 mM ammonium acetate (60/20/20, v/v/v). Mobile phase B consisted of 100% ethanol containing 1 mM ammonium acetate. A Zorbax SB-C8 reversed-phase column (5 μm, 2.1 × 50 mm) was obtained from Agilent (Palo Alto, CA). The MS operating conditions were as above.
2.4. LC-Multiple reaction monitoring
Normal phase LC coupled with multiple reaction monitoring (MRM) was performed using an Agilent 1200 Quaternary LC system interfaced to a 4000 Q-Trap hybrid triple quadrupole linear ion-trap mass spectrometer equipped with a Turbo V ion source (Applied Biosystems). The LC conditions are same as described above. The post-column splitter diverted ~10% of the LC flow to the Turbo V ion source. MRM was performed in the negative ion mode with MS settings as follows: CUR = 20 psi; GS1 = 20 psi; Ion source gas 2 (GS2) = 30 psi; IS = −4500 V; Heater temperature (TEM) = 350 °C; Interface heater = ON; DP = −40 V; Entrance potential (EP) = −10 V; Collision cell exit potential (CXP) = −5 V. The MRM pairs are as follows: 719.6/79.0 (C45 DolP from S. acidocaldarius), 917.8/79.0 (C60 DolP from Hfx. volcanii), 1392.2/79.0 (C95 DolP from human fibroblasts), and 845.6/79.0 (C55 UndP from Escherichia coli).
3. RESULTS
3.1. S. acidocaldarius contains short, highly saturated DolP
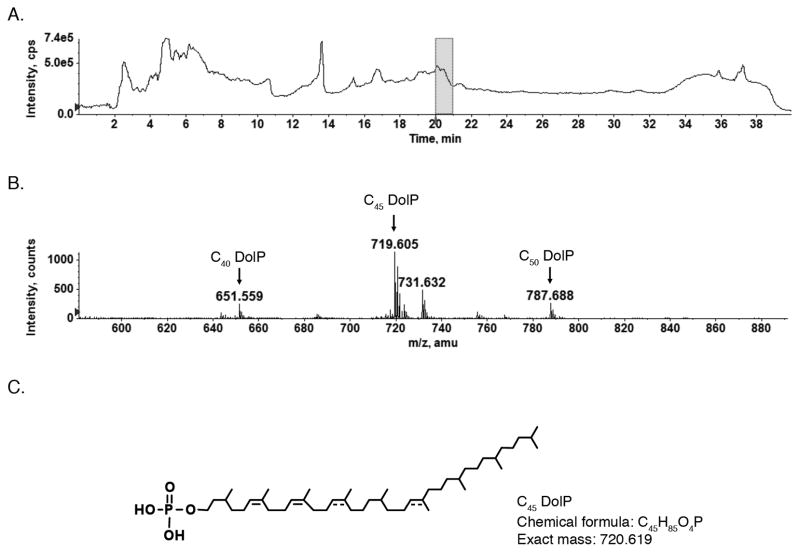
To begin analysis of S. acidocaldarius DolP, normal phase LC-ESI/MS of a lipid extract was performed in the negative ion mode using a high resolution QSTAR XL quadrupole time-of-flight tandem mass spectrometer (Fig 1A). The mass spectrum averaged from those acquired during the retention time of 20–21 min shows prominent monoisotopic ion peaks of m/z 651.559, 719.605 and 787.688, corresponding to the [M-H]− ions of C40, C45 and C50 DolP containing five saturated and three, four and five unsaturated isoprene units, respectively (Fig 1B). These measured ion masses are in agreement with the calculated values of the [M-H]− ion of C40, C45 and C50 DolP, namely m/z 651.549, 719.611 and 787.674, respectively. In addition, a m/z 731.632 peak corresponding to archaetidic acid (calculated value of m/z 731.632) was observed. The proposed structure of S. acidocaldarius C45 DolP is presented in Fig 1C.
Fig 1.
Identification of a novel DolP by normal phase LC/ESI-MS/MS analysis of a S. acidocaldarius lipid extract. A. The total ion chromatogram of normal phase LC-ESI/MS analysis performed in the negative ion mode. B. The [M-H]− ions of C40, C45 and C50 DolP detected at m/z 651.559, 719.605 and 787.688, assigned as C40 DolP, C45 DolP and C50 DolP, respectively. The mass spectrum shown is averaged from spectra acquired during the 20–21 min window, indicated by the shaded area in A. C. The proposed structure of S. acidocaldarius C45DolP. Where the position of a double bond is only speculated, a dotted line is drawn.
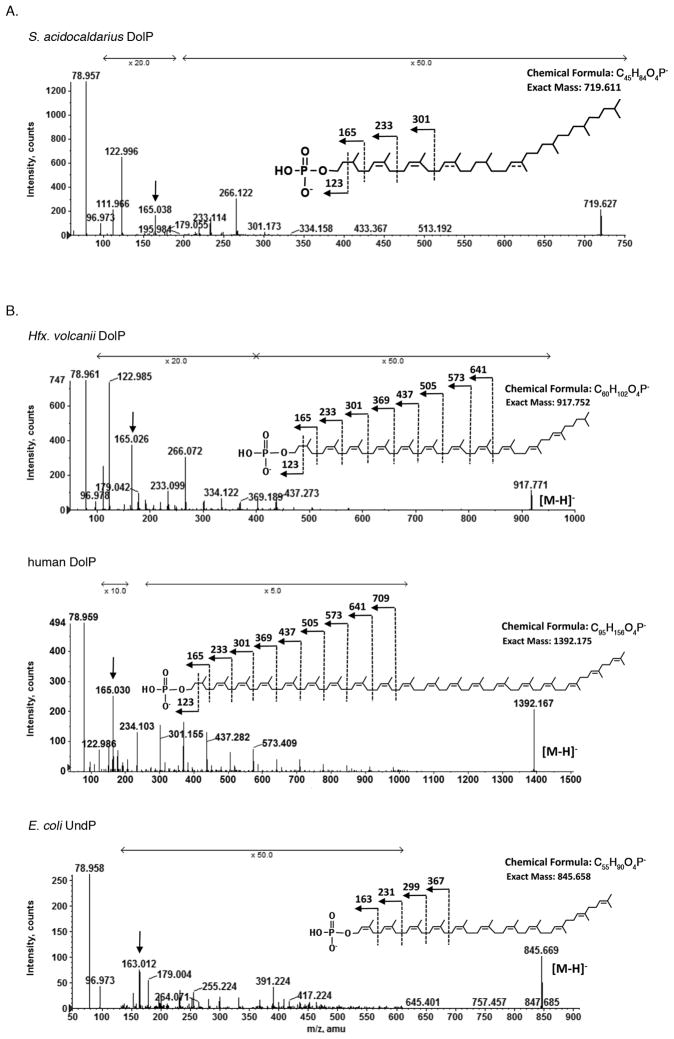
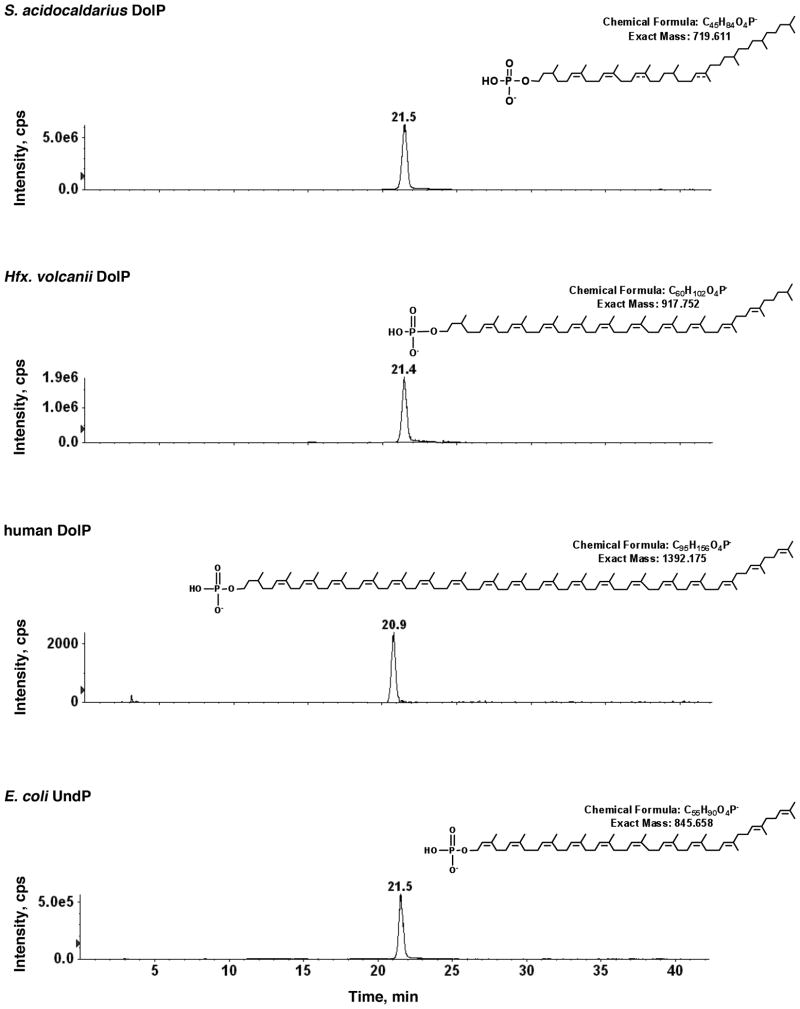
When MS/MS was performed on the [M-H]− ion of C45 DolP at m/z 719.63 (Fig 2A), a fragmentation pattern consistent with a DolP molecule presenting a saturated isoprene subunit at the α- as well as multiple saturated isoprene subunits along the polyisoprenoid chain, including that found at the ω-position. The same saturation patterns were obtained for C40 and C50 dolichyl phosphate, except that one less or one more isoprene unit was detected, respectively (not shown). As the site of some double bonds could not be defined based on the fragmentation data, these were tentatively assigned taking into consideration indirect evidence discussed below. The interpretation of the MS/MS data for S. acidocaldarius DolP was supported by the results of MS/MS analysis performed on the [M-H]− ions of Hfx. volcanii DolP, human DolP and E. coli UndP (Fig 2B). Both the haloarchaeal and human lipids are known to contain a saturated α-position isoprene subunit [12,20,21,25], reflected here by the product ion peaks at m/z 165.026 and 165.030, respectively (Fig 2B, arrows, upper and middle panels, respectively). The same peak (m/z 165.038) was observed in the MS/MS spectrum of the [M-H]− ion of S. acidocaldarius DolP (Fig 2A, arrow). By contrast, a m/z 163.012 peak was seen in the E. coli UndP MS/MS spectrum (Fig 2B, arrow, lower panel), corresponding to the unsaturated isoprene subunit at the αposition expected for this molecule [26]. Confirmation of the mono-phosphorylated nature of the S. acidocaldarius molecule also came from a comparison of normal phase LC-MRM chromatographic peaks showing the retention time of. acidocaldarius C45 DolP (MRM pair: 719.6/79.0) with those of Hfx. volcanii DolP (MRM pair: 917.8/79.0), human DolP (MRM pair: 1392.2/79.0) and E. coli UndP (MRM pair: 845.6/79.0). The similar retention times of these species are reflective of them each carrying the same charge (−1) (Fig 3).
Fig 2.
Structural elucidation of S. acidocaldarius DolP by MS/MS. A. Collision-induced dissociation (CID) MS/MS analysis of the m/z 719.6 [M-H]− ion peak corresponding to S. acidocaldarius C45 DolP. B. CID MS/MS analysis of the [M-H]− ion peaks at m/z 917.8, 1392.2, and 845.6, corresponding to Hfx. volcanii C55 DolP (top panel), human C95 DolP (middle panel) and E. coli C55 UndP (bottom panel), respectively. In each MS/MS spectrum, the arrow denotes the mass of the α-position isoprene subunit-containing fragment ion, while the inset shows the fragmentation scheme and lists the chemical formula and expected mass of the starting material. The arrows marked x5.0, x10.0, x20.0, x25.0 and x50.0 reflect magnification of ion peaks in the corresponding m/z region.
Fig 3.
Normal phase LC of S. acidocaldarius DolP and other mono-phosphorylated polyisoprenoids. LC-MRM chromatographic peaks portraying the LC retention times of S. acidocaldarius C45 DolP, Hfx. volcanii C55 DolP, human C95 DolP and E. coli C55 UndP are presented as listed. In each panel, the inset shows the chemical structure, chemical formula and expected mass of each [M-H]− ion.
Finally, while the data presented here were obtained using S. acidocaldarius samples prepared from stationary-phase cells, consistent results were acquired from cells grown to mid-logarithmic phase (not shown).
3.2. S. acidocaldarius contains hexose-charged DolP
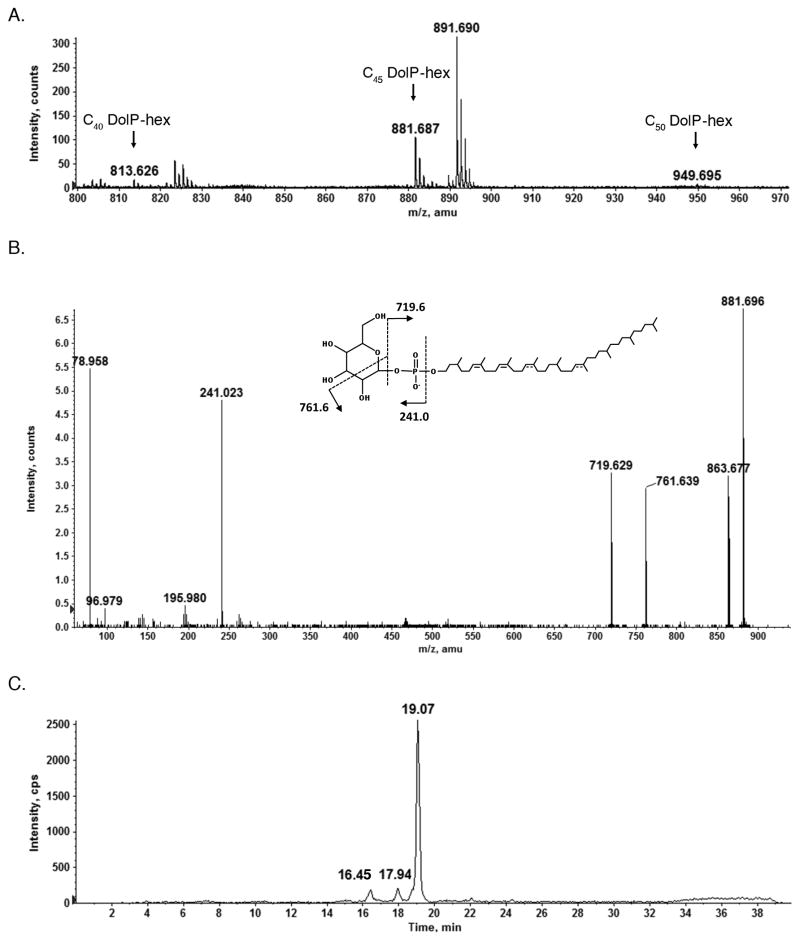
Like Eukarya and Bacteria, Archaea also perform N-glycosylation. In S. acidocaldarius, the surface layer glycoprotein is modified by a heterogeneous group of N-linked glycans, the largest of which corresponds to a tribranched hexasaccharide comprising two N-acetylglucosamine, two mannose, one glucose and one 6-sulfoquinovose (6-deoxy-6-sulfoglucose) residues [27]. The same N-linked glycan had been previously reported to decorate S. acidocaldarius cytochrome b558/566 [28]. Towards determining whether DolP participates in the S. acidocaldarius N-glycosylation pathway, the normal phase LC-ESI/MS/MS profile of the S. acidocaldarius Bligh-Dyer lipid extract was scanned for glycan-charged DolP species. Such examination revealed the presence of [M-H]− ions at m/z 813.626, 881.687 and 949.695, respectively corresponding to C40, C45 and C50 DolP modified by a hexose residue (Fig 4A). This assignment was confirmed upon MS/MS analysis of the C45 DolP-hexose [M-H]− peak at m/z 881.7 (Fig 4B). Furthermore, the extracted ion chromatogram of the m/z 881.7 revealed three distinct C45 DolP-hexose species, retained at 16.45, 17.94 and 19.07 min, respectively (Fig 4C). Similarly, three DolP-hexose species were also found in Hfx. volcanii [22].
Fig 4.
Normal phase LC/ESI-MS analysis of a S. acidocaldarius lipid extract reveals DolP-hexose. A. The [M-H]− ions of C40, C45 and C50 DolP-hexose detected at m/z 813.626, 881.687 and 949.695, indicated by C40 DolP-hex, C45 DolP-hex and C50 DolP-hex, respectively. The mass spectrum shown is averaged from spectra acquired during the 18.9–19.3 min window. B. CID MS/MS of the [M-H]− ion of hexose-charged C45 DolP (m/z 881.687). The inset shows the predicted chemical structure of DolP-hexose and the MS/MS fragmentation scheme. Where the position of a double bond is only speculated, a dotted line is drawn. C. Normal phase LC-extracted ion chromatogram of the hexose-charged C45 DolP [M-H]− ion at m/z 881.7, revealing three stereoisomeric species.
3.3. S. acidocaldarius contains both dolichol and DolP presenting varying degrees of reduction
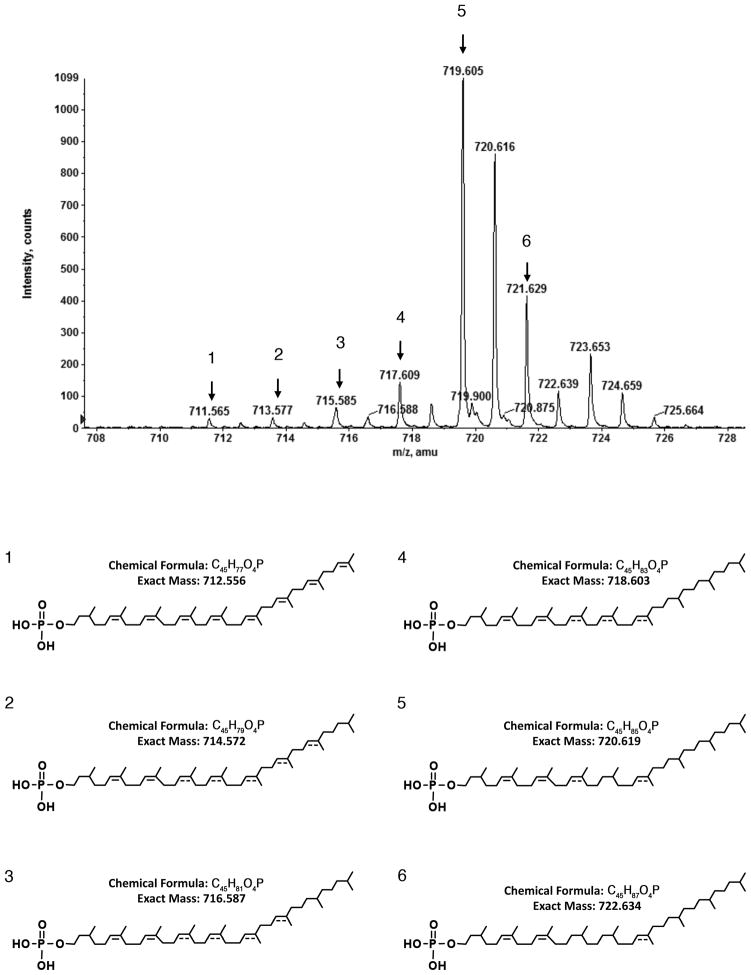
The unusually high degree of double bond saturation in S. acidocaldarius DolP could be an adaptation to the high temperatures encountered by this organism, as suggested for S. acidocaldarius phospholipids, which contain almost fully saturated isoprenoid chains [29]. Hence, with the aim of gaining insight into the pathway of S. acidocaldarius DolP biosynthesis, the normal phase LC-ESI/MS profile of the S. acidocaldarius lipid extract was analyzed for the presence of precursors/derivatives of the C45 species. In addition to the major C45 DolP peak observed at m/z 719.605 and containing saturated isoprene subunits at the α-position, at the ω-position and at three additional internal positions, such analysis also revealed [M -H]− ion peaks at m/z 711.565, 713.577; 715.585, 717.609 and 721.629 (Fig 5A). These correspond to versions of C45 DolP containing saturated isoprene subunits at the α-position alone, at the α- and ω-positions and at the α-position, the ω-position and at one, two and four internal positions (Fig 5B). No fully unsaturated species was detected.
Fig 5.
S. acidocaldarius DolP presents varying degrees of isoprene saturation. S. acidocaldarius C45 DolP containing saturated isoprene subunits at the α-position alone, at the α- and ω-positions and at the α-position, the ω-position and at one to four internal positions are revealed by normal phase LC-ESI/MS. The structure of each numbered [M-H]− ion peak is given below, as is the chemical formula and expected ion mass. Where the position of a double bond is only speculated, a dotted line is drawn. The mass spectrum shown is averaged from spectra acquired during the 20–21 min window.
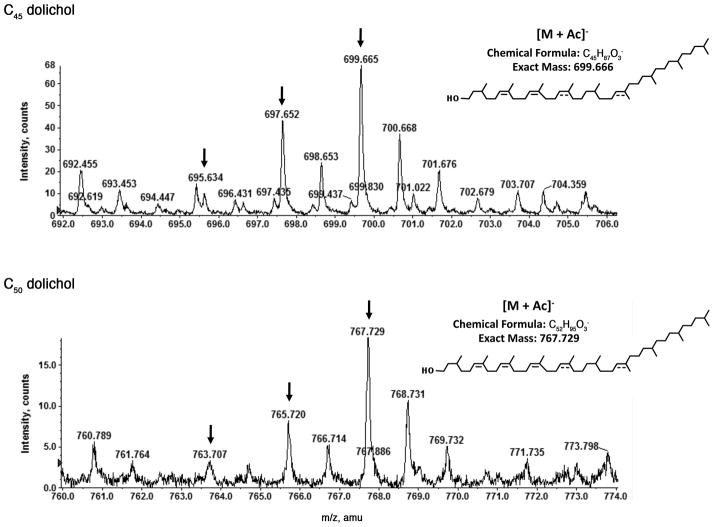
When the S. acidocaldarius lipid extract was subjected to reserve phase LC-ESI/MS analysis, acetate (Ac) adduct [M + Ac]− ions of free C45 dolichols (m/z 695.634, 697.652 and 699.655) and C50 dolichols (m/z 763.707, 765.720 and 767.792) were observed (Fig 6, arrows in the top and bottom panels, respectively). The various versions of C40 dolichol were undetectable, as overlapping peaks derived from other lipid groups in the sample obscured their signals.
Fig 6.
Detection of S. acidocaldarius dolichol by reverse phase LC-ESI/MS. The acetate adduct [M + Ac]− ions of C45 dolichols (m/z 695.634, 697.652 and 699.655) and C50 dolichols (m/z 763.707, 765.720 and 767.792) are observed. The mass spectra shown are averaged from spectra acquired during the 15.1–15.6 min window.
4. DISCUSSION
One of the hallmarks that distinguish Archaea as a distinct domain of life is the composition of their membrane phospholipids. Rather than comprising fatty acyl groups ester-linked to sn-glycerol-3-phosphate as in Eukarya and Bacteria, archaeal phospholipids are composed of isoprenoid chains ether-linked to sn-glycerol-1-phosphate [30,31]. Moreover, the membranes of thermophilic archaea, such as S. acidocaldarius, contain membrane-spanning tetraether isoprenoid-based lipids [32, 33]. As such, the study of polyprenol biosynthesis in Archaea may reveal aspects of the process unique to this life form or alternatively, elucidate aspects of the process not evident using other models. The present analysis of S. acidocaldarius DolP reveals this potential. S. acidocaldarius DolP is unlike its counterparts in other Archaea or in Eukarya. Comprising only eight to ten isoprene subunits, S. acidocaldarius DolPs are shorter than the C70-C110 species found in Eukarya [2] or the C55 and C60 species observed in Hfx. volcanii [20,21]. C45-C60 polyisoprenes are, however, detected in the Gram-negative bacterium, Campylobacter jejuni, where they serve as the platform upon which N-linked glycans are assembled [34]. Moreover, whereas S. acidocaldarius DolP contains saturated α- and ω-position isoprene subunits as do their Hfx. volcanii counterparts, the major S. acidocaldarius DolP species include an additional two-four saturated isoprene subunits, thus presenting a degree of saturation not previously observed elsewhere. This high degree of saturation may reflect the need for a molecule stable in the face of elevated temperatures and acidity, conditions naturally encountered by S. acidocaldarius DolP. By comparison, only the α-isoprene subunit is saturated in eukaryal DolP [1,2,25].
Although the pathway used for S. acidocaldarius DolP biogenesis has yet to be delineated, previous efforts point to several aspects of the process apparently unique to this organism. In contrast to almost all other known Archaea, S. acidocaldarius apparently relies on the classic MVA pathway to generate the building blocks of polyprenols, i.e. IPP and DMAPP, as this species lacks isopentenyl phosphate kinase, one of the two enzymes unique to the archaeal version of the pathway [35]. Secondly, in vitro analysis of S. acidocaldarius cis-polyprenyl diphosphate synthase revealed that GGPP is the preferred substrate of the enzyme [36]. Accordingly, the S. acidocaldarius DolP structures presented in this report contain the branching point after a geranylgeranyl moiety, although this remains to be confirmed experimentally. In most Eukarya and Bacteria, by contrast, farnesyl pyrophosphate serves as the primary substrate for cis-polyprenyl diphosphate synthase in DolP and UndP biogenesis [1,2].
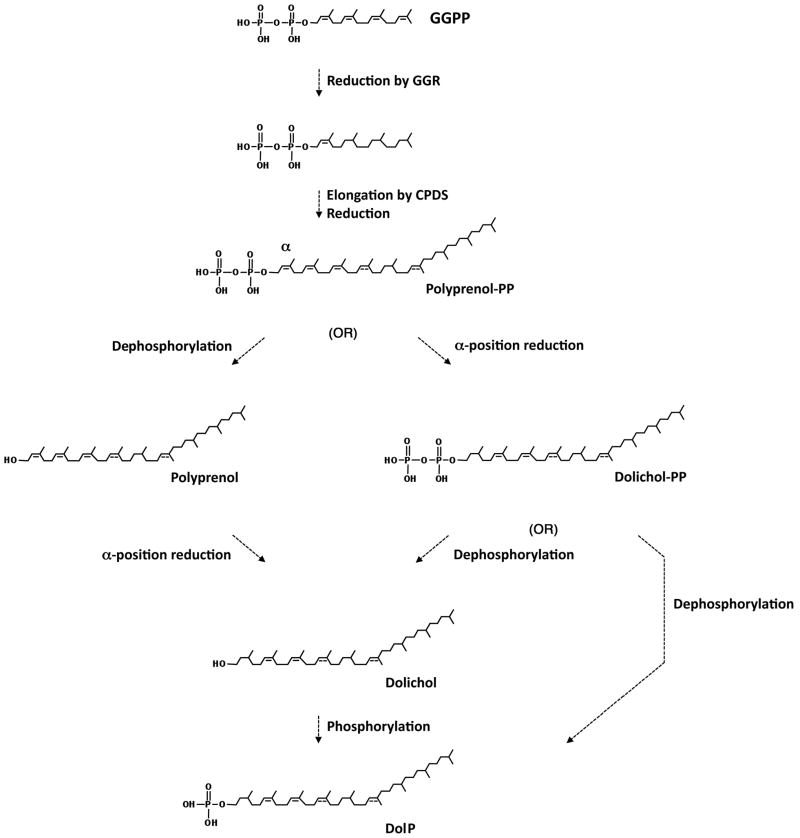
Based on the present study, S. acidocaldarius DolP also presents a degree of saturation never seen before. Although the agents responsible for this high degree of saturation are not currently known, a gernaylgeranyl reductase (GGR) thought to catalyze the hydrogenation of geranylgeranyl groups in S. acidocaldarius phospholipid precursors has been identified [37]. In vitro characterization revealed this S. acidocaldarius GGR to be capable of reducing only three of the four double bonds of geranylgeranyl diphosphate (GGPP), activity also seen in plant and bacterial GGRs. In this case, the α-isoprene subunit of GGPP is not reduced and thus remains accessible for further prenyl transfer reactions. It remains, however, to be determined whether this GGR, encoded by the Saci_0986 gene, also participates in DolP biogenesis. It is conceivable that the multiple double bond reduction steps involved in S. acidocaldarius DolP biogenesis rely on another one or more of the six putative GGRs reportedly encoded by the S. acidocaldarius genome [19]. Since, as the various versions of S. acidocaldarius dolichol and DolP observed in the current study all presented a reduced isoprene subunit at the α-position, it is reasonable to assume that saturation at this position relies on a distinct enzyme not involved in the saturation of other double bonds found in S. acidocaldarius DolP precursors. While an α-isoprene reductase responsible for converting polyprenol to dolichol in Eukarya has only recently been identified, it is clear that more than one reductase can catalyze this activity [12]. Based on the results presented here as well as earlier findings, a proposed pathway for S. acidocaldarius DolP biosynthesis is presented in Fig 7.
Fig 7.
A proposed S. acidocaldarius DolP biosynthesis pathway. In S. acidocaldarius, three IPP subunits and DMAPP, all synthesized via the classic MVA pathway [34], combine to form GGPP (not shown). Three of the four double bonds in this species are reduced by GGR; the α-position isoprene subunit remains unsaturated [36]. Four-six isoprene subunits are then added at the α-position of the GGPP backbone by cis-polyprenyl diphosphate synthase(s) (CPDS) [35] to generate C40, C45 and C50 polyprenol diphosphate (Polyprenol PP). The polyprenol diphosphate αposition isoprene subunit is indicated. At this point, removal of the two phosphate groups, followed by multiple reduction reactions and phosphorylation may occur to yield DolP, in a process reminiscent of what is proposed to occur in Eukarya [1,2]. Alternatively, C40, C45 and C50 polyprenol diphosphate may undergo multiple reduction reactions followed by removal of one phosphate group or removal of both phosphate groups and re-phosphorylation, in both cases yielding DolP. The order in which the α-position and other isoprene subunits of the polyprenol diphosphate precursor are reduced is not known. Furthermore, where the position of a double bond is only speculated, a dotted line is drawn.
Finally, the detection of S. acidocaldarius DolP modified by distinct hexoses implies that DolP serves as the glycan lipid carrier in N-glycosylation and that multiple glycan-modified DolP are involved in S. acidocaldarius N-glycosylation. This is the case in Hfx. volcanii, where the first four saccharide subunits of the S-layer glycoprotein N-linked pentasaccharide (i.e. hexose, two hexuronic acids and a methyl ester of hexuronic acid [21]) are sequentially assembled on a common dolichyl phosphate carrier, whereas the final pentasaccharide residue, mannose, is derived from a distinct dolichyl phosphate [22]. In the present study, no DolP species bearing higher ordered glycans were detected. This could be because such species are present only at low levels at steady state conditions or that a different extraction protocol than the typical Blight-Dyer method [24] used here is required for their detection. Indeed, short DolPs, such as detected here, are less hydrophobic than are longer DolPs. When linked to multiple sugars, the short S. acidocaldarius DolP may not be sufficiently hydrophobic to partition into the chloroform-based lower phase formed in the Bligh-Dyer extraction employed.
In conclusion, the thermacidophile S. acidocaldarius contains DolP that is not only shorter than what is found elsewhere but that also presents a degree of saturation not previously reported. With a completed genome sequence and protocols for gene deletion now available [38,39], it may be possible to fully delineate the pathway of S. acidocaldarius DolP biogenesis and identify those reductases responsible for the high degree of saturation detected in this molecule. Such findings may shed light on the enigmatic mechanisms involved in the reduction of polyprenol to dolichol in Eukarya, in particular, aiding in the identification of the alternative, as yet unknown eukaryal polyprenol reductase(s) [12].
Highlights.
A novel dolichol phosphate was identified in the thermoacidophilic archaeon, Sulfolobus acidocaldarius using LC-ESI/MS/MS.
The unusually short S. acidocaldarius dolichol phosphate presents a degree of saturation not previously reported.
S. acidocaldarius dolichol phosphate - and -position isoprenes are saturated, as are intra-chain isoprenes.
Hexose-charged dolichol phosphate species were also detected.
Acknowledgments
The authors thank Dr. Christian R. H. Raetz for advice and support. The mass spectrometry facility in the Department of Biochemistry of the Duke University Medical Center and Z.G. are supported by the LIPID MAPS Large Scale Collaborative Grant number GM-069338 from NIH. J.E. is supported by the Israel Science Foundation (grant 30/07). B.H.M. and S.V.A. are supported by a VIDI grant from the Dutch Science Organization (NWO). S.V.A. received intramural funds from the Max Planck Society.
Footnotes
Abbreviations used: Acetate, Ac; chloroform, CHCl3; cis-polyprenyl diphosphate synthase, CPDS; collision induced dissociation, CID; deoxyxylulose phosphate, DOXP; dimethylallyl diphosphate, DMAPP; dolichyl phosphate, DolP; geranylgeranyl diphosphate, GGPP; geranylgeranyl reductase, GGR; isopentenyl diphosphate, IPP; methanol, MeOH; mevalonate, MVA; multiple reaction monitoring, MRM; tandem mass spectrometry, MS/MS; undecaprenol phosphate, UndP.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Swiezewska E, Danikiewicz W. Polyisoprenoids: Biosynthesis, structure and function. Prog Lipid Res. 2005;44:235–258. doi: 10.1016/j.plipres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Jones MB, Rosenberg JN, Betenbaugh MJ, Krag SS. Structure and synthesis of polyisoprenoids used in N-glycosylation across the three domains of life. Biochim Biophys Acta. 2009;1790:485–494. doi: 10.1016/j.bbagen.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ericsson J, Dallner G. Distribution, biosynthesis, and function of mevalonate pathway lipids. Subcell Biochem. 1993;21:229–272. doi: 10.1007/978-1-4615-2912-5_11. [DOI] [PubMed] [Google Scholar]
- 4.McGarvey DJ, Croteau R. Terpenoid metabolism. Plant Cell. 1995;7:1015–1026. doi: 10.1105/tpc.7.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajda A, Konopka-Postupolska D, Krzymowska M, Hennig J, Skorupinska-Tudek K, Surmacz L, Wójcik J, Matysiak Z, Chojnacki T, Skorzynska-Polit E, Drazkiewicz M, Patrzylas P, Tomaszewska M, Kania M, Swist M, Danikiewicz W, Piotrowska W, Swiezewska E. Role of polyisoprenoids in tobacco resistance against biotic stresses. Physiol Plant. 2009;135:351–364. doi: 10.1111/j.1399-3054.2009.01204.x. [DOI] [PubMed] [Google Scholar]
- 6.Burda P, Aebi M. The dolichol pathway of N-linked glycosylation. Biochim Biophys Acta. 1999;1426:239–257. doi: 10.1016/s0304-4165(98)00127-5. [DOI] [PubMed] [Google Scholar]
- 7.Bloch K. The biological synthesis of cholesterol. Science. 1965;150:19–28. doi: 10.1126/science.150.3692.19. [DOI] [PubMed] [Google Scholar]
- 8.Miziorko HM. Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch Biochem Biophys. 2011;505:131–143. doi: 10.1016/j.abb.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohmer M, Knani M, Simonin P, Sutter B, Sahm H. Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J. 1993;295:517–524. doi: 10.1042/bj2950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohmer M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep. 1999;16:565–574. doi: 10.1039/a709175c. [DOI] [PubMed] [Google Scholar]
- 11.Schenk B, Fernandez F, Waechter CJ. The ins(ide) and out(side) of dolichyl phosphate biosynthesis and recycling in the endoplasmic reticulum. Glycobiology. 2001;11:61R–70R. doi: 10.1093/glycob/11.5.61r. [DOI] [PubMed] [Google Scholar]
- 12.Cantagrel V, Lefeber DJ, Ng BG, Guan Z, Silhavy JL, Bielas SL, Lehle L, Hombauer H, Adamowicz M, Swiezewska E, De Brouwer A, Bluemel P, Cegielska J, Houliston SR, Swistun D, Ali BR, Babovic-Vuksanovic D, van Bokhoven H, Wevers RA, Raetz CRH, Freeze HH, Morava E, Al-Gazali L, Gleeson JG. SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. Cell. 2010;142:1–15. doi: 10.1016/j.cell.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pace NR. A molecular view of microbial diversity and the biosphere. Science. 1997;276:734–740. doi: 10.1126/science.276.5313.734. [DOI] [PubMed] [Google Scholar]
- 15.DeLong EF. Everything in moderation: archaea as ‘non-extremophiles’. Curr Opin Genet Dev. 1998;8:649–654. doi: 10.1016/s0959-437x(98)80032-4. [DOI] [PubMed] [Google Scholar]
- 16.Rothschild LJ, Mancinelli RL. Life in extreme environments. Nature. 2001;409:1092–1101. doi: 10.1038/35059215. [DOI] [PubMed] [Google Scholar]
- 17.Smit A, Mushegian A. Biosynthesis of isoprenoids via mevalonate in Archaea: the lost pathway. Genome Res. 2000;10:1468–1484. doi: 10.1101/gr.145600. [DOI] [PubMed] [Google Scholar]
- 18.Grochowski LL, Xu H, White RH. Methanocaldococcus jannaschii uses a modified mevalonate pathway for biosynthesis of isopentenyl diphosphate. J Bacteriol. 2006;188:3192–3198. doi: 10.1128/JB.188.9.3192-3198.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumi R, Atomi H, Driessen AJ, van der Oost J. Isoprenoid biosynthesis in Archaea--biochemical and evolutionary implications. Res Microbiol. 2011;162:39–52. doi: 10.1016/j.resmic.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Kuntz C, Sonnenbichler J, Sonnenbichler I, Sumper M, Zeitler R. Isolation and characterization of dolichol-linked oligosaccharides from Haloferax volcanii. Glycobiology. 1997;7:897–904. doi: 10.1093/glycob/7.7.897. [DOI] [PubMed] [Google Scholar]
- 21.Guan Z, Naparstek S, Kaminski L, Konrad Z, Eichler J. Distinct glycan-charged phosphodolichol carriers are required for the assembly of the pentasaccharide N-linked to the Haloferax volcanii S-layer glycoprotein. Mol Microbiol. 2010;78:1294–1303. doi: 10.1111/j.1365-2958.2010.07405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaminski L, Abu-Qarn M, Guan Z, Naparstek S, Ventura VV, Raetz CRH, Hitchen PG, Dell A, Eichler J. AglJ adds the first sugar of the N-linked pentasaccharide decorating the Haloferax volcanii S-layer glycoprotein. J Bacteriol. 2010;192:5572–5579. doi: 10.1128/JB.00705-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brock TD, Brock KM, Belly RT, Weiss RL. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Microbiol. 1972;84:54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- 24.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 25.Ekström TJ, Chojnacki T, Dallner G. The alpha-saturation and terminal events in dolichol biosynthesis. J Biol Chem. 1987;262:4090–4097. [PubMed] [Google Scholar]
- 26.Wright A, Dankert M, Fennessey P, Robbins PW. Characterization of a polyisoprenoid compound functional in O-antigen biosynthesis. Proc Natl Acad Sci USA. 1967;57:1798–1803. doi: 10.1073/pnas.57.6.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peyfoon E, Meyer B, Hitchen PG, Panico M, Morris HR, Haslam SM, Albers SV, Dell A. The S-layer glycoprotein of the crenarchaeote Sulfolobus acidocaldarius is glycosylated at multiple sites with chitobiose-linked N-glycans. Archaea. 2010:754101. doi: 10.1155/2010/754101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zahringer U, Moll H, Hettmann T, Knirel YA, Schafer G. Cytochrome b558/566 from the archaeon Sulfolobus acidocaldarius has a unique Asn-linked highly branched hexasaccharide chain containing 6-sulfoquinovose. Eur J Biochem. 2000;267:4144–4149. doi: 10.1046/j.1432-1327.2000.01446.x. [DOI] [PubMed] [Google Scholar]
- 29.Dannenmuller O, Arakawa K, Eguchi T, Kakinuma K, Blanc S, Albrecht AM, Shmutz M, Nakatani Y, Ourisson G. Membrane properties of archaeal macrocyclic diether phospholipids. Chemistry. 2000;6:645–54. doi: 10.1002/(sici)1521-3765(20000218)6:4<645::aid-chem645>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 30.Kates M. Biology of halophilic bacteria, Part II. Membrane lipids of extreme halophiles: biosynthesis, function and evolutionary significance. Experientia. 1993;49:1027–1036. doi: 10.1007/BF01929909. [DOI] [PubMed] [Google Scholar]
- 31.Koga Y, Morii H. Biosynthesis of ether-type polar lipids in archaea and evolutionary considerations. Microbiol Mol Biol Rev. 2007;71:97–120. doi: 10.1128/MMBR.00033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Rosa M, Gambacorta A, Bu’lock JD. Extremely thermophilic acidophilic bacteria convergent with Sulfolobus acidocaldarius. J Gen Microbiol. 1975;86:156–164. doi: 10.1099/00221287-86-1-156. [DOI] [PubMed] [Google Scholar]
- 33.Langworthy TA. Comparative lipid composition of heterotrophically and autotrophically grown Sulfolobus acidocaldarius. J Bacteriol. 1977;130:1326–1332. doi: 10.1128/jb.130.3.1326-1332.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reid CW, Stupak J, Szymanski CM, Li J. Analysis of bacterial lipid-linked oligosaccharide intermediates using porous graphitic carbon liquid chromatography-electrospray ionization mass spectrometry: Heterogeneity in the polyisoprenyl carrier revealed. Anal Chem. 2009;81:8472–8478. doi: 10.1021/ac9013622. [DOI] [PubMed] [Google Scholar]
- 35.Boucher Y, Kamekura M, Doolittle WF. Origins and evolution of isoprenoid lipid biosynthesis in archaea. Mol Microbiol. 2004;52:515–527. doi: 10.1111/j.1365-2958.2004.03992.x. [DOI] [PubMed] [Google Scholar]
- 36.Hemmi H, Yamashita S, Shimoyama T, Nakayama T, Nishino T. Cloning, expression, and characterization of cis-polyprenyl diphosphate synthase from the thermoacidophilic archaeon Sulfolobus acidocaldarius. J Bacteriol. 2001;183:401–404. doi: 10.1128/JB.183.1.401-404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato S, Murakami M, Yoshimura T, Hemmi H. Specific partial reduction of geranylgeranyl diphosphate by an enzyme from the thermoacidophilic archaeon Sulfolobus acidocaldarius yields a reactive prenyl donor, not a dead-end product. J Bacteriol. 2008;190:3923–3929. doi: 10.1128/JB.00082-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L, Bruegger K, Skovgaard M, Redder P, She Q, Torarinsson E, Greve B, Awayez M, Zibat A, Klenk HP, Garrett RA. The genome of Sulfolobus acidocaldarius, a model organism of the Crenarchaeota. J Bacteriol. 2005;187:4992–4999. doi: 10.1128/JB.187.14.4992-4999.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner M, Berkner S, Ajon M, Driessen AJM, Albers SV. Expanding and understanding the genetic toolbox of the hyperthermophilic genus Sulfolobus. Biochem Soc Trans. 2009;37:97–101. doi: 10.1042/BST0370097. [DOI] [PubMed] [Google Scholar]