Abstract
Purpose
In previous cancer vaccine clinical trials targeting Survivin, induction of specific CD8+ T cell responses did not consistently lead to clinical responses. Considering the critical role of CD4+ T cell help in generating anti-tumor immunity, integration of anti-Survivin CD4+ T cell responses may enhance the efficacy of anti-Survivin cancer immunotherapy. HLA-DP4 is emerging as an attractive MHC target allele of CD4+ T cell-mediated immunotherapy, since it is one of the most frequent HLA alleles in many ethnic groups. In this paper, we aimed to elucidate DP4-restricted CD4+ T cell responses against Survivin in cancer patients.
Experimental Design
We generated a human cell-based artificial antigen-presenting cell (aAPC) expressing HLA-DP4, CD80, and CD83 and induced DP4-restricted antigen-specific CD4+ T cells. The number, phenotype, effector function, and in vitro longevity of generated CD4+ T cells were determined.
Results
We first determined previously unknown DP4-restricted CD4+ T cell epitopes derived from CMV pp65, to which sustained Th1-biased recall responses were induced in vitro using DP4-aAPC. In contrast, DP4-aAPC induced in vitro both Th1 and Th2 long-lived anti-Survivin CD4+ T cells from cancer patients. Both Survivin-specific Th1 and Th2 cells were able to recognize Survivin-expressing tumors in a DP4-restricted manner. Neither Survivin-specific IL-10 secreting Tr1 cells nor Th17 cells were induced by DP4-aAPC.
Conclusions
DP4-restricted anti-Survivin Th1 and Th2 immunity with sufficient functional avidity can be induced from cancer patients. The development of strategies to concurrently induce both CD4+ and CD8+ T cell responses against Survivin is warranted for optimal anti-Survivin cancer immunotherapy.
Keywords: HLA-DP4, Survivin, CD4+ T cells, artificial APC, K562
Introduction
Survivin, a member of the inhibitor of apoptosis protein family, has been implicated as playing a critical role in both cell survival and regulation of mitosis in cancer (1). While Survivin is expressed in a variety of normal fetal tissues, expression is absent in most adult tissues. In contrast, Survivin is highly expressed in many types of tumors (2). This widespread expression appears to be clinically important as high expression is correlated with poor prognosis. Based on these observations, great interest has emerged in targeting this molecule in anti-cancer therapeutic approaches. Clinical strategies targeting Survivin include the use of the antisense oligonucleotide LY2181308, the low molecular weight molecule inhibitor YM155, and vaccine therapy (3, 4).
Intriguingly, both in vivo and in vitro data have shown that Survivin is immunogenic. Spontaneous CTL responses directed against Survivin-derived HLA class I-restricted T-cell epitopes have been detected in cancer patients (5–7). Survivin-reactive CTL were shown to kill HLA-matched tumors of different tissue types (8, 9). Moreover, dendritic cells (DC) pulsed with Survivin mRNA have been shown to induce Survivin-specific CTL that lyse both Survivin-positive tumor cell lines and primary leukemic and lymphoma cells in vitro (10). Based on these results, clinical vaccine trials using DC pulsed with HLA class I-restricted Survivin peptides and full-length Survivin mRNA have been conducted (11–13). Although some patients developed HLA class I-restricted anti-Survivin CD8+ T cell responses in these trials, clinical response rates were unsatisfactory. HLA class I-restricted CD8+ T cells are primary mediators of adaptive immunity due to their potent direct effector function. However, many studies have demonstrated that CD4+ T cells provide help which is critical for generating optimal CD8+ T cell responses and long-lasting memory (14, 15). Therefore, vaccine-induced anti-Survivin CD8+ T cell responses may be enhanced by simultaneous induction of anti-Survivin CD4+ helper T cell responses.
In humans, HLA-DR-restricted antigen-specific CD4+ T cell responses have been intensively studied (http://www.cancerimmunity.org/peptidedatabase/Tcellepitopes.htm). However, the fact that there is no common HLA-DR allele with a frequency greater than 25% makes these studies difficult to translate to the clinic. In contrast, HLA-DP4 is a particularly well-suited HLA class II target since it is one of the most prevalent HLA alleles among many races and ethnic groups (i.e. ~75% of Caucasians). Recently, DP4-restricted CD4+ T cell antigenic peptides have been identified from infectious pathogens and tumor-associated antigens including Survivin (16–21).
Previously, we generated K562-based artificial APC (A2-aAPC) by transducing HLA-A2, CD80, and CD83 (22). A2-aAPC can generate large numbers of antigen-specific CD8+ CTL with a central/effector memory phenotype and potent effector function (23). These CTL are surprisingly long-lived and can be maintained in vitro without any feeder cells or cloning for greater than one year. Moreover, they lack an exhausted phenotype (23).
In this study, we have applied our experience with A2-aAPC and developed a novel standardized and renewable K562-derived HLA-DP4-aAPC. After demonstrating the induction of responses to the viral antigen CMV pp65 for comparison, we elucidated HLA-DP4-restricted CD4+ T cell responses against the tumor-associated antigen, Survivin.
Materials and Methods
Cells
Peripheral blood mononuclear cells (PBMC) were obtained from healthy donors and cancer patients. Appropriate informed consent and institutional review board approval were obtained. K562-derived aAPC and lymphoblastoid T2 cells were cultured in RPMI 1640 supplemented with 10% FCS and gentamycin (Invitrogen, Carlsbad, CA). Renal cancer cell lines were maintained in DMEM containing 10% FCS and gentamycin. All cell lines were obtained from ATCC (Manassas, VA).
cDNAs
HLA-DPA1*0103 and DPB1*0401 cDNA were cloned from normal PBMC using RT-PCR based upon the published sequence. All cDNA were cloned into the pMX vector and the sequence was verified.
Generation of aAPC and other transfectants
A retrovirus system was employed to establish K562-based HLA-DP0401 (DP4)-aAPC as described previously (22). Briefly, K562, which is deficient for HLA-class I and class II expression, was sequentially transduced with DPA1*0103, DPB1*0401, CD80, and CD83 and positive cells were isolated by mAb staining and subsequent magnetic-bead guided sorting. T2 cells defective for HLA class II expression were similarly transduced with DPA1*0103 and DPB1*0401 to establish T2 stably expressing HLA-DP4 (T2/DP4). Renal cancer cell lines negative for HLA-DR and DP were retrovirally transduced with HLA-DP4. Positive cells were isolated as described above.
Flow cytometry analysis
mAbs recognizing the following surface antigens were used: pan HLA class II, HLA-DR from Beckman Coulter, Brea, CA; HLA-DP from Abcam, Cambridge, MA; HLA-DR, CD80, CD83 and mouse isotype controls from BD Biosciences, San Diego, CA; PD-1 from eBioscience, San Diego, CA; CTLA-4 from R&D Systems, Minneapolis, MN; LAG-3 from United States Biological, Swampscott, MA. Surface molecule staining was performed as described elsewhere (22). Intracellular cytokine staining of IL-4, IFN-γ and Foxp3 was performed as described elsewhere (24). T2/DP4 cells were pulsed with peptide and used as a stimulator.
Production of HLA-DP4 restricted CD4+ T cells
High resolution HLA class II genotyping was performed by the American Red Cross, Dedham, MA. CD4+ T cells were purified using a positive or negative isolation kit (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instruction.
Purified HLA-DP0401 matched CD4+ T cells were plated in 24 well plates at 2×106 cells/well in serum-free X-Vivo 20 (Lonza, Walkersville, MD). An aAPC was pulsed with peptide or protein antigen overnight at 37°C unless otherwise noted. These cells were then irradiated (200 Gy) and added to the responder cells at a responder to stimulator ratio of 20:1 (day 0). When only small numbers of CD4+ T cells were available, 96 well plates were utilized and input numbers of cells were reduced accordingly. On day 3 following stimulation, heat inactivated, human AB serum was added to a final concentration of 2%. Starting on day 3, 10 IU/ml IL-2 (Chiron, Emeryville, CA) and 10 ng/ml IL-15 (Peprotech, Rocky Hill, NJ) were added to the cultures every three days.
Recombinant full-length CMV (cytomegalovirus) pp65 protein was purchased from Miltenyi Biotec. CMV Ab titer was measured using a CMV IgG ELISA kit purchased from Immuno-Biological Laboratories, Minneapolis, MN according to the manufacturer's manual. Peptides used were pp65 peptide mix (BD Biosciences), fifty-seven 20-mer pp65 peptides overlapping by 10 amino acids (JPT, Acton, MA), pp65495 (495NLVPMVATV503), tetanus toxin TT947 (947FNNFTVSFWLRVPKVSASHLE967), Oxy271 (271EKKYFAATQFEPLAARL287), HIV pol476 (476ILKEPVHGV484), HIV env31 (31TEKLWVTVYYGVPVW45), and Survivin#90 peptide (90KKQFEELTLGEFLKL104). Synthetic peptides were obtained from New England Peptides (Gardner, MA) and JPT Peptide Technologies (Acton, MA). Unless otherwise noted, HIV pol476 and HIV env31 peptides were used as control peptides for HLA-A2 and HLA-DP4 molecules, respectively.
IFN-γ, IL-2, IL-4, and IL-10 ELISPOT
IFN-γ, IL-2, IL-4, and IL-10 ELISPOT assays were performed as described before (24, 25).
IL-17 and IL-21 ELISA
IL-17 and IL-21 ELISA kits were obtained from R&D Systems and eBioscience, respectively, and were used according to the manufacturer's instruction. The limits of IL-17 and IL-21 detection were 15.0 pg/ml and 31.3 pg/ml, respectively.
Cell-based competitive binding assay
T2/DP4 cells were pulsed with graded concentrations of competitor peptides in the presence of 0.5 μM biotin-conjugated reference peptide, Oxy271. After intensive washing, the cells were stained by PE-conjugated streptavidin, washed and fluorescence intensity was measured by flow cytometry analysis. Binding was expressed as mean fluorescence intensity. % inhibition of binding by competitor peptide was calculated by the mean fluorescence intensity (MFI), using the formula: %inhibition=[1−(MFI with competitor peptide/MFI without competitor peptide)] × 100.
Western blot analysis
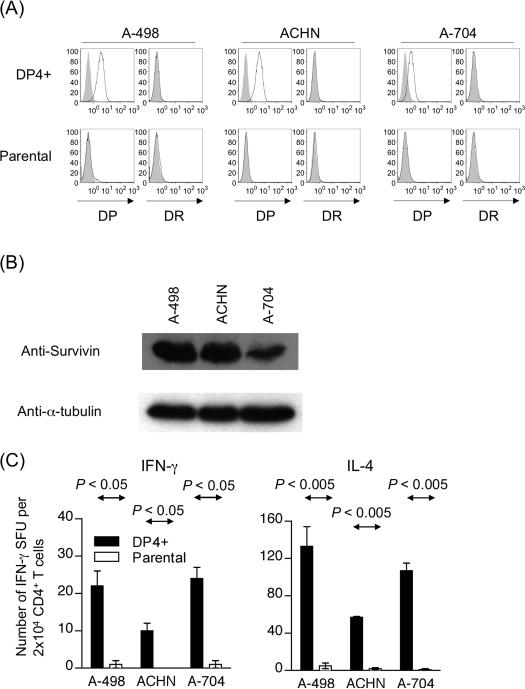
Survivin protein expression was detected by Western blot analysis as described previously (24, 26). Briefly, duplicate PVDF membranes were loaded with 30 μg cell lysates of three renal cancer cell lines, A-498, ACHN, and A-704, and were probed with rabbit anti-Survivin mAb (R&D Systems) or mouse anti-α-tubulin mAb (Sigma, St. Louis, MO).
Statistical Analysis
The function of DP4+ CD4+ T cell lines was determined using ELISPOT and ELISA assays to measure antigen-specific cytokine production. Statistical analysis was performed using Microsoft Excel 2004 for Mac and GraphPad Prism 5.0c. Unpaired, two-tailed Welch's t-test was used for two-sample comparisons. P values of < 0.05 were considered significant.
Results
Generation of K562-based aAPC expressing HLA-DP4 as a single HLA allele
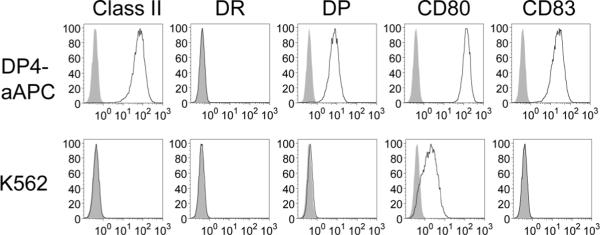
Previously, we and others reported the generation of human cell-based artificial APC using HLA class I−, class II−, CD54+, CD58+ K562 cells as a backbone (27). We serially introduced HLA-DPA1*0103, DPB1*0401, CD80, and CD83 to K562 cells using a retrovirus system in order to establish DP0401 (DP4)-aAPC. Stable expression of transduced molecules was confirmed by flow cytometry analysis following staining with specific mAbs (Figure 1). Transduction of DPA1*0103 or DPB1*0401 alone did not induce HLA-DP surface expression, indicating that K562 expresses neither DPα nor β endogenously (data not shown). Since parental K562 lacks the expression of any HLA allele, generated DP4-aAPC expresses DP4 as a single HLA allele.
Figure 1. Generation of K562-based aAPC that expresses DP4 as a single HLA allele.
The surface expression of HLA-DR, DP, CD80, and CD83 on DP4-aAPC and parental K562 was analyzed by flow cytometry following staining with specific mAbs. Note that K562 endogenously expresses a modest level of CD80 on the cell surface.
Identification of DP4-restricted immunogenic CD4+ T cell peptides derived from CMV pp65
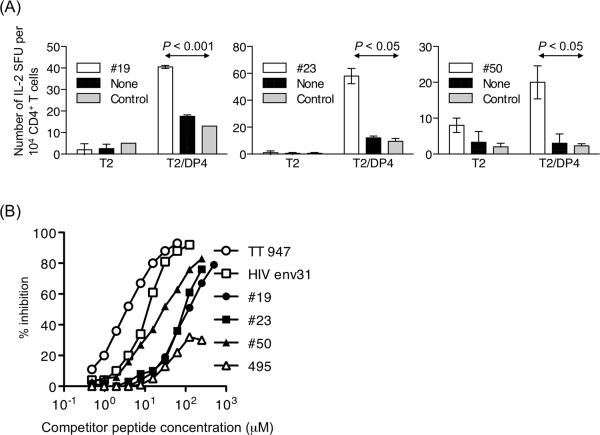
CMV pp65 is an immunodominant target of CD4+ and CD8+ T cell responses to CMV. And yet, to the best of our knowledge, DP4-restricted pp65-derived CD4+ T cell epitopes have not previously been reported. In order to identify pp65-derived CD4+ T cell epitopes naturally processed and presented by HLA-DP4 molecules, pp65-specific CD4+ T cells were generated using autologous monocytes pulsed with full-length pp65 protein in DP4+ CMV Ab+ healthy donors followed by restimulation with DP4-aAPC pulsed with overlapping pp65 peptides. Then, using T2/DP4 cells pulsed with pp65-derived overlapping peptides in an ELISPOT assay, we identified three DP4-restricted pp65-derived immunogenic peptides, pp65#19 (181YYTSAFVFPTKDVALRHVVC200), pp65#23 (221DQYVKVYLESFCEDVPSGKL240), and pp65#50 (491ILARNLVPMVATVQGQNLKY510) (data not shown). To confirm the immunogenicity of these three peptides, we stimulated CD4+ T cells purified from other DP4+ donors using autologous monocytes pulsed with full-length pp65 protein and subsequently using DP4-aAPC pulsed with synthetic peptides. IL-2 ELISPOT revealed that all three peptides were indeed immunogenic and were able to stimulate functionally competent CD4+ T cells (Figure 2A).
Figure 2. Identification of DP4-restricted immunogenic CD4+ T cell epitopes derived from CMV pp65.
(A) CD4+ T cells purified from DP4+ donors were initially stimulated with autologous monocytes pulsed with recombinant full-length pp65 protein. T cells were then restimulated with DP4-aAPC pulsed with pp65 peptide mix. Generated pp65-specific CD4+ T cells were analyzed for peptide specificity by IL-2 ELISPOT using T2/DP4 pulsed with pp65#19, pp65#23, or pp65#50 as stimulators. Parental T2 cells pulsed with each peptide were used as control stimulators. (B) A cell-based competitive binding assay was conducted to demonstrate the binding of pp65#19, pp65#23, or pp65#50 peptides to DP4 molecules. % inhibition when 0.5 μM Oxy271 was used as a reference peptide is depicted. While TT947 and HIV env31 peptides were used as positive controls, pp65495 served as a negative control.
Using a cell-based competitive binding assay, we demonstrated that these three pp65-derived peptides bind DP4 molecules in vitro (Figure 2B). Among the three, pp65#50 was the most potent binder. However, the binding was weaker than previously identified DP4-restricted peptides such as TT947 and HIV env31.
DP4-aAPC generates long-lived pp65#23-specific CD4+ T cells that secrete Th1 cytokines
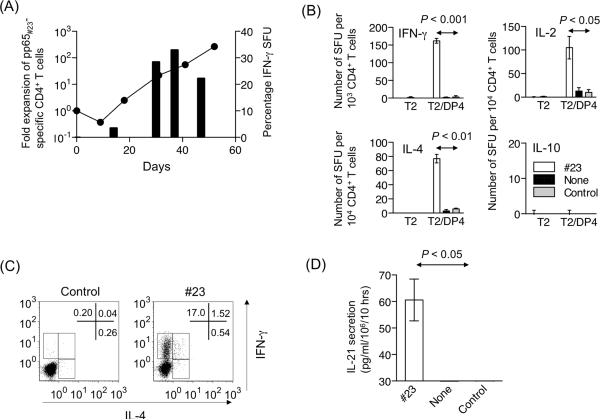
Using DP4-aAPC pulsed with pp65#23 peptide, we were able to readily generate polyfunctional pp65#23-specific CD4+ T cells from all five DP4+ CMV Ab+ donors tested, suggesting that pp65#23 is an immunoprevalent epitope. Purified DP4+ CD4+ T cells were initially stimulated using autologous monocytes pulsed with full-length recombinant pp65 protein. Subsequently, T cells were repeatedly stimulated using pp65#23 peptide-pulsed DP4-aAPC every 9 days. Between stimulations, 10 IU/ml IL-2 and 10 ng/ml IL-15 were added to the T cell cultures every three days. According to this standardized protocol, we were able to maintain DP4-restricted pp65#23-specific CD4+ T cells for greater than 50 days without any allogeneic feeder cells or T cell cloning (Figure 3A). The antigen specificity determined by IFN-γ ELISPOT reached over 30%.
Figure 3. DP4-restricted pp65#23-specific CD4+ T cells generated using DP4-aAPC are long-lived and functionally competent.
(A) DP4-aAPC can generate long-lived pp65#23-specific CD4+ T cells. Purified DP4+ CD4+ T cells were repeatedly stimulated using peptide-pulsed and irradiated DP4-aAPC every 9 days. Between stimulations, 10 IU/ml IL-2 and 10 ng/ml IL-15 were added to the T cell cultures. Representative data is shown where fold expansion of the total cell number of a pp65#23-specific CD4+ T cell line from a healthy donor is depicted with a line (left axis) and percentage of IFN-γ SFU is shown with bars (right axis). We were able to readily generate pp65#23-specific CD4+ T cells from all five DP4+ CMV Ab+ donors tested (data not shown). (B) The cytokine profile of pp65#23-specific CD4+ T cells were also studied for IFN-γ, IL-2, IL-4, and IL-10 by ELISPOT as described in Figure 2A. Note that the IFN-γ assay depicts an input of 103 CD4+ T cells, while 104 cells were input for assays of other cytokines. (C) IFN-γ and IL-4 production by pp65#23-specific CD4+ T cells were analyzed by intracellular cytokine staining using T2/DP4 pulsed with control or pp65#23 peptide as stimulators. (D) Antigen-specific IL-21 production was measured by ELISA using T2/DP4 pulsed with control or pp65#23 peptide as the APC. Parental T2 cells did not induce any IL-21 secretion regardless of the peptides pulsed (data not shown). The limit of detection was 31.3 pg/ml.
ELISPOT assays demonstrated that DP4-restricted pp65#23-specific CD4+ T cells predominantly secreted IFN-γ and much less IL-4 in an antigen-specific manner (Figure 3B). Upon stimulation, they also produced IL-2, but no IL-10 or IL-17 was detected (Figure 3B, Supplementary Figure 1, and data not shown). Intracellular cytokine staining confirmed that pp65#23-specific CD4+ T cells predominantly secreted IFN-γ and that a small fraction of CD4+ T cells produced both IFN-γ and IL-4 in a pp65#23-specific manner (Figure 3C).
Previously, we and others demonstrated that IL-21, predominantly secreted by activated CD4+ T cells, can enhance CD8+ T cell responses and may be a mediator of CD4+ T cell help (24, 28, 29). Using an IL-21 ELISA, we showed that pp65#23-specific CD4+ T cells generated using DP4-aAPC secreted IL-21 in an antigen-specific manner (Figure 3D). No detectable IL-21 was secreted when stimulated by DP4-restricted control peptide, HIV env31. Unfortunately, intracellular staining of human IL-21 is not yet available, and we therefore have not yet determined whether pp65#23-specific CD4+ T cells can secrete IL-21 and other cytokines simultaneously.
Long-lived Survivin-specific CD4+ T cells generated using DP4-aAPC secrete both IFN-γ and IL-4
Survivin is an attractive target antigen for tumor immunotherapy, since it is expressed by many tumor types and is indispensable for tumor growth. It has been demonstrated that Survivin is immunogenic both in vivo and in vitro (30). Recently, seven DP4-restricted Survivin-specific CD4+ T cell epitopes have been identified by other investigators (21). Using the cell-based competitive binding assay described above, we confirmed that, among these peptides, Survivin#90 peptide binds DP4 molecules with the highest potency (Supplementary Table 1).
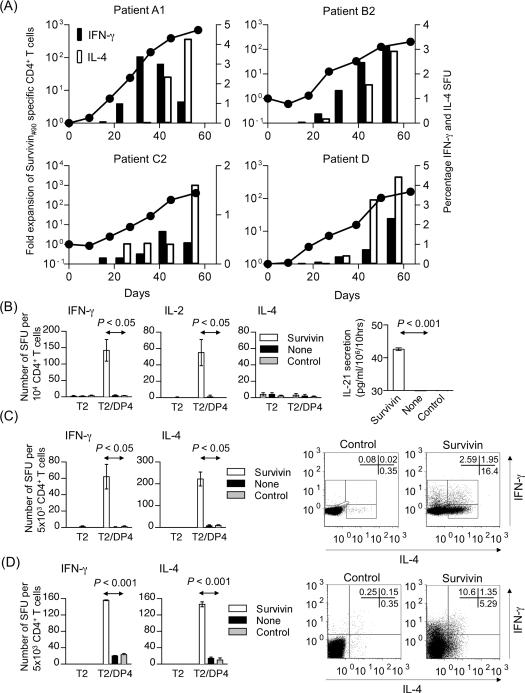
We have successfully generated long-lived Survivin#90-specific DP4-restricted CD4+ T cells in four of six patients with advanced melanoma using DP4-aAPC and characterized their cytokine secretion profile (Supplementary Table 2). While Survivin#90-specific IFN-γ secretion was demonstrated in all generated CD4+ T cell lines, none of the Survivin#90-specific CD4+ T cell lines secreted IL-10 nor IL-17. The secretion of IL-2, IL-4 and IL-21 was detectable in some lines. Longitudinal ELISPOT assays demonstrated that long-lived Survivin#90-specific CD4+ T cells (Patients A1, B2, C2 and D) secreted both IFN-γ and IL-4 in an antigen-specific manner (Figure 4A). In contrast, Survivin#90-specific CD4+ T cells from Patient B1 were capable of secreting IFN-γ, IL-2, and IL-21 but not IL-4 in an antigen-specific way on Day 42 (Figure 4B). Intracellular cytokine staining on Day 51 revealed that Survivin#90-specific CD4+ T cells from Patient A1 were biased towards the Th2 lineage, secreting more IL-4 than IFN-γ (Figure 4C), while Survivin#90-specific CD4+ T cells from Patient B2 were biased towards the Th1 lineage, secreting more IFN-γ than IL-4 on Day 54 (Figure 4D). It should be noted that a small population of cells secreted both IFN-γ and IL-4 simultaneously. These results suggest that both Th1 and Th2 CD4+ T cell responses can be induced against Survivin using DP4-aAPC in vitro.
Figure 4. DP4-restricted Survivin-specific CD4+ T cells generated using DP4-aAPC are long-lived and secrete both IFN-γ and IL-4.
(A) Long-lived DP4-restricted Survivin#90-specific CD4+ T cells were generated from cancer patients as described in Figure 3A. Representative data is shown for four Survivin-specific CD4+ T cell lines (Patients A1, B2, C2 and D) where fold expansion of the total cell number is depicted with solid circles connected by a line (left axis). These cell lines secreted both IFN-γ and IL-4 in a Survivin#90-specific manner. IFN-γ (black bar) and IL-4 (white bar) SFU percentage is shown with bars (right axis). (B) IFN-γ, IL-2, and IL-4 secretion by Survivin#90-specific CD4+ T cells from Patient B1 was studied using ELISPOT on Day 42 as described in Figure 3B. IL-21 production on Day 64 was measured by ELISA as described in Figure 3D. The limit of detection was 31.3 pg/ml. Parental T2 cells did not induce any IL-21 secretion regardless of the peptides pulsed (data not shown). (C) IFN-γ and IL-4 secretion by Survivin-specific CD4+ T cells on Day 51 (Patient A1) was studied by ELISPOT. Intracellular staining of IFN-γ and IL-4 was also conducted by stimulating with T2/DP4 pulsed with control or Survivin#90 peptide. (D) IFN-γ and IL-4 secretion by Survivin-specific CD4+ T cells on Day 54 (Patient B2) was studied by ELISPOT. Intracellular staining was also conducted as described in Figure 4C.
Both Survivin#90-specific Th1 and Th2 cells were able to recognize Survivin-expressing tumors in a DP4-restricted manner
Survivin expression is indispensable for tumor cells, and, to the best of our knowledge, no tumor cell line exists that lacks Survivin expression (31). Furthermore, since the half-life of peptides on MHC class II molecules can be up to two weeks in living cells, it is impractical to block processing and presentation of Survivin-derived peptides via HLA-DP4 molecules by knocking down endogenous expression of Survivin protein (32). Therefore, to demonstrate that Survivin-specific CD4+ T cells can recognize tumor cells, we transduced HLA-DP4 to Survivin-positive renal cancer cell lines (A-498, ACHN, and A-704), which do not express any HLA-DR or DP alleles endogenously and used these tumor cells as targets (Figure 5A). All three cell lines expressed Survivin at the protein level (Figure 5B). As shown in Figure 5C, Patient A1 Survivin-specific CD4+ T cells recognized DP4-transduced but not parental renal cancer cell lines and secreted IFN-γ and IL-4. Patient B1 Survivin-specific CD4+ T cells also recognized all three DP4-transduced but not parental renal cancer cell lines (data not shown). These results indicate that Survivin-specific CD4+ T cells generated using DP4-aAPC can recognize tumor cells with sufficient avidity to induce an anti-tumor response.
Figure 5. Both Survivin-specific Th1 and Th2 cells are able to recognize Survivin-expressing tumors in a DP4-restricted manner.
(A) The renal cancer cell lines, A-498, ACHN, and A-704, are endogenously defective for HLA-DP and DR expression. These cells were retrovirally transduced with HLA-DP4. HLADP and DR expression on parental and DP4-transduced was analyzed by flow cytometry analysis using specific mAbs. (B) Survivin protein expression by renal cancer cell lines, A-498, ACHN, and A-704, was analyzed by immunoblot analysis using specific mAb. (C) DP4-restricted Survivin#90-specific CD4+ T cells recognize DP4+ Survivin+ tumor cells. Survivin#90-specific CD4+ T cells (Patient A1) were incubated with DP4-transduced or parental renal cancer cell lines, A-498, ACHN, and A-704 in IFN-γ and IL-4 ELISPOT. Similar results for IFN-γ secretion were obtained with Survivin#90-specific CD4+ T cells (Patient B1) (data not shown).
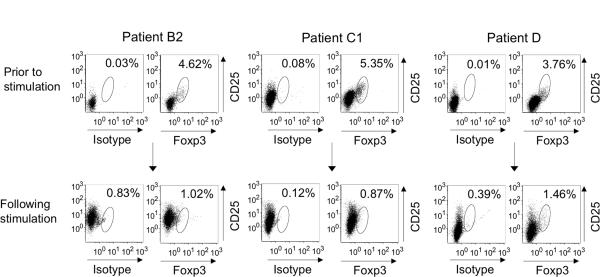
Long-term in vitro culture using DP4-aAPC does not induce the expression of Foxp3 and exhaustion markers
Previously we demonstrated that both HLA-DR-restricted antigen-specific CD4+ T cells and A2-restricted antigen-specific CD8+ T cells expanded using K562-based aAPC lacked the expression of Foxp3 (23, 33). We analyzed Foxp3 expression in Survivin#90-specific CD4+ T cell cultures generated using DP4-aAPC (Figure 6). Before stimulation, CD4+ T cells from Patients B2, C1 and D demonstrated 4.62%, 5.71% and 3.76% Foxp3 positivity respectively. After repeated stimulations, Survivin#90-specific CD4+ T cell cultures were only 1.02%, 1.41% and 1.46% positive for Foxp3 on Day 59, Day 77, and Day 75 respectively. These results suggest that under the T cell culture conditions employed, K562-based-aAPC did not induce the expansion of Foxp3+ Treg.
Figure 6. Long-term in vitro culture using DP4-aAPC does not induce the expression of Foxp3.
DP4-restricted Survivin-specific CD4+ T cells (Patients B2, C1 and D) were analyzed for the intracellular expression of Foxp3. Total CD4+ T cells in culture were gated. Purified CD4+ T cells from the same donor prior to stimulation were also studied for comparison.
It has been shown that chronic stimulation can exhaust antigen-specific T cells as manifested by the cessation of cytokine secretion and the induction of exhaustion marker expression (34). Previously, we demonstrated that long-lived T cells cultured in vitro using K562-derived A2-aAPC were functionally competent and displayed a memory phenotype without inducing exhaustion marker expression (23, 35). We have also studied whether Survivin#90-specific CD4+ T cells, which were long-lived and preserved the ability to secrete cytokine, expressed exhaustion molecules after prolonged culture (Supplementary Figure 2). DP4-restricted Survivin#90-specific CD4+ T cells kept in culture for 76 days (Patient B1) and 92 days (Patient C1) using DP4-aAPC were subjected to flow cytometry after staining with specific mAbs. These long-lived T cells induced very low or no expression of PD-1, CTLA-4, and LAG-3. In contrast, CD25 expression was well preserved on these long-lived CD4+ T cells. These results suggest that long-term in vitro culture using DP4-aAPC induced minimal or no expression of exhaustion molecules on antigen-specific CD4+ T cells.
Discussion
Using a novel K562-derived HLA-DP4 expressing artificial APC, we induced and characterized DP4-restricted anti-viral and tumor antigen-specific CD4+ T cell responses in vitro. We were able to identify three novel, immunogenic pp65-derived DP4-restricted epitope peptides. Among them, pp65#23 appeared to be the most immunoprevalent, since we were able to generate specific CD4+ T cells from all five donors tested. Another newly identified CD4+ T cell epitope, pp65#50, is intriguing since it encompasses the well-known immunodominant A2-restricted CD8+ T cell epitope, pp65495 (495NLVPMVATV503). The overlapping of CD8+ and CD4+ T cell epitopes has been reported for many other antigens (15). In fact, the previously described Survivin-derived A2 peptide, ELTLGEFLKL, also overlaps with the DP4-restricted Survivin#90 peptide, 90KKQFEELTLGEFLKL104, used in this study (21, 24).
HLA class II/peptide multimers have been generated for a limited number of HLA class II alleles and high binding peptides (36, 37). However, unlike HLA class I/peptide multimers, class II tetramers have been much more difficult to generate. It is believed that this is due, at least partially, to the generally weaker binding of peptides to class II compared to class I molecules. In fact, our attempt to generate HLA-DP4/pp65#23 and Survivin#90 peptide tetramers failed. There is no denying that HLA/peptide multimers are critical reagents. Multimer staining will enable the detailed characterization as well as the isolation of the whole or subpopulations of peptide-specific T cells. Development of novel methodologies to efficiently produce class II multimers for less strongly binding peptides is warranted.
We have shown that both CMV and Survivin-specific CD4+ T cells generated using DP4-aAPC can secrete IL-21 in an antigen-specific manner. We and others previously demonstrated ex vivo that IL-21 can enhance antigen-specific CD8+ T cell responses and may be a mediator of CD4+ T cell help (24, 29). Recently, in mouse models of chronic infection, it has been demonstrated that IL-21 is a mediator of CD4+ T cell help for CD8+ T cells in vivo (38). IL-2, another cytokine that shares the common cytokine receptor γ chain with IL-21, has also been shown to transmit CD4+ T cell help in various models (39). Since antigen-specific CD4+ T cells generated using our DP4-aAPC can secrete both IL-2 and IL-21, it is conceivable that these cytokines could independently or cooperatively mediate CD4+ T cell help to CD8+ CTL. It should be also noted that, unlike IL-2, which is indispensable for the maintenance and expansion of Foxp3+ Treg, IL-21 suppresses the expansion of Foxp3+ Treg, which is in accordance with our data that DP4-aAPC do not expand Treg cells (Figure 6) (40).
Accumulating evidence indicates that Survivin is expressed in normal adult cells, particularly hematopietic cells, and may regulate their proliferation and survival (41). This low level expression may induce tolerance by deletion of the Survivin-specific T cell repertoire, especially T cells with high avidity. However, we were able to generate long-lived Survivin-specific CD4+ T cells from 4 out of 6 cancer patients that expanded over 100–1,000-fold, produced IL-2, IL-4, IFN-γ and IL-21 in an antigen-specific way, and recognized tumor cells. These results suggest that the Survivin-specific T cell repertoire is not completely deleted and that functionally competent Survivin-specific CD4+ T cells with sufficient avidity can be expanded in vitro.
There are several splice variants for Survivin (42). The immunogenic peptide Survivin#90 is encoded by wild type Survivin as well as the 2B and 3B splice variants. In contrast, the variant ΔEx3 lacks this peptide sequence. It has been reported that expression of the ΔEx3 isoform correlates with poor survival and increased tumor aggressiveness (43–46). However, it is very rare that tumor cells express the ΔEx3 isoform in the absence of wild type Survivin (47). Co-expression with wild type Survivin appears to be necessary for proliferation of cells expressing ΔEx3 (48). While the expression of wild type Survivin could restore proliferation of HeLa and U2OS cells depleted of pan-Survivin expression by RNAi, expression of the ΔEx3 variant alone could not. In fact, it has been suggested that the ΔEx3 variant's function is dependent on its ability to form a heterodimer with wild type Survivin (48, 49). Finally, in virtually all tumors studied, the wild type Survivin expression level is much higher than the splice variants (44, 50). Taken all together, these observations suggest that the Survivin#90 epitope, encoded by wild type Survivin, is desirable as a CD4+ T cell epitope for Survivin-targeted immunotherapy.
The mechanism which causes T cell exhaustion is still largely unknown. We previously reported that long-lived CD8+ T cells generated in vitro with A2-aAPC in the absence of CD4+ T cells did not express PD-1 or CTLA-4 (23). In this study, we demonstrated that DP4-aAPC also did not induce an exhausted immunophenotype on antigen-specific CD4+ T cells after in vitro culture for more than 2 months, while preserving their ability to secrete cytokines. These results suggest that there exists a common mechanism whereby our T cell culture system, based on K562-derived aAPC, prevents the induction of an exhausted immunophenotype to both CD4+ and CD8+ T cells. In this context, it should be pointed out that we have yet to observe a conversion of the phenotype of antigen-specific T cells from CD45RO+/CD45RA− memory to CD45RO−/CD45RA+ terminally differentiated effector after long-term in vitro culture. It is possible that K562-based aAPC may deliver a signal, which halts the differentiation of T cells at the late memory stage, thus preventing terminal differentiation and/or exhaustion. Whether these antigen-specific T cells, cultured in vitro with K562-based aAPC, are exhausted or not, however, can be only determined in humans, i.e. in clinical trials. We have recently conducted a clinical trial where large numbers of MART1-specific CTL generated in vitro using A2-aAPC, IL-2 and IL-15 were infused to patients with advanced melanoma without lymphodepletion or IL-2 therapy (35). It is very encouraging that MART1-specific CTL with a memory phenotype persisted in patients as phenotypically and functionally memory T cells longer than 16 months after infusion.
It is intriguing that Survivin-specific CD4+ T cells generated in vitro with DP4-aAPC can secrete both IFN-γ and IL-4. In principle, IFN-γ, a Th1 cytokine, and IL-4, a Th2 cytokine, enhance cellular and humoral responses, respectively. However, the role of IL-4 in anti-tumor immunity has been paradoxical and controversial. While IL-4 exhibits potent anti-tumor ability in some mouse models, it can promote tumor growth and inhibit anti-tumor Th1 responses in others. Therefore, the role of anti-Survivin Th2 responses has yet to be established in anti-tumor immunity. If it is concluded that IL-4 secreting anti-Survivin CD4+ T cells negatively impact anti-tumor responses, we may need to modify our DP4-aAPC based CD4+ T cell culture system to favor the generation of IFN-γ secreting Th1 cells for the purpose of adoptive therapy. Furthermore, anti-Survivin vaccine therapy may need to be modified so that a Th1-skewed anti-Survivin response is induced in humans.
Supplementary Material
To demonstrate that CD4+ T cells freshly purified from cancer patients (Patients B and C) were able to secrete IL-10, they were maximally stimulated with PHA and were subjected to IL-10 ELISPOT assay.
The protein expression of PD-1, CTLA-4, LAG-3, and CD25 on Survivin#90-specific CD4+ T cells from Patient B1 expanded using DP4-aAPC for 76 days was analyzed by flow cytometry. Results for Survivin#90-specific CD4+ T cells from Patient C1 after 92 days of culture are also shown. Resting CD4+ T cells and PHA-activated CD4+ T cells were also stained as negative and positive controls.
Statement of translational relevance.
Adoptive transfer of in vitro generated anti-tumor lymphocytes can establish clinically effective anti-tumor memory. Previously, we reported a clinical grade, K562-derived HLA-A2 artificial antigen-presenting cell (aAPC) that generates in vitro large numbers of long-lived anti-tumor CD8+ T cells (CTL) with a memory phenotype from cancer patients. Recent clinical trials demonstrated that, upon adoptive transfer, autologous anti-tumor CTL generated in vitro using this aAPC, IL-2, and IL-15 can persist as memory T cells and induce biologic responses in cancer patients without lymphodepletion or IL-2 therapy. Herein, we developed a K562-derived HLA-DP4 aAPC and studied HLA-DP4-restricted anti-Survivin CD4+ T cell responses in cancer patients. Our data suggests that cancer patients can mount both Th1 and Th2 responses against Survivin with sufficient avidity to recognize tumor cells. Considering our previous success in translating K562-derived A2-aAPC to the clinic and the high prevalence of HLA-DP4, development of a clinical grade, K562-derived DP4-aAPC is warranted.
Acknowledgments
Grant support: MT, MOB, OI, and LMN were supported with funds from the Cancer Research Institute. NH was funded by NIH grants, K22CA129240 and R01CA148673, and the American Society of Hematology Scholar Award.
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
References
- 1.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 2.Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy: fulfilled promises and open questions. Carcinogenesis. 2007;28:1133–9. doi: 10.1093/carcin/bgm047. [DOI] [PubMed] [Google Scholar]
- 3.Tolcher AW, Mita A, Lewis LD, Garrett CR, Till E, Daud AI, et al. Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J Clin Oncol. 2008;26:5198–203. doi: 10.1200/JCO.2008.17.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan BM, O'Donovan N, Duffy MJ. Survivin: a new target for anti-cancer therapy. Cancer Treat Rev. 2009;35:553–62. doi: 10.1016/j.ctrv.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Andersen MH, Pedersen LO, Capeller B, Brocker EB, Becker JC, thor Straten P. Spontaneous cytotoxic T-cell responses against survivin-derived MHC class I-restricted T-cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res. 2001;61:5964–8. [PubMed] [Google Scholar]
- 6.Hirohashi Y, Torigoe T, Maeda A, Nabeta Y, Kamiguchi K, Sato T, et al. An HLA-A24-restricted cytotoxic T lymphocyte epitope of a tumor-associated protein, survivin. Clin Cancer Res. 2002;8:1731–9. [PubMed] [Google Scholar]
- 7.Reker S, Becker JC, Svane IM, Ralfkiaer E, Straten PT, Andersen MH. HLA-B35-restricted immune responses against survivin in cancer patients. Int J Cancer. 2004;108:937–41. doi: 10.1002/ijc.11634. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt SM, Schag K, Muller MR, Weck MM, Appel S, Kanz L, et al. Survivin is a shared tumor-associated antigen expressed in a broad variety of malignancies and recognized by specific cytotoxic T cells. Blood. 2003;102:571–6. doi: 10.1182/blood-2002-08-2554. [DOI] [PubMed] [Google Scholar]
- 9.Siegel S, Wagner A, Schmitz N, Zeis M. Induction of antitumour immunity using survivin peptide-pulsed dendritic cells in a murine lymphoma model. Br J Haematol. 2003;122:911–4. doi: 10.1046/j.1365-2141.2003.04535.x. [DOI] [PubMed] [Google Scholar]
- 10.Pisarev V, Yu B, Salup R, Sherman S, Altieri DC, Gabrilovich DI. Full-length dominant-negative survivin for cancer immunotherapy. Clin Cancer Res. 2003;9:6523–33. [PubMed] [Google Scholar]
- 11.Tsuruma T, Hata F, Torigoe T, Furuhata T, Idenoue S, Kurotaki T, et al. Phase I clinical study of anti-apoptosis protein, survivin-derived peptide vaccine therapy for patients with advanced or recurrent colorectal cancer. J Transl Med. 2004;2:19. doi: 10.1186/1479-5876-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wobser M, Keikavoussi P, Kunzmann V, Weininger M, Andersen MH, Becker JC. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother. 2005:1–5. doi: 10.1007/s00262-005-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weide B, Pascolo S, Scheel B, Derhovanessian E, Pflugfelder A, Eigentler TK, et al. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J Immunother. 2009;32:498–507. doi: 10.1097/CJI.0b013e3181a00068. [DOI] [PubMed] [Google Scholar]
- 14.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54:721–8. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev. 2008;222:129–44. doi: 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- 16.Schultz ES, Lethe B, Cambiaso CL, Van Snick J, Chaux P, Corthals J, et al. A MAGE-A3 peptide presented by HLA-DP4 is recognized on tumor cells by CD4+ cytolytic T lymphocytes. Cancer Res. 2000;60:6272–5. [PubMed] [Google Scholar]
- 17.Zeng G, Wang X, Robbins PF, Rosenberg SA, Wang RF. CD4(+) T cell recognition of MHC class II-restricted epitopes from NY-ESO-1 presented by a prevalent HLA DP4 allele: association with NY-ESO-1 antibody production. Proc Natl Acad Sci U S A. 2001;98:3964–9. doi: 10.1073/pnas.061507398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castelli FA, Buhot C, Sanson A, Zarour H, Pouvelle-Moratille S, Nonn C, et al. HLA-DP4, the most frequent HLA II molecule, defines a new supertype of peptide-binding specificity. J Immunol. 2002;169:6928–34. doi: 10.4049/jimmunol.169.12.6928. [DOI] [PubMed] [Google Scholar]
- 19.de Graaff PM, Heidema J, Poelen MC, van Dijk ME, Lukens MV, van Gestel SP, et al. HLA-DP4 presents an immunodominant peptide from the RSV G protein to CD4 T cells. Virology. 2004;326:220–30. doi: 10.1016/j.virol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Cohen WM, Pouvelle-Moratille S, Wang XF, Farci S, Munier G, Charron D, et al. Scanning the HIV genome for CD4+ T cell epitopes restricted to HLA-DP4, the most prevalent HLA class II molecule. J Immunol. 2006;176:5401–8. doi: 10.4049/jimmunol.176.9.5401. [DOI] [PubMed] [Google Scholar]
- 21.Wang XF, Kerzerho J, Adotevi O, Nuyttens H, Badoual C, Munier G, et al. Comprehensive analysis of HLA-DR- and HLA-DP4-restricted CD4+ T cell response specific for the tumor-shared antigen survivin in healthy donors and cancer patients. J Immunol. 2008;181:431–9. doi: 10.4049/jimmunol.181.1.431. [DOI] [PubMed] [Google Scholar]
- 22.Hirano N, Butler MO, Xia Z, Ansen S, von Bergwelt-Baildon MS, Neuberg D, et al. Engagement of CD83 ligand induces prolonged expansion of CD8+ T cells and preferential enrichment for antigen specificity. Blood. 2006;107:1528–36. doi: 10.1182/blood-2005-05-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler MO, Lee JS, Ansen S, Neuberg D, Hodi FS, Murray AP, et al. Long-lived antitumor CD8+ lymphocytes for adoptive therapy generated using an artificial antigen-presenting cell. Clin Cancer Res. 2007;13:1857–67. doi: 10.1158/1078-0432.CCR-06-1905. [DOI] [PubMed] [Google Scholar]
- 24.Ansen S, Butler MO, Berezovskaya A, Murray AP, Stevenson K, Nadler LM, et al. Dissociation of its opposing immunologic effects is critical for the optimization of antitumor CD8+ T-cell responses induced by interleukin 21. Clin Cancer Res. 2008;14:6125–36. doi: 10.1158/1078-0432.CCR-08-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirano N, Butler MO, Xia Z, Berezovskaya A, Murray AP, Ansen S, et al. Identification of an immunogenic CD8+ T-cell epitope derived from gamma-globin, a putative tumor-associated antigen for juvenile myelomonocytic leukemia. Blood. 2006;108:2662–8. doi: 10.1182/blood-2006-04-017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirano N, Butler MO, Xia Z, Berezovskaya A, Murray AP, Ansen S, et al. Efficient presentation of naturally processed HLA class I peptides by artificial antigen-presenting cells for the generation of effective antitumor responses. Clin Cancer Res. 2006;12:2967–75. doi: 10.1158/1078-0432.CCR-05-2791. [DOI] [PubMed] [Google Scholar]
- 27.Hirano N, Takahashi T, Ohtake S, Hirashima K, Emi N, Saito K, et al. Expression of costimulatory molecules in human leukemias. Leukemia. 1996;10:1168–76. [PubMed] [Google Scholar]
- 28.Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–33. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Bleakley M, Yee C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J Immunol. 2005;175:2261–9. doi: 10.4049/jimmunol.175.4.2261. [DOI] [PubMed] [Google Scholar]
- 30.Andersen MH, Svane IM, Becker JC, Straten PT. The universal character of the tumor-associated antigen survivin. Clin Cancer Res. 2007;13:5991–4. doi: 10.1158/1078-0432.CCR-07-0686. [DOI] [PubMed] [Google Scholar]
- 31.Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–20. [PubMed] [Google Scholar]
- 32.Lanzavecchia A, Reid PA, Watts C. Irreversible association of peptides with class II MHC molecules in living cells. Nature. 1992;357:249–52. doi: 10.1038/357249a0. [DOI] [PubMed] [Google Scholar]
- 33.Butler MO, Ansen S, Tanaka M, Imataki O, Berezovskaya A, Mooney MM, et al. A panel of human cell-based artificial APC enables the expansion of long-lived antigen-specific CD4+ T cells restricted by prevalent HLA-DR alleles. Int Immunol. 2010;22:863–73. doi: 10.1093/intimm/dxq440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed R, Bevan MJ, Reiner SL, Fearon DT. The precursors of memory: models and controversies. Nat Rev Immunol. 2009;9:662–8. doi: 10.1038/nri2619. [DOI] [PubMed] [Google Scholar]
- 35.Butler MO, Friedlander P, Milstein MI, Mooney MM, Metzler G, Murray AP, et al. Establishment of Antitumor Memory in Humans Using in Vitro-Educated CD8+ T Cells. Sci Transl Med. 2011;3:80ra34. doi: 10.1126/scitranslmed.3002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James EA, LaFond R, Durinovic-Bello I, Kwok W. Visualizing antigen specific CD4+ T cells using MHC class II tetramers. J Vis Exp. 2009 doi: 10.3791/1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vollers SS, Stern LJ. Class II major histocompatibility complex tetramer staining: progress, problems, and prospects. Immunology. 2008;123:305–13. doi: 10.1111/j.1365-2567.2007.02801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson LD, Jameson SC. Immunology. A chronic need for IL-21. Science. 2009;324:1525–6. doi: 10.1126/science.1176487. [DOI] [PubMed] [Google Scholar]
- 39.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–40. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Yee C. IL-21 mediated Foxp3 suppression leads to enhanced generation of antigen-specific CD8+ cytotoxic T lymphocytes. Blood. 2008;111:229–35. doi: 10.1182/blood-2007-05-089375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukuda S, Pelus LM. Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther. 2006;5:1087–98. doi: 10.1158/1535-7163.MCT-05-0375. [DOI] [PubMed] [Google Scholar]
- 42.Sampath J, Pelus LM. Alternative splice variants of survivin as potential targets in cancer. Curr Drug Discov Technol. 2007;4:174–91. doi: 10.2174/157016307782109652. [DOI] [PubMed] [Google Scholar]
- 43.Yamada Y, Kuroiwa T, Nakagawa T, Kajimoto Y, Dohi T, Azuma H, et al. Transcriptional expression of survivin and its splice variants in brain tumors in humans. J Neurosurg. 2003;99:738–45. doi: 10.3171/jns.2003.99.4.0738. [DOI] [PubMed] [Google Scholar]
- 44.Ling X, Yang J, Tan D, Ramnath N, Younis T, Bundy BN, et al. Differential expression of survivin-2B and survivin-DeltaEx3 is inversely associated with disease relapse and patient survival in non-small-cell lung cancer (NSCLC) Lung Cancer. 2005;49:353–61. doi: 10.1016/j.lungcan.2005.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taubert H, Kappler M, Bache M, Bartel F, Kohler T, Lautenschlager C, et al. Elevated expression of survivin-splice variants predicts a poor outcome for soft-tissue sarcomas patients. Oncogene. 2005;24:5258–61. doi: 10.1038/sj.onc.1208702. [DOI] [PubMed] [Google Scholar]
- 46.Li XN, Shu Q, Su JM, Adesina AM, Wong KK, Perlaky L, et al. Differential expression of survivin splice isoforms in medulloblastomas. Neuropathol Appl Neurobiol. 2007;33:67–76. doi: 10.1111/j.1365-2990.2006.00782.x. [DOI] [PubMed] [Google Scholar]
- 47.O'Driscoll L, Linehan R, S MK, Cronin D, Purcell R, Glynn S, et al. Lack of prognostic significance of survivin, survivin-deltaEx3, survivin-2B, galectin-3, bag-1, bax-alpha and MRP-1 mRNAs in breast cancer. Cancer letters. 2003;201:225–36. doi: 10.1016/s0304-3835(03)00518-4. [DOI] [PubMed] [Google Scholar]
- 48.Noton EA, Colnaghi R, Tate S, Starck C, Carvalho A, Ko Ferrigno P, et al. Molecular analysis of survivin isoforms: evidence that alternatively spliced variants do not play a role in mitosis. The Journal of biological chemistry. 2006;281:1286–95. doi: 10.1074/jbc.M508773200. [DOI] [PubMed] [Google Scholar]
- 49.Caldas H, Jiang Y, Holloway MP, Fangusaro J, Mahotka C, Conway EM, et al. Survivin splice variants regulate the balance between proliferation and cell death. Oncogene. 2005;24:1994–2007. doi: 10.1038/sj.onc.1208350. [DOI] [PubMed] [Google Scholar]
- 50.Ryan B, O'Donovan N, Browne B, O'Shea C, Crown J, Hill AD, et al. Expression of survivin and its splice variants survivin-2B and survivin-DeltaEx3 in breast cancer. British journal of cancer. 2005;92:120–4. doi: 10.1038/sj.bjc.6602314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
To demonstrate that CD4+ T cells freshly purified from cancer patients (Patients B and C) were able to secrete IL-10, they were maximally stimulated with PHA and were subjected to IL-10 ELISPOT assay.
The protein expression of PD-1, CTLA-4, LAG-3, and CD25 on Survivin#90-specific CD4+ T cells from Patient B1 expanded using DP4-aAPC for 76 days was analyzed by flow cytometry. Results for Survivin#90-specific CD4+ T cells from Patient C1 after 92 days of culture are also shown. Resting CD4+ T cells and PHA-activated CD4+ T cells were also stained as negative and positive controls.