Abstract
Phenolic compounds were extracted from defatted flaxseeds using ethanol-dioxane (1:1, v/v). The crude extract obtained was purified using Amberlite XAD-16 column chromatography with water and methanol as mobile phases. RP-HPLC and SE-HPLC showed a lignan macromolecule (LM) as a dominant phenolic compound in the purified extract. After the alkaline hydrolysis of LM caffeic acid glucoside (CaAG) was isolated using a semi-preparative HPLC and its structure was confirmed by LC-ESI-MS. In LM of the investigated flaxseed, one molecule of caffeic acid corresponded with five molecules of p-coumaric acid and two molecules of ferulic acid. The presence of caffeic acid in the lignan molecule might be very beneficial due to its high antioxidant activity.
Keywords: Flaxseed, Phenolic compounds, Lignan macromolecule, Caffeic acid
Introduction
Flaxseed (Linum usitatissimum L.) is the richest known source of plant lignans [1]. Secoisolariciresinol diglucoside (SDG) is the main lignan of flaxseed and represents over 95% of the total lignans in this plant [2, 3]. Plant lignans are converted by colon bacteria to mammalian lignans: enterodiol and enterolactone. Health benefits of flaxseed lignans have been linked to the prevention of CVD, hypercholesterolemia, menopausal symptoms, and breast, prostate and colon cancers [4–8]. Additionally, the beneficial effects of flaxseed on human health may result from its antioxidant capacity. Results of numerous studies confirmed antioxidant activity of flaxseed lignans in different experimental model systems [9–13]. However, also non-lignan phenolic compounds present in flaxseed may contribute to its antioxidant potential.
Lignans in flaxseed are present as an oligomeric structure, called lignan macromolecule (LM) [14]. Recently, the structure and size of lignan macromolecule is a subject of careful investigation [14–19]. Based on NMR analysis a straight chain oligomeric structure composed of five SDG residues interconnected by four HMGA residues (molecular weight of about 4,000 Da) was revealed by Kamal-Eldin et al. [16]. Within LM, SDG is ester linked by 3-hydroxy-3-methylglutaric acid (HMGA). Herbacetin diglucoside and hydroxycinnamic acids glucosides: 4-O-β-glucopyranosyl-p-coumaric acid (CouAG), 4-O-β-glucopyranosyl-ferulic acid (FeAG) were reported to be part of LM, since they are released from flaxseed extract after alkali treatment [14–18]. Struijs et al. [19] reported that CouAG and FeAG were incorporated into the lignan macromolecule via ester-linkage of their carboxyl groups to glucosyl moieties of SDG. The attachment of HMGA to the glucosyl moiety of CouAG or FeAG was not observed, therefore it was suggested that glucosides of hydroxycinnamic acids were terminal units of the lignan macromolecule. The assumption that caffeic acid glucoside might be a part of the lignan macromolecule has also surfaced [14, 19]. To our knowledge, however, it has not been confirmed so far. The aim of the presented study was to verify whether caffeic acid is one of the components constituting lignan macromolecule of flaxseed.
Materials and Methods
Chemicals and Standards
All solvents and reagents were of analytical (ACS) grade or better, unless otherwise specified. Methanol, acetonitrile and acetic acid of HPLC grade were purchased form Sigma-Aldrich Co. Ltd. (Poznań, Poland) whereas ethanol, dioxane and hexane were acquired from P.O.Ch. Company (Gliwice, Poland). Phenolic acid standards (including p-coumaric acid, ferulic acid, caffeic acid) as well as Amberlite XAD-16 were obtained from Sigma-Aldrich Co. Ltd. (Poznań, Poland). RP-18 gel was purchased from Merck (Darmstad, Germany).
Plant Material
Partially defatted flaxseeds available on organic food market in Poland were acquired from “Ekoprodukt” company (Częstochowa, Poland).
Extraction Procedure
Flaxseeds were ground and defatted with hexane. Then phenolic compounds were extracted using a mixture of dioxane and ethanol (1:1, v/v) [20]. Extraction was carried out for 16 h, at 60 °C with continuous shaking in a water bath (357 Elpan, Lubawa, Poland). Then, solvent was evaporated at 40 °C using a Büchi Rotavapor R-200 (Büchi Labortechnik, Flawil, Switzerland). The crude extract obtained was purified in order to remove co-extracted carbohydrates using a column chromatography on Amberlite XAD-16 with water and methanol as mobile phases [21].
Release of Monomeric Compounds from Lignan Macromolecule
Alkaline Hydrolysis
The purified extract was suspended in 0.3 M NaOH, and left for two days at room temperature under continuous shaking. The obtained hydrolysate was acidified to pH 3 using 2 M HCl [20] and subjected to column chromatography on RP-18 gel. Water soluble compounds were eluted with distilled water and discarded, whereas phenolic compounds liberated from the lignan macromolecule were eluted with methanol. The solvent was removed using rotary evaporator.
Acid Hydrolysis
A part of alkaline hydrolysate was subsequently subjected to acid hydrolysis. Briefly, hydrolysate was suspended in 2 M HCl and heated for 2 h at 100 °C [22]. Then the hydrolysate was cooled down, neutralized with NaOH and subjected to column chromatography on RP-18 gel according to the procedure described above.
Chromatographic Analyses
Analytical Reversed-phase HPLC
The purified extract was analyzed using a prepacked Luna C18 (250 × 4.6 mm, 5 μm particle size, Phenomenex, Torrance, CA) column coupled to a Shimadzu system (Shimadzu Co., Kyoto, Japan) consisting of LC-10ADVp pumps, SCL 10AVp system controller, SPD-M10AVp diode array detector. A flow rate of 1 ml/min, and gradient elution of acetonitrile-water-acetic acid (5:93:2, v/v/v) [solvent A] and of acetonitrile-water-acetic acid (40:58:2, v/v/v) [solvent B], 0–50 min solvent B from 0 to 100% were applied [23]. The concentration of sample dissolved in methanol was 2 mg/ml; injection volume 20 μl; separation of compounds was monitored at 280 nm. The samples were also analyzed applying an isocratic elution of acetonitrile-water-acetic acid (10:88:2, v/v/v) using the same Shimadzu system [24]. The chromatograms were recorded at 320 nm.
Size Exclusion HPLC
The purified extract was analyzed using a TSK G2000SWXL column (300 × 7.8 mm, 5 μm; TosoHaas) and the same Shimadzu system. The mobile phase, consisting of 45% (v/v) acetonitrile and 0.1% TFA (v/v) was delivered at a rate of 0.2 ml/min [25]. Samples (20 μl) at a concentration of 2 mg/ml in methanol were injected manually onto the column. Separation was monitored at 280 nm.
Semi-preparative RP-HPLC
The compound tentatively identified as CaAG was separated from the alkaline hydrolysate on a Luna C18 column (250 × 10 mm, 5 μm; Phenomenex) using the Shimadzu system described above. The separation was performed in an isocratic mode with acetonitrile-water-acetic acid (5:92:2, v/v/v) as a mobile phase. The flow rate was 4 ml/min and injection volume of 500 μl was applied.
LC-ESI-MS
The compound isolated from the flaxseed hydrolysate and tentatively identified as CaAG was dissolved in 80% (v/v) methanol and injected on a Luna C18(2) column (150 × 2 mm; 3 μm particle size; Phenomenex) coupled to a Shimadzu LC MS—QP 8000 system (Kyoto, Japan). A mobile phase of 80% methanol (v/v) was delivered isocratically at a flow rate of 0.2 ml/min. The mass spectrum was obtained in the negative ESI ionisation mode over an m/z range of 100–1,200. The temperature and voltage of the curved desolvation line were set to 230 °C; and +85 V, respectively. The probe voltage and nitrogen nebulizer gas flow were set at +4.5 kV and 4.5 l/min, respectively. The defragmentation voltage was +45 V.
Results
RP-HPLC and SE-HPLC chromatograms of the purified extract of flaxseed (Fig. 1) showed only one broad peak suggesting the presence of heterogenic macromolecule of lignans. Free phenolic acids or their glucosides were not detected in the extract within the structure of native LM. In order to get insight into the composition of the molecule structure, the alkaline hydrolysis was performed. Under alkaline conditions the ester-linkages are degraded while the glycosidic bonds are stable. The compounds released by alkaline hydrolysis were analyzed by RP-HPLC applying an isocratic separation with a mobile phase of acetonitrile-water-acetic acid (10:88:2, v/v/v). The chromatogram recorded at 320 nm revealed the presence of three peaks (Fig. 2b). Based on the literature data and our previous studies we expected the occurrence of peaks 2 and 3 assigned as CouAG and FeAG, respectively. Yet, the presence of compound (peak 1) with higher polarity than CouAG was surprising. The subsequent acid hydrolysis of the alkaline-hydrolyzed flaxseed extract released phenolic compounds from the glycosidic bonds. The compounds present in alkaline and acid hydrolysate were tentatively identified as caffeic, p-coumaric and ferulic acids (Fig. 2c) by retention time mapping with external commercial standards. Those findings led to the assumption that the unidentified peak observed in the separation of alkaline hydrolysate with retention time of 4.8 min might be originated from caffeic acid glucoside. To confirm this assumption this polar compound was separated from the alkaline hydrolysate using a semi-preparative HPLC, and analyzed using LC-ESI-MS. In its ESI-MS spectrum, a negative molecular ion [M-H]- at m/z 341 was recorded which corresponds to caffeic acid linked to glucose (CaAG), and one fragment ion [M-H]- at m/z 179, corresponding to deprotonated glucose or caffeic acid (Fig. 3).
Fig. 1.
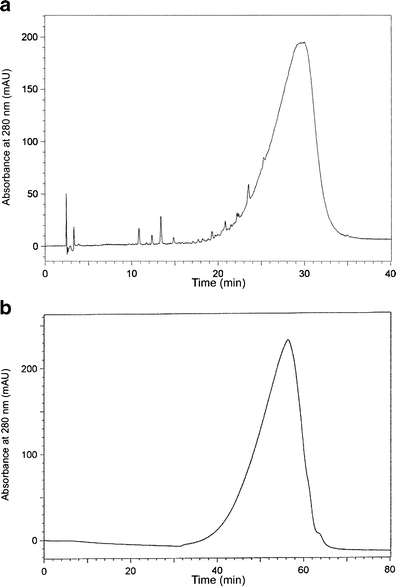
Chromatograms of (a) RP-HPLC (gradient system) and (b) SE-HPLC separations of lignan macromolecule recorded at 280 nm
Fig. 2.
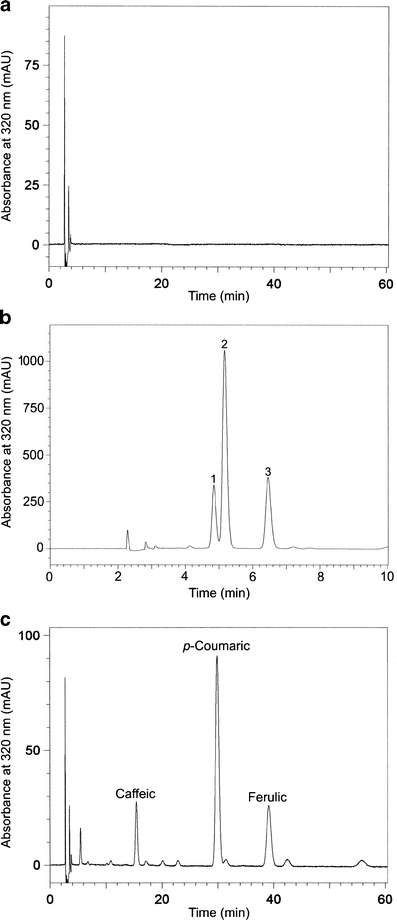
RP-HPLC chromatograms of (a) lignan macromolecule; (b) alkaline hydrolysate and (c) alkaline and acid-hydrolysates of flaxseed lignan macromolecule recorded at 320 nm. The separations were performed using an isocratic elution of acetonitrile-water-acetic acid (10:88:2, v/v/v)
Fig. 3.
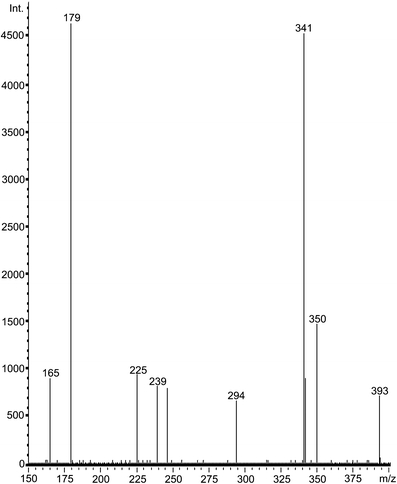
ESI mass spectrum of CaAG isolated from alkaline hydrolysate of flaxseed lignan macromolecule
Contents of caffeic, p-coumaric, and ferulic acids in flaxseed lignan molecule were quantified based on peak areas in reference to those obtained for corresponding external standards. The data presented in Table 1 was recalculated to obtain the molar ratio between individual phenolic acids in LM of the investigated flaxseed. The molar ratio of caffeic acid : p-coumaric acid:ferulic acid amounted to 1:4.71:2.14, which suggests that one molecule of caffeic acid corresponds to five molecules of p-coumaric acid and two molecules of ferulic acid in flaxseed lignan macromolecule.
Table 1.
Contents of phenolic acids in hydrolysates of lignan macromolecule
| Type of hydrolysate | Caffeic acid | p-Coumaric acid | Ferulic acid |
|---|---|---|---|
| Alkaline-hydrolysate | – | – | – |
| Alkaline and acid-hydrolysate | 3.23 | 13.88 | 7.46 |
Expressed as mg per g of preparation
–Not detected
Discussion
Up to date investigations of the structure of flaxseed lignan macromolecule were performed using RP-HPLC in gradient systems [14–20, 22, 23]. However, the systems applied did not reveal the presence of caffeic acid glucoside. To our knowledge, in the literature data the assumption that CaAG might be a part of flaxseed lignan macromolecule was mentioned only by Struijs et al. [14, 19]. The authors reported that the semi-preparative separation of CouAG enabled to obtain the compound of 97% purity, and its MS analysis showed that besides CouAG a compound with an m/z ratio of 340.9 ([M-H]-) was present. This compound was identified as caffeic acid glucoside. However, the subsequent NMR analysis confirmed the presence and structure of SDG, CouAG, FeAG, and HDG only, released from the lignan macromolecule after alkali treatment. In the presented study, the isocratic elution applied enabled the separation of CaAG, CouAG and FeAG in the alkaline hydrolysate of flaxseed lignan macromolecule as well as cafffeic, p-coumaric and ferulic acids after subsequent acid hydrolysis of the alkaline hydrolysate. The semi-preparative separation of CaAG and LC-ESI-MS analysis of that compound confirmed its structure.
The presence of caffeic acid in lignan molecule increases its antioxidant potential. The caffeic acid glucoside liberated from the LM after alkaline hydrolysis possesses a free hydroxyl group which can act as a free radical scavenger, in contrast to 4-O-β-glucopyranosyl-p-coumaric acid (CouAG) and 4-O-β-glucopyranosyl-ferulic acid (FeAG). The application of an additional acid hydrolysis results in the presence of free caffeic acid in the preparation obtained. The antiradical activity of this compound is very strong. Karamać et al. [26] determined ARP (antiradical power) defined as 1/EC50 for several phenolic acids. The ARP of caffeic acid (20.92) was much stronger than those of ferulic acid (10.79) and p-coumaric acid (0.015). Similar relationship between antioxidant activity of caffeic, ferulic, and p-coumaric acids was reported by Brand-Williams et al. [27].
Acknowledgements
We gratefully acknowledge financial support of the Polish Ministry of Science and Higher Education for 2008–2010 (N N312 237435).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- ARP
antiradical power
- CaAG
caffeic acid glucoside
- CouAG
p-coumaric acid glucoside
- CVD
cardiovascular disease
- FeAG
ferulic acid glucoside
- HDG
herbacetin diglucoside
- HMGA
3-hydroxy-3-methylglutaric acid
- LC-ESI-MS
liquid chromatography electrospray ionization mass spectrometry
- LM
lignan macromolecule
- MW
molecular weight
- m/z
mass to charge ratio
- NMR
nuclear magnetic resonanse
- RP-HPLC
reversed-phase high performance liquid chromatography
- RT
retention time
- SDG
secoisolaricerisinol diglucoside
- SE-HPLC
size exclusion high performance liquid chromatography
- TFA
trifluoroacetic acid
- UV-VIS DAD
ultraviolet-visible diode array detector
References
- 1.Thompson LU, Robb P, Serraino M, Cheung F. Mammalian lignan production from various foods. Nutr Cancer. 1991;16:43–52. doi: 10.1080/01635589109514139. [DOI] [PubMed] [Google Scholar]
- 2.Meagher LP, Beecher GR, Flanagan VP, Li W. Isolation and characterization of lignans, isolariresinol and pinoresinol, in flaxseed meal. J Agric Food Chem. 1999;47:3173–3180. doi: 10.1021/jf981359y. [DOI] [PubMed] [Google Scholar]
- 3.Liu Z, Sarrinen NM, Thompson LU. Sesamin is one of the major precursor of mammalian lignans in sesame seed (Sesamum indicum) as observed in vitro and in rats. J Nutr. 2006;136:906–912. doi: 10.1093/jn/136.4.906. [DOI] [PubMed] [Google Scholar]
- 4.Prasad K. Dietary flax in prevention of hypercholesterolemic atherosclerosis. Atherosclerosis. 1999;132:69–76. doi: 10.1016/S0021-9150(97)06110-8. [DOI] [PubMed] [Google Scholar]
- 5.Boccardo F, Lunardi G, Guglielmini P, Parodi M, Murialdo R, Schettini G, Rubagotti A. Serum enterolactone levels and the risk of breast cancer in woman with palpable cysts. Eur J Cancer. 2004;40:84–89. doi: 10.1016/S0959-8049(03)00576-8. [DOI] [PubMed] [Google Scholar]
- 6.Nesbitt P, Thompson L. Human metabolism of mammalian lignans precursors in raw and processed flaxseed. Am J Clin Nutr. 1999;69:549–555. doi: 10.1093/ajcn/69.3.549. [DOI] [PubMed] [Google Scholar]
- 7.Adlercreutz H. Lignans and human health. Crit Rev Clin Lab Sci. 2007;44:483–525. doi: 10.1080/10408360701612942. [DOI] [PubMed] [Google Scholar]
- 8.Touré A, Xueming X. Flaxseed lignans: Source, biosynthesis, metabolism, antioxidant activity, Bio-active components, and health benefits. Compr Rev Food Sci. 2010;9:261–269. doi: 10.1111/j.1541-4337.2009.00105.x. [DOI] [PubMed] [Google Scholar]
- 9.Amarowicz R, Wanasundara U, Wanasundara J, Shahid F. Antioxidant activity of ethanolic extracts of flaxseed in a β-carotene-linoleate model system. J Food Lipids. 1993;1:111–117. doi: 10.1111/j.1745-4522.1993.tb00239.x. [DOI] [Google Scholar]
- 10.Amarowicz R, Karamać M, Wanasundara JPD, Shahidi F. Antioxidant activity of hydrophobic fractions of flaxseed. Nahrung. 1997;41:178–180. doi: 10.1002/food.19970410314. [DOI] [Google Scholar]
- 11.Hosseinian FS, Muir AD, Westcott ND, Krol ES. Antioxidant capacity of flaxseed lignans in two model systems. J Am Oil Chem Soc. 2006;83:835–840. doi: 10.1007/s11746-006-5034-x. [DOI] [Google Scholar]
- 12.Hu C, Yuan YV, Kitts DD. Antioxidant activities of the flaxseed lignin secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem Toxicol. 2007;45:2219–2227. doi: 10.1016/j.fct.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Lorenc-Kukuła K, Amarowicz R, Oszmiański J, Dermann P, Starzycki M, Skała J, Żuk M, Kulma A, Szopa J. Pleiotropic effect of phenolic compounds content increases in transgenic flax plant. J Agric Food Chem. 2005;53:3685–3692. doi: 10.1021/jf047987z. [DOI] [PubMed] [Google Scholar]
- 14.Struijs K, Vincken JP, Verhoef RP, Oostveen WHM, Voragen AGJ, Gruppen H. The flavonoid herbacetin diglucoside as a constituent of the lignan macromolecule from flaxseed hulls. Phytochem. 2007;68:1227–1235. doi: 10.1016/j.phytochem.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Johnsson P, Peerlkamp N, Kamal-Eldin A, Andersson RE, Andersson R, Lundgren LN, Åman P. Polymeric fractions containing phenol glucosides in flaxseed. Food Chem. 2002;76:207–212. doi: 10.1016/S0308-8146(01)00269-2. [DOI] [Google Scholar]
- 16.Kamal-Eldin A, Peerlkamp N, Johnsson P, Andersson R, Andersson RE, Lundgren LN, Åman P. An oligomer from flaxseed composed of secoisolariciresinol diglucoside and 3-hydroxy-3-methyl glutaric acid residues. Phytochem. 2001;58:587–590. doi: 10.1016/S0031-9422(01)00279-5. [DOI] [PubMed] [Google Scholar]
- 17.Strandås C, Kamal-Eldin A, Andersson R, Åman P. Composition and properties of flaxseed phenolic oligomers. Food Chem. 2008;110:106–112. doi: 10.1016/j.foodchem.2008.01.064. [DOI] [PubMed] [Google Scholar]
- 18.Struijs K, Vincken JP, Verhoef R, Voragen AGJ, Gruppen H. Hydroxycinnamic acids are ester-linked directly to glucosyl moieties within the lignan macromolecule from flaxseed hulls. Phytochem. 2008;69:1250–1260. doi: 10.1016/j.phytochem.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Struijs K, Vincken JP, Doeswijk TG, Voragen AGJ, Gruppen H. The chain length of lignan macromolecule from flaxseed hulls is determined by the incorporation of coumaric acid and glucosides and ferulic acid glucosides. Phytochem. 2009;70:262–269. doi: 10.1016/j.phytochem.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Johnsson P, Kamal-Eldin A, Lundgren LN, Åman P. HPLC method for analysis of secoisolariciresinol diglucoside in flaxseeds. J Agric Food Chem. 2000;48:5216–5219. doi: 10.1021/jf0005871. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava A, Greenspan P, Hartle DK, James L, Hargrove JL, Amarowicz R, Pegg RB. Antioxidant and anti-inflammatory activities of polyphenolics from southeastern U.S. range blackberry cultivars. J Agric Food Chem. 2010;58:6102–6109. doi: 10.1021/jf1004836. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Yuan JP, Xu SP, Wang JH, Liu X. Separation and determination of secoisolariciresinol diglucoside oligomers and their hydrolysates in the flaxseed extract by high-performance liquid chromatography. J Chromatogr A. 2008;1185:223–232. doi: 10.1016/j.chroma.2008.01.066. [DOI] [PubMed] [Google Scholar]
- 23.Craft BD, Kosińska A, Amarowicz R, Pegg RB. Antioxidant properties of extracts obtained from raw, dry-roasted, and oil-roasted US peanuts of commercial importance. Plant Foods Hum Nutr. 2010;65:311–318. doi: 10.1007/s11130-010-0160-x. [DOI] [PubMed] [Google Scholar]
- 24.Weidner S, Amarowicz R, Karamać M, Frączek E. Changes in endogenous phenolic acids during development of Secale cereal caryopses and after dehydration treatment of unripe rye grains. Plant Physiol Biochem. 2000;38:595–602. doi: 10.1016/S0981-9428(00)00774-9. [DOI] [Google Scholar]
- 25.Kosińska A, Urbalewicz A, Penkacik K, Karamać M, Amarowicz R. SE-HPLC-DAD analysis of the flaxseed lignan macromolecule and its hydrolysates. Pol J Food Nutr Sci. 2011;61:263–271. [Google Scholar]
- 26.Karamać M, Kosińska A, Pegg R. Comparison of radical-scavenging activities for selected phenolic acids. Pol J Food Nutr Sci. 2005;55:165–170. [Google Scholar]
- 27.Brand-Williams W, Cuvelier ME, Berest C. Use of a free radical method to evaluate antioxidant activity. LWT – Food. Sci Technol. 1995;28:25–30. [Google Scholar]


