Abstract
Accumulating evidence indicates that neutralizing antibodies play an important role in protection from chronic hepatitis C virus (HCV) infection. Efforts to elicit such responses by immunization with intact heterodimeric E1E2 envelope proteins have met with limited success. To determine whether antigenic sites, which are not exposed by the combined E1E2 heterodimer structure, are capable of eliciting neutralizing antibody responses, we expressed and purified each as separate recombinant proteins E1 and E2, from which the immunodominant hypervariable region (HVR-1) was deleted. Immunization of chimpanzees with either E1 or E2 alone induced antigen-specific T-helper cytokines of similar magnitude. Unexpectedly, the capacity to neutralize HCV was observed in E1 but not in animals immunized with E2 devoid of HVR-1. Furthermore, in vivo only E1-vaccinated animals exposed to the heterologous HCV-1b inoculum cleared HCV infection.
The hepatitis C virus (HCV) envelope glycoproteins E1 and E2 are heterodimerically exposed on the surface of the virion and play a crucial role in receptor binding, viral fusion, and entry of HCV [1]. Combined, E1E2 heterodimers have long been considered and evaluated as candidate HCV vaccine antigens, primarily, but not exclusively, for the induction of neutralizing antibodies [2]. Due to the different functions of the E1E2 heterodimer, it is safe to assume that it will undergo conformational changes during the virus life cycle [3]. The E2 molecule in this complex is known to bind specifically to cell-surface molecules such as CD81, scavenger receptor class B type 1 (SR-B1), Claudin-1, and others [1, 4]. However, in both E1 and E2, structural homologies to fusion mediating proteins of related viral families have been described [5]. E2 also possesses a major determinant of isolate-specific neutralizing antibodies located near its N terminus called the hypervariable region (HVR-1). However, due to its immunodominance and the consequential selective pressure on this region, it rapidly accumulates nonsynonymous mutations making it hypervariable, which is an undesirable attribute for a candidate vaccine antigen. In contrast, the role of E1 in HCV infection and immunity is still unclear, yet several antibodies directed against E1 were found to prevent cell entry [6, 7].
We rationalized that by removing the HVR-1 from E2, and separating the 2 components of the heterodimer, that novel structural features might be revealed, creating new targets for the induction of broad neutralizing antibodies. To test this hypothesis, chimpanzees were immunized with either recombinant E2 protein with the HVR-1 deleted or the intact recombinant E1 protein alone. To determine whether the vaccine-induced antibody responses were sufficient to protect from persistent HCV infection, all animals were exposed to a 1b inoculum, which has the propensity to cause chronic infection. By 18 weeks, the 2 E1-immunized animals had cleared HCV infection, whereas RNA viremia persisted in the 2 E2-immunized animals and the control animal. Vaccine-induced protection from persistent HCV infection correlated with E1-induced neutralizing antibodies, demonstrating a previously unrecognized role for E1 subunit in immunization.
MATERIALS AND METHODS
Animals
This study was critically reviewed and approved and undertaken by the institute’s animal ethical committee and performed in accordance with Dutch law and international guidelines for the use of animals in research (BPRC IACUC ID 253) in consultation and prior to amended Dutch legislation. Five mature, captive bred chimpanzees (Pan troglodytes verus), naive for retroviruses and hepatitis viruses, were socially housed and monitored multiple times per day by experienced veterinary staff (BPRC, Rijswijk, The Netherlands).
Throughout the study, animals were checked twice daily for general behavior, appetite, and stool consistency. Body weight, rectal temperature, hematology, and clinical chemistry were assessed during each sedation for immunization, blood collection, or liver biopsy. Serum and peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood samples collected using aseptic techniques as described elsewhere [8].
HCV Immunogens and Assay Antigens
Recombinant HCV envelope proteins E1s (aa192–aa326) and E2ΔHVR-1 (E2 aa412–aa715 with deletion of HVR-1 aa384–aa411) were used for immunizations and in the cellular assays. Both proteins were derived from the European HCV 1b BE11 strain and were expressed by recombinant vaccinia virus transfected Vero cells; purity of all proteins was estimated to be >95% by SDS-PAGE [9]. For immunization, E1 and E2 proteins were formulated with Alum (Alhydrogel, Superfos Biosector), 1.3% Al(OH)3 in phosphate-buffered solution, and diluted with physiological saline to a final protein concentration of 50 μg/mL and 0.13% Al(OH)3. Although both the vaccine proteins as well as the challenge virus were derived from genotype HCV 1b, the challenge was considered heterologous because in E1 7% and in E2 ∼11% of the amino acid residues were different between the vaccine and challenge sequence.
To analyze virus-induced immune responses after challenge, HCV NS3 (NS3 1071–1084 and 1181–1465), an Eschericia coli fusion protein based on a HCV 1b isolate (BE8309), was used.
Experimental Design, Immunizations, and HCV Exposure
Chimpanzee E1-Ma and E1-Yo were immunized with 50 μg of E1, whereas E2-Jo and E2-Ka received 50 μg of E2 recombinant protein. One animal, Ctrl-Hu, served as a challenge control and did not receive any HCV immunogen or adjuvant before challenge.
Animals were given intramuscular immunizations in the biceps at weeks 0, 3, 6, 9, 12, and 15 at a dose of 50 μg of protein per mL diluted Alum (Figure 1). At week 18, the animals were intravenously exposed to 100 CID of an in vitro-titrated HCV 1b inoculum J4.91’01, diluted in saline.
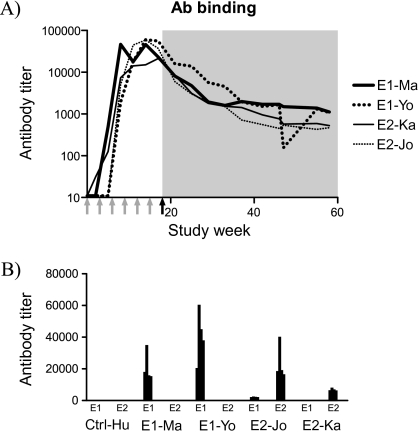
Figure 1.
Humoral responses. A, Vaccine-specific antibody titers over time, ie, E1-specific antibodies were measured in E1-Ma and E1-Yo, and E2-specific antibodies in serum from E2-Ka and E2-Jo. Time points of immunizations are indicated by gray arrows under the X-axis, and the time point of challenge is indicated by a black arrow. The shaded area represents the post-challenge follow-up period. B, Evaluation of cross-reactivity. Serum samples collected at weeks 0, 13, 14, 15, and 16 (separate bars) were tested for binding to E1 and E2 with standard ELISA.
Humoral Responses
Vaccine-specific antibody titers were determined by limiting dilution ELISA [8] or INNO-LIA HCV (Innogenetics).
HCV-neutralizing capacity of longitudinal serum samples was determined before and during the immunization period at weeks 7 and 17 and at week 107, which is 89 weeks after infection. The assay was performed as described elsewhere [10–12] with HCVpp-expressing E1 and E2 envelope glycoproteins of the isolates CG (J) (AF333324) and UKN1B 12.6 (AY734975)
Virus Quantification
Quantification of HCV RNA in serum was monitored using Amplicor (Roche Diagnostics) [13].
Cellular Immune Responses
The number of HCV-specific PBMCs was determined by lymphoproliferation and ELISPOT assay at regular time points during the immunization period and after experimental HCV exposure as described elsewhere [8].
Analysis of HCV E1 and E2 Sequence Diversity
Viral RNA was isolated at weeks 1, 3, 6, 12, 31, 36, and 41 weeks after challenge for as long as the virus was detectable in serum, making use of the QIAamp Viral RNA Mini Spin protocol (QIAGEN). DNA synthesis and outer polymerase chain reaction (PCR) was performed in one step using the QIAGEN OneStep RT-PCR kit, followed by a conventional nested PCR. The following primer sets were used to sequence E1; outer primer set HCPr638 (5′CTG TCY TGY TTG ACC RTC CCA GC3′ nt 544–566 when aligned–H77) and HCPr639 (5′CCC GTC AAC GCC GGC RAA GAG TAR C3′ nt 1149–1125) inner primer set HCPr640 (5′GTY TGA CCA YCC CAG CTT CCG CT3′ nt 551–573) and HCPr641 (5′TCA ACG CCG GCR AAR AGT ARC RYC AC3′ nt 1145–1120). To completely cover the E2 region, the following 3 primer sets were used. Fragment I: outer primer HCPr642 (5′TGG CGC TAC TCT TTG CCG GCG TTG A3′ nt 1121–1145) and HCPr643 (5′AGG CAC CTA GGT GTC AAC CA3′ nt 1844–1825); inner PCR HCPr644 (5′CGG CGT TGA CGG GGC GAC CTA3′ nt 1137–1157) and HCPr645 (5′CCC GAG CCA CAT TTT GTG TA3′ nt 1820–1801). Fragment II: outer HCPr691 (5′GTG TGY GGY CCA GTG TAY TG3′ nt 1510–1529) and HCPr692 (5′CCA CTC YGT YGT RGA CAG YA3′ nt 2037–2018); inner HCPr693 (5′GAY GTG RTG CTY CTY AAC AA3′ nt 1609–1628) and HCPr694 (5′TCC TCC AAG TYR CAR CGC TC3′ nt 1988–1969). Fragment III: outer HCPr646 (5′TGA TCT GCC CCA CGG ACT GC3′ nt 1757–1776) and HCPr647 (5′TCC CGG TCC AAG GCG TAA GC3′ nt 2459–2440); inner primers HCPr648 (5′ACG GAC TGC TTC CGG AAG CA3′ nt 1768–1787) and HCPr649 (5′GGC GTA AGC TCG TGG TGG TA3′ nt 2448–2429).
RESULTS
Vaccine-Specific Antibody Responses
The induction of vaccine-specific antibodies in serum was determined by ELISA. As shown in Figure 1, all 4 vaccinees developed envelope-specific antibodies with similar magnitude and kinetics, increasing following each immunization and peaking 14–17 weeks following the first immunization. After experimental HCV exposure at week 18, HCV-specific antibody responses gradually declined, yet persisted at low levels until the end of the study. Similar declines have been noted previously by us and others as a frequent sequelae of HCV infection in chimpanzees. Antibody formation directly after experimental infection is low and often falling below the detection limits of standard ELISA assays [14, 15]. In the control animal HCV-specific antibodies were only observed after challenge (data not shown).
Induction of HCV-Specific T-Cell Responses by Immunization
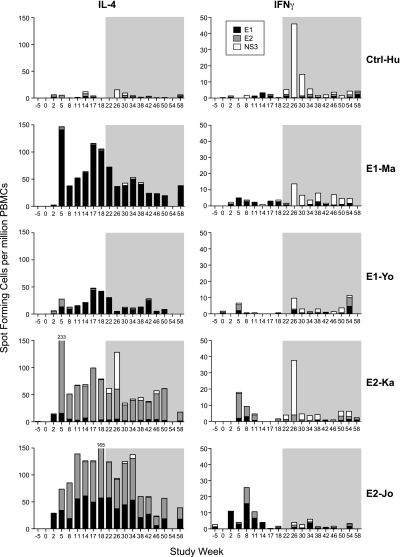
Induction of cellular immune responses against the vaccine proteins (E1 or E2), as well as an HCV protein not included in the vaccine (NS3), were assessed by lymphocyte proliferation and ELISPOT analysis. As shown in Figure 2, both E1-immunized animals and the 2 E2-immunized animals developed vaccine-induced interleukin 4 (IL-4) secreting cells (140 IL-4 sfu/106 PBMCs in E1-Ma, 44 IL-4 sfu/106 in E1-Yo, 217 IL-4 sfu/106 in E2-Ka, and 106 IL-4 sfu/106 in E2-Jo). Instead, the number of vaccine-induced interferon γ (IFN-γ) secreting cells was much lower in all 4 animals (<10 sfu/106 PBMCs in each animal). These data implied a Th2-dominant immune response, which correlated with high vaccine-induced antibody levels in serum at the time point of challenge. As expected, only weak cellular immune responses were observed against the nonvaccine viral protein NS3 following HCV exposure in all 5 animals. Expectedly, NS3-specific responses were IFN-γ biased, whereas the envelope-specific responses preserved their Th2-like characteristics. T-cell responses were further substantiated by lymphoproliferation and IL-2 ELISPOT (Supplementary Figure 1). Although E2-Jo was only immunized with recombinant E2 protein and not with E1, in vitro stimulation of PBMCs with E1 also induced cytokine production. This cross-reaction between the 2 envelope proteins was also observed in the humoral response (Figure 1B). Serum samples collected at weeks 0, 13, 14, 15, and 16 from E2-Jo were found to have some reactivity against E1, which was low compared with the E2 binding. Neither E1- nor E2-specific T-cell responses were observed prior to challenge in Ctrl-Hu.
Figure 2.
HCV-specific IL-4 and IFN-γ responses. Magnitude over time of IL-4 and IFN-γ cytokine responses of individual animals were measured against recombinant proteins HCV E1 (black bars), E2 (gray bars), and NS3 (white bars). Responses are shown for the 2 E1 protein-immunized animals E1-Ma and E1-Yo, the 2 E2 protein-immunized animals E2-Ka and E2-Jo, and the naive control animal Ctrl-Hu. Immunizations were given at weeks 0, 3, 6, 9, 12, and 15, followed by HCV exposure at week 18. The shaded area represents the postchallenge follow-up period. Shown are the numbers of antigen-specific spot-forming cells per million PBMCs minus the background (mean of triplicate medium controls).
Neutralizing Capacity of Vaccine-Induced Antibodies
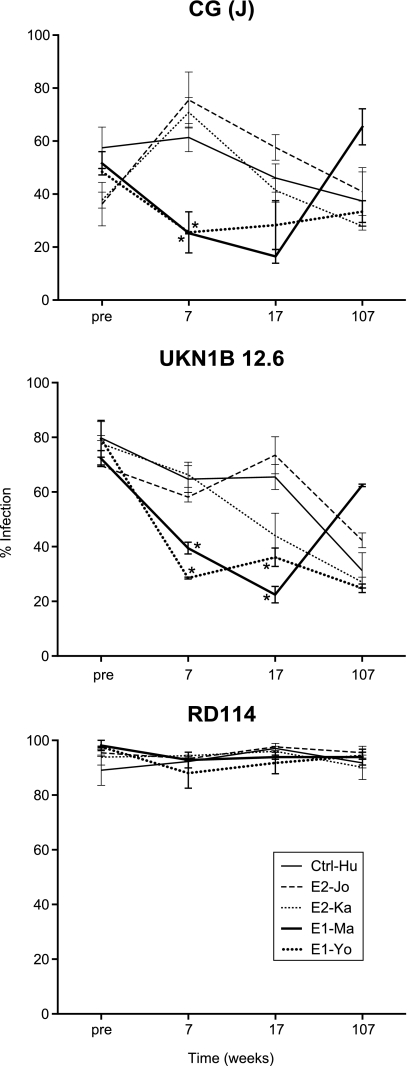
To determine whether the antibodies induced by the vaccine proteins E1 or E2 were functional in neutralizing HCV, pseudoparticle assays (HCVpp) were performed on serum obtained before and 7, 17, and 107 weeks after initial immunization. The HCVpp were pseudotyped with E1E2 glycoproteins derived from strain UKN1B 12.6, which has a closely related amino acid sequence to the vaccine strain, or CG (J), an isolate most similar to the challenge inoculum [16]. As a control, the glycoprotein from RD114, the cat endogenous virus, was used. Figure 3 displays the percentage of target cells infected by HCVpp, and the inhibition of infection by serum from vaccinees obtained at different time points. Comparison of the percentage of infection between the immunized animals and the control animal Ctrl-Hu showed more efficient inhibition by sera obtained from the E1-immunized animals than by sera from the E2-immunized animals. At week 7, both of the E1-immunized animals developed antibodies that strongly inhibited CG (J) HCVpp infection (P < .01; 2-way ANOVA), whereas no inhibition was observed by sera from the 2 E2-immunized animals. This CG (J) neutralization was also observed, but at a somewhat reduced level, at week 17 during the pause between immunization and viral exposure. In both E1-immunized animals, significant neutralization (P < .01) was also observed against HCVpp exposing the UKN1B 12.6 envelope both at weeks 7 and 17. As expected, persistent HCV infection induced neutralizing antibodies inhibiting HCVpp infection with both CG (J), as well as UKN1B 12.6 isolates (week 107; Figure 3). Vaccine-induced levels of neutralization declined in the absence of HCV in E1-Ma and E1-Yo.
Figure 3.
Neutralizing antibodies. Serum samples collected before immunization and at weeks 7, 17, and 107 were tested for the inhibition of infection of Huh-7 target cells by HCVpp. Serum was tested against CG (J) and UKN1B 12.6 HCV isolate envelopes, whereas RD114, a cat endogenous virus-derived envelope, served as a control. Results are expressed as percentage of infected cells. Plotted in the graphs are the results of 2 independent assays, both performed in duplicate. *Reduction of percentage of infected cells compared with the control animal (P < .01).
Neutralizing Antibody Titers Correlate With Clearance of Infection
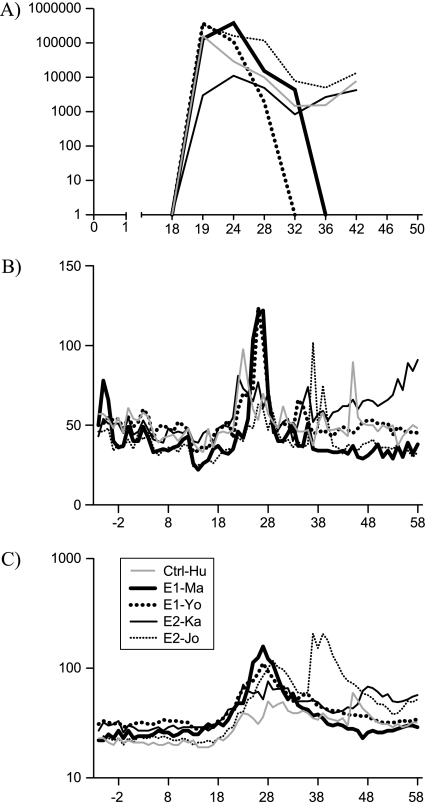
Three weeks after the final protein immunization, efficacy against viral challenge was evaluated by intravenous exposure to 100 CID HCV 1b J4.91’01. All 4 vaccinees and the naive control animal became acutely infected. A peak virus load between 1 and 5 × 105 IU mL−1 was reached 1–6 weeks after challenge in all animals except E2-Ka, where the peak viremia was 1 log lower (Figure 4A). Subsequently, the virus load decreased to levels between 103 and 104 IU mL−1 in the control and the 2 E2-immunized animals. Instead, in the 2 E1-immunized animals virus became undetectable in serum and liver biopsies by quantitative PCR 18 weeks after challenge. Furthermore, liver enzyme levels ALT and γGT returned to prechallenge values in these 2 animals (Figures 4B and 4C). In contrast to the E1-immunized animals, all others remained persistently infected and retained slightly elevated liver γGT (Figure 4C) (P = .039, 2-tailed Student t test).
Figure 4.
Viral load and liver enzymes. Shown are (A) the plasma viral load (copies mL−1), (B) ALT levels (IU mL−1), and (C) γGT (U mL−1) in individual animals observed over time.
No Evidence for Selective Immune Pressure Directed Against Envelope Regions
To rule out that development of chronic infection in the E2-vaccinated animals was either due to an ineffective E2-induced vaccine response, or due to viral evolution and vaccine escape, we performed longitudinal sequence analysis of the E1 and E2 regions of the predominant viral variants in plasma. Furthermore, we compared these sequences to the challenge inoculum J4.91’01, as well as a parental virus isolate from which it was derived HC-J4/91 (D10750) [17]. As shown in Figure 5, all the nonsynonymous mutations relative to the inoculum aligned. In the acute phase, only 1 amino acid residue change was observed in the E1 region (G232A or G232D) and 3 in the E2 region (F438L, A466V, and V496I). These changes seem to be unrelated to the vaccination strategy, because they are observed in all animals irrespective of the antigen with which they were immunized.
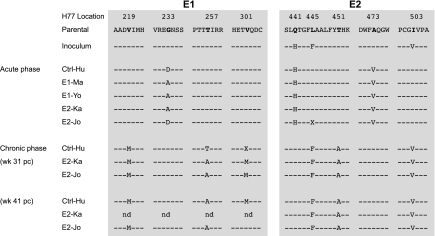
Figure 5.
Sequence alignments from the peripheral blood of the envelope regions E1 and E2 from the indicated animals following persistent viremia (viral escape or evasion). Nucleotides are represented by standard single letter codes, dashes indicate identity with the reference sequence HC-J4/91, and substitutions or mutations are represented by single letter codes. Sequences were derived from time points after virus escaped the immune responses. Samples from the following time points were used to isolate viral sequences: Ctrl-Hu, 41 weeks; E1-Ma, 6 weeks; E1-Yo, 12 weeks; E2-Ka, 31 weeks; and E2-Jo, 41 weeks (last time points from which amplification of sequences was routine) after HCV exposure.
In the chronic phase of infection in the animals not immunized with E1, 3 de novo mutations occurred in putative E1 epitopes (V219M, T256A, and V301M) and 2 in E2 (H434Q and T444A). Surprisingly, all residues that became established during the acute phase reverted to the residue found in the original inoculum. Interestingly, with the exception of position 301 in the E1 region (valine in E2-Jo and methionine in the other animals), the dominant viral variants (in both E1 an E2 regions) that emerged at the end of the study were found to be identical in the 3 animals that became persistently infected.
DISCUSSION
The partial characterization of the immune correlates of clearance of HCV infection and the identification of the targeted viral antigens facilitated the rational development of a vaccine for the prevention of chronic HCV infection [8, 18, 19]. HCV vaccine studies directed at the induction of virus neutralizing antibodies have focused on the use of either the E1E2 heterodimer or the E2 protein alone [2, 15, 20, 21]. However, 2 lines of evidence have suggested a role for E1. First, broadly neutralizing human monoclonal antibodies to the E1 glycoprotein have been identified [6, 7]. Second, functional analysis has identified several membrane active regions in E1 (as well as E2) involved in virus-cell membrane fusion [5].
In this study, we tested the hypothesis that the independent use of HCV E1 subunit as a vaccine immunogen would be capable of inducing neutralizing antibodies and facilitating protection from HCV infection. Interestingly, although immunization with E1 was successful in inducing neutralizing antibodies, these did not have a direct blunting of the acute viral peak after a relatively high dose, intravenous HCV exposure. Ultimately, E1-neutralizing antibodies, together with recruited Th responses, appear to have facilitated HCV 1b clearance. However, additional mechanisms such as antibody-dependent cellular cytotoxicity, recruited innate responses in the liver, or late induction of cytotoxic T lymphocyte activity may have contributed to clearance.
Although antibodies were also present in the serum of the E2-immunized animals, these antibodies lacked the ability to neutralize HCV, and the virus persisted in these animals. It could be speculated that the E2 protein devoid of its HVR-1 was misfolded and incapable of inducing NAbs. However, comparison of E2 proteins with and without HVR-1 for reactivity with a range of patient sera showed no clear differences, suggesting that the conformational structure of the E2 protein lacking the HVR-1 as used in this study was antigenically intact (data not shown).
Although the immune responses observed in the E1-immunized animals were restricted to the vaccine component, chimpanzee E2-Jo not only responded to E2 but also to E1 in both cellular as well as humoral immune assays. Although this observation is somewhat unexpected, it must be noted that shared immunoreactivity between the 2 envelope glycoproteins was reported elsewhere [22] and may be elicited in animals that carry the appropriate major histocompatibility complex makeup.
The majority of the HCV vaccine efficacy studies in chimpanzees were done before the development of suitable HCV neutralization assays; therefore, only limited data have been available on the correlation of HCV neutralization and vaccine efficacy. Furthermore, in the majority of HCV protein vaccine efficacy studies to date, E1 and E2 were administered as heterodimer [21] and as the HCVpp also express E1 and E2, it is impossible to determine the role of the separate envelope components. To or knowledge, this is the first study to demonstrate a direct correlation with vaccine-induced E1-neutralizing antibodies and early clearance of HCV infection. Earlier studies have focused on E2 as the lead vaccine target given the observations of E2-binding inhibition assays and the characterization of several E2-specific virus-neutralizing mAb [23, 24]. The E2 antigen used in this study was devoid of the HVR-1, and therefore the outcome does not allow direct comparison with earlier protein vaccine efficacy studies. Our efforts to focus the immune response to the more conserved regions of E2 failed because the antibodies generated with this strategy did not inhibit infection with HCVpp. The lack of the HVR-1 may possibly have reduced binding to one of the SR-B1 regions [11, 25]. Because SR-B1 also mediates HCV uptake and cross-presentation by dendritic cells in humans [26], this may have resulted in suboptimal antigen presentation and the formation of lower affinity antibodies with reduced neutralizing capacity, despite induction of high T-cell and binding antibody responses.
After immunization, all vaccinees developed an antigen-specific Th-2--dominant immune response directed toward the vaccine components as shown by increased lymphoproliferation, the high number of HCV-specific IL-4--secreting cells, and by the formation of HCV-specific antibodies before challenge. The degree of protection observed against this heterologous 1b variant is different from the complete protection obtained with E1E2 heterodimers against HCV-1a challenge of chimpanzees [15]. However, our data concur with the outcome of reexposure to HCV in humans and chimpanzees, where viral clearance is observed much later, after the acute phase [2].
Although vaccine-mediated formation of viral escape variants is of great concern, this activity was not observed in this study.
This proof of principle study reveals the previously unrecognized importance of E1 alone as a candidate HCV vaccine antigen. Moreover, these findings suggest that further insights into the structure-function relationship of the E1E2 heterodimer are required. Improved understanding of the dynamics and structural changes on receptor binding and membrane fusion may reveal conserved domains that could be exploited as neutralizing antibody targets for immunization.
Supplementary Data
Supplementary Data are available at The Journal of Infectious Diseases online.
Funding
The part of the work in the laboratory of FL Cosset was supported by Agence Nationale pour la Recherche contre le SIDA et les Hépatites Virales (ANRS) and by the European Research Council (ERC-2008-AdG-233130-HEPCENT).
Supplementary Material
Acknowledgments
We are indebted to Bob Purcell, NIAID, NIH, for advice and comments as well as for the titrated HCV 1b challenge stock. We thank the veterinary care staff of the BPRC for their kind and professional care of the animals in this study and David Davis for help with statistics.
References
- 1.Sabahi A. Hepatitis C Virus entry: the early steps in the viral replication cycle. Virol J. 2009;6:117. doi: 10.1186/1743-422X-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houghton M, Abrignani S. Prospects for a vaccine against the hepatitis C virus. Nature. 2005;436:961–6. doi: 10.1038/nature04081. [DOI] [PubMed] [Google Scholar]
- 3.Op De Beeck A, Cocquerel L, Dubuisson J. Biogenesis of hepatitis C virus envelope glycoproteins. J Gen Virol. 2001;82:2589–95. doi: 10.1099/0022-1317-82-11-2589. [DOI] [PubMed] [Google Scholar]
- 4.Cocquerel L, Voisset C, Dubuisson J. Hepatitis C virus entry: potential receptors and their biological functions. J Gen Virol. 2006;87:1075–84. doi: 10.1099/vir.0.81646-0. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Berna AJ, Moreno MR, Guillen J, Bernabeu A, Villalain J. The membrane-active regions of the hepatitis C virus E1 and E2 envelope glycoproteins. Biochemistry. 2006;45:3755–68. doi: 10.1021/bi0523963. [DOI] [PubMed] [Google Scholar]
- 6.Keck ZY, Sung VM, Perkins S, et al. Human monoclonal antibody to hepatitis C virus E1 glycoprotein that blocks virus attachment and viral infectivity. J Virol. 2004;78:7257–63. doi: 10.1128/JVI.78.13.7257-7263.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meunier JC, Russell RS, Goossens V, et al. Isolation and characterization of broadly neutralizing human monoclonal antibodies to the E1 glycoprotein of hepatitis C virus. J Virol. 2008;82:966–73. doi: 10.1128/JVI.01872-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rollier C, Depla E, Drexhage JA, et al. Control of heterologous hepatitis C virus infection in chimpanzees is associated with the quality of vaccine-induced peripheral T-helper immune response. J Virol. 2004;78:187–96. doi: 10.1128/JVI.78.1.187-196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leroux-Roels G, Depla E, Hulstaert F, et al. A candidate vaccine based on the hepatitis C E1 protein: tolerability and immunogenicity in healthy volunteers. Vaccine. 2004;22:3080–6. doi: 10.1016/j.vaccine.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Sandrin V, Boson B, Salmon P, et al. Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and nonhuman primates. Blood. 2002;100:823–32. doi: 10.1182/blood-2001-11-0042. [DOI] [PubMed] [Google Scholar]
- 11.Bartosch B, Dubuisson J, Cosset FL. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J Exp Med. 2003;197:633–42. doi: 10.1084/jem.20021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartosch B, Verney G, Dreux M, et al. An interplay between hypervariable region 1 of the hepatitis C virus E2 glycoprotein, the scavenger receptor BI, and high-density lipoprotein promotes both enhancement of infection and protection against neutralizing antibodies. J Virol. 2005;79:8217–29. doi: 10.1128/JVI.79.13.8217-8229.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerken G, Pontisso P, Roggendorf M, et al. Clinical evaluation of a single reaction, diagnostic polymerase chain reaction assay for the detection of hepatitis C virus RNA. J Hepatol. 1996;24:33–7. doi: 10.1016/s0168-8278(96)80183-8. [DOI] [PubMed] [Google Scholar]
- 14.Rollier CS, Paranhos-Baccala G, Verschoor EJ, et al. Vaccine-induced early control of hepatitis C virus infection in chimpanzees fails to impact on hepatic PD-1 and chronicity. Hepatology. 2007;45:602–13. doi: 10.1002/hep.21573. [DOI] [PubMed] [Google Scholar]
- 15.Choo QL, Kuo G, Ralston R, et al. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci U S A. 1994;91:1294–8. doi: 10.1073/pnas.91.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavillette D, Tarr AW, Voisset C, et al. Characterization of host-range and cell entry properties of the major genotypes and subtypes of hepatitis C virus. Hepatology. 2005;41:265–74. doi: 10.1002/hep.20542. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto H, Kojima M, Okada S, et al. Genetic drift of hepatitis C virus during an 8.2-year infection in a chimpanzee: variability and stability. Virology. 1992;190:894–9. doi: 10.1016/0042-6822(92)90933-g. [DOI] [PubMed] [Google Scholar]
- 18.Rehermann B. Cellular immune response to the hepatitis C virus. J Viral Hepat. 1999;6(Suppl 1):31–5. doi: 10.1046/j.1365-2893.1999.00008.x. [DOI] [PubMed] [Google Scholar]
- 19.Diepolder HM, Gruener NH, Heeney J. Of chimps and men–finding the path to an HCV vaccine. Hepatology. 2003;37:1472–4. doi: 10.1002/hep.510370634. [DOI] [PubMed] [Google Scholar]
- 20.Youn JW, Park SH, Lavillette D, et al. Sustained E2 antibody response correlates with reduced peak viremia after hepatitis C virus infection in the chimpanzee. Hepatology. 2005;42:1429–36. doi: 10.1002/hep.20934. [DOI] [PubMed] [Google Scholar]
- 21.Dahari H, Feinstone SM, Major ME. Meta-analysis of hepatitis C virus vaccine efficacy in chimpanzees indicates an importance for structural proteins. Gastroenterology. 2010;139:965–74. doi: 10.1053/j.gastro.2010.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petit MA, Jolivet-Reynaud C, Peronnet E, Michal Y, Trepo C. Mapping of a conformational epitope shared between E1 and E2 on the serum-derived human hepatitis C virus envelope. J Biol Chem. 2003;278:44385–92. doi: 10.1074/jbc.M304047200. [DOI] [PubMed] [Google Scholar]
- 23.Owsianka AM, Tarr AW, Keck ZY, et al. Broadly neutralizing human monoclonal antibodies to the hepatitis C virus E2 glycoprotein. J Gen Virol. 2008;89:653–9. doi: 10.1099/vir.0.83386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosa D, Campagnoli S, Moretto C, et al. A quantitative test to estimate neutralizing antibodies to the hepatitis C virus: cytofluorimetric assessment of envelope glycoprotein 2 binding to target cells. Proc Natl Acad Sci U S A. 1996;93:1759–63. doi: 10.1073/pnas.93.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voisset C, Callens N, Blanchard E, Op De Beeck A, Dubuisson J, Vu-Dac N. High density lipoproteins facilitate hepatitis C virus entry through the scavenger receptor class B type I. J Biol Chem. 2005;280:7793–9. doi: 10.1074/jbc.M411600200. [DOI] [PubMed] [Google Scholar]
- 26.Barth H, Schnober EK, Neumann-Haefelin C, et al. Scavenger receptor class B is required for hepatitis C virus uptake and cross-presentation by human dendritic cells. J Virol. 2008;82:3466–79. doi: 10.1128/JVI.02478-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.